Abstract
Objective
Liraglutide (Lir) protects cardiomyocytes against high glucose-induced myocardial damage. This study investigated whether Notch signaling participated in the antiapoptotic effects of Lir on rat H9c2 cardiomyocytes subjected to hypoxia followed by reoxygenation (H/R).
Methods
We used H9c2 rat cardiomyocytes as a model of H/R and measured viability, apoptosis, and expression of the apoptotic genes Bax and Bcl-2 and Notch signaling genes Notch1 and Jagged1. Notch1 was depleted by siRNA to test the effect of Notch1 deficiency on the antiapoptotic effects of Lir on H/R-treated H9c2 cardiomyocytes.
Results
After H/R treatment, viability was significantly decreased, and the apoptosis rate was greater in the H/R group than in the control (CT). Lir at 50, 100, and 200 nM significantly increased viability and decreased apoptosis in H/R-treated H9c2 cells. Treatment with 50 nM Lir for 2 hours before H/R significantly increased the expression levels of Notch1, Jagged1, and Bcl-2 compared with the CT levels. Bax was downregulated, which indicated that Lir activated Notch signaling and inhibited apoptosis. Notch1 depletion partially abolished the antiapoptotic effect of Lir on H/R-treated H9c2 cells by altering apoptotic gene expression.
Conclusion
Lir activated Notch signaling, which was responsible for the antiapoptotic effect of Lir on H9c2 cardiomyocytes.
Keywords: Liraglutide, glucagon-like peptide-1, cardiomyocyte, apoptosis, Notch signaling, hypoxia/reoxygenation model, myocardial infarction, ischemia/reperfusion injury
Introduction
Myocardial reperfusion is the chief strategy for preventing ischemic injury; however, it can also induce myocardial ischemia/reperfusion (I/R) injury (MIRI). I/R-induced cardiomyocyte apoptosis is a vital pathological process. Increasing evidence indicates that glucagon-like peptide 1 (GLP-1), an incretin hormone in the gastrointestinal tract that stimulates insulin secretion, can activate antiapoptotic signaling pathways to protect the heart against MIRI.1–3
Liraglutide (Lir) is a GLP-1 receptor agonist that protects cardiomyocytes against injury associated with high glucose levels in the infarcted heart.4,5 At present, the underlying molecular mechanism of the antiapoptotic effects of Lir on cardiomyocytes has not been fully elucidated. Previous studies demonstrated that the cardioprotective effects of GLP-1 were mediated through the classical GLP-1 receptor (GLP-1R) and included reduced I/R-induced damage, increased energy substrate utilization, and reduced oxidative stress and apoptosis.6 GLP-1 activates GLP-1R and increases intracellular cAMP levels, which lead to activation of the protein kinase A (PKA) pathway and the exchange protein activated by cAMP (Epac-1) pathway.7,8 Previous research suggested that both the PKA and Epac-1 pathways were required for the GLP-1– and Lir-mediated inhibition of oxidative stress and apoptosis in cardiomyocytes.2,3 Recent studies indicated that Notch signaling plays an important role in the protective response to I/R-induced oxidative stress and myocardial damage.9 Notch1 prevents cardiomyocyte apoptosis by activating the PI3K/AKT pro-survival pathway and regulating apoptotic gene expression.10–13 Consequently, we hypothesized that Notch signaling may be involved in the antiapoptotic effects of Lir on cardiomyocytes.
The present study investigated whether the Notch signaling pathway was involved in the cardioprotective effects of Lir against H/R-induced apoptosis in rat cardiomyocytes. We focused on the effects of Lir on H/R-induced H9c2 cell apoptosis. Our results demonstrated that Lir could promote Notch1 expression, which inhibited H9c2 cell apoptosis.
Materials and Methods
Ethics approval was not required because no humans or animals were used in this study.
H9c2 cell culture and Lir treatment
The rat cardiomyocyte cell line H9c2 was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (TransGen Biotech, Beijing, China), 100 units/mL penicillin (Lukang Pharmaceutical, Shandong, Jinan, China), and 100 µg/mL streptomycin (Lukang Pharmaceutical) in 5% CO2 in air (v/v) at 37°C. The cells were seeded in six-well plates and grown to approximately 70% confluence before incubation with Lir at 0, 50, 100, 200, or 500 nM for 2 hours before H/R.
The hypoxia/reoxygenation (H/R) cell model
The H/R cell model was established as previously described.13 Briefly, H9c2 cells were cultured in deoxygenated DMEM in a hypoxic atmosphere of 95% N2 and 5% CO2 at 37°C for 3 hours. The hypoxic medium was then replaced with reoxygenated medium, and the cells were cultured in 95% O2 and 5% CO2 for 3 hours. After the treatment, cell viability was determined using an MTT cell viability assay kit (Biolab Technology Co., Ltd, Beijing, China) following the manufacturer’s instructions.
Apoptosis assay
The apoptosis rate was measured by flow cytometry using an annexin V-FITC apoptosis detection kit (Beyotime, Beijing, China). Approximately 1 × 105 cells were collected and suspended in 200 µL of binding buffer. After adding 5 µL of annexin V-FITC and 10 µL of propidium iodide (PI), the cultures were incubated at room temperature in the dark for 15 min. The samples were analyzed via flow cytometry (Beckman Coulter). The apoptosis rate was calculated as the number of annexin V-positive cells divided by the number of PI-negative (living) cells.
Western blot analysis
Cells were lysed in protein lysis buffer (Beyotime) at 4°C, and aliquots were separated by 10% SDS-PAGE and transferred to a PVDF membrane (Millipore, Bedford, MA, USA)). The membranes were blocked with 5% milk for 1 hour at room temperature and incubated overnight at 4°C with the following primary antibodies: Bcl-2 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bax (1:500, Santa Cruz), Notch1 (1:200, Abcam, Cambridge, UK), Jagged1 (1:200, Abcam), GLP-1R (1:500, Abcam), and β-actin (1:1000, Abcam). After washing, the membranes were incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies (Beyotime) at a 1:1000 dilution. The signals were detected using an enhanced chemiluminescence system, and protein band densities were determined using ImageJ software (US National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/download.html). Relative protein levels were normalized to those of β-actin.
Small interfering RNA (siRNA) and RT-qPCR
w?>Three siRNAs targeting Notch1 gene transcription were designed and synthesized by Genepharma (Shanghai, China) (Table 1). The siRNAs were transfected into H9c2 cells, and the interference efficiency of siRNA was determined using qPCR. Total RNA was extracted from the cultured cells using TRIzol (Invitrogen, Thermo Fisher Scientific) according to the instructions and reverse-transcribed into cDNA using PrimeScript™ II cDNA synthesis kit (Takara, Dalian, China). SYBR® Premix Ex Taq™ II (Takara, Dalian, China) was used for RT-qPCR. The sequences of the three siRNAs and the primer sequences are listed in Table 1. GAPDH was used as the internal control for normalization. Relative gene expression was determined by the 2−ΔΔCt method.
Table 1.
Primers for qPCR and siRNA sequences.
| Name | Sequence (5′–3′) | Size (bp) |
|---|---|---|
| GAPDH | F: CCAGGGGTGCCTTCTCTTR: CCGTGGGTAGAGTCATACTGG | 124 |
| Notch1 | F: GAGGCGTGGCAGACTATGCR: CTTGTACTCCGTCAGCGTGA | 140 |
| Notch1 siRNA1 | CAGCAAACATCCAGCAGCAGCAAAG | |
| Notch1 siRNA2 | CAAACATCCAGCAGCAGCAAAGCCT | |
| Notch1 siRNA3 | CCAGCAAACATCCAGCAGCAGCAAA |
Experimental design
Five H9c2 cell groups were used for analysis. The control (CT) group was transfected with control siRNA. The H/R group was transfected with control siRNA and subjected to H/R. The H/R+siRNA1 group was transfected with 100 µM siRNA1 for 24 hours before H/R. The H/R+Lir group was transfected with control siRNA and treated with 50 nM Lir for 2 hours before H/R. The H/R+siRNA1+Lir group was transfected with siRNA1 and treated with 50 nM Lir for 2 hours before H/R.
Data analysis
Data were analyzed by one-way ANOVA using SPSS 19.0 (IBM, Chicago, IL, USA). The data were expressed as the mean ± SEM, and differences were considered statistically significant at P < 0.05.
Results
Lir reduced H/R-induced injury
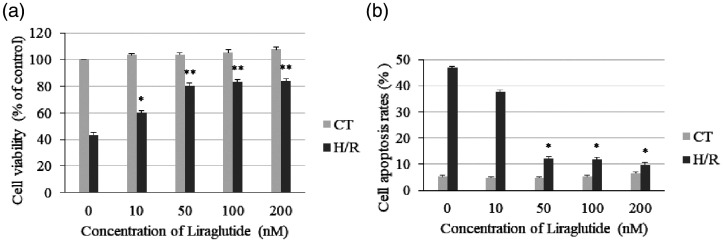
The H9c2 cell viability and apoptosis rates were determined after Lir and H/R treatment. As presented in Figure 1a, cell viability was significantly decreased in the H/R group with that in the CT group (P < 0.001), and Lir significantly increased the cell viability in the H/R groups (10 nM, P < 0.05; 50, 100, and 200 nM, P < 0.001). The level of apoptosis was significantly higher in the H/R group than in the CT group (P < 0.001), and 50, 100, and 200 nM Lir significantly decreased apoptosis after H/R treatment (all P < 0.05, Figure 1b). Moreover, there were no significant differences in cell viability and apoptosis rates after treatment with 50, 100, and 200 nM Lir in both the CT and H/R groups. The results indicated that 50 nM Lir was sufficient to protect H9c2 cells against H/R-induced injury and was used in the following experiments.
Figure 1.
Effects of liraglutide (Lir) on viability and apoptosis in H9c2 cardiomyocytes exposed to hypoxia followed by reoxygenation (H/R). (a) Cell viability was determined using the MTT cell viability assay. (b) The apoptosis rate was measured using an annexin V-FITC assay. Data are expressed as the mean ± SEM (n = 3). *P < 0.05 and **P < 0.001 vs. 0 nM Lir in the H/R group. CT, control group.
Lir enhanced Notch signaling activity after H/R treatment
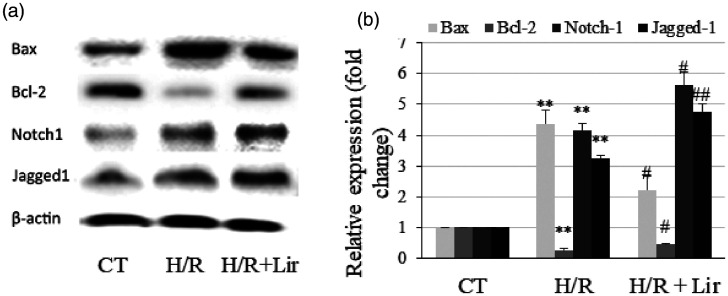
The protein expression levels of the apoptosis genes Bax and Bcl-2 and the Notch signaling genes Notch1 and Jagged1 were determined using western blotting. As presented in Figure 2a and b, Bax, Notch1, and Jagged1 expression was significantly upregulated after H/R treatment, whereas the expression of the antiapoptotic protein Bcl-2 was downregulated (P < 0.001 vs. CT). After treatment with Lir, the expression levels of Notch1, Jagged1, and Bcl-2 were significantly increased compared with those in the H/R group (all P < 0.05), and Bax was downregulated (P < 0.05). This result indicated that Lir activated Notch signaling and inhibited apoptosis.
Figure 2.
Effects of liraglutide (Lir) on the expression of Bax, Bcl-2, Notch1, and Jagged1. (a) The protein expression levels were determined using western blotting. (b) The protein bands were analyzed using ImageJ software. Protein levels were normalized to that of β-actin. Data are expressed as the mean ± SEM (n = 3). **P < 0.001 vs. the control (CT) group, #P < 0.05 and ##P < 0.001 vs. hypoxia/reoxygenation (H/R) group. For the H/R+Lir groups, H9c2 cells were treated with 50 nM Lir for 2 hours before H/R.
Notch1 is involved in the antiapoptotic effect of Lir on H/R-induced H9c2
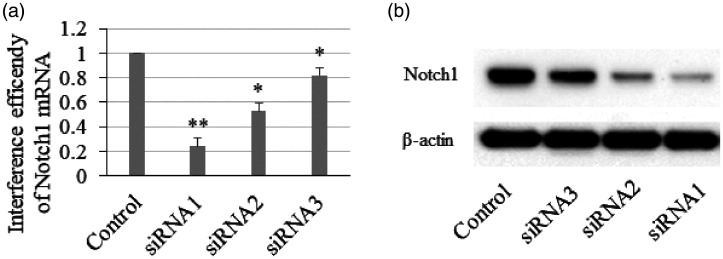
To determine whether Notch1 was involved in the antiapoptotic effect of Lir on H9c2 cardiomyocytes, we used siRNA to deplete Notch1 in H9c2 cells. The interference efficiencies of the three Notch1 siRNAs were measured by qPCR and western blotting. As presented in Figure 3a and b, siRNA1 was the most efficient siRNA, and it was used in subsequent experiments.
Figure 3.
Interference efficiency of Notch1 small interfering RNA in H9c2 cells. (a) The mRNA level of Notch1 was determined by RT-qPCR. (b) The Notch1 protein level was determined using western blotting (n = 3). *P < 0.05 and **P < 0.001 vs. control group.
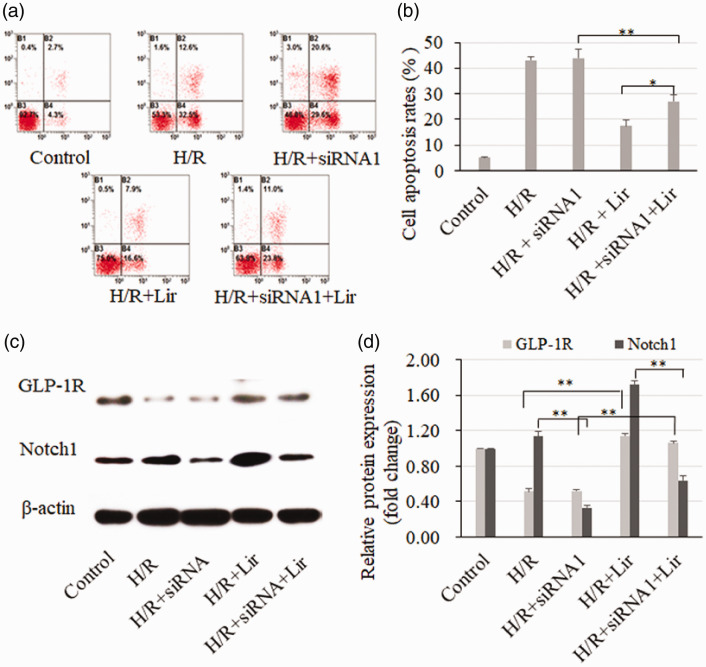
FACS was used to determine the apoptosis rates in the different groups (Figure 4a and b). Apoptosis was not affected after Notch1 depletion in H/R treatment groups. However, in the H/R and Lir treatment groups, apoptosis was significantly increased after the depletion of Notch1 (H/R+Lir vs. H/R+siRNA1+Lir, P < 0.05). Moreover, the western blot analysis confirmed that GLP-1R was activated by Lir and that Notch1 was significantly downregulated by siRNA (Figure 4c and d, P < 0.001). In combination with the results in Figure 1a, these data suggest that Notch1 enhanced the antiapoptotic effect of Lir on H/R-induced H9c2 cells.
Figure 4.
The apoptosis rate was measured using annexin V-FITC after liraglutide (Lir) treatment and hypoxia/reoxygenation (H/R) in H9c2 cells with Notch1 depletion (a). Apoptosis rate = annexin V-positive cells/PI-negative (living) cells (b). The GLP-1R and Notch1 protein levels were determined by western blotting (c and d). n = 3, *P < 0.05, **P < 0.001.
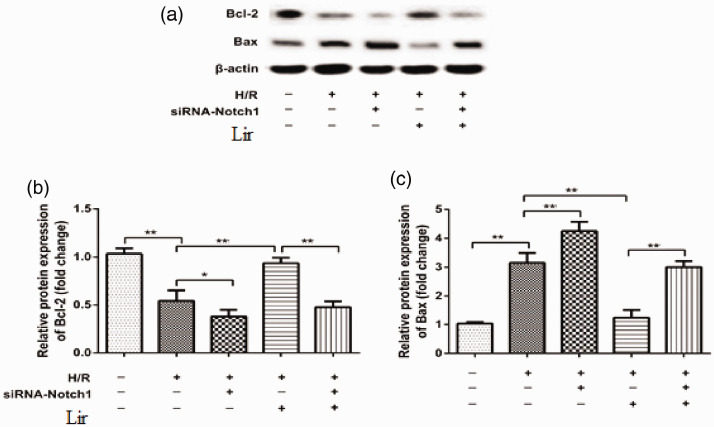
To further confirm that Notch1 was involved in the Lir antiapoptotic effect against H/R, we measured the protein expression of Bax and Bcl-2 by western blotting (Figure 5a). As presented in Figure 5b and c, Bcl-2 expression was decreased after the depletion of Notch1 in the H/R treatment groups (H/R vs. H/R+siRNA1, P < 0.05), and Bax protein expression was increased (H/R vs. H/R+siRNA1, P < 0.001). In the H/R and Lir treatment groups, the upregulation of Bax and downregulation of Bcl-2 were also obvious (H/R+siRNA1 vs. H/R+siRNA1+Lir, both P < 0.001). Taken together, our results demonstrated that Notch1 was involved in the Lir-mediated antiapoptotic effect on H9c2 cardiomyocytes.
Figure 5.
The protein expression levels of Bax and Bcl-2 were determined by western blotting after liraglutide (Lir) treatment and hypoxia/reoxygenation (H/R) in H9c2 cells with Notch1 depletion (a). The protein bands of Bax (b) and Bcl-2 (c) were quantitated using ImageJ software. Protein levels were normalized to that of β-actin. Data are expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.001).
Discussion
It has been reported that GLP-1 activates an antiapoptotic signaling pathway to protect cardiomyocytes against I/R-induced apoptosis.1,2,14 Notch signaling pathway activation is important for the protective response to I/R-induced cardiomyocyte apoptosis.10,13,15 The present study demonstrated that the GLP-1R agonist Lir could promote Notch1 expression to protect cardiomyocytes against H/R-induced apoptosis by altering Bax and Bcl-2 expression in H9c2 cells.
Several studies have reported that GLP-1 and GLP-1R agonists have cardioprotective effects and play an important role in the regulation of heart functions.6 Amal et al.1 reported for the first time that GLP-1 protected the infarcted heart against MIRI by activating PI3K and mitogen-activated protein kinase signaling. Subsequently, several studies found that the PKB/Akt, ERK1/2, PKA, and Epac-dependent pathways were involved in antiapoptotic activity.2,14,16 Increasing evidence indicates that in addition to reducing blood glucose levels and blood pressure, GLP-1 and GLP-1R agonists also improve lipid profiles and directly reduce the risks of heart failure and myocardial infarction.6
The GLP-1R agonist Lir is used to treat patients with type 2 diabetes mellitus, and its pharmacological action is similar to that of GLP-1. Some studies have demonstrated that Lir protects cultured cardiomyocytes against high glucose-induced cell apoptosis and oxidative stress and that the Epac-1/Akt and AMPK pathways may be involved in these effects.3,4,17 In addition, a previous study demonstrated that Lir could reduce cardiac fibrosis and myocardial death in a rat model of myocardial infarction.5 In this study, we found that treatment with 50 nM Lir for 2 hours before H/R could increase the viability and decrease the apoptosis rate in H/R-treated H9c2 cells by regulating Bcl-2 and Bax expression, demonstrating that Lir could protect cardiomyocytes against H/R-induced apoptosis.
Notch signaling has been revealed to play an important role in protecting cardiomyocytes against MIRI.9 Activation of the Notch1 pathway significantly reduced cardiomyocyte apoptosis, infarct size, and myocardial fibrosis and promoted coronary neo-angiogenesis and revascularization of the ischemic myocardium.18–20 In the present study, we found that Lir increased Notch1 and Jagged1 expression in H/R-exposed H9c2 cells, and the antiapoptotic effect of Lir was partially abolished after Notch1 depletion. This result indicated that Notch signaling may be involved in the protective effects of Lir on cardiomyocytes and further expands our understanding of the molecular mechanisms underlying the protective effects of Lir on the heart.
In conclusion, our findings indicated that Notch signaling was involved in the prevention of H/R-induced damage to cardiomyocytes through the action of Lir, which enhanced Notch1 expression. The Lir-mediated antiapoptotic effect was inhibited by Notch1 depletion. Our findings will contribute to elucidating the molecular mechanisms underlying the protective effects of Lir on the heart.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the Shaanxi Province Key Research and Development Program (2017SF-093) and the project of the Second Affiliated Hospital of Xi’an Medical University (18KY0107).
ORCID iD
Zihua Yang https://orcid.org/0000-0003-1725-9820
References
- 1.Bose AK, Mocanu MM, Carr RD, et al. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 2005; 54: 146–151. [DOI] [PubMed] [Google Scholar]
- 2.Mangmool S, Hemplueksa P, Parichatikanond W, et al. Epac is Required for GLP-1R-Mediated Inhibition of Oxidative Stress and Apoptosis in Cardiomyocytes. Mol Endocrinol 2015; 29: 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu XM, Ou QY, Zhao W, et al. The GLP-1 Analogue Lir Protects Cardiomyocytes from High Glucose-induced Apoptosis by Activating the Epac-1/Akt Pathway. Exp Clin Endocr Diab 2014; 122: 608–614. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LH, Li CG, Zhu QX, et al. Lir, a glucagon-like peptide-1 analog, inhibits high glucose-induced oxidative stress and apoptosis in neonatal rat cardiomyocytes. Exp Ther Med 2019; 17: 3734–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao HY, Ren HY, Du H, et al. Lir repairs the infarcted heart: The role of the SIRT1/Parkin/mitophagy pathway. Mol Med Rep 2018; 17: 3722–3734. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Poudyal H. Mechanisms for the cardiovascular effects of glucagon-like peptide-1. Acta Physiol 2016; 216: 277–313. [DOI] [PubMed] [Google Scholar]
- 7.Insel PA, Zhang L, Murray F, et al. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol 2012; 204: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Z, Jin T. New insights into the role of cAMP in the production and function of the incretin hormone glucagon-like peptide-1 (GLP-1). Cell Signal 2010; 22: 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Nistri S, Sassoli C, Bani D. Notch Signaling in Ischemic Damage and Fibrosis: Evidence and Clues from the Heart. Front Pharmacol 2017; 8: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Pang S, Huang J, et al. Blockade of RBP-J-Mediated Notch Signaling Pathway Exacerbates Cardiac Remodeling after Infarction by Increasing Apoptosis in Mice. Biomed Res Int 2018; 2018: 5207031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kratsios P, Catela C, Salimova E, et al. Distinct Roles for Cell-Autonomous Notch Signaling in Cardiomyocytes of the Embryonic and Adult Heart. Circ Res 2010; 106: 559–572. [DOI] [PubMed] [Google Scholar]
- 12.Pei H, Yu Q, Xue Q, et al. Notch1 cardioprotection in myocardial ischemia/reperfusion involves reduction of oxidative/nitrative stress. Basic Res Cardiol 2013; 108: 373. [DOI] [PubMed] [Google Scholar]
- 13.Zhou XL, Wan L, Xu QR, et al. Notch signaling activation contributes to cardioprotection provided by ischemic preconditioning and postconditioning. J Transl Med 2013; 11: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huisamen B, Genade S, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7-36) amide in the rat heart and their role in protection against ischaemia. Cardiovasc J Afr 2008; 19: 77–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B, Song B. Notch 1 Signalling Inhibits Cardiomyocyte Apoptosis in ischaemic Postconditioning. Heart Lung Circ 2014; 23: 152–158. [DOI] [PubMed] [Google Scholar]
- 16.Ravassa S, Zudaire A, Carr RD, et al. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am J Physiol-Heart C 2011; 300: H1361–H1372. [DOI] [PubMed] [Google Scholar]
- 17.Ma GQ, Liu YW, Wang Y, et al. Lir reduces hyperglycemia-induced cardiomyocyte death through activating glucagon-like peptide 1 receptor and targeting AMPK pathway. J Recept Sig Transd 2020; 40: 133–140. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari R, Rizzo P. The Notch pathway: a novel target for myocardial remodelling therapy? Eur Heart J 2014; 35: 2140–2145. [DOI] [PubMed] [Google Scholar]
- 19.Gude NA, Emmanuel G, Wu W, et al. Activation of Notch-mediated protective signaling in the myocardium. Circ Res 2008; 102: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol-Heart C 2003; 285: H579–H588. [DOI] [PubMed] [Google Scholar]