Abstract
We assessed prevalence of HIV risk behaviors and depressive symptoms among adolescent girls and young women (AGYW) aged 15–24 years attending 4 public family planning clinics in Western Kenya from January to June 2019. Moderate-to-severe depression (MSD) was defined as a Center for Epidemiologic Studies Depression Scale (CESD-10) score ≥10. Among 487 AGYW, the median age was 22 years (IQR 20–23), and 59 (12%) AGYW reported MSD. MSD was more prevalent among AGYW without a current partner p=0.001 and associated with HIV risk factors including partner ≥10 years older, recent transactional sex, forced sex, intimate partner violence, and alcohol use (each p≤0.005). Thirty-four percent of AGYW with MSD had a high HIV risk score corresponding to 5 to 15 incident HIV cases per 100 person-years. Overlapping high prevalence of depression and HIV risk among AGYW underscores the need for integrated mental health and HIV services in family planning clinics.
Keywords: Depression, HIV, adolescent, women, girls
INTRODUCTION
Adolescence and young adulthood are characterized by developmental and hormonal changes [1], increasing vulnerability to psychiatric distress [2]. Worldwide, 10–20% of adolescents experience depression [3]. A majority (90%) of the world’s adolescents live in low- and middle-income countries (LMICs) [3, 4] where <30% of the population has access to mental health services [5]. Globally, adolescent girls and young women (AGYW) are disproportionally burdened by depression, with double the lifetime risk compared to their male counterparts [6] and evidence suggests that AGYW in LMICs frequently experience depression [6–8].
In African settings with high HIV-burden, AGYW have high risk for HIV acquisition and are a priority population for HIV prevention efforts [9]. The recently completed “Evidence for Contraceptive Options and HIV Outcomes (ECHO)” trial found an HIV incidence of 4.3% among African AGYW within family planning (FP) clinics [10]. These findings highlight the urgent need for HIV prevention among AGYW who seek FP services in SSA. Evaluating potential intersections between the dual epidemics of depression and HIV incidence among AGYW could inform strategies that concurrently address mental wellbeing and HIV prevention within this group.
FP services are some of the most readily accessed health services by AGYW in sub-Saharan Africa (SSA) with over a third of reproductive aged women using modern contraceptive methods in the region [11–13]. HIV prevention counseling is increasingly implemented via FP services within high HIV-burden settings [14–16], and Kenya is one of the first countries in SSA to programmatically offer PrEP to AGYW in FP clinics [16, 17]. AGYW in SSA who regularly access FP could similarly be reached with clinic-based mental health programs; yet, no studies to date have evaluated depression among AGYW seeking FP services.
Few studies have evaluated depression among AGYW overall in SSA. Available data is derived from participants in household surveys or research cohorts [18–24] who may differ from the general population of AGYW seeking FP in public sector settings. Data on depression and HIV behavioral risk among AGYW accessing FP services in SSA would be particularly helpful for planning interventions that could be integrated within FP clinics.
We evaluated the prevalence of depression and its relationship with HIV risk behaviors among AGYW seeking FP services and offered PrEP for HIV prevention within public sector FP clinics in Western Kenya.
METHODS
Study setting and population
The PrIYA Program was a two-year implementation project to reach AGYW at high risk for HIV acquisition through integrated delivery of PrEP within routine maternal child health (MCH) and FP systems [16, 25]. Western Kenya has adult HIV prevalence of 19.9% (up to 28% among pregnant women). PrIYA was conducted in collaboration with the Department of Health and Sanitation, Kisumu County, and the National AIDS and STI Control Programme (NASCOP). PrIYA was implemented from June 2017 to December 2018 in 16 predominantly public sector facilities in Kisumu County [26–28]. We conducted a follow-on study to evaluate psychosocial characteristics, behavioral risk factors for HIV, and PrEP uptake among AGYW seeking FP services at former PrIYA sites. The follow-up study was conducted at 4 public sector facilities purposively selected based on the highest monthly enrollment of new FP clients. The current analysis focuses on prevalence of depression among AGYW seeking FP services and its relationship to HIV risk behaviors.
We approached all HIV-uninfected women after receipt of their FP services at the 4 selected facilities from October 2018 to June 2019. Women were eligible for enrollment if they were between 15 and 24 years, had ever been screened for PrEP, were receiving FP services at the facility, and were able to provide consent. Women were included in this current analysis if they had complete information on depressive symptoms.
Data collection procedures
Surveys were administered in Kiswahili, Dholuo, or English by trained study nurses using tablet-based questionnaires in REDCap—a secure web application for survey and database management. Participants were surveyed about demographics, partner characteristics, sexual and reproductive behaviors, perceived HIV risk, HIV risk behaviors, psychosocial factors, and experiences being offered and/or using PrEP. Prior to survey implementation, the data collection instrument was field tested by study staff and questionnaire items or translations were refined as needed.
Assessment of depressive symptoms and psychosocial factors
Guided by a modified HIV risk environment framework as described by Rhodes et al, we aimed to identify social environment factors associated with depression and HIV risk behaviors [29]. The ‘risk environment’ is a conceptual framework that explores how physical, social, political, and economic environments impact vulnerability to HIV [29]. We assessed participants for depressive symptoms using the Center for Epidemiologic Studies Depression scale (CESD-10) [30], an instrument validated for use in sub-Saharan Africa. The CESD-10 has similar psychometric properties to the commonly used Patient Health Questionnaire-9 depressive symptom screening tool and has been previously used in HIV prevention studies among AGYW in African settings [31, 32]. Each scale item in the 10-item scale depicts a discrete depressive symptom (e.g., “I felt depressed”, “I felt lonely”, etc.). Participants rate each item between 0 to 3 based on past-week frequency. Higher total scores indicate higher severity of depressive symptoms, with scores ranging from 0–30. We defined having symptoms of moderate-to-severe depression (MSD) as having a CESD-10 score of 10 or greater (binary outcome [yes/no]), the validated cutoff by Andresen et al. denoting high likelihood of moderate-to-severe depression [30, 33].
Intimate partner violence was assessed with the four-item Hurt, Insult, Threaten, and Scream scale (HITS) [34, 35], using a cutoff of 10 or greater to define intimate partner violence (absolute range: 4–20). Social support was evaluated with the 18-item Medical Outcomes Study social support scale (MOS-SSS) [36] which asks participants to identify how often they find various types of support when they need it, with higher scores denoting higher social support (absolute range: 18–90). We defined low social support as scores below 72 since these scores meant participants felt they were unable to receive social support at least “most of the time” for each scenario [37, 38].
Assessment of behavioral HIV risk
HIV risk factors were assessed using a standardized assessment tool developed by the Kenya Ministry of Health to screen for PrEP eligibility, which includes the following behavioral characteristics in the last 6 months: sex without a condom, engagement in transactional sex, being forced to have sex, sharing needles while using intravenous drugs, and using post-exposure prophylaxis more than twice [39]. We also evaluated HIV risk behaviors using a validated risk score that was developed to predict risk of HIV acquisition among young women in SSA [40], including age <25 years old (risk score of 2), not living with a spouse/partner (1), any alcohol use within the past 30 days (1), receiving financial support from a partner (1), having a partner with other sexual partners or not knowing if a partner has other sexual partners (2), and having a curable STI (1). Because the study did not assess sexually transmitted infections (STIs), we utilized the modified version of this HIV risk score which excludes information about STI diagnosis [40]. An HIV risk score of ≥5 is considered “high”, corresponding to 5 to 15 incident HIV cases per 100 person-years, whereas risk scores of ≤4 correspond to <5 incident HIV cases per 100 person-years [40]. To assess self-perceived risk for HIV acquisition, we asked participants “What is your gut feeling about how likely you are to get infected with HIV?”, with possible responses of very likely, somewhat likely, very unlikely, or extremely unlikely [41].
Statistical analysis
Descriptive statistics were used to determine the prevalence of depression. We used Chi-squared tests to compare frequency of depression by demographic and behavioral characteristics and to compare frequency of high HIV risk and individual HIV risk behaviors among women with and without depression. We identified correlates of depression using Poisson regression models, clustering by facility. We used similar models to calculate prevalence ratios for HIV risk factors by depression status. Potential correlates of depression identified in univariable models were adjusted for age and prior pregnancy in multivariable models [7, 8]; adjustment variables were determined a priori. Analyses were performed in STATA 13.0 (College Station, TX).
Considerations for human subjects
The study protocol, informed consent forms, and data collection tools were reviewed and approved by the Kenyatta National Hospital-University of Nairobi Ethics Research Committee and University of Washington Human Subjects Review Committee. Approval was additionally obtained by the Kisumu County Department of Health and health administrators in the health facilities involved. All participants provided written informed consent. All participants were welcome to end the interview at any point during the survey and received a KSH 300 (approximately USD 3) reimbursement for their time.
RESULTS
Participant characteristics
This analysis included 487 AGYW who had complete CESD-10 data (80% of total study participants, Figure 1). Median age was 22 years (interquartile range [IQR] 20–23), 21% (102/487) were currently in school, and the median completed education was 12 years (IQR 10–12, Table I). Most participants had a current partner (402/487, 83%) and over half were married or cohabiting (285/487, 59%). Most AGYW reported at least one prior pregnancy (398/487, 82%), and two thirds of those who had at least one prior pregnancy and provided information about the delivery date (255/379, 67%) had given birth in the last 12 months. Among these AGYW seeking FP services, the most common contraceptive method currently used was injectables (45%) followed by implants (38%). Fewer AGYW used oral contraceptive pills (11%), intrauterine contraceptive devices (IUDs, 3%), or condoms alone (2%).
Figure 1.
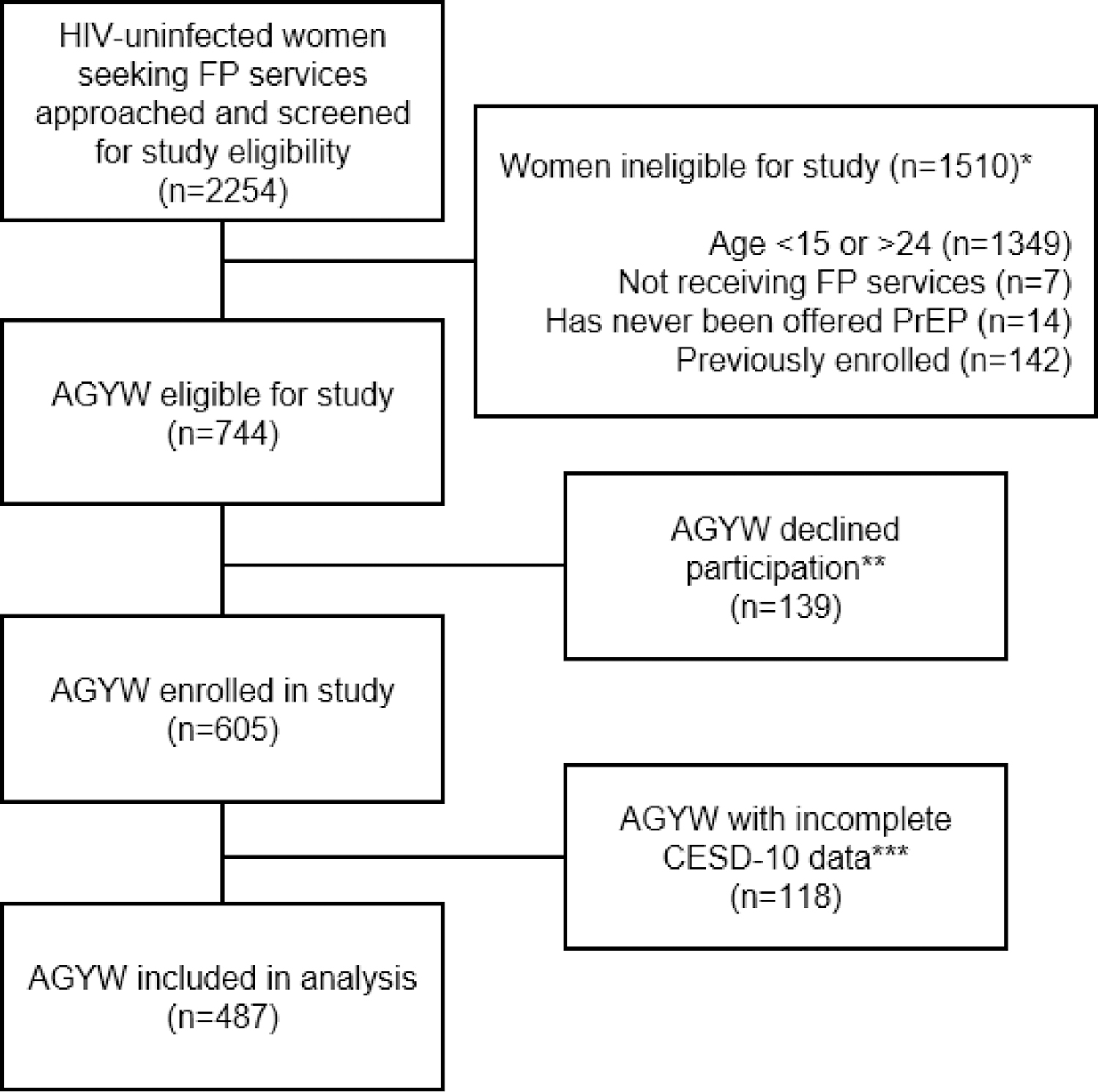
Flow chart of participant inclusion in the present analysis among HIV-uninfected AGYW seeking FP services in Western Kenya
*Categories describing reasons for ineligibility are not mutually exclusive
**Reasons for declination were not captured systematically. Anecdotally, the most common reason for declining was lack of time. Other common reasons included infant crying/fussing and male partner refusal.
***118 women had incomplete data for CESD-10 depressive symptom scale items, thus were excluded from the present analysis.
Table I.
Characteristics of adolescent girls and young women (ages 15–24) seeking family planning services in Western Kenya (N=487)
| Characteristic | N or Median (% or IQR) | |
|---|---|---|
| Demographic characteristics | ||
| Age | 22.0 | 20.0, 23.0 |
| ≤ 18 years | 48 | 9.9% |
| > 18 years | 439 | 90.1% |
| Currently in school (n=485) | ||
| No | 383 | 79.0% |
| Yes | 102 | 21.0% |
| Educational attainment (years) | 12.0 | 10.0, 12.0 |
| Regularly employed (n=486) | ||
| No | 416 | 85.6% |
| Yes | 70 | 14.4% |
| Household crowding | ||
| ≤ 3 people per room | 430 | 88.3% |
| > 3 people per room | 57 | 11.7% |
| Pregnancy history and family planning use | ||
| Ever been pregnant before | ||
| No | 89 | 18.3% |
| Yes | 398 | 81.7% |
| Number of pregnancies (n=398) | 1.0 | 1.0, 2.0 |
| Number of children (n=396) | 1.0 | 1.0, 2.0 |
| Time since most recent pregnancy (n=379) | ||
| < 12 months | 255 | 67.3% |
| ≥ 12 months | 124 | 32.7% |
| Family planning use | ||
| Initiating FP today | 257 | 52.8% |
| Refilling/continuing FP | 206 | 42.3% |
| Removing FP today | 24 | 4.9% |
| Family planning methoda (n=463) | ||
| Injectableg | 206 | 44.5% |
| IUCD | 13 | 2.8% |
| Implant | 175 | 37.8% |
| Condoms | 10 | 2.2% |
| OCP | 51 | 11.0% |
| Other | 8 | 1.7% |
| Partnership characteristics | ||
| Has current partner | ||
| No | 83 | 17.0% |
| Yes | 404 | 83.0% |
| Partner age difference (n=366) | 5.0 | 3.0, 7.0 |
| <10 years | 328 | 89.6% |
| ≥10 years | 38 | 10.4% |
| Psychosocial characteristics | ||
| Social support score (median, IQR) | 72.0 | 63.0, 80.0 |
| Low social support scoreb (n=486) No | 274 | 56.4% |
| Yes | 212 | 43.6% |
| Intimate partner violencec (HITS Score) (N=404) No | 391 | 96.8% |
| Yes | 13 | 3.2% |
| Depression score (Median CESD Score, IQR) | 4.0 | 2.0, 6.0 |
| Symptoms of moderate-to-severe depressiond No | 428 | 87.9% |
| Yes | 59 | 12.1% |
| HIV risk factors | ||
| Total lifetime sexual partners (median, IQR) | 2.0 | 2.0, 3.0 |
| <4 partners | 408 | 83.8% |
| ≥4 partners | 79 | 16.2% |
| Sex without a condom (last 6 months) | ||
| No | 108 | 22.2% |
| Yes | 379 | 77.8% |
| Transactional sex (last 6 months) | ||
| No | 472 | 96.9% |
| Yes | 15 | 3.1% |
| Forced sex (last 6 months) | ||
| No | 461 | 94.7% |
| Yes | 26 | 5.3% |
| High self-perceived HIV riske | ||
| No | 417 | 85.6% |
| Yes | 70 | 14.4% |
| HIV risk score factors (Balkus et. al.) | ||
| Marital status | ||
| Unmarried/not living with a partner | 285 | 58.5% |
| Married/living with a partner | 202 | 41.5% |
| Any alcohol use (past 30 days) | ||
| No | 419 | 86.0% |
| Yes | 68 | 14.0% |
| Partner provides financial support | ||
| No | 12 | 2.5% |
| Yes | 475 | 97.5% |
| Primary partner has other partners (n=402) | ||
| No | 223 | 55.5% |
| Yes | 143 | 35.6% |
| Don’t know | 36 | 9.0% |
| Balkus et al. HIV risk scoref ≥ 5 | ||
| No | 382 | 78.4% |
| Yes | 105 | 21.6% |
Family planning method currently in use after receipt of FP services today
We evaluated social support using the 18-item Medical Outcomes Study social support score (MOS-SSS); scores of 72 and above indicate respondents feel they are able to receive social support “most of the time” for each scenario item (Low social support: MOS-SSS score <72 = “Yes”, MOS-SSS ≥72 = “No”,)
We evaluated intimate partner violence using the 4-item Hurt, Insult, Threaten, and Scream scale (HITS), defining intimate partner violence as scores of 10 and above (IPV: HITS score ≥10 = “Yes”, HITS score <10 = “No”)
We evaluated depressive symptoms using the 10-item Center for Epidemiologic Studies Revised Depression Scale (CESD-10); scores of 10 and above denote high likelihood of moderate-to-severe depression (Symptoms of moderate to severe depression: CESD-10 score ≥10 = “Yes”, CESD-10 score <10 = “No”)
We evaluated self-perceived HIV risk by asking “What is your gut feeling about how likely you are to get infected with HIV?”, with possible responses of “very likely”, “somewhat likely”, “very unlikely”, “extremely unlikely”. (High self-perceived HIV risk: Very/somewhat likely = “Yes”, Extremely/very unlikely = “No”)
We evaluated HIV risk using the Balkus et al. HIV risk scoring: Age <25 = 1, Married = 2, any alcohol = 1, partner provides financial support = 1, partner has other partners: yes = 2, do not know = 2. Scores of ≥5 correspond to 5–15 incident HIV cases per 100 person-years in cohorts of African women; risk scores of ≤4 correspond to <5 incident HIV cases per 100 person-years. (High HIV risk: HIV risk score ≥ 5 = “Yes”, HIV risk score <5 = “No”)
Women in Kenya using injectable contraceptives almost exclusively use Depomedroxyprogesterone acetate (DMPA)(12)
Prevalence and correlates of depression
Overall, 59/487 (12%, 95% CI:9.5–15.3) of AGYW had CESD-10 scores ≥10 and were classified as having moderate-to-severe depression (MSD); the median CESD-10 score was 4 (IQR 2–6; absolute range 0–25). Compared to those with a current stable partner, MSD was nearly three times as frequent among AGYW without a partner, after adjusting for age and prior pregnancy (25% vs. 9%, aPR=2.7, 95%CI:1.5–4.8, p=0.001) (Table II). Among AGYW with a current partner (n=366), MSD was twice as prevalent in those with an older partner compared to those with partners more similar in age (≥10 years age difference vs. <10 years, aPR:2.3, 95%CI:1.1–4.6, p=0.022). MSD was more prevalent among AGYW with low social support (i.e., did not feel supported “all” or “most of the time” for all social support scenarios) than among those with high social support (19% vs. 7%, aPR:2.6, 95% CI:1.5–4.5, p=0.001). Frequency of MSD among AGYW who had a prior pregnancy was four times higher than in those without a prior pregnancy after adjusting for age (14% vs. 3.4%, adjusted prevalence ratio[aPR]=4.2, 95%CI:3.1–5.6, p-value<0.001). Among AGYW who had a prior pregnancy, frequency of MSD was higher if they had given birth in the prior 12 months (16% vs. 11%, aPR=1.5, 95% CI:1.1–2.1, p-value<0.018). No other characteristics were associated with depression.
Table II.
Prevalence of depression by characteristics of AGYW seeking FP services in Western Kenya (N=487)
| Characteristic | Depressed (CESD≥10) N(%) N=59 |
Not depressed (CESD<10) N(%) N=428 |
Prevalence ratio PR (95% CI) |
p-value | aPRa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age | ||||||
| >18 years | 54 (12.3%) | 385 (87.7%) | ref | |||
| ≤ 18 years | 5 (10.4%) | 43 (89.6%) | 0.85 (0.38 – 1.88) | 0.683 | ||
| Marital status | ||||||
| Married/living with a partner | 32 (11.2%) | 253 (88.8%) | ref | 0.590 | ||
| Unmarried/not living with a partner | 27 (13.4%) | 175 (86.6%) | 1.19 (0.63 – 2.24) | |||
| Currently in school (N=485) | ||||||
| No | 47 (12.3%) | 336 (87.7%) | ref | |||
| Yes | 12 (11.8%) | 90 (88.2%) | 0.96 (0.66 – 1.39) | 0.823 | ||
| Regularly employed (N=486) | ||||||
| No | 49 (11.8%) | 367 (88.2%) | ref | |||
| Yes | 9 (12.9%) | 61 (87.1%) | 1.09 (0.75 – 1.58) | 0.644 | ||
| Household crowding | ||||||
| ≤ 3 people per room | 53 (12.3%) | 377 (87.7%) | ref | |||
| > 3 people per room | 6 (10.5%) | 51 (89.5%) | 0.85 (0.42 – 1.72) | 0.658 | ||
| Pregnancy history and family planning use | ||||||
| Ever been pregnant before | ||||||
| No | 3 (3.4%) | 86 (96.6%) | ref | ref | ||
| Yes | 56 (14.1%) | 342 (85.9%) | 4.17 (2.97 – 5.87) | <0.001* | 4.16 (3.09 – 5.61) | <0.001* |
| Time since most recent pregnancy (n=379) | ||||||
| ≥ 12 months | 13 (10.5) | 111 (89.5%) | ref | |||
| < 12 months | 40 (15.7%) | 215 (84.3%) | 1.50 (1.05 – 2.14) | 0.026* | 1.49 (1.07 – 2.07) | 0.018* |
| Family planning services sought today (N=643) | ||||||
| Initiating FP method today | 28 (10.9%) | 229 (89.1%) | ref | |||
| Refilling/continuing FP | 27 (13.1%) | 179 (86.9%) | 1.20 (0.86 – 1.69) | 0.287 | ||
| Removing FP method today | 4 (16.7%) | 20 (83.3%) | 1.53 (0.50 – 4.66) | 0.454 | ||
| Family planning methodb (N=463) | ||||||
| Injectable | 22 (10.7%) | 184 (89.3%) | ref | |||
| Intra-uterine device | 2 (15.4%) | 11 (84.6%) | 1.44 (0.65 – 3.20) | 0.370 | ||
| Implant | 25 (14.3%) | 150 (85.7%) | 1.34 (0.81 – 2.22) | 0.260 | ||
| Condoms | 1 (10.0%) | 9 (90.0%) | 0.94 (0.11 – 8.09) | 0.952 | ||
| Oral contraceptive pills | 5 (8.5%) | 54(91.5%) | 0.79 (0.39 – 1.62) | 0.525 | ||
| Partner and psychosocial characteristics | ||||||
| Has current partner | ||||||
| Yes | 38 (9.4%) | 366 (90.6%) | ref | ref | ||
| No | 21 (25.3%) | 62 (74.7%) | 2.69 (1.68 – 4.30) | <0.001* | 2.70 (1.52 – 4.78) | 0.001* |
| Partner age difference (n=366) | ||||||
| <10 years | 27 (8.2%) | 301 (91.8%) | ref | ref | ||
| ≥10 years | 8 (21.1%) | 30 (78.9%) | 2.56 (1.49 – 4.40) | 0.001* | 2.27 (1.12 – 4.58) | 0.023 |
| Social support score (N=486) | ||||||
| High social support (≥72) | 19 (6.9%) | 255 (93.1%) | ref | ref | ||
| Low social support (<72) | 40 (18.9%) | 172 (81.1%) | 2.72 (1.47 – 5.05) | 0.002* | 2.60 (1.52– 4.47) | 0.001* |
Prevalence ratios (PR) were adjusted for age and prior pregnancy. PR for prior pregnancy was adjusted for age. PR for time since prior pregnancy was adjusted for age.
After receipt of FP services today
Significance level ≤ 5%
Depression and behavioral risk factors for HIV
One in five (105/487, 22%) AGYW had HIV risk scores ≥5 and were defined as having high risk for HIV acquisition. Frequency of high HIV risk was nearly two-fold higher among AGYW with MSD compared to those without MSD after adjustment for prior pregnancy (34% vs 20%, aPR:1.7 95% CI:1.3–2.2, p<0.001). Frequencies of all individual factors contributing to the HIV risk score were also higher among AGYW with MSD after adjustment for age and prior pregnancy, except for marital status and primary partner having other partners (Table III, Figure 2).
Table III.
Prevalence of HIV risk factors by depression status among AGYW seeking FP services in Western Kenya (n=487)
| Characteristic | Depressed (CESD≥10) N(%) |
Not depressed (CESD<10) N(%) |
Prevalence ratio PR (95% CI) |
p-value | aPRa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| HIV risk factors | ||||||
| Total lifetime sexual partners ≥ 4 | 21 (35.6%) | 58 (13.6%) | 2.63 (1.14 – 6.05) | 0.023 | 2.37 (1.16 – 4.82 | 0.017* |
| Sex without a condomc | 42 (71.2%) | 337 (78.7%) | 0.90 (0.69 – 1.19) | 0.473 | – | – |
| Transactional sexc | 5 (8.5%) | 10 (2.3%) | 3.63 (1.30 – 10.11) | 0.014* | 3.78 (1.31 – 10.95) | 0.014* |
| Forced sexc | 10 (16.9%) | 16 (3.7%) | 4.53 (1.56 – 13.16) | 0.005* | 4.59 (1.56 – 13.54) | 0.006* |
| Intimate partner violenced | 7 (18.4%) | 6 (1.6%) | 11.24 (2.44 – 51.70) | 0.002* | 10.25 (3.09 – 33.98) | <0.001* |
| High self-perceived HIV riske | 29 (49.2%) | 41 (9.6%) | 5.13 (2.73 – 9.66) | <0.001* | 4.47 (2.53 – 7.91) | <0.001* |
| HIV risk score factors (Balkus et. al.b) | ||||||
| High HIV risk (Balkus risk score: ≥ 5)^ | 20 (33.9%) | 85 (19.9%) | 1.71 (1.31 – 2.22 | <0.001* | 2.14 (1.80 – 2.54) | <0.001* |
| Unmarried/not living with partner | 27 (45.8%) | 175 (40.9%) | 1.12 (0.74 – 1.70) | 0.599 | – | – |
| Alcohol use (any in the past 30 days) | 14 (23.7%) | 54 (12.6%) | 1.88 (1.36 – 2.60) | <0.001* | 2.13 (1.44 – 3.14) | <0.001* |
| Partner does not provide financial support | 3 (5.1%) | 9 (2.1%) | 2.42 (1.04 – 5.64) | 0.041* | 1.96 (0.78 – 4.92) | 0.153 |
| Primary partner has other partners (Yes/Do not know) |
28 (47.5%) | 151 (35.3%) | 1.35 (0.97 – 1.87) | 0.076 | – |
Prevalence ratios were adjusted for age and prior pregnancy. The prevalence ratio for high HIV risk score was adjusted for prior pregnancy.
Balkus risk scoring: Age <25 = 1 (all participants in this analysis are <25, thus we have excluded age from the table but included the age score in the risk score calculation), Married = 2, any alcohol = 1, partner provides financial support = 1, partner has other partners: yes = 2, do not know = 2
Within the last 6 months
Intimate partner violence = HiTS score ≥10 (n=404)
High self-perceived HIV risk = Somewhat/very likely to acquire HIV; Low self-perceived HIV risk = Extremely/very unlikely to acquire HIV
Significance level ≤ 5%
Figure 2.
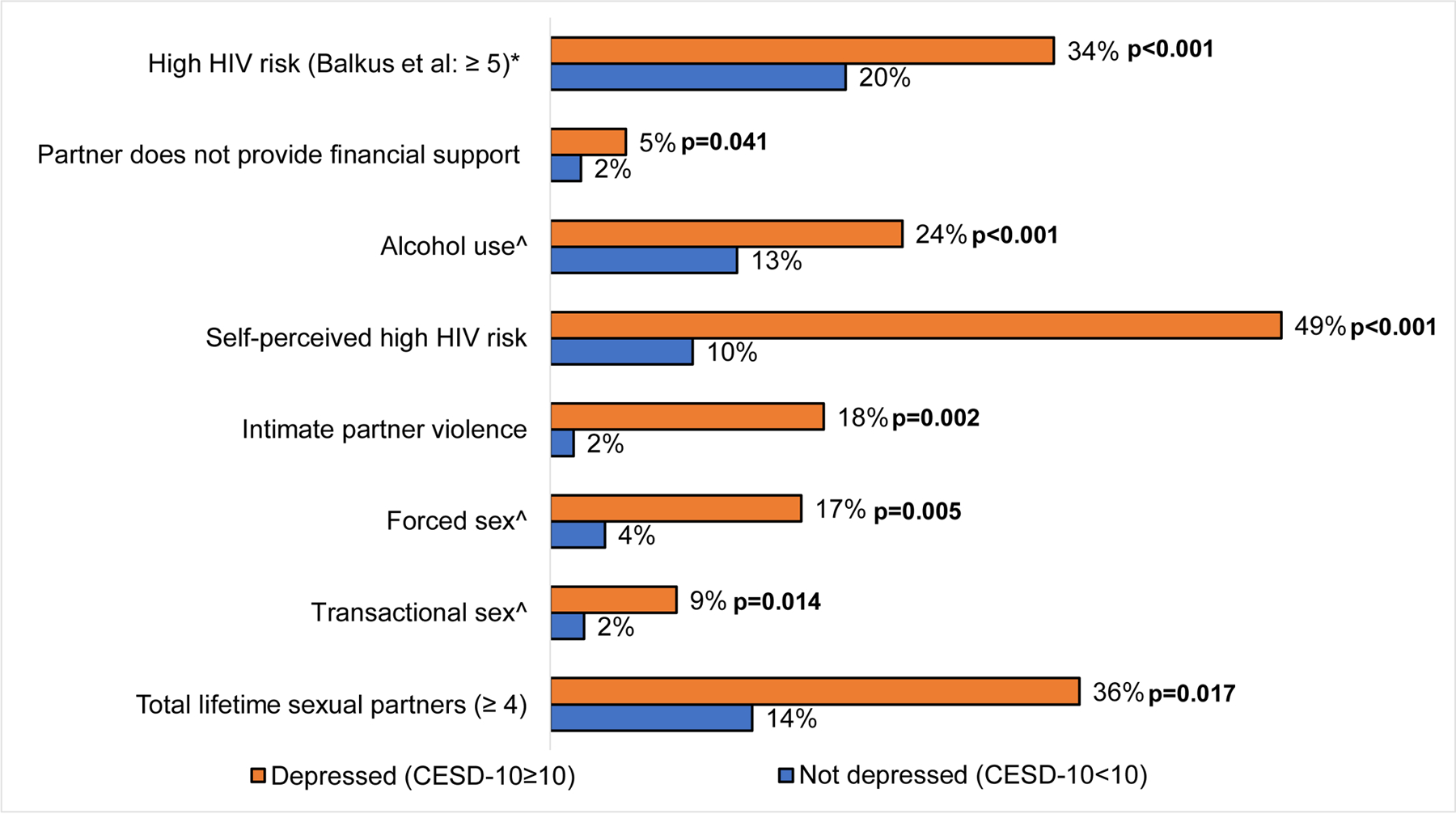
Frequency distribution of behavioral HIV risk factors associated with depression among AGYW seeking FP services in Western Kenya (n=487)**
**P-values are from unadjusted prevalence ratios
*Balkus risk scoring: Age <25 = 1 (all participants in this analysis are <25, thus we have excluded age from the figure but included the age score in the risk score calculation), Married = 2, any alcohol = 1, partner provides financial support = 1, partner has other partners: yes = 2, do not know = 2
^Experience reported for the last 6 months
Among other HIV risk factors not included in the risk score, having ≥4 lifetime sexual partners was more frequently reported among depressed AGYW than non-depressed AGYW after adjustment for age and prior pregnancy (36% vs. 14%, aPR:2.4, 95% CI:1.2–4.8, p=0.017). Frequency of IPV was 10 times higher among AGYW with MSD than those without MSD (18% vs 2%, aPR:10.3, 95% CI: 3.1–33.9, p<0.001). Women with MSD also more frequently had transactional sex in the last 6 months (aPR:3.8, 95% CI:1.3–10.9, p=0.014) and more frequently were forced to have sex in the last 6 months (PR:4.6, 95%CI:1.6–13.5, p=0.006) than women without MSD. AGYW with MSD self-perceived they were “somewhat or very likely” to acquire HIV in the next year five times more frequently than those without MSD (49% vs. 10%, aPR:4.5, 95% CI: 2.5–7.9, p<0.001). Frequency of having sex without a condom in the last 6 months did not differ by depression status (Table III, Figure 2).
DISCUSSION
In this study of Kenyan AGYW seeking FP services, more than one in ten AGYW had symptoms of moderate-to-severe depression (MSD) and having MSD was associated with high frequency of HIV risk behaviors. MSD was more frequent among AGYW without a current stable partner, those with a >10 year age difference with their partners, those with low social support, and those with a prior pregnancy. To our knowledge, this is the first assessment of the relationship between MSD and HIV risk behaviors among AGYW attending FP clinics. Sixty percent of Kenyan women using contraception attend public sector FP clinics [13], presenting an accessible platform for reaching young women in need of mental health and HIV services. Our study contributes data on the overlapping burden of depression and HIV behavioral risk among women seeking FP services.
We found that 12% of AGYW seeking FP services had symptoms of moderate-to-severe depression, consistent with global estimates of 10–20% among adolescents and young adults [3, 8]. This is also within the range of depression prevalence found in prior studies among AGYW in SSA, including East Africa (12–36%) [18–20, 22, 42]. The higher prevalence of depression in some previous studies may be due to the influence of pregnancy [43, 44], HIV [45–47], or household sampling that reached depressed individuals missed at facilities [48].
Recent data from HIV prevention trials conducted among AGYW with high risk of HIV acquisition in FP clinics suggest HIV incidence is high in this setting [49]. In our study, AGYW seeking FP services frequently had HIV risk factors (22%). This was consistent with findings from the study populations used to derive (VOICE study) [50] and validate (HPTN 035 study) [51] the Balkus et al. HIV risk score, where frequency of having a HIV risk score >5 was 46% and 29%, respectively [40]. Our findings that frequency of multiple HIV risk factors was higher among AGYW with depression was consistent with results from similar studies in Uganda and South Africa [13, 18, 20, 42, 52]. Our study is the first to apply an empiric HIV risk assessment tool to assess the relationship between depression and HIV risk in AGYW [40]. Depression was associated with having a high HIV risk score which has been specifically associated with high HIV incidence among AGYW in SSA. Overall, 21% of all AGYW and 34% of AGYW with MSD attending FP services in our study had a HIV risk score >5 which has been associated with HIV incidence of 5–15 per 100 person-years [40]. These young women are particularly vulnerable and need to be prioritized for HIV prevention interventions.
In our study, 68% of AGYW with MSD were within 12 months of a prior pregnancy and recent pregnancy was associated with higher risk of MSD. Depression after childbirth is common [44, 53, 54] as pregnancy and postpartum are periods of major physiologic and psychosocial change [55, 56]. These changes are compounded by the hormonal and biological developments of adolescence [3] in young mothers. Postpartum women may be a group to target for depression screening within FP clinics. Depression may modify adherence to FP interventions and have negative effects on other health outcomes. Thus, depression detection and management during FP services could have broad health benefits for women. Given frequent access to FP services by AGYW in Kenya, FP clinics could be a strategic venue to diagnose depression.
Similar to a study from South Africa, we found a relationship between depression and having older male sexual partners [18]. Some studies suggest that HIV incidence among AGYW may be partially driven by partnerships with older men, who are more likely than younger men to be HIV-infected [9, 57, 58]. Age-disparate relationships among young women may pose both a concurrent mental health and HIV risk. Screening for depressive symptoms among AGYW with older partners could enable psychosocial support concurrent with PrEP. Our finding that low social support and not having a current partner is associated with depression is widely evidenced across diverse settings and mental disorders [8, 59], including studies in Africa [19, 60]. Mental health interventions that provide social support, such as peer counseling and support groups [61–64], have been successful in other African clinic-based settings and could potentially be integrated into FP clinics to reach AGYW who regularly access health facilities.
The World Health Organization advocates for integration of mental health services into routinely attended care settings [65]. FP services are well-attended by AGYW in Africa [11]; 60% of Kenyan women access FP from the public sector [13]. Health care workers delivering FP services routinely counsel patients about sensitive topics such as sexual health and relationship issues. Despite high prevalence of depression risk in adolescence, currently most cases go undetected and untreated in SSA due to a dearth of mental health professionals and services [7, 66]. Expanding counseling topics in FP clinics to include depression screening and discussion of HIV prevention methods is a natural next step to address these intersecting health issues [14, 15]. Tailored counseling may be required to support AGYW with MSD to navigate HIV prevention, including PrEP use and adherence, directly addressing their depression as a potential barrier to motivation and self-efficacy.
Limitations
Our study was limited by its cross-sectional design and therefore we are unable to assess temporal relationships between depression and HIV risk factors. Some behavioral HIV risk factors, such as transactional sex, could be an antecedent or consequence of depression. We sampled AGYW seeking FP services in public sector clinics [13]. Our results may not be generalizable to AGYW who seek FP in private facilities or pharmacies. However, a substantial proportion of Kenyan women access FP services from public sector clinics and we intentionally designed our study to gather evidence within this high coverage setting. We used self-reported information from participants, including information on partner and relationship characteristics which could introduce reporting bias. Future evaluations could incorporate male partners or confirm information with clinical records (e.g., male partner HIV status). Finally, depressive symptoms were identified using a psychosocial scale validated in SSA settings, including among youth in Kenya [32], but did not include formal diagnosis of depression by a clinician.
CONCLUSION
We found that AGYW seeking FP services in Western Kenya frequently had symptoms of depression. One third of AGYW with MSD had high risk of HIV acquisition based on a validated HIV risk score. AGYW with MSD also had higher frequency of individual HIV risk factors than AGYW without depression. AGYW routinely access FP clinics, yet mental health services are typically unavailable in this context. Depression screening and HIV prevention interventions should be integrated into FP clinics that are routinely attended by AGYW in SSA.
Acknowledgements:
We thank the PrIYA study team and clients for their time and contributions. We thank the Kenyan Ministry of Health nationally and the Kisumu County Department of health, as well as the facility heads and in-charges for their collaboration.
Funding: This study was funded by the US National Institutes of Health (R01HD094630). The PrEP Implementation for Young Women and Adolescents (PrIYA) Program was funded by the United States Department of State as part of the DREAMS Innovation Challenge (Grant # 37188–1088 MOD01), managed by JSI Research & Training Institute, Inc. The PrIYA Team was supported by the University of Washington’s Center for AIDS Research (CFAR) (P30 AI027757) and the Global Center for Integrated Health of Women, Adolescents, and Children (Global WACh).
Footnotes
Publisher's Disclaimer: Disclaimer: This work was funded by a grant from the United States Department of State as part of PEPFAR’s DREAMS Partnership, managed by JSI Research & Training Institute, Inc. (JSI). The opinions, findings, and conclusions stated herein are those of the authors and do not necessarily reflect those of the United States Department of State or JSI.
Conflicts of Interest: The authors have no financial conflicts of interest to declare.
References
- [1].World Health Organization. Adolescent health. World Health Organization, https://www.who.int/maternal_child_adolescent/adolescence/en/ (2019, accessed 22 March 2019).
- [2].Patton GC, Sawyer SM, Santelli JS, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet (London, England) 2016; 387: 2423–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. Adolescent mental health, https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health (2018, accessed 23 March 2019).
- [4].Nagata JM, Ferguson BJ, Ross DA. Research Priorities for Eight Areas of Adolescent Health in Low- and Middle-Income Countries. J Adolesc Health 2016; 59: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kohn R, Saxena S, Levav I, et al. The treatment gap in mental health care. Bull World Health Organ 2004; 82: 858–66. [PMC free article] [PubMed] [Google Scholar]
- [6].World Health Organization. Gender and women’s mental health. World Health Organization, https://www.who.int/mental_health/prevention/genderwomen/en/ (2013, accessed 24 August 2019). [Google Scholar]
- [7].Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012; 90: 139–149H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kieling C, Baker-Henningham H, Belfer M, et al. Child and adolescent mental health worldwide: evidence for action. Lancet 2011; 378: 1515–1525. [DOI] [PubMed] [Google Scholar]
- [9].Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: Key populations for HIV epidemic control. J Int AIDS Soc 2015; 18: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shannon K, Strathdee SA, Goldenberg SM, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet 2015; 385: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].African Population and Health Research Center. Family Planning in East Africa: Trends and Dynamics, http://aphrc.org/wp-content/uploads/2018/01/Family-Planning-in-East-Africa-Report_January-2018.pdf (2018, accessed 16 August 2019).
- [12].DSW. Family Planning in Kenya: A review of national and district policies and budgets, https://www.dsw.org/uploads/tx_aedswpublication/family-planning-kenya_update.pdf (2014, accessed 11 September 2019).
- [13].National Bureau of Statistics Nairobi K. Republic of Kenya Kenya Demographic and Health Survey 2014, www.DHSprogram.com (2015, accessed 1 June 2019).
- [14].Brant A, Dhillon P, Hull S, et al. Integration of HIV preexposure prophylaxis (PrEP) services with family planning services: an evaluation using the RE-AIM framework. Contraception 2018; 98: 368. [Google Scholar]
- [15].Seidman D, Weber S, Carlson K, et al. Family planning providers’ role in offering PrEP to women. Contraception 2018; 97: 467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mugwanya KK, Pintye J, Kinuthia J, et al. Integrating preexposure prophylaxis delivery in routine family planning clinics: A feasibility programmatic evaluation in Kenya. PLOS Med 2019; 16: e1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cowan FM, Delany-Moretlwe S, Sanders EJ, et al. PrEP implementation research in Africa: what is new? In: J Int AIDS Soc. Department of International Public Health, Liverpool School of Tropical Medicine, Liverpool, UKWits Reproductive Health and HIV Institute, University of Witwatersrand, Johannesburg, South AfricaKenya Medical Research Institute, Nairobi, KenyaCenter for Cl: Epub ahead of print 2016 DOI: 10.7448/ias.19.7.21101. [DOI] [Google Scholar]
- [18].Nduna M, Jewkes RK, Dunkle KL, et al. Associations between depressive symptoms, sexual behaviour and relationship characteristics: a prospective cohort study of young women and men in the Eastern Cape, South Africa. J Int AIDS Soc 2010; 13: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Osok J, Kigamwa P, Vander Stoep A, et al. Depression and its psychosocial risk factors in pregnant Kenyan adolescents: a cross-sectional study in a community health Centre of Nairobi. BMC Psychiatry 2018; 18: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lundberg P, Rukundo G, Ashaba S, et al. Poor mental health and sexual risk behaviours in Uganda: A cross-sectional population-based study. BMC Public Health 2011; 11: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kuringe E, Materu J, Nyato D, et al. Prevalence and correlates of depression and anxiety symptoms among out-of-school adolescent girls and young women in Tanzania: A cross-sectional study. PLoS One 2019; 14: e0221053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bonful HA, Anum A. Sociodemographic correlates of depressive symptoms: a cross-sectional analytic study among healthy urban Ghanaian women. BMC Public Health 2019; 19: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Atuhaire C, Cumber SN. Factors associated with postpartum depression among adolescents in Uganda. Pan Afr Med J 2018; 30: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Velloza J, Baeten JM, Haberer J, et al. Effect of Depression on Adherence to Oral PrEP Among Men and Women in East Africa. J Acquir Immune Defic Syndr 2018; 79: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kinuthia J, Pintye J, Abuna F, et al. PrEP uptake among pregnant and postpartum women: Results from a large implementation program within routine maternal child health (MCH) clinics in Kenya (Abstract #1366). Conf Retroviruses Opportunistic Infect (CROI), http://programme.aids2018.org/Abstract/Abstract/7484 (2018, accessed 8 July 2019). [Google Scholar]
- [26].National AIDS & STI Control Program. Kenya HIV Country Profiles 2016, http://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf (2016, accessed 8 July 2019).
- [27].Gumbe A, McLellan-Lemal E, Gust DA, et al. Correlates of prevalent HIV infection among adults and adolescents in the Kisumu incidence cohort study, Kisumu, Kenya. Int J STD AIDS 2015; 26: 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Akinyi B, Odhiambo C, Otieno F, et al. Prevalence, incidence and correlates of HSV-2 infection in an HIV incidence adolescent and adult cohort study in western Kenya. PLoS One 2017; 12: e0178907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rhodes T The ‘risk environment’: a framework for understanding and reducing drug-related harm. Int J Drug Policy 2002; 13: 85–94. [Google Scholar]
- [30].Center for Epidemiologic Studies Depression Scale Revised (CESD-R-10), https://www.brandeis.edu/roybal/docs/CESD-10_website_PDF.pdf (accessed 14 December 2018).
- [31].Baron EC, Davies T, Lund C. Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry 2017; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kilburn K, Prencipe L, Hjelm L, et al. Examination of performance of the Center for Epidemiologic Studies Depression Scale Short Form 10 among African youth in poor, rural households. BMC Psychiatry 2018; 18: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med; 10: 77–84. [PubMed] [Google Scholar]
- [34].Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med 2009; 36: 439–445.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hurt, Insulted, Threatened with Harm and Screamed (HITS) Domestic Violence Screening Tool.
- [36].Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32: 705–714. [DOI] [PubMed] [Google Scholar]
- [37].Kornblith AB, Herndon JE, Zuckerman E, et al. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer 2001; 91: 443–454. [DOI] [PubMed] [Google Scholar]
- [38].Gold KJ, Spangenberg K, Wobil P, et al. Depression and risk factors for depression among mothers of sick infants in Kumasi, Ghana. Int J Gynecol Obstet 2013; 120: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].National AIDS & STI Control Program. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2016 Edition, https://aidsfree.usaid.gov/sites/default/files/kenya_art_2016.pdf (2016, accessed 8 July 2019).
- [40].Balkus JE, Brown E, Palanee T, et al. An Empiric HIV Risk Scoring Tool to Predict HIV-1 Acquisition in African Women. JAIDS J Acquir Immune Defic Syndr 2016; 72: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Napper LE, Fisher DG, Reynolds GL. Development of the perceived risk of HIV scale. AIDS Behav 2012; 16: 1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smit J, Myer L, Middelkoop K, et al. Mental health and sexual risk behaviours in a South African township: A community-based cross-sectional study. Public Health 2006; 120: 534–542. [DOI] [PubMed] [Google Scholar]
- [43].O’hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry 1996; 8: 37–54. [Google Scholar]
- [44].Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord 2017; 219: 86–92. [DOI] [PubMed] [Google Scholar]
- [45].Boarts JM, Buckley-Fischer BA, Armelie AP, et al. The Impact of HIV Diagnosis-Related vs. Non-Diagnosis Related Trauma on PTSD, Depression, Medication Adherence, and HIV Disease Markers. J Evid Based Soc Work 2009; 6: 4–16. [DOI] [PubMed] [Google Scholar]
- [46].Endeshaw M, Walson J, Rawlins S, et al. Stigma in Ethiopia: association with depressive symptoms in people with HIV. AIDS Care 2014; 26: 935–939. [DOI] [PubMed] [Google Scholar]
- [47].Nyirenda M, Chatterji S, Rochat T, et al. Prevalence and correlates of depression among HIV-infected and -affected older people in rural South Africa- ClinicalKey. J Affect Disord 2013; 151: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Andersson LMC, Schierenbeck I, Strumpher J, et al. Help-seeking behaviour, barriers to care and experiences of care among persons with depression in Eastern Cape, South Africa. J Affect Disord 2013; 151: 439–448. [DOI] [PubMed] [Google Scholar]
- [49].Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium K, Baeten JM, Beksinska M, et al. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet (London, England) 2019; 394: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med 2015; 372: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Van Damme L, Corneli A, Ahmed K, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med 2012; 367: 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jewkes R, Dunkle K, Nduna M, et al. Factors associated with HIV sero-status in young rural South African women: connections between intimate partner violence and HIV. Int J Epidemiol 2006; 35: 1461–1468. [DOI] [PubMed] [Google Scholar]
- [53].World Health Organization. Maternal mental health. WHO, http://www.who.int/mental_health/maternal-child/maternal_mental_health/en/ (2015, accessed 1 November 2018). [Google Scholar]
- [54].O’hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry 1996; 8: 37–54. [Google Scholar]
- [55].World Health Organisation (WHO). Adolescent pregnancy, https://www.who.int/en/news-room/fact-sheets/detail/adolescent-pregnancy (2018, accessed 23 March 2019).
- [56].Reid V, Meadows-Oliver M. Postpartum Depression in Adolescent Mothers: An Integrative Review of the Literature. J Pediatr Heal Care 2007; 21: 289–298. [DOI] [PubMed] [Google Scholar]
- [57].Stoner MCD, Nguyen N, Kilburn K, et al. Age-disparate partnerships and incident HIV infection in adolescent girls and young women in rural South Africa. AIDS 2018; 33: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Haines C, Loades ME, Coetzee BJ, et al. Which HIV-infected youth are at risk of developing depression and what treatments help? A systematic review focusing on Southern Africa. Int J Adolesc Med Health; 0. Epub ahead of print 6 August 2019 DOI: 10.1515/ijamh-2019-0037. [DOI] [PubMed]
- [59].Grav S, Hellzèn O, Romild U, et al. Association between social support and depression in the general population: the HUNT study, a cross-sectional survey. J Clin Nurs 2012; 21: 111–120. [DOI] [PubMed] [Google Scholar]
- [60].Heyningen T van, Myer L, Onah M, et al. Antenatal depression and adversity in urban South Africa. J Affect Disord 2016; 203: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kaaya SF, Blander J, Antelman G, et al. Randomized controlled trial evaluating the effect of an interactive group counseling intervention for HIV-positive women on prenatal depression and disclosure of HIV status. AIDS Care 2013; 25: 854–862. [DOI] [PubMed] [Google Scholar]
- [62].Chibanda D, Weiss HA, Verhey R, et al. Effect of a Primary Care–Based Psychological Intervention on Symptoms of Common Mental Disorders in Zimbabwe. JAMA 2016; 316: 2618. [DOI] [PubMed] [Google Scholar]
- [63].Chibanda D, Mesu P, Kajawu L, et al. Problem-solving therapy for depression and common mental disorders in Zimbabwe: piloting a task-shifting primary mental health care intervention in a population with a high prevalence of people living with HIV. BMC Public Health 2011; 11: 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nakimuli-Mpungu E, Wamala K, Okello J, et al. Group support psychotherapy for depression treatment in people with HIV/AIDS in northern Uganda: a single-centre randomised controlled trial. Lancet HIV 2015; 2: e190–e199. [DOI] [PubMed] [Google Scholar]
- [65].WHO | Integrating mental health into primary care: a global perspective. WHO. [Google Scholar]
- [66].World Health Organization. World Health Organization Mental Health Atlas 2011, http://www.who.int/about/licensing/copyrig (2011, accessed 4 November 2018).


