Abstract
Introduction:
This study aims at probing into the expression and biological function of long non-coding RNA (lncRNA) TMPO-AS1 in non-small cell lung cancer (NSCLC), and exploring its regulatory role for miR-204-3p and erb-b2 receptor tyrosine kinase 2 (ERBB2).
Methods:
In this study, paired NSCLC samples were collected, and the expression levels of TMPO-AS1, miR-204-3p and ERBB2 were examined by quantitative real-time polymerase chain reaction (qRT-PCR); proliferative ability and colony formation ability were detected by CCK-8 assay and plate colony formation assay, respectively; flow cytometry was performed to detect the effect of TMPO-AS1 on apoptosis; Transwell assay was used to detect the changes of migration and invasion; qRT-PCR and Western blot were utilised to analyse the changes of miR-204-3p and ERBB2 regulated by TMPO-AS1; luciferase reporter gene assay and RNA immunoprecipitation assay were employed to determine the regulatory relationship between TMPO-AS1 and miR-204-3p.
Results:
We demonstrated that TMPO-AS1 was significantly up-regulated in cancerous tissues of NSCLC samples, and positively correlated with the expression of ERBB2, while negatively correlated with miR-204-3p. After transfection of TMPO-AS1 shRNAs into NSCLC cells, the malignant phenotypes of NSCLC cells were significantly inhibited, while overexpression of TMPO-AS1 had opposite effects; TMPO-AS1 was also demonstrated to regulate the expression of miR-204-3p by sponging it, and indirectly modulate the expression of ERBB2.
Conclusion:
Collectively, we conclude that TMPO-AS1 has the potential to be the ‘ceRNA’ to regulate the expression of ERBB2 by sponging miR-204-3p in NSCLC.
Keywords: ERBB2, miR-204-3p, NSCLC, TMPO-AS1
Introduction
The incidence of lung cancer ranks the first in the world among all of the cancers. As the main type of lung cancer, non-small cell lung cancer (NSCLC) accounts for 80%–85% of the total cases of lung cancer.1,2 Despite the continuous development of therapeutic method such as surgical techniques, new chemotherapeutics, radiotherapy and targeted drugs, the treatment effect for NSCLC is still limited, and the 5-year survival rate of NSCLC is still only 15%–30%.3,4 Exploring detailed molecular mechanisms of NSCLC is essential for the development of new anti-cancer therapies to improve the survival of NSCLC patients in the future.
Long non-coding RNA (lncRNA) refers to a class of RNA molecules with more than 200 nucleotides in length and no protein coding ability. Although lncRNAs cannot be translated into proteins, they play an important role in various biological processes, such as cell growth and death, cell differentiation, inflammatory response, migration and so on.5,6 Numerous studies show that lncRNAs can affect the progression of many types of tumours.7 TMPO antisense RNA 1 (TMPO-AS1), a lncRNA, is reported to be associated with the cancer progression and adverse prognosis of prostate cancer.8 Existing studies authenticate that TMPO-AS1 promotes the progression of NSCLC through regulating its natural antisense transcript TMPO, and is associated with poor prognosis of lung adenocarcinoma.9,10 However, the functional role and underlying mechanism of TMPO-AS1 in NSCLC have not been clarified clearly.
MicroRNAs (miRNAs), which also belong to non-coding RNAs, are a class of non-coding single-stranded small RNA with length of 21–25 nt. Similar with lncRNA, miRNAs participate in a series of important processes in cell biology.11 Besides, they are also involved in the pathological process of many malignancies.12 MiR-204 is found to be a tumour suppressor in glioma, renal cell carcinoma and hepatocellular carcinoma, and it inhibits the proliferation and metastasis of cancer cells.13–15 However, the role of miR-204-3p in the progression of NSCLC needs further study.
Erb-b2 receptor tyrosine kinase 2 (ERBB2, also known as Her-2) is a member of the human epidermal growth factor receptor family, whose gene is located on chromosome 17q12, and it belongs to transmembrane tyrosine kinase receptor protein and has tyrosine kinase activity. Previous studies find that the overexpression of ERBB2 is related to the unfavourable prognosis of multiple cancers including NSCLC.16–18
In this study, we tried to explore the expression, clinical implication, function and underlying mechanism of TMPO-AS1 in NSCLC. Gain-of-function and loss-of-function experiments confirmed the biological effects of TMPO-AS1 on NSCLC progression. We also proved that TMPO-AS1 regulated the phenotypes of NSCLC cells via miR-204-3p/ERBB2 axis, providing a new theoretical basis for the tumorigenesis and progression of this deadly disease.
Method and materials
Tissue samples
Paired cancerous tissues/adjacent normal tissues (at least 3 cm away from the surgical margin) of 30 patients with NSCLC (18 males, 12 females; 14 cases of squamous carcinoma, 16 cases of adenocarcinoma; 36–72 years old) who had underwent surgery were randomly selected in Shanghai General Hospital from January 2017 to February 2018. Considering a confidence level of 95%, a significance level of 5%, and a minimum power of 80%, a sample size of 30 would suffice. No patients received neoadjuvant therapy such as chemotherapy or radiotherapy prior to surgery. All specimens were immediately stored in –196°C liquid nitrogen until they were used for RNA extraction. All biological samples were obtained with patients written informed consent. Our study was endorsed by the Research Ethics Committee of the Shanghai General Hospital (Ethical No: SHGH-20160411).
Cell culture and transfection
Human bronchial epithelial cell line BEAS-2B and human NSCLC cell lines A549, H226, H522 and H1299 were purchased from American type culture collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS, Gibco, Life Technologies, Carlsbad, CA), 100 U/ml penicillin G and 100 μg/ml streptomycin (Gibco, Life Technologies, Carlsbad, CA) in a 5% CO2 incubator at 37°C. The medium was replaced at the intervals of every 3 to 4 d.
For cell transfection, A549 and H226 cells were seeded on culture plate at a density of 1×106/ml and incubated at 37°C in 5% CO2 for 24 h before transfection.
MiR-204-3p mimics (5’-GCUGGGAAGGCAAAG”GGACGU-3’),
miR-204-3p inhibitors (5’-ACGUCCCUUUGCC”UUCCCAGC-3’),
control miRNA (5’-CGAGGGUAUAG”UGCUGUAUGU-3’),
TMPO-AS1 shRNA (sh-TMPO-AS1#1: 5’-CCGCCAAACGCCCGCCTTT-3’; sh-TMPO-AS1#2: 5’-GCAGGCACTCATATTCCTT-3’);
sh-control: 5’-CCGCAAACCCGCCGCCTTT-3’ and TMPO-AS1 overexpression plasmids were purchased from RiboBio Co., Ltd. (Guangzhou, China). LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA) was used to transiently transfect the miRNA mimics, shRNAs or plasmids into A549 and H226 cells according to the supplier’s instructions.
RNA extraction and quantitative Real-Time PCR Analysis (qRT-PCR)
Primer 6.0 software was used to design primers according to the sequence of each gene in GenBank. Total RNA was extracted from frozen tissue and cultured cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). MMLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) was used for reversing transcription reaction to produce cDNA. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on ABI 7500 real-time PCR system (Applied Biosystems, San Francisco, CA, USA) using SYBR premix EX TAQ II (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The conditions of qRT-PCR were as follows: pre-denaturation at 95°C for 10 min, denaturation at 95°C for 15 s, denaturation at 60°C for 15 s, 45 cycles, and acquisition of fluorescence signal at 60°C. GAPDH and U6 were used as the standard internal references of TMPO-AS1 and ERBB 2, and miR-204-3p, respectively. The results of gene expression were analysed by 2(-ΔΔCt) method. We used NormFinder to evaluate gene expression stability via grouped analysis. The specific primer sequence information was shown in the Table 1.
Table 1.
qRT-PCR primer sequences.
| Name | Primer sequences |
|---|---|
| TMPO-AS1 | Forward:5’-AGCCCACACACTACAGGCA-3’ |
| Reverse:5’-GCACAAAAGCAGTACGACCT-3’ | |
| ERBB2 | Forward:5’-TGACACCTAGCGGAGCGAT-3’ |
| Reverse:5’-GGGGGATGTGTTTTCCCTCAA-3’ | |
| GAPDH | Forward:5’-TATGATGATATCAAGAGGGTAGT-3’ |
| Reverse:5’-TGTATCCAAACTCATTGTCATAC-3’ | |
| miR-204-3p | Forward:5’-AGCTGTACAAGTAAGCCTGATCATGTACCCATAGG-3’ |
| Reverse:5’-GGGAGAGGGGCTTAGCTTATGGGACAGTTATGGGC-3’ | |
| U6 | Forward:5’-GCTTCGGCAGCACATATACTAAAAT-3’ |
| Reverse:5’-CGCTTCACGAATTTGCGTGTCAT-3’ |
Cell counting kit-8 (CCK-8) assay
Cell growth was measured by CCK-8 method. A549 and H226 cells transfected for 24 h (3000 cells per well) were placed in 96-well plates and incubated for 1, 2, 3 and 4 d respectively. On each day, 10 ml CCK-8 solution (Dojindo, Kumamoto, Japan) was added and the cells were incubated at 37°C for 1 h. Absorption was measured at 450 nm with a microplate reader.
Plate colony formation
Each group of cells in logarithmic growth phase was trypsinised with 0.25% trypsin and resuspended in DMEM containing 10% FBS. 1000 cells in each group were inoculated into the wells of a 6-well palte and cultured in 37°C at 5% CO2 incubators for 2 weeks. The supernatant was discarded, and the wells were washed twice with PBS and fixed with 4% paraformaldehyde. Then GIMSA solution was added to stain the colonies for 10 min. After washing the wells again, the number of colonies was counted under microscope.
Transwell assay
For migration and invasion test, serum-free DMEM was used to prepare cell suspension and the cell density was modulated to 2×105 cells/ml. 200 μL cell suspension was inoculated into Transwell chamber (Corning, 8 micron pore diameter) and 600 μL DMEM equipped with 10% FBS was added into the lower chamber. After 36 h of continuous culture, the cells in the upper chamber were carefully wiped off with a cotton swab. Then the migrated cells were fixed with 4% polyformaldehyde solution for 30 min before air-drying. After 30 min of dyeing in crystal violet solution, PBS solution was used to wash the chamber for three times. After that, the number of cells passing through the membrane in five visual fields was counted under an inverted microscope, and the average value was taken to reflect the migration or invasion ability of cells in each group.
Apoptosis assay
The apoptotic rate of NSCLC cells in each group was detected according to the instructions of Annexin V-FITC/PI apoptotic detection kit (BDingen, San Diego, CA, USA) by flow cytometry (Becton, Dickinson, Mountain View, USA).
Luciferase reporter assay
Dual-luciferase reporter assay system (Promega, Madison, WI, USA) was used to determine the targeting binding relationship between miRNA and lncRNA. In short, the target fragments of wild-type (WT) TMPO-AS1 and mutant (MUT) TMPO-AS1 were constructed (Promega, Madison, WI, USA). Afterwards, TMPO-AS1-wt or TMPO-AS1-mut were co-transfected with miR-204-3p mimics or negative control substances into A549 and H226 cells. After 48 h of transfection, luciferase activity was determined according to the manufacturer’s instructions.
RNA immunoprecipitation (RIP) assay
According to the instructions, Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was applied in RIP assay to verify the interaction between TMPO-AS1 and miR-204-3p. Control mimics and miR-204-3p mimics were transfected into A549 cells and H226 cells, and the cells were lysed in RIP cleavage buffer. 100 μL cell lysate was incubated with magnetic beads coupled with human anti-Argonaute2 (Ago2) antibody or negative control IgG in RIP buffer. The sample was then incubated with protease K to digest protein, and then the precipitation of RNA was obtained by TRIzol method. The purified RNA was processed by reverse transcription and qRT-PCR to detect the expression of TMPO-AS1.
Western blot
After collecting the cells, RIPA lysate (containing 1% PMSF) was added to extract the total protein, and the concentration of the total protein was determined according to the instructions of BCA kit (Beyotime Biotechnology, Hangzhou, China). The mixture of 5 × loading buffer and protein sample was denatured in a water bath at 100°C for 5 min. The protein samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF, Millipore, MA, USA) membrane. After being blocked with defatted milk for 30 min, the membrane was incubated with primary antibody of ERBB2 (ab31889, Abcam, 1:1000) at 4°C overnight. Afterwards, TBST buffer was used to wash the membranes, and the secondary antibody was added to the membranes for incubation at 37°C for 2 h. At last, chemiluminescence was developed using a hypersensitive ECL (Baiaosi Bioscience Co., Ltd., Wuhan, China).
Statistical analysis
SPSS statistical software (version 22.0) was used for data analysis in this study. All data are expressed as mean ± SD. Whether the data were normally distributed was examined by One-Sample Kolmogorov–Smirnov test. For normally distributed data, t test was used to make the comparison between two groups. One-way ANOVA test was used to compare three or more groups. If there was significant difference, Newman–Keuls analysis was used to make the comparison between two groups. For skewed distributed data, Wilcoxon signed rank test was used to make comparison between two groups. For survival analysis, Kaplan–Meier method was used with kmplotter database (http://www.kmplot.com). P < 0.05 was considered to have statistical significance.
Results
Expression of TMPO-AS1 in NSCLC
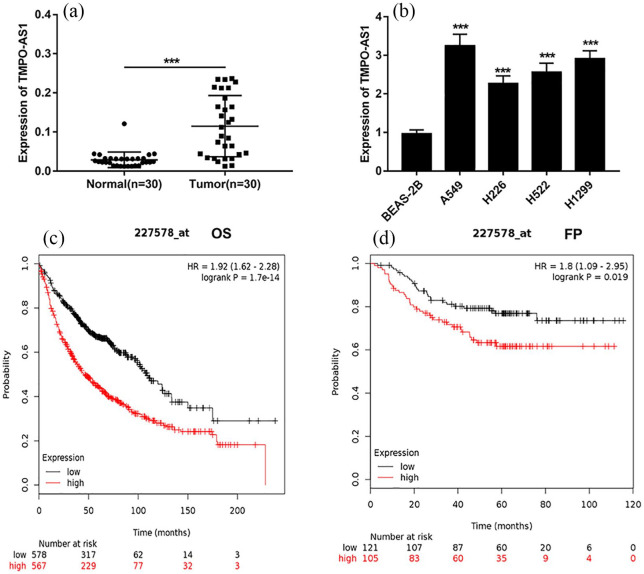
To confirm the expression level of TMPO-AS1 in NSCLC, firstly, we detected the expression of TMPO-AS1 in 30 patients’ NSCLC tissues and their corresponding adjacent tissues by qRT-PCR, the results of which showed that the expression of TMPO-AS1 was significantly increased in NSCLC tissues compared with in normal tissues (Figure 1(a)). In addition, the expression of TMPO-AS1 in NSCLC cell lines was significantly higher than that in BEAS-2B cells (Figure 1(b)). In addition, Kaplan–Meier survival analysis was performed to detect the relationship between the expression of TMPO-AS1 and the prognosis of NSCLC patients. The results showed that compared with the patients form low expression group of TMPO-AS1, the overall survival time of patients in the high expression group of TMPO-AS1 was significantly shorter (Figure 1(c)). Additionally, patients with high expression of TMPO-AS1 suffered from earlier disease progression (Figure 1(d)). These data indicated that TMPO-AS1 could be involved in the progression of NSCLC, and might be used as a biomarker.
Figure 1.
The expression of TMPO-AS1 in NSCLC tissues and cell lines. (a) qRT-PCR was used to detect the expression level of TMPO-AS1 in NSCLC tissues and adjacent normal tissues. (b) qRT-PCR was used to detect the expression level of TMPO-AS1 in normal bronchial epithelial cell line and NSCLC cell lines. (c) The overall survival time (OS) of patients with high and low expression of TMPO-AS1. (d) The first progression time (FP) of patients with high and low expression of TMPO-AS1.
**P < 0.01 and ***P < 0.001.
Effects of TMPO-AS1 on proliferation, metastasis and apoptosis of NSCLC cells
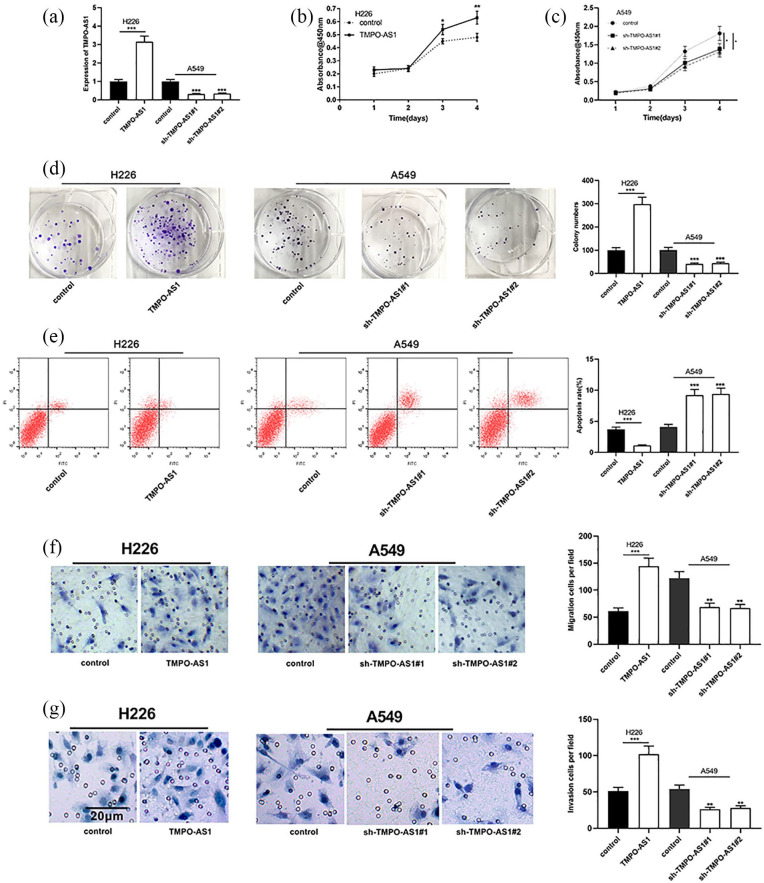
Among NSCLC cell lines, the expression of TMPO-AS1 was lowest in H226 cells, so H226 cells were selected for subsequent TMPO-AS1 overexpression experiments; the expression of TMPO-AS1 was highest in A549 cells, so A549 cells were selected for subsequent TMPO-AS1 knockdown experiments (Figure 2(a)). Further studies showed that over-expression of TMPO-AS1 could significantly promote the proliferation, migration and invasion of NSCLC cell H226 and inhibit its apoptosis, while knocking down TMPO-AS1 could exert the opposite function on NSCLC cell A549 (Figure 2(b)–(g)). These data implied that TMPO-AS1 could modulate the malignant phenotypes of NSCLC cells.
Figure 2.
Effects of TMPO-AS1 NSCLC cells on proliferation, migration, invasion and apoptosis. (a) The expression levels of TMPO-AS1 in cells were detected by qRT-PCR after transfection. (b and c) Proliferation of H226 and A549 cells was detected by CCK-8 assay respectively. (d)Proliferation of H226 cells and A549 was detected by colony formation assay respectively. (e) Apoptosis of H226 and A549 cells was detected by flow cytometry after overexpression and knockdown of TMPO-AS1. (f and g) Transwell method was used to determine the migration and invasion of H226 and A549 cells respectively.
*P < 0.05, **P < 0.01, ***P < 0.001.
MiR-204-3p was one target of TMPO-AS1
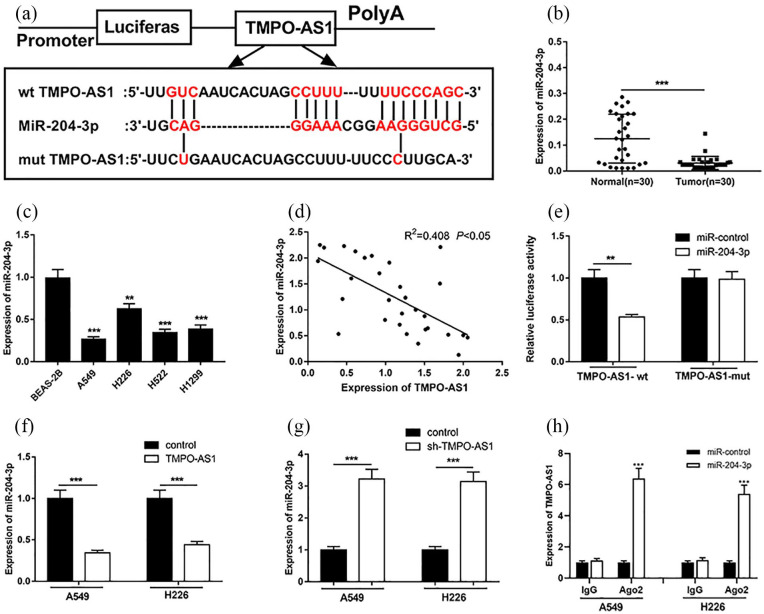
In order to pinpoint the potential relationship between TMPO-AS1 and miR-204-3p, we conducted a bioinformatics analysis using LncBase Predicted v.2 database (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php). The data showed that TMPO-AS1 contained a potential conserved binding site for miR-204-3p (Figure 3(a)). The results of qRT-PCR showed that the expression of miR-204-3p was significantly down-regulated in NSCLC tissues and cells (Figure 3(b and c)). Meanwhile, the results showed that the expression level of TMPO-AS1 was negatively correlated with that of miR-204-3p in NSCLC samples (Figure 3(d)). In order to further verify the binding relationship between TMPO-AS1 and miR-204-3p, dual luciferase reporter assay was conducted. It revealed that the miR-204-3p mimics could decrease the luciferase activity of luciferase reporter containing wide type TMPO-AS1 sequence, but had no significant effect on the luciferase activity of mutated TMPO-AS1 sequence (Figure 3(e)). In addition, overexpressing and downregulating TMPO-AS1 decreased and increased the expression of miR-204-3p in NSCLC cells A549 and H226, respectively (Figure 3(f) and (g)). Moreover, RIP assay was performed, showing that miR-204-3p and TMPO-AS1 were markedly enriched in Ago2 immunoprecipitate compared with IgG (Figure 3(h)). These results suggested that TMPO-AS1 could specifically inhibit the expression of miR-204-3p.
Figure 3.
TMPO-AS1 targeted miR-204-3p. (a) TMPO-AS1 had complementary pairing relationship with miR-204-3p. (b) qRT-PCR was used to detect miR-204-3p expression in NSCLC tissues. (c) qRT-PCR was used to detect the expression level of miR-204-3p in normal bronchial epithelial cell line and NSCLC cell lines. (d) The correlation between TMPO-AS1 and miR-204-3p expression in NSCLC tissues. (e) Dual luciferase report assay was conducted to determine the targeting relationship between TMPO-AS1 and miR-204-3p. (f) The effect of overexpression of TMPO-AS1 on the expression levels of miR-204-3p in A549 and H226. (g) The effect of knocking down TMPO-AS1 on the expression levels of A549 and H226 cell lines. (h) RIP assay was performed to detect the interaction between TMPO-AS1 and miR-204-3p.
**P < 0.01, ***P < 0.001.
Effects of miR-204-3p on proliferation, metastasis and apoptosis of NSCLC cells
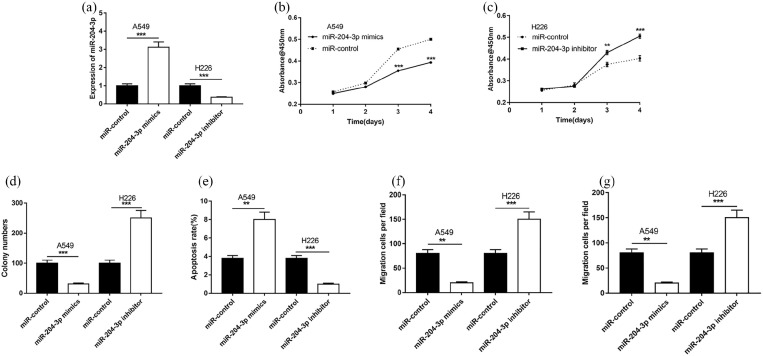
To further fathom the function of miR-204-3p, miR-204-3p mimics and inhibitors were transfected into A549 and H226 cells, respectively (Figure 4(a)). As shown, transfection of miR-204-3p mimics could significantly inhibit the proliferation, migration and invasion of A549 cells and induce its apoptosis, while transfection of miR-204-3p inhibitors could exert opposite functions in H226 cells (Figure 4(b)–(g)).
Figure 4.
Effects of miR-204-3p on NSCLC cells on proliferation, migration, invasion and apoptosis. (a) miR-204-3p in cells were detected by qRT-PCR after transfection. (b and c) Proliferation of H226 and A549 cells was detected by CCK8 respectively. (d) Proliferation of H226 cells and A549 was detected by colony formation assay respectively. (e) Apoptosis of H226 and A549 cells was detected by flow cytometry. (f and g) Transwell method was used to determine the migration and invasion of H226 and A549 cells respectively.
**P < 0.01, ***P < 0.001.
MiR-204-3p reversed the effects of TMPO-AS1 on promoting proliferation, metastasis and inhibiting apoptosis of NSCLC cells
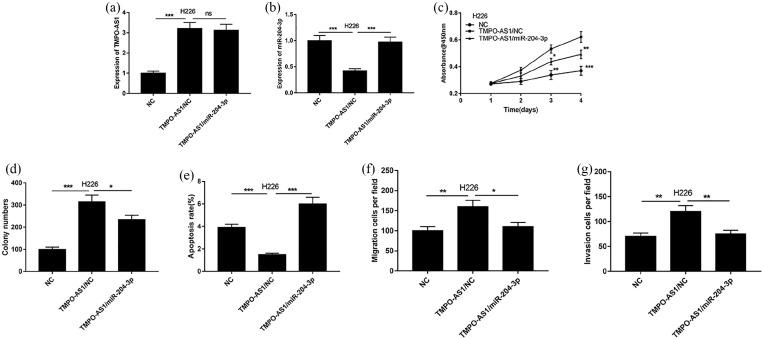
To further, validate the role of TMPO-AS1/miR-204-3p axis in NSCLC progression, we co-transfected TMPO-AS1 overexpression plasmid and miR-204-3p mimics in H226 cells. The results of qRT-PCR showed that the transfection of miR-204-3p mimics increased the expression of miR-204-3p, but had no significant effect on the expression of TMPO-AS1 (Figure 5(a) and (b)). As shown, overexpression of TMPO-AS1 promoted the malignant phenotypes of H226 cells, and the co-transfection of miR-204-3p mimics partly reversed the function of TMPO-AS1 (Figure 5(c)–(g)).
Figure 5.
The effects of miR-204-3p on proliferation, migration, invasion and apoptosis of H226 cells overexpressed with TMPO-AS1. (a and b) The expression of TMPO-AS1 and miR-204-3p in H226 cells was detected by qRT-PCR respectively. (c and d) The proliferation of H226 cells was examined by CCK-8 and colony formation assay respectively. (e and f) The migration and invasion of H226 cells was tested by Transwell assay respectively. (g) The apoptosis of H226 cells was detected by flow cytometry.
*P < 0.05, **P < 0.01, ***P < 0.001.
MiR-204-3p targeted ERBB2 in NSCLC cells
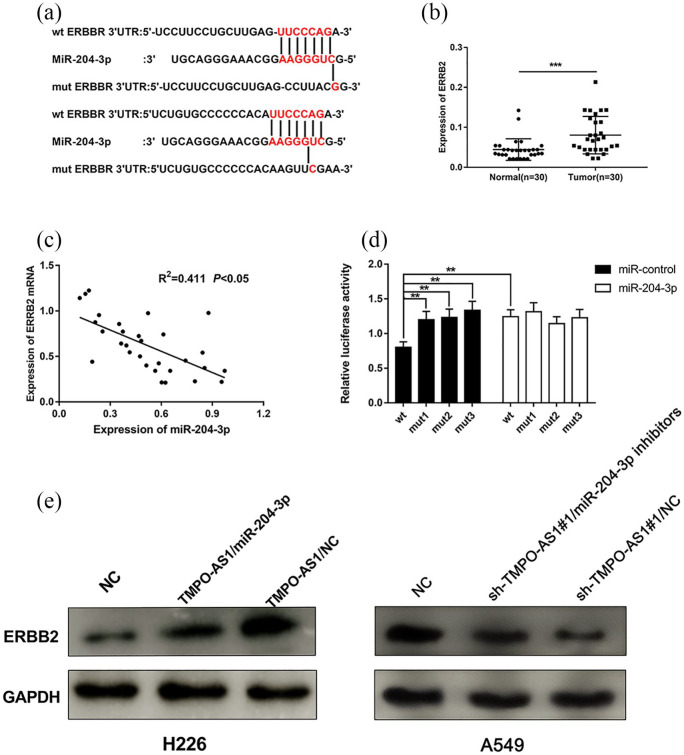
In order to study the downstream mechanism of miR-204-3p on NSCLC cells, we searched the Targetscan database (http://www.targetscan.org/vert_72) to select the candidate targets of miR-204-3p. As shown, the 3’UTR of ERBB2 contained two binding sequences for miR-204-3p (Figure 6(a)). The results of qRT-PCR showed that ERBB2 expression was significantly up-regulated in NSCLC tissues (Figure 6(b)), which was consistent with previous report,18 and was negatively correlated with the expression level of miR-204-3p (Figure 6(c)). In order to further verify the binding relationship between miR-204-3p and ERBB2, dual luciferase reporter assay was performed. As shown, miR-204-3p mimics could decrease the luciferase activity of luciferase reporter containing wide type 3’UTR of ERBB2, but no significant effect on the luciferase activity of mutated 3’UTR of ERBB2 could be found (Figure 6(d)). Additionally, Western blot showed that the expression level of ERBB2 was significantly increased after overexpression of TMPO-AS1 in H226 cells, and this effect was partly reversed when miR-204-3p was co-transfected; additionally, TMPO-AS1 knockdown suppressed the expression of ERBB2, and co-transfection of miR-204-3p inhibition counteracted this effect in A549 cells (Figure 6(e)). Collectively, these data indicated that TMPO-AS1 could indirectly modulate the expression level of ERBB2 as a competing endogenous RNA (ceRNA) by sponging miR-204-3p.
Figure 6.
miR-204-3p targeted ERBB2 in NSCLC. (a) Complementary base pairing relationship between miR-204-3p and the 3’UTR of ERBB2. (b) qRT-PCR was used to detect ERBB2 expression in NSCLC tissues. (c) The correlation between the expression levels of miR-204-3p and ERBB2 in NSCLC tissues. (d) Dual luciferase report test was used to ensure the relationship between miR-204-3p and the 3’UTR of ERBB2; E: Western blot was used to detect ERBB2 expression after transfection of TMPO-AS1 overexpression plasmid with/without miR-204-3p mimics.
**P < 0.01 ***P < 0.001.
Discussion
NSCLC poses a serious threat to human health, with mortality rate increasing year by year.19 Many genes are abnormally expressed in NSCLC tissues and play a key role in the tumorigenesis and progression of NSCLC by acting as oncogenes or tumor suppressors.20,21 An increasing number of studies demonstrate that lncRNAs play prominent roles in cancer progression. For example, the up-regulation of SNHG1 promotes the malignant phenotype of cervical cancer22; DLX6-AS1 facilitates proliferation and metastasis of lung adenocarcinoma cells through activating Notch signalling pathway.23 LncRNAs also play an essential regulatory role in the tumorigenesis and progression of NSCLC. For example, it is reported that MIAT can enhance the proliferation and metastasis of NSCLC cells by up-regulating TDP43, suggesting that it may be a potential therapeutic target for NSCLC24; PVT1 promotes invasion of NSCLC cells by up-regulating matrix metalloprotein 9.25 In this study, we demonstrated the significant up-regulation of TMPO-AS1 expression in NSCLC cancer tissues and cells. Further, gain-of-function and loss-of-function experiments confirmed that TMPO-AS1 modulated the malignant phenotypes of NSCLC cells. These data indicated that TMPO-AS1 exerted cancer-promoting effects in NSCLC.
MiRNAs are also essential for the occurrence and development of tumours. For example, miRNA-449a inhibits the proliferation and metastasis of endometrial cancer by targeting CDC25A, and miRNA-10a promotes the proliferation of cancer cells in oral squamous cell carcinoma by up-regulating GLUT1 and promoting glucose metabolism.26,27 In NSCLC, miR-187-3p inhibits cancer progression by downregulating BCL6; miR-221 plays a carcinogenic role by directly targeting TIMP2.28,29 In this study, we first found that the expression of miR-204-3p was down-regulated in NSCLC tissues and cells. Furthermore, we confirmed that miR-204-3p inhibited the proliferation, migration and invasion of NSCLC cells, and promoted cell apoptosis. These data suggested that miR-204-3p played an anti-cancer role in NSCLC.
It is reportedly found that lncRNAs play the role of ceRNA or miRNA sponge by interacting with miRNAs via microRNA recognition elements, and the highly expressed ceRNA will adsorb miRNAs to reduce their availability. For instance, LINC00963 promotes osteosarcoma proliferation, migration and invasion by targeting miR-204-3p.30 In colorectal cancer, HOTAIR facilitates 5-FU resistance by inhibiting miR-218 and activating downstream NF-κB signalling.31 In NSCLC, SNHG1 boosts cancer progression by sponging miR-101-3p and activation of Wnt/β-catenin signalling pathway.32 Inspired by the ceRNA regulatory network and emerging evidence that lncRNAs may be involved in this regulation, in this study, we hypothesised that TMPO-AS1 might also be a ceRNA, so we explored the potential interaction between TMPO-AS1 and miR-204-3p. Bioinformatcis analysis and dual luciferase reporter assay confirmed that TMPO-AS1 could specifically bind with miR-204-3p and reduce its expression. Additionally, overexpression of miR-204-3p could reverse the effect of TMPO-AS1 on promoting the malignant phenotype of NSCLC.
Membrane overexpression of ERBB2 constitutes a therapeutic target in cancer therapy. In breast cancer and gastric cancer, ERBB2 targeted therapies significantly improve patients’ clinical outcome.33,34 In NSCLC, ERBB2 regulates cancer stem-like cell phenotype via AKT and ERK1/2 signalling, and its knockdown blocks the proliferation and metastasis of NSCLC cells.35,36 In this study, bioinformatics and dual luciferase reporter analysis confirmed that miR-204-3p directly targeted ERBB2. Western blot results showed that over-expression of TMPO-AS1 could increase the expression of ERBB2, while the transfection of mimics of miR-204-3p reversed the effect of TMPO-AS1 on the expression of ERBB2. These data suggested that in NSCLC, ERBB2 was modulated by TMPO-AS1 and miR-204-3p, which partly explained the dysregulation of ERBB2 in some cases of NSCLC.
Conclusion
Our study demonstrates that the expression of TMPO-AS1 is up-regulated in NSCLC tissues and cells. Functional experiments confirm that TMPO-AS1 can promote the proliferation, migration, invasion and inhibit apoptosis of NSCLC cells by regulating the miR-204-3p/ERBB2 axis, which implies valuable and promising therapeutic targets for NSCLC. There are still some shortcomings of this study, such as fewer samples collected and no in vivo data, which needs to be improved in the future. In addition, whether TMPO-AS1 can promote other malignant phenotypes of NSCLC cells such as chemoresistance and radiation resistance needs further investigation. Moreover, many other potential downstream miRNAs of TMPO-AS1 are needed to be screened and validated. It is also interesting to explore whether TMPO-AS1 can regulate the progression of NSCLC via repressing other miRNAs, besides miR-204-3p.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from *the Research Ethics Committee of the Shanghai General Hospital (Ethical No: SHGH-20160411)*.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: Xi Chen  https://orcid.org/0000-0002-5751-0875
https://orcid.org/0000-0002-5751-0875
Data availability statement: The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1. Alcantud JCR, Varela G, Santos-Buitrago B, et al. (2019) Analysis of survival for lung cancer resections cases with fuzzy and soft set theory in surgical decision making. PLoS One 14(6): e0218283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu DJ, Li YH, Zhong M. (2019) LncRNA FBXL19-AS1 promotes proliferation and metastasis via regulating epithelial-mesenchymal transition in non-small cell lung cancer. European Review for Medical and Pharmacological Sciences 23(11): 4800–4806. [DOI] [PubMed] [Google Scholar]
- 3. Yu H, Han Z, Xu Z, et al. (2019) RNA sequencing uncovers the key long non-coding RNAs and potential molecular mechanism contributing to XAV939-mediated inhibition of non-small cell lung cancer. Oncology Letters 17(6): 4994–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheng J, Wang L, Han Y, et al. (2018) Dual roles of protein as a template and a sulfur provider: A general approach to metal sulfides for efficient photothermal therapy of cancer. Small 14(1). [DOI] [PubMed] [Google Scholar]
- 5. Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease Cell 152(6): 1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponting CP, Oliver PL, Reik W. (2009) Evolution and Functions of Long Noncoding RNAs. Cell 136(4): 629–641. [DOI] [PubMed] [Google Scholar]
- 7. Kim MY. (2019) Long non-coding RNAs in cancer. Non-Coding RNA Research 4(2): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang W, Su X, Yan W, et al. (2018) Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate 78(16): 1248–1261. [DOI] [PubMed] [Google Scholar]
- 9. Peng F, Wang R, Zhang Y, et al. (2017) Differential expression analysis at the individual level reveals a lncRNA prognostic signature for lung adenocarcinoma. Molecular Cancer 16(1): 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qin Z, Zheng X, Fang Y. (2019) Long noncoding RNA TMPO-AS1 promotes progression of non-small cell lung cancer through regulating its natural antisense transcript TMPO. Biochemical and Biophysical Research Communications 516(2): 486–493. [DOI] [PubMed] [Google Scholar]
- 11. Sayed D, Abdellatif M. (2011) MicroRNAs in development and disease. Physiological Reviews 91(3): 827–87. [DOI] [PubMed] [Google Scholar]
- 12. Grenda A, Nicoś M, Szczyrek M, et al. (2019) MicroRNAs aid the assessment of programmed death ligand 1 expression in patients with non-small cell lung cancer. Oncology Letters 17(6): 5193–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han Z, Zhang Y, Sun Y, et al. (2018) ERβ-Mediated Alteration of circATP2B1 and miR-204–3p signaling promotes invasion of clear cell renal cell carcinoma. Cancer Research 78(10): 2550–2563. [DOI] [PubMed] [Google Scholar]
- 14. Chen PH, Chang CK, Shih CM, et al. (2016) The miR-204-3p-targeted IGFBP2 pathway is involved in xanthohumol-induced glioma cell apoptotic death. Neuropharmacology 110(Pt A): 362–375. [DOI] [PubMed] [Google Scholar]
- 15. Cui ZH, Shen SQ, Chen ZB, et al. (2014) Growth inhibition of hepatocellular carcinoma tumor endothelial cells by miR-204-3p and underlying mechanism. World Journal of Gastroenterology 20(18): 5493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Griguolo G, Pascual T, Dieci MV, et al. (2019) Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. Journal for Immunotherapy of Cancer 7(1): 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xin X, Gu Y, Chen Y, et al. (2017) Functional analysis implicating the SNP rs61552325 in ERBB2 as an effector for androgen-insensitive prostate cancer cell invasion. Oncotarget 8(20): 33745–33755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ricciardi GR1, Russo A, Franchina T, et al. (2014) NSCLC and HER2: between lights and shadows. Journal of Thoracic Oncology 9(12): 1750–162. [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Shen XB, Jia W, et al. (2019) The p53/miR-193a/EGFR feedback loop function as a driving force for non-small cell lung carcinoma tumorigenesis. Therapeutic Advances in Medical Oncology 11: 1758835919850665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie Y, Zhang Y, Du L, et al. (2018) Circulating long noncoding RNA act as potential novel biomarkers for diagnosis and prognosis of non-small cell lung cancer. Molecular Oncology 12(5): 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li P, Xing W, Xu J, et al. (2019) microRNA-301b-3p downregulation underlies a novel inhibitory role of long non-coding RNA MBNL1-AS1 in non-small cell lung cancer. Stem Cell Research & Therapy 10(1): 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Yang Y, Li L, et al. (2018) LncRNA SNHG1 enhances cell proliferation, migration and invasion in cervical cancer. Biochemistry and Cell Biology 96(1): 38–43. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Li P, Zhao W, et al. (2015) Expression of long non-coding RNA DLX6-AS1 in lung adenocarcinoma. Cancer Cell International 15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao HL, Xu SQ, Li Q, et al. (2019) Long noncoding RNA MIAT promotes the growth and metastasis of non-small cell lung cancer by upregulating TDP43. European Review for Medical and Pharmacological Sciences 23(8): 3383–3389. [DOI] [PubMed] [Google Scholar]
- 25. Chen W, Zhu H, Yin L, et al. (2017). lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA and Cell Biology 36(9): 787–793. [DOI] [PubMed] [Google Scholar]
- 26. Ye W, Xue J, Zhang Q, et al. (2014) MiR-449a functions as a tumor suppressor in endometrial cancer by targeting CDC25A. Oncology Reports 32(3): 1193–1199. [DOI] [PubMed] [Google Scholar]
- 27. Chen YH, Song Y, Yu YL, et al. (2019) miRNA-10a promotes cancer cell proliferation in oral squamous cell carcinoma by upregulating GLUT1 and promoting glucose metabolism. OncologyLletters 17(6): 5441–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun C, Li S, Yang C, et al. (2016) MicroRNA-187-3p mitigates non-small cell lung cancer (NSCLC) development through down-regulation of BCL6. Biochemical and Biophysical Research Communications 471(1): 82–88. [DOI] [PubMed] [Google Scholar]
- 29. Yin Z, Xu M, Li P. (2017) miRNA-221 acts as an oncogenic role by directly targeting TIMP2 in non-small-cell lung carcinoma. Gene 620: 46–53. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Y, Yin L, Li H, et al. (2019) The LncRNA LINC00963 facilitates osteosarcoma proliferation and invasion by suppressing miR-204-3p/FN1 axis. Cancer Biology & Therapy 20(8): 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li P, Zhang X, Wang L, et al. (2017) lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-κB/TS signaling in colorectal cancer. Molecular Therapy. Nucleic Acids 8: 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui Y, Zhang F, Zhu C, et al. (2017) Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/β-catenin signaling pathway. Oncotarget 8(11): 17785–17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asif HM, Sultana S, Ahmed S, et al. (2016) HER-2 Positive Breast Cancer - a Mini-Review. Asian Pacific Journal of Cancer Prevention: APJCP 17(4): 1609–1615. [DOI] [PubMed] [Google Scholar]
- 34. Jomrich G, Schoppmann SF. (2016) Targeting HER 2 and angiogenesis in gastric cancer. Expert Review of Anticancer Therapy 16(1): 111–122. [DOI] [PubMed] [Google Scholar]
- 35. Honkanen T, Wilenius E, Koivunen P, et al. (2017) HER2 regulates cancer stem-like cell phenotype in ALK translocated NSCLC. International Journal of Oncology 51(2): 599–606. [DOI] [PubMed] [Google Scholar]
- 36. Lu Y, Wang Y, Zhang M, et al. (2016) HER2-siRNA delivered by EGFR-specific single chain antibody inhibits NSCLC cell proliferation and tumor growth. Oncotarget 7(17): 23594–23607. [DOI] [PMC free article] [PubMed] [Google Scholar]