Abstract
Pelvic congestion syndrome (PCS) typically causes chronic non-cyclical abdominal pain with a considerable negative effect on the quality of life of women. However, pediatric cases with PCS are limited and non-invasive therapy for adolescent patients has not been reported. We report here a 13-year-old girl who presented with intermittent abdominal pain since the age of 2 years and her symptoms further deteriorated after breast development at 6 years and 9 months old. PCS and coexistent idiopathic central precocious puberty were finally diagnosed on the basis of tortuous ovarian and pelvic veins, and a pubertal response to a gonadotropin-releasing hormone (GnRH) test without hypothalamic–pituitary lesions. After treatment with the GnRH agonist, the pain score was greatly reduced and there was increased prediction of adult height. This case highlights the occurrence of PCS in adolescents and also indicates the role of non-invasive GnRH agonists in young patients with PCS before surgical intervention.
Keywords: Pelvic congestion syndrome, chronic abdominal pain, precocious puberty, adolescent, gonadotropin-releasing hormone agonist, predicted adult height, estrogen
Introduction
Chronic pelvic pain (CPP) is defined as intermittent or continuous discomfort of the lower abdomen and pelvis for longer than 6 months.1 CPP is a common problem in Western and oriental societies, and even in pediatric patients.2,3 Among the diverse etiologies, pelvic congestion syndrome (PCS) is a common cause in approximately one third of patients with CCP.1 While PCS is mostly diagnosed in women with a multiparous history, only a few cases of PCS have been described in children.1 The diagnostic criteria for PCS with computed tomography (CT) includes the presence of at least four ipsilateral pelvic veins differing in caliber, with at least one measuring > 4 mm in maximum diameter or an ovarian vein caliber > 8 mm.1 Currently, embolization of the ovarian vein is the first-line therapy for PCS1 while non-invasive treatment, such as a gonadotropin-releasing hormone agonist (GnRHa), has been reported in some studies limited to adults.4 We report here an adolescent with PCS coexistent with idiopathic central precocious puberty (ICPP) who was treated with a GnRHa, which successfully attenuated her abdominal pain and restored her predicted adult height.
Case report
A 13-year-old girl presented with intermittent abdominal pain since the age of 2 years. During acute episodes, she had to visit the Emergency Department twice a month. All laboratory tests, including blood analysis, urinalysis, and stool analysis, were unremarkable. Abdominal sonography showed no major abnormalities. She showed breast development at 6 years and 9 months old, but her parents did not pay much attention to this development. She was brought to our hospital at the age of 9 years and 4 months because of obvious breast enlargement. At that time, her height was 134.7 cm (50th–85th percentiles), weight was 25.0 kg (15th percentile), breasts were at Tanner stage III, and pubic hair was at Tanner stage I. Baseline endocrine function showed a follicle-stimulating hormone level of 6.18 mIU/mL, luteinizing hormone level of 3.10 mIU/mL, estradiol (E2) level of 68.76 pg/mL, and prolactin level of 6.10 ng/mL. Her bone age was 10 years. Regular follow-up visits were recommended, but she was lost to follow-up in subsequent years. However, the severity of non-cyclical dull abdominal pain became aggravated. When she was affected by unbearable pain, she had to visit the Emergency Department twice a week during the pubertal period. We used the visual analogue scale score to quantify the patient’s pain intensity (Table 1). In this scale, the intensity of pain ranges from 0 to 10. Namely, 0 indicates no pain, while 10 indicates the most unbearable pain.
Table 1.
Clinical presentations of our patient during gonadotropin-releasing hormone agonist treatment.
| Date | Height (cm) | Bone age (CA) | PAH (cm) | Visual analogue scale pain score |
|---|---|---|---|---|
| 03-28-2015 | 134.7 | 10–11 years (9 years, 4 months) | ||
| 09-07-2016 | 143.4 | 12–13 years (10 years 9 months) | 152.4 | 8–10 |
| 12-28-2016 | 146.1 | |||
| 03-22-2017 | 146.8 | 12–13 years (11 years 4 months) | 156.0 | 2–3 |
| 06-07-2017 | 147.9 | |||
| 08-30-2017 | 148.7 | 12–13 years (11 years, 9 months) | 158.0 | 0–1 |
| 11-15-2017 | 149.6 | |||
| 02-07-2018 | 150.8 | 12–13 years (12 years, 2.5 months) | 160.2 | 0–1 |
| 05-02-2018 | 151.2 | |||
| 07-25-2018 | 152.5 | 12–13 years (12 years, 8 months) | 162.0 | 0–1 |
CA, chronological age; PAH, predicted adult height.
She returned to our pediatric endocrine clinic at the age of 10 years and 9 months. At that time, a physical examination showed that her height was 143.4 cm (50th percentile), weight was 31.0 kg (15th–50th percentiles), breasts were at Tanner stage V, and pubic hair was at Tanner stage II. A gonadotropin-releasing hormone (GnRH) stimulation test showed a peak follicle-stimulating hormone level of 16.89 mIU/mL, peak luteinizing hormone level of 22.03 mIU/mL, and baseline E2 level of 74.99 pg/mL. Other endocrine function tests showed a free thyroxine level of 15.19 pmol/L, total thyroxine level of 95.38 nmol/L, thyroid-stimulating hormone level of 1.72 µIU/ml, and insulin-like growth factor-I level of 210 ng/mL. Her bone age was close to 13 years. Pelvic sonography showed no abnormalities of the uterus or ovaries. Magnetic resonance imaging of the sella showed a normal hypothalamic–pituitary structure, thus confirming the diagnosis of ICCP.
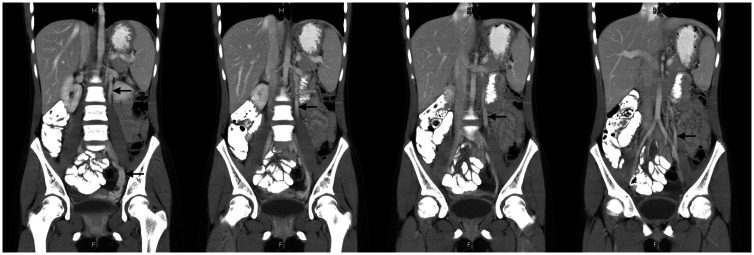
To examine the cause behind the worsening abdominal pain, tumor markers (free beta-human chorionic gonadotropin, alpha-fetoprotein, carcinoembryonic antigen, and cancer antigen 125) were measured and all of the data were within the normal range. Further abdominal CT showed tortuous ovarian and pelvic veins with dilatation at the left adnexa, which measured up to 8.4 mm in the maximal caliber of the ovarian vein (Figure 1). This met the diagnostic criteria of PCS.1 According to the clinical presentations and imaging findings, the diagnosis of left-sided PCS was ultimately made.
Figure 1.
Abdominal computed tomography shows tortuous dilated ovarian and pelvic veins (indicated by arrows).
Although ovarian venous embolization is the mainstream treatment for PCS, GnRHa therapy was chosen for the patient because of her parents’ refusal for surgical intervention owing to her young age, risk of anesthesia, and radiation exposure during venous embolization. At the age of 10 years and 10 months, she started receiving a leuprorelin acetate 11.25 mg injection every 3 months. After the third course of treatment, the symptom of dull abdominal pain had considerably subsided. A physical examination, which was performed at the age of 12 years and 8 months, showed that her height was 152.5 cm (15th–50th percentiles) and weight was 34.5 kg (3rd–15th percentiles). Her bone age remained at 13 years during the 2-year treatment (Table 1). Follow-up abdominal CT showed no further progress in the tortuous ovarian veins.
Discussion
High serum estrogen levels are associated with venous distensibility and varicose vein formation.1 Long-term exposure of excessive estrogen might increase the risk of venous wall degeneration and subsequently cause PCS. We report the first adolescent case of PCS coexistent with ICPP. Although determining which diagnosis occurs first is difficult, a clinical association between PCS and ICPP should be considered because of the strong effect of estrogen in both diseases. A previous study showed that elevated serum E2 levels might activate matrix metalloproteinases and consequently accelerate varicose vein formation, suggesting that the vascular effect of estrogen depends on its concentration.5 Expression of estrogen receptors and G protein-coupled estrogen receptor is increased in patients with venous disease and shows a positive correlation with the severity of symptoms.6 Furthermore, estrogen receptors and G protein-coupled estrogen receptor show high expression in patients with low-grade varicosity, implying that estrogen elicits a primary action in the early stage of venous disease.6
Although the pathophysiology of intractable pain in patients with PCS is not fully understood, it is thought to be caused by three mechanisms. These mechanisms include stasis of engorged pelvic veins, which activates nociceptors within the venous wall, release of neurotransmitters from dilated pelvic veins, and compression from contiguous anatomical structures to nearby nerves.1 In the current case, marked improvement of abdominal pain with no further progress of dilated pelvic veins during GnRHa therapy indicated that the patient’s alleviation of pain was not likely to be related to a structural venous change. The most likely explanation for the pathophysiology in our patient might be a hormone-modulating nociceptive transmission pathway in consideration of the coexistent ICPP.
Previous research has postulated the “low estrogen hypothesis” and suggested that severity of pain is also increased in a low estrogen milieu.7 However, accumulating evidence has shown that E2 levels in painful varicose veins are much higher than those in unaffected veins, suggesting that E2 levels are proportional to pain perception.8 Furthermore, fluctuations in E2 levels have an effect on nociceptive sensitivity.7 Importantly, numerous studies have shown that the estrogen-mediated endogenous opioid system might induce hyperalgesia and lead to idiopathic painful states.9 The findings in the present case support the notion that high serum estrogen levels in ICPP increase the severity of pain in PCS. Notably, GnRHa therapy contributed to the considerable reduction in pelvic pain by decreasing variation in estrogen levels. However, further studies are warranted to support such conjecture.
GnRHa can downregulate GnRH receptors and decrease secretion of gonadotropins, which in turn suppress ovarian hormone synthesis. Therefore, GnRHa therapy should be a useful treatment in patients with PCS and an alternative to invasive interventions. While the drug effect of GnRHa therapy usually fades with time in adults,1 our adolescent patient was successfully treated with GnRHa for at least 2 years and showed no intolerance effect. This finding suggests different metabolic backgrounds in old and young patients. In view of the positive clinical response, our findings suggest a new dimension of GnRHa as a viable option before surgical intervention for adolescent girls with PCS and ICPP.
Acknowledgment
The authors thank the patient and her family for their participation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics statement
This article was approved by the Ethics Committee of the Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center. The patient and her parents agreed to the use of her imaging and clinical data for publication and academic research and provided written informed consent.
Funding
This study was supported in part by a grant from the Research Fund of Tri-Service General Hospital (TSGH-E-109204).
ORCID iDs
Jwo-Huey Yu https://orcid.org/0000-0001-9854-2681
Chien-Ming Lin https://orcid.org/0000-0001-7525-5743
References
- 1.Phillips D, Deipolyi AR, Hesketh RL, et al. Pelvic congestion syndrome: etiology of pain, diagnosis, and clinical management. J Vasc Interv Radiol 2014; 25: 725–733. [DOI] [PubMed] [Google Scholar]
- 2.Sznajder KK, Harlow SD, Burgard SA, et al. Gynecologic pain related to occupational stress among female factory workers in Tianjin, China. Int J Occup Environ Health 2014; 20: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korterink JJ, Diederen K, Benninga MA, et al. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One 2015; 10: e0126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soysal ME, Soysal S, Vicdan K, et al. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Human Reprod (Oxford, England) 2001; 16: 931–939. [DOI] [PubMed] [Google Scholar]
- 5.MacColl E, Khalil RA. Matrix Metalloproteinases as Regulators of Vein Structure and Function: Implications in Chronic Venous Disease. J Pharmacol Exp Ther 2015; 355: 410–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serra R, Gallelli L, Perri P, et al. Estrogen Receptors and Chronic Venous Disease. Eur J Vasc Endovasc Surg 2016; 52: 114–118. [DOI] [PubMed] [Google Scholar]
- 7.Hassan S, Muere A, Einstein G. Ovarian hormones and chronic pain: A comprehensive review. Pain 2014; 155: 2448–2460. [DOI] [PubMed] [Google Scholar]
- 8.Asciutto G, Mumme A, Asciutto KC, et al. Oestradiol levels in varicose vein blood of patients with and without pelvic vein incompetence (PVI): diagnostic implications. Eur J Vasc Endovasc Surg 2010; 40: 117–121. [DOI] [PubMed] [Google Scholar]
- 9.Smith YR, Stohler CS, Nichols TE, et al. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 2006; 26: 5777–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]