Abstract
Objective
Cardiac diseases lead to heart failure (HF), but the progression can take several years. Using blood samples to monitor changes in the heart before clinical symptoms begin may help to improve patient management.
Methods
Microarray data GSE42955 and GSE9128 were used as study datasets and GSE16499, GSE57338, and GSE59867 were used as validation groups. The “limma” package from R Language was used to identify differentially expressed genes. Functional enrichment analyses of gene ontology terms and Kyoto Encyclopedia of Genes and Genomes pathways were performed using Database for Annotation, Visualization and Integrated Discovery. We also investigated the correlation between the heart and blood using the mRNA expression level.
Results
Three hub genes, CD14, CD163, and CCR1, were identified. Functional enrichment analyses showed their involvement in the immune response and in the inflammatory response, which are the critical biochemical processes in ischemic HF. The mRNA expression level further demonstrated that a special model may exist to help to predict the mRNA level in the heart based on that in blood.
Conclusions
Our study identified three biomarkers that can connect the heart and blood in ischemic heart diseases, which may be a new approach to help better manage ischemic cardiac disease patients.
Keywords: Heart and blood communication, heart failure, ischemic cardiomyopathy, ST-elevation myocardial infarction, differentially expressed genes, biomarker, heart disease
Introduction
Heart failure (HF) is a major public health issue, and approximately 50% of patients die within 5 years after diagnosis. It is estimated that 20% to 46% of people have a lifetime risk of HF, and the incidence is increasing.1–3 Many cardiac diseases eventually lead to HF.4 Because the heart has compensatory mechanisms, patients undergo the cardiac remodeling process, which leads to changes in the ionic channel, pump, exchanger densities, and kinetics, and this results in inevitable HF.5 If we can slow or even stop any of the stages, it may help to better manage HF. Thus, a simple, inexpensive, and easy-to-use method is required to determine the dynamic processes that occur in the heart, especially with a simple method.
Ischemic cardiomyopathy (ICM) is a main and lethal cause of HF,6 and is also a leading cause of death worldwide.7 Patients have a higher in-hospital mortality rate and more complications (which are further complicated by more symptoms and the need for more care) than non-ICM patients.8 Additionally, ICM can result in left ventricular dysfunction. The left ventricular ejection fraction (LVEF) was reported to predict the prognosis of these patients. However, it could not distinguish between different outcomes when the EF was ≤5%. Thus, right ventricular EF and the end-systolic volume index were also applied.9,10 However, all of these measurements do not represent the biochemical processes (BPs) in the heart, which is more closely related to the prognosis.
ICM involves chronic immune system activation, and CD4+ T-cells were globally activated and expanded in ICM patients.11 Microarray data also indicated that activation of the immune system and the inflammatory system may be critical processes in ICM.12,13 Both inflammatory responses and immune responses exist in ICM patients when the immune system can maintain immune homeostasis and tolerance, and this can suppress inflammatory injury and ameliorate cardiac remodeling.14 However, regulatory T-lymphocyte cells (Tregs), which are key suppressors of the immune response, were dysfunctional in ICM.11 When the balance is destroyed, inflammation can contribute to left ventricular remodeling.15,16 Thus, anti-inflammation can improve cardiac remodeling,17 and this is a treatment strategy for ICM patients.18
Acute myocardial infarction (AMI) can also result in cardiac remodeling, and it is another cardiac disease that can lead to HF. Similar to ICM, the progression of HF after AMI is complex and affected by multiple factors.2 To clarify the dynamic progression of HF, studies using heart samples will be the best and most straightforward method, but the heart tissue is usually unavailable unless it is obtained during a heart transplant operation.
Tissues interact with each other. Recently, a study revealed that DPP4 can connect heart and blood in healthy people,19 and, thus, inferring the status of the heart on the basis of the blood may be possible. However, DPP4 is a serine protease that is responsible for cleaving selected N-terminal penultimate amino acids, and it seems not to have a direct impact on cardiac diseases. In this study, we aimed to identify if there were any shared differentially expressed genes (DEGs) between the heart and the blood using microarray data on the cardiac diseases that were downloaded from Gene Expression Omnibus (GEO) database. We also investigated the correlation of their mRNA expression level between the heart and blood.
Materials and methods
Microarray data
In this study, the datasets were downloaded from the public database GEO. GSE42955 was based on the GPL6244 platform, which is also known as the [HuGene‐1_0‐st] Affymetrix Human Gene 1.0 ST Array (transcript [gene] version), and it measures expression data that have different statuses in the human heart, including ICM (n = 12), dilated cardiomyopathy (n = 12), and control (n = 5). Peripheral blood mononuclear cell (PBMC) expression data were obtained from GSE9128, which was based on the [HG-U133A] Affymetrix Human Genome U133A Array (GPL96 platform). It was performed in three categories of patients, including ICM (n = 4), non-ICM (n = 4), and control patients (n = 3). Here, we obtained ICM and control patient data from these two datasets. Because the data were downloaded from a GEO database and analyzed using bioinformatic methods, an ethics review and informed consent were not required for this study.
Data preprocessing
After the series matrix files were downloaded, we used the “impute” package in R language (https://www.r-project.org/) to impute the missing data. Then, the probe IDs were converted into gene symbols on the basis of their annotation packages using R Bioconductor (https://www.bioconductor.org/). When more than one probe corresponded to the same gene, we used the probe with the highest expression to represent the gene, and no gene names were omitted. To ensure that the ICM group and control group were comparable and that the comparation was meaningful, hierarchical clustering and the principal component analysis (PCA) plots were constructed to ensure the distinction between two groups before screening the DEGs. Finally, the “limma” package20 was used to screen out DEGs with the cut-off criteria of |log2(fold change)| >1.5 and a P-value <0.05. The DEGs were visualized by a heatmap using the “pheatmap” package (cran.r-project.org/web/packages/pheatmap/index.html). The heatmaps were constructed using TOP100 genes with the lowest p-value.
Protein–protein interaction network analysis and identified candidate hub genes
This part contained the following three steps: 1) A Venn diagram was applied to show if shared DEGs between heart tissue and PBMC existed; 2) Protein–protein interaction (PPI) network analysis of the shared genes was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) with medium confidence (interaction score >0.4); and 3) After the PPI networks of DEGs in heart tissue and PBMC were constructed, these two large PPI networks were further analysis using Cytoscape 3.7.1 software (https://cytoscape.org/). The large network was simplified into the TOP50 genes network on the basis of the connective degree with the help of the cytoHubba App.21 Finally, the shared DEGs were identified, and these genes were considered to be candidate hub genes that were used for further analysis.
Function enrichment analysis
The function enrichment analysis of was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID). This analysis included gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). This process also contained the following two steps: (1) Enrichment of DEGs in the heart tissue and PBMC; and (2) Enrichment of the shared DEGs. A P-value <0.05 was defined as the significance threshold.
Validation and mRNA expression
Because small sample sizes may cause unstable outcomes, the validation datasets GSE16499 and GSE57338 for heart and GSE59867 for PBMCs were used. Both GSE16499 and GSE57338 were used as the test datasets to investigate ischemic HF and the normal human heart. However, we could not identify a more suitable dataset than GSE59867 to test the results, which contained 111 ST-elevation myocardial infarction (STEMI) patients and 46 controls with stable coronary artery disease (CAD); nine of 111 developed HF and eight of 111 were non-HF after follow-up. These 111 patients were followed-up for 6 months and their blood samples were collected at four time points. Data analyses were performed as mentioned above. The mRNA expression levels were presented after log2 management.
Results
Screening out differentially expressed genes
There were 222 DEGs that were screened out from the heart tissue samples (GSE42955), including 166 downregulated and 56 upregulated genes. In PBMC samples (GSE9128), there were 386 DEGs between ICM and the control group, including 219 downregulated genes and 167 upregulated genes. The heatmap and PCA plots showed a significant difference between ICM and controls, which indicates that the DEGs were meaningful.
Protein–protein interaction network analysis and identification of candidate hub genes
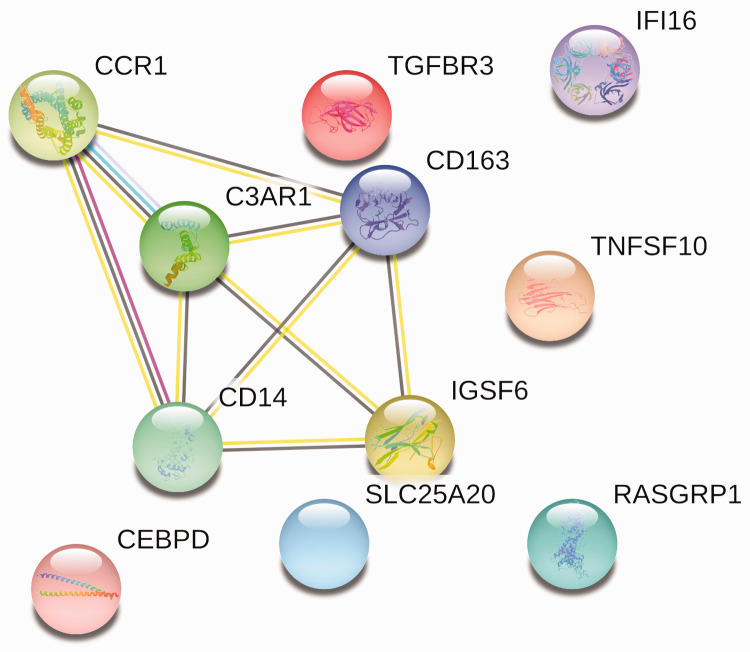
A Venn diagram was used to identify shared genes between the two datasets. Twelve genes were identified, including C3AR1, CD14, CCR1, IGSF6, IFI16, TGFBR3, DDX3Y, RASGRP1, TNFSF10, CD163, SLC25A20, and CEBPD.
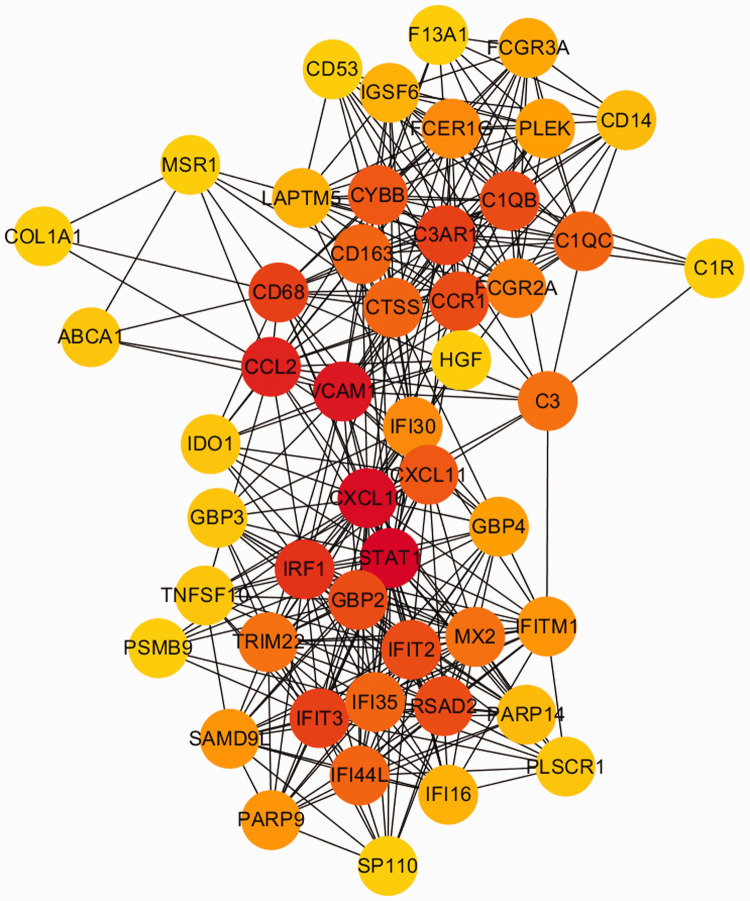
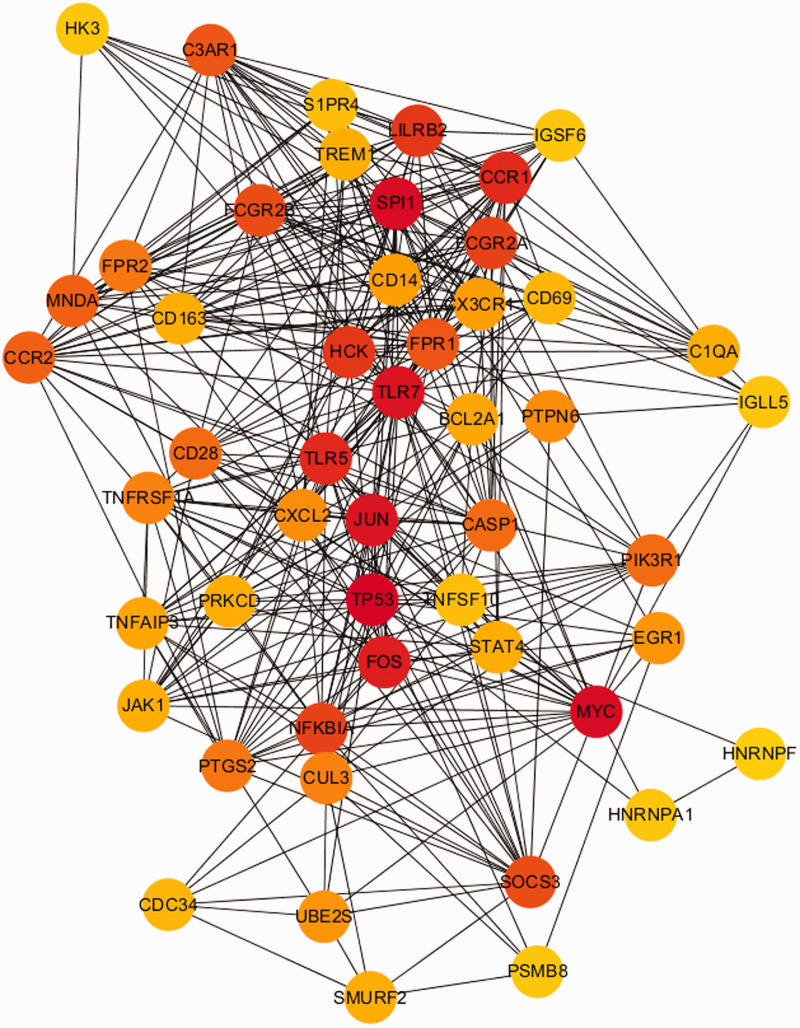
Then, the PPI networks were constructed using the STRING database with the shared genes and the DEGs in two datasets. The network of shared genes showed that there may be five important genes, as shown in Figure 1. To further confirm the importance of these genes, we identified if they were in the TOP50 DEG gene networks in the two datasets, and we found that these five important genes were in these networks (Figures 2 and 3). Thus, these five genes (CCR1, C3AR1, CD163, CD14, and IGSF6) were considered to be candidate hub genes, and they were further analyzed.
Figure 1.
PPI network of 12 shared genes. Eleven of 12 genes were enriched.
PPI, protein–protein interaction.
Figure 2.
PPI network of heart tissue (GSE42955), which was simplified into TOP50 on the basis of the connectivity degree.
PPI, protein–protein interaction.
Figure 3.
PPI network of PBMC (GSE9128), simplified into TOP50 on the basis of the connectivity degree.
PPI, protein–protein interaction; PBMC, peripheral blood mononuclear cells.
Functional enrichment analysis
After functional enrichment analyses using DAVID, the results of gene ontology–biochemical processes (GO-BP) terms showed that the DEGs in heart tissue, PBMCs, and the shared genes were involved in the following same biological processes: immune response, inflammatory response, and positive regulation of tumor necrosis factor production. The candidate hub genes C3AR1, CD14, and CCR1 were involved in the inflammatory response, and IGSF6 and CCR1 were involved in the immune response. Although the KEGG pathways did not contain any of the shared genes, the pathways in heart tissue and PBMCs are mostly present in infectious diseases, including Staphylococcus aureus infection, influenza A, malaria, and herpes simplex infection, and in immune system signaling pathways, including the tumor necrosis factor (TNF) signaling pathway, T cell receptor signaling pathway, and B cell receptor signaling pathway.
Validation and mRNA expression
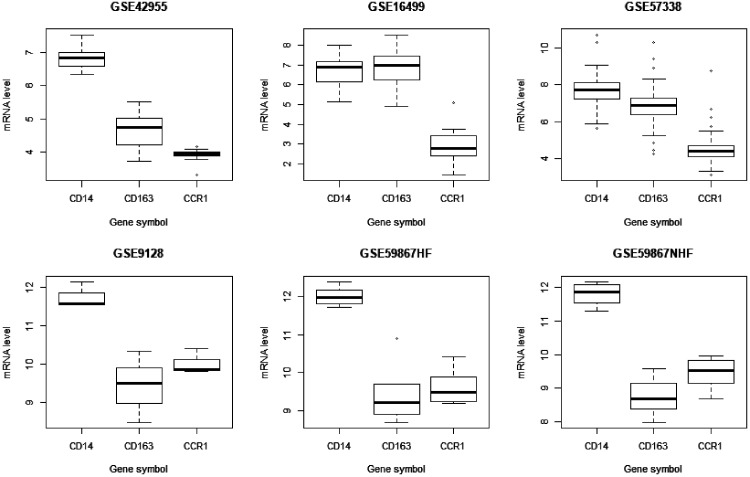
To confirm our finding, three datasets were used as validation groups. After data processing, we found that CD14, CD163, and CCR1 were also found on the list of DEGs in these datasets. This is because the GSE59867 dataset contains samples that were collected at four time points, but we did not find any candidate hub genes in the lists of DEGs at other time point except for “admission”, and thus, the following data were all from the “admission” time point. We noticed that mRNA expression of these genes was lower in the heart than in PBMCs, as shown in Figure 4. Additionally, we screened out DEGS in the direction of “experimental group expression level – control/normal group expression level”, and the results showed that mRNA expression levels in the heart were lower in the experimental groups than in the controls. However, we obtained the opposite results in PBMCs, as shown in Table 1.
Figure 4.
mRNA level of three hub genes. The data has gone through log2 management.
Table 1.
log2FC value of each genes.
| Symbol | CD14 | CD163 | CCR1 |
|---|---|---|---|
| GSE42955 | −1.383002 | −0.731100 | −0.650646 |
| GSE16499 | −1.380755 | −1.053452 | −1.007141 |
| GSE57338 | −0.812935 | −1.432626 | −0.775009 |
| GSE9128 | 0.681245 | 1.099868 | 0.665574 |
| GSE59867HF | 0.6985875 | 1.256895 | 0.809402 |
| GSE59867NHF | NS | NS | NS |
We used the direction “experimental – control”, and here, “–” means that mRNA expression in experimental groups were lower than that of control by >1.5-fold.
NS, not significant.
Discussion
A previous study demonstrated that tissue in multicellular organisms cannot work in isolation.19 In this study, we used microarray data to infer DEGs between ICM and control, and we found there were 12 shared DEGs between the heart and PBMCs, which demonstrated that there may be shared biochemical processes between them. Five of 12 genes were regarded as candidate hub genes after PPI network analysis. The function enrichment results were consistent with previous studies, which showed that the immune response and inflammatory response play very important roles in ICM in both the heart and blood.11–13 All the candidate hub genes were involved in these BPs except CD163, which was shown to play a role in inflammation.22
To confirm our results, validation groups that included three datasets were used. After analysis, CD14, CD163, and CCR1 were found in the DEGs lists of the three datasets, which means they may play a critical role in heart and blood communication, and they were regarded as hub genes. Additionally, we found the following interesting mRNA expression results that were related to these genes: 1) mRNA expression levels were lower in the heart than in PBMCs; 2) mRNA expression levels were lower in experimental groups than in controls in the heart, but the opposite results were found in PBMCs; and 3) In GSE59867, the three DEGs were found in patients who developed HF, but they were not found in those who did not develop HF. In this study, these three DEGs can be found in ICM patients, STEMI patients who developed HF later, and ischemic HF patients. We suggest that they are the key factors in the processes of developing HF, especially ischemic HF.
CD14 is recognized as a glycosylphosphatidylinositol (GPI)-anchored protein, and it is involved in microbial recognition, which plays an important role in the innate immune response. The innate immune system is reported to be related to cardiomyopathy outcomes.23 CD14 also participates in regulating metabolism, insulin resistance, obesity, and neurodegenerative diseases.24,25 A recent study also found out that the CD14 level was higher in CAD patients than in normal controls, and that it may help to diagnose patients with stable CAD.26
CD163 is a hemoglobin (Hb) scavenger receptor related to the anti-inflammatory response.22,27 It was found to significantly increase in the peripheral blood of ischemic stroke patients, and it is involved in ischemic brain injury.28,29 CD163-expressing macrophages were involved in regeneration of ischemic injured tissue.30 Because the macrophages can be found in both blood and tissue under ischemic conditions, the role of CD163 in ischemic heart disease requires further study.
CC-type chemokine receptor 1 (CCR1) is expressed on monocytes, neutrophils, and T cells, and it has been demonstrated to be involved in various inflammatory responses, including ischemia–reperfusion injury.31,32 Mesenchymal stem cell (MSC) injection is a therapeutic approach to ICM that can improve left ventricular function,33 and previous studies indicated that MSCs with overexpression of CCR1 may show a better treatment effect, which could be partly related to systemic anti-inflammatory activities.34,35 In our study, CCR1 was enriched in the inflammatory response and in the immune response.
Monitoring the BPs in the heart may help to better manage patients with heart disease, but the current overall picture of tissue–tissue interactions is lacking.19 The heart has compensatory mechanisms, and patients with cardiac diseases will undergo a series of changes, which results in inevitable HF.5 If we can understand the process before the clinical symptoms begin, we can probably slow or stop its progression. In this study, we identified three hub genes that may help to improve our understanding of the dynamic status of the heart through the peripheral blood. However, this study has some limitations, as follows: this study was performed based on bioinformatics analysis of microarray data that were downloaded from the GEO database, which means that the patients’ baseline could not be strictly controlled. Because of the inconsistent baseline, we could not construct a model showing the relationship of mRNA levels in the heart and in the blood. Additionally, the sample size for blood is too small. Thus, proof-of-concept studies are needed to confirm the results.
Conclusion
In this study, we identified three biomarkers that can connect the heart and blood using a bioinformatics method and that probably play a critical role in the development of HF. This finding may help to monitor the dynamic biochemical processes in the heart and to help to better manage patients with ischemic heart disease. Because all the data were from a GEO database, further studies are needed to support this finding.
Acknowledgments
The co-first authors Chengcong Chen and Hong Peng have made substantial contributions to this work. Other authors participated in revising the manuscript and data extraction. All authors mentioned in the manuscript agreed to be mentioned in the manuscript.
Ethics and consent
The data were downloaded from a GEO database and analyzed using bioinformatic methods, and thus, an ethics review and informed consent were not required for this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Sanming Project of Medicine in Shenzhen, SZSM201812056
ORCID iD
Guoqing Dong https://orcid.org/0000-0002-3791-1700
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 2.Maciejak A, Kiliszek M, Michalak M, et al. Gene expression profiling reveals potential prognostic biomarkers associated with the progression of heart failure. Genome Med 2015; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Huang Y, Zeng Y, et al. Targeting the DPP-4-GLP-1 pathway improves exercise tolerance in heart failure patients: a systematic review and meta-analysis. BMC Cardiovasc Disord 2019; 19: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016; 13: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattoni S, Roe AT, Aronsen JM, et al. Compensatory and decompensatory alterations in cardiomyocyte Ca(2+) dynamics in hearts with diastolic dysfunction following aortic banding. J Physiol 2017; 595: 3867–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panza JA, Ellis AM, Al-Khalidi HR, et al. Myocardial viability and long-term outcomes in ischemic cardiomyopathy. N Engl J Med 2019; 381: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborators GCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F, Li P. Patient characteristics and in-hospital outcomes of heart failure with ischemic versus non-ischemic cardiomyopathy. J Card Fail 2019; 25: S70. [Google Scholar]
- 9.Pai RG, Varadarajan P, Rouleau JL, et al. Value of cardiovascular magnetic resonance imaging-derived baseline left ventricular ejection fraction and volumes for precise risk stratification of patients with ischemic cardiomyopathy: insights from the Surgical Treatment for Ischemic Heart Failure (STICH) trial. JAMA Cardiol 2017; 2: 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabe MA, Sabe SA, Kusunose K, et al. Predictors and prognostic significance of right ventricular ejection fraction in patients with ischemic cardiomyopathy. Circulation 2016; 134: 656–665. [DOI] [PubMed] [Google Scholar]
- 11.Bansal SS, Ismahil MA, Goel M, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 2019; 139: 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Jiang Q, Ding Z, et al. Identification of a common different gene expression signature in ischemic cardiomyopathy. Genes (Basel) 2018; 9: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweet ME, Cocciolo A, Slavov D, et al. Transcriptome analysis of human heart failure reveals dysregulated cell adhesion in dilated cardiomyopathy and activated immune pathways in ischemic heart failure. BMC Genomics 2018; 19: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Z, Yu K, Chen L, et al. Interleukin-2/anti-interleukin-2 immune complex attenuates cardiac remodeling after myocardial infarction through expansion of regulatory T cells. J Immunol Res 2016; 2016: 8493767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westman PC, Lipinski MJ, Luger D, et al. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 2016; 67: 2050–2060. [DOI] [PubMed] [Google Scholar]
- 16.Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 2018; 186: 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung M, Ma Y, Iyer RP, et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 2017; 112: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sliwa K, Woodiwiss A, Kone VN, et al. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: results of a randomized study. Circulation 2004; 109: 750–755. [DOI] [PubMed] [Google Scholar]
- 19.Long Q, Argmann C, Houten SM, et al. Inter-tissue coexpression network analysis reveals DPP4 as an important gene in heart to blood communication. Genome Med 2016; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43: e47. doi:10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin CH, Chen SH, Wu HH, et al. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 2014; 8 Suppl 4: S11. doi:10.1186/1752-0509-8-S4-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 2013; 18: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frantz S, Falcao-Pires I, Balligand JL, et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail 2018; 20: 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol 2013; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingham V, Williams A, Bate C. Glimepiride reduces CD14 expression and cytokine secretion from macrophages. J Neuroinflammation 2014; 11: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norata GD, Lee MY, Huang CH, et al. Clinical proteomics identifies urinary CD14 as a potential biomarker for diagnosis of stable coronary artery disease. PLoS One 2015; 10: e0117169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarado-Vazquez PA, Bernal L, Paige CA, et al. Macrophage-specific nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology 2017; 222: 900–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell GC, Petrone AB, Treadway MB, et al. Machine-learning approach identifies a pattern of gene expression in peripheral blood that can accurately detect ischaemic stroke. NPJ Genom Med 2016; 1: 16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connell GC, Tennant CS, Lucke-Wold N, et al. Monocyte-lymphocyte cross-communication via soluble CD163 directly links innate immune system activation and adaptive immune system suppression following ischemic stroke. Sci Rep 2017; 7: 12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akahori H, Karmali V, Polavarapu R, et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat Commun 2015; 6: 7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SW, Hildebrandt GC, Olkiewicz KM, et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood 2007; 110: 3447–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichel CA, Khandoga A, Anders HJ, et al. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol 2006; 79: 114–122. [DOI] [PubMed] [Google Scholar]
- 33.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 2014; 311: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luger D, Lipinski MJ, Westman PC, et al. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res 2017; 120: 1598–1613. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Zhang Z, Guo J, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 2010; 106: 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]