Abstract
Objective.
To examine the effectiveness of topical imiquimod therapy for vulvar Paget’s disease.
Methods.
A systematic literature search was conducted using three public search engines with entry keywords “Paget’s disease” and “imiquimod”. Case reports describing imiquimod treatment for vulvar Paget’s disease were examined for demographics, treatment patterns, and outcome (63 cases).
Results.
Median age was 68, and nearly a half of cases were recurrent disease (50.8%) with surgical resection being the most common prior treatment modality (62.5%). All cases used 5% imiquimod and the median treatment duration was 4 months. The most common initial treatment frequency was 3–4 times/week (68.3%) followed by 5–7 (17.4%) and 1–2 times/week (14.3%). Frequency-reduction due to adverse effects was seen in 9.5% with the initial 5–7 times/week regimen being associated with the highest reduction rate (1–2, 3–4, and 5–7 times/week: 0%, 2.3%, and 81.8%, p b 0.01). In 46 (73.0%) cases, a complete remission (CR) to imiquimod therapy was reported, with 2, 4, and 6-month cumulative CR rates being 9.8%, 31.1%, and 71.6%, respectively. With median follow-up duration of 12 months after the completion of imiquimod treatment, 2 (5.7%) of the 35 women who had a CR developed disease recurrence. Age, disease status (primary versus recurrent), and treatment frequency after dose reduction were not associated with CR rates (all, p > 0.05).
Conclusion.
Our results suggested that imiquimod therapy may be an effective possible treatment option for vulvar Paget’s disease, especially for women who have experienced recurrence after multiple surgical resections or who are with poor surgical candidates.
Keywords: Paget disease, Imiquimod, Review
1. Introduction
Extramammary Paget’s disease (EMPD) is a rare non-invasive intraepithelial adenocarcinoma. EMPD is commonly seen in the vulva and accounts for up to 60% of EMPD and 1% of vulvar neoplasm cases [1,2]. EMPD is most commonly seen in postmenopausal Caucasian women and appears as an erythematous, eczematoid, or pruriginous lesion. Approximately 10–20% of cases are associated with a coexisting malignancy within the vulva or at sites such as the breast, rectum, bladder, cervix and skin [3–5].
The origin of EMPD remains controversial. It may be viewed as a carcinoma of adnexal stem cells [6], as a sweat gland carcinoma arising from the intraepidermal portion of the gland [7], or as a carcinoma derived from the toker cells of mammary-like glands of the vulva [8]. EMPD has a multifocal nature and because of the occult fashion of spread it has a chronic and relapsing course and is difficult to treat. Standard treatments for vulvar Paget’s disease include surgical excision with vulvectomy, laser ablation, photodynamic therapy, radiotherapy, or topical chemotherapy [9]. However, relapse occurs in over 30% of patients [10].
Imiquimod has emerged as a promising drug for the treatment of EMPD. It is an immune response modulator that targets toll-like receptors of dendritic and Langerhans cells which results in the release of multiple cytokines and can directly induce apoptosis of transformed epithelial cells [11,12]. Imiquimod is a drug of choice for genital warts, vulvar and vaginal intraepithelial neoplasia, and actinic keratosis [13]. However, available evidence supporting imiquimod use in vulvar Paget’s disease is mainly in case reports, and exact statistics for treatment efficiency of imiquimod have not been well described. The aim of this study was to conduct a systematic review of the literature to describe the effectiveness of topical imiquimod therapy in vulvar Paget’s disease.
2. Materials and methods
2.1. Source and study selection
A literature search was conducted per the MOOSE guidelines for systematic review by using PubMed/MEDLINE and ScienceDirect with entry keywords “Paget’s disease” and “imiquimod” conducted on March 6, 2015 [14]. Eligibility criteria were case reports or case series for vulvar Paget’s disease in the English literature that had an adequate description of patient demographics, treatment type and response, and follow-up. References of each article were reviewed and articles that met inclusion criteria were also added.
2.2. Clinical information
Among the eligible cases, the following variables were abstracted from the selected papers: chronologic year and country of publication, age at diagnosis, presenting symptoms, personal history of malignancy other than Paget’s disease, disease status of vulvar Paget’s disease (initial versus persistent/recurrent), size (described or measured by utilizing clitoral index if image pictures of the vulvar was presented) [15], anatomical location of Paget’s disease, past treatment history for persistent/recurrent vulvar Paget’s disease, details of imiquimod treatment (dose, frequency, and duration), dose reduction and its reason, response to imiquimod treatment, and last, follow-up duration after the completion of imiquimod treatment and disease status [14].
2.3. Definitions
Outcome of treatment response was grouped into the following: complete response (CR) defined as no residual disease in posttreatment biopsy or complete remission of clinical symptoms and no visible lesion; persistent disease (PD) defined by residual, stable, or persistent disease based on a post-treatment biopsy or sustained clinical symptoms or a visible stable vulvar lesion; and progressive disease defied by worsening symptoms or increase in the size of any visible lesion. Outcome response for treatment was obtained after the completion of imiquimod therapy. Time duration for imiquimod therapy was abstracted from the record and defined as treatment duration. Post-treatment follow-up course was also abstracted and defined as follow-up duration. Among the cases for whom follow-up duration was obtainable, the presence or absence of disease recurrence and treatment pattern for recurrence were abstracted.
2.4. Statistical analysis
The primary focus of the analysis was to examine the cumulative response rate of imiquimod therapy for vulvar Paget’s disease. The secondary focus of the analysis was to identify the clinical–pathological factors associated with response to the imiquimod therapy. Continuous variables were assessed for normality by the Kolmogorov-Smirnov test expressed as mean (±standard deviation) or median (range). Statistical significance of continuous variables was determined by Student t test or Mann–Whitney U test as appropriate. Categorical or ordinal variables were assessed by chi-square test or the Fisher’s exact test as appropriate, expressed as odds ratio (OR) with 95% confidence interval (CI). Because response to imiquimod treatment is a time-dependent event based on treatment duration or follow-up time, survival analysis with log-rank test for univariate analysis and Cox proportional hazard regression model for multivariate analysis were performed in this study, and statistical significance was expressed with hazard ratio (HR) and 95% CI. Cumulative response rate after the initiation of imiquimod therapy was estimated at 2, 4, and 6 months of treatment course, respectively. All statistical tests were two-tailed, and p value of less than 0.05 was considered as statistically significant. Statistical Package for the Social Sciences (SPSS, version 22.0, Chicago, IL) was used for the analysis.
3. Results
A schema of the study selection is shown in Fig. 1. During the study period, there were 57 articles identified by the search, of which the title and abstract were screened. Of those, 33 articles were not relevant to the study and were excluded. The remaining 24 articles were then reviewed for the full contents [10,16–38]. This comprises 66 cases, and there were 3 cases excluded due to inadequate information. The remaining 63 cases of vulvar Paget’s disease treated with imiquimod comprised the study population.
Fig. 1.
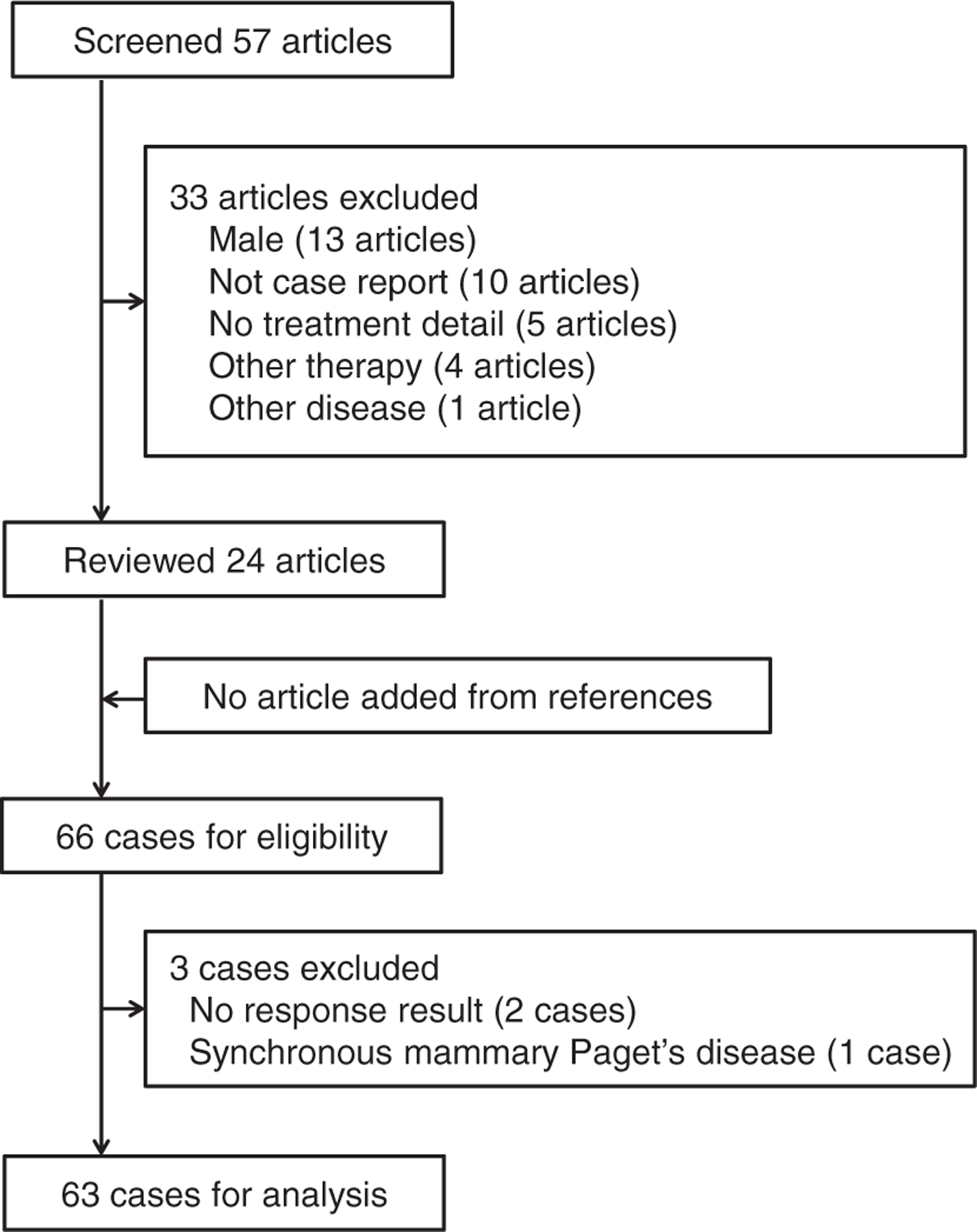
Searching criteria for vulvar Paget’s disease treated with imiquimod. PubMed/MEDLINE and ScienceDirect were searched with entry keywords “Paget’s disease” and “imiquimod” (March 6, 2015).
Patient demographics are shown in Table 1. The majority of papers were published in 2011 or later (76.2%) and emanated from centers located in Europe (66.7%). The median age of patients was 68 years (range: 41–92). All cases with localized vulvar Paget’s disease did not have underlying malignancy at the time of diagnosis. Of 63 cases, 49.2% were primary and 50.8% were recurrences. Among 32 cases of recurrent Paget’s disease, the recurrence was diagnosed at a median 66 months (range: 4–360) from the end of prior treatment. The majority of prior treatment was surgery (62.5%). The median size of the lesion was 6.5 cm (range: 2–9). All cases were treated with 5% imiquimod. The most common initial treatment frequency was 3–4 times per week, followed by 5–7, and 1–2 times per week (68.3%, 17.4%, 14.3% prospectively). The median treatment duration was 4 months (range: 1–13).
Table 1.
Patient demographics for vulvar Paget’s disease.
| N = 63 (100%) | |
|---|---|
| Year of publication | |
| <2005 | 1 (1.6%) |
| 2006–2010 | 14 (22.2%) |
| ≥ 2011 | 48 (76.2%) |
| Area of publication | |
| Europe | 42 (66.7%) |
| South America | 15 (23.8%) |
| North America | 5 (7.9%) |
| Asia | 1 (1.6%) |
| Age (years) | 68 (41–92) |
| <60 | 9 (14.3%) |
| 60–69 | 21 (33.3%) |
| ≥ 70 | 27 (42.9%) |
| Missing | 6 (9.5%) |
| Disease type | |
| Primary | 31 (49.2%) |
| Recurrent | 32 (50.8%) |
| Time from end of prior treatmenta | 66 (4–360) |
| Prior treatment typeb | |
| Surgery | 20 (62.5%) |
| Imiquimod | 2 (6.3%) |
| Laser therapy | 1 (3.1%) |
| Photodynamic therapy | 1 (3.1%) |
| Missing | 8 (25.0%) |
| Size | 6.5 (2–9) |
| <5 cm | 18 (28.6%) |
| ≥ 5 cm | 13 (20.6%) |
| Missing | 32 (50.8%) |
| IQ dose, 5% | 63 (100%) |
| IQ treatment frequency | |
| 1–2/week | 9 (14.3%) |
| 3–4/week | 43 (68.3%) |
| 5–7/week | 11 (17.4%) |
| Duration of IQ treatment | 4 (1–13) |
| <2 months | 10 (15.8%) |
| 2–3.9 months | 16 (25.4%) |
| 4–5.9 months | 26 (41.3%) |
| ≥ 6 months | 11 (17.5%) |
| Frequency-reduction of IQ treatment | |
| No | 53 (84.2%) |
| Yes | 10 (15.8%) |
Median (range) or number (%) is shown. Abbreviation: IQ, imiquimod.
Expressed as month.
No prior chemotherapy or radiotherapy.
Details of treatment outcome of imiquimod therapy are shown in Table 2. Among the reported cases, 46 (73.0%) achieved a CR and 17 (27.0%) cases had PD. There were no reported cases of disease progression. Follow-up after the completion of imiquimod treatment was documented in the 35 patients who had a CR with the median time being 12 months (range: 0.5–53). Of those, 2 (5.7%) patients recurred. In addition, there was one recurrence reported in 11 patients without documentation for follow-up period making 3 (6.5%) total recurrences among 46 patients who had a CR, and 2 of those 3 were treated with imiquimod therapy again, resulting in another CR. When the CR and PD groups were compared, there was no difference in area of publication (p = 0.32), age (p = 0.58), disease type (primary versus recurrent, p = 0.44), tumor size (p = 0.37), treatment duration (p = 0.72), and frequency-reduction of imiquimod treatment (p = 0.19).
Table 2.
Outcome of imiquimod therapy for vulvar Paget’s disease.
| No. (%) | Complete | Persistent | Progress | p-Value | |
|---|---|---|---|---|---|
| Total | 63 (100%) | 46 (73.0%) | 17 (27.0%) | 0 | |
| Year of publication | < 0.01 | ||||
| <2011 | 15 (23.8%) | 15 (100%) | 0 (0%) | 0 | |
| ≥ 2011 | 48 (76.2%) | 31 (64.6%) | 17 (35.4%) | 0 | |
| Area of publication | 0.32 | ||||
| Europe | 42 (66.7%) | 29 (69.0%) | 13 (31.0%) | 0 | |
| Non-Europe | 21 (33.3%) | 17 (81.0%) | 4 (19.0%) | 0 | |
| Age (years) | 0.58 | ||||
| <70 | 30 (52.6%) | 22 (73.3%) | 8 (26.7%) | 0 | |
| ≥ 70 | 27 (47.4%) | 18 (66.7%) | 9 (33.3%) | 0 | |
| Disease type | 0.44 | ||||
| Primary | 31 (49.2%) | 24 (77.4%) | 7 (22.6%) | 0 | |
| Recurrent | 32 (50.8%) | 22 (68.8%) | 10 (31.2%) | 0 | |
| Size | 0.37 | ||||
| <5 cm | 18 (58.1%) | 16 (88.9%) | 2 (11.1%) | 0 | |
| ≥ 5 cm | 13 (41.9%) | 10 (76.9%) | 3 (23.1%) | 0 | |
| Initial treatment frequency | 0.058 | ||||
| 1–2/week | 9 (14.3%) | 5 (55.6%) | 4 (44.4%) | 0 | |
| 3–4/week | 43 (68.3%) | 30 (69.8%) | 13 (30.2%) | 0 | |
| 5–7/week | 11 (17.5%) | 11 (100%) | 0 (0%) | 0 | |
| Duration of treatment | 0.72 | ||||
| <2 months | 16 (25.4%) | 10 (62.5%) | 6 (37.5%) | 0 | |
| 2–3.9 months | 10 (15.9%) | 8 (80.0%) | 2 (20.0%) | 0 | |
| 4–5.9 months | 26 (41.3%) | 20 (76.9%) | 6 (23.1%) | 0 | |
| ≥ 6 months | 11 (17.5%) | 8 (72.7%) | 3 (27.3%) | 0 | |
| Frequency reduction | 0.19 | ||||
| No | 53 (84.1%) | 37 (69.8%) | 16 (30.2%) | 0 | |
| Yes | 10 (15.9%) | 9 (90.0%) | 1 (10.0%) | 0 |
Chi-square test for p-values.
The treatment reduction patterns are shown in Table 3. In 10 (15.8%) of 63 women, the frequency of treatment was reduced. Between the three treatment groups that started off with 1–2, 3–4, and 5–7 applications per week, the group initially treated with 5–7 applications showed a significantly greater probability of frequency-reduction of imiquimod treatment, with 9 (81.8%) out of 11 women requiring a dose reduction. In contrast, none of the women treated with 1–2 applications per week and 2.3% of the women treated with 3–4 applications per week required a dose reduction (p b 0.01). Among 10 women requiring a frequency-reduction, 6 (9.5% of study population) were due to an adverse reaction from imiquimod. The common reasons of reduction were skin irritation (40%), erosion (40%), and pain (20%). Of those 6 women who required frequency-reduction, all eventually completed the scheduled treatment. The median time from beginning of the treatment to the frequency-reduction due to an adverse effect of imiquimod was 4 weeks (range: 1–8).
Table 3.
Reduction of imiquimod therapy.
| No. (%) | Reduction (−) | Reduction (+) | Time to reductiona | p-Value | |
|---|---|---|---|---|---|
| Reduction of initial schedule | |||||
| Total | N = 63 | 53 (84.2%) | 10 (15.8%) | < 0.001 | |
| 1–2/week | 9 (14.3%) | 9 (100%) | 0 (0%) | ||
| 3–4/week | 43 (68.3%) | 42 (97.7%) | 1 (2.3%) | ||
| 5–7/week | 11 (17.4%) | 2 (18.2%) | 9 (81.8%) | ||
| Post-reduction schedule | |||||
| 1–2 → 1–2/week | 9 (14.3%) | ||||
| 3–4 → 3–4/week | 42 (67.7%) | ||||
| 3–4 → 1–2/week | 1 (1.6%) | ||||
| 5–7 → 5–7/week | 2 (3.2%) | ||||
| 5–7 → 3–4/week | 8 (12.7%) | ||||
| 5–7 → 1–2/week | 1 (1.6%) | ||||
| Reason for reductionb | n = 10 | ||||
| Irritation | 4 (40%) | 3 (1–5) | |||
| Erosion | 4 (40%) | 3.5 (1–5) | |||
| Pain | 2 (20%) | 4.5 (2–7) | |||
| Ulceration | 1 (10%) | 7 | |||
| Scheduled reduction | 3 (30%) | 3 | |||
| Not specified | 1 (10%) | 8 | |||
Chi-square test for p-value.
Median (range).
Total number exceeds 10 due to cases with multiple adverse reactions to imiquimod.
Results of the time-dependent analysis for imiquimod therapy are shown in Table 4 and Fig. 2. Cumulative CR rates of imiquimod therapy at 2, 4, and 6-months were 9.8%, 31.1%, and 71.6%, respectively (Fig. 2A). The cumulative CR rate did not differ significantly by age (6-month CR rate, ≥ 70 versus b 70, 69.1% versus 78.7%, p = 0.90, Fig. 2B), or by disease type (primary versus recurrent, 74.2% versus 68.9%, p = 0.32, Fig. 2C). An initial treatment frequency of 5–7 times per week was associated with a significantly better CR rate than the other groups (1–2, 3–4, and 5–7 times per week, 71.9%, 65.6%, and 90.9%, p = 0.011, Fig. 2D). However, the post-frequency-reduction regimen showed that the CR rates of 3–7 per weeks group and 1–2 group per weeks did not differ significantly (72.4% versus 62.6%, p = 0.79, Fig. 2E). The reduction of treatment frequency did not affect the CR rates significantly (non-reduction versus any reduction, 70.1% versus 80.0%, p = 0.15, Fig. 2F). In multivariate analysis controlling for year and area of publication, age, disease type, and treatment frequency (initial and final), age and disease pattern (primary versus recurrent) were not associated with CR rate.
Table 4.
Risk factor for cumulative complete remission rate of vulvar Paget’s disease.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| No. | 6 M (%) | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Year of publication | <0.01 | 0.02 | ||||
| <2011 | 15 | 100% | 1 | 1 | ||
| ≥ 2011 | 48 | 60.6% | 0.30 (0.15–58) | 0.30 (0.14–0.65) | ||
| Area of publication | 0.086 | 0.16 | ||||
| Non-Europe | 21 | 66.5% | 1 | 1 | ||
| Europe | 42 | 73.6% | 1.63 (0.89–3.01) | 1.81 (0.79–4.13) | ||
| Age (years) | 0.90 | 0.41 | ||||
| <70 | 30 | 78.7% | 1 | 1 | ||
| ≥ 70 | 27 | 69.1% | 1.04 (0.55–1.95) | 0.75 (0.38–1.49) | ||
| Disease type | 0.32 | 0.48 | ||||
| Primary | 31 | 74.2% | 1 | 1 | ||
| Recurrence | 32 | 68.9% | 0.75 (0.42–1.37) | 0.76 (0.34–1.66) | ||
| Initial IQ treatment frequency | 0.011 | 0.48 | ||||
| 1–2/week | 9 | 71.9% | 1 | 1 | ||
| 3–4/week | 43 | 65.6% | 0.72 (0.27–1.93) | 0.67 (0.07–6.68) | ||
| 5–7/week | 11 | 90.9% | 1.88 (0.62–5.68) | 1.09 (0.11–11.06) | ||
| Final IQ treatment frequencya | 0.79 | 0.88 | ||||
| 1–2/week | 11 | 62.6% | 1 | 1 | ||
| 3–7/week | 52 | 72.4% | 1.12 (0.45–2.72) | 1.18 (0.14–9.86) | ||
| Reduction of IQ treatment frequency | 0.15 | |||||
| No | 53 | 70.1% | 1 | |||
| Yes | 10 | 80.0% | 1.64 (0.79–3.41) | |||
Log-rank test for univariate analysis and Cox proportional hazard regression test for multivariate analysis. Abbreviations: IQ, imiquimod; 6 M (%), 6-month cumulative complete response rate; HR, hazard ratio: and CI, confidence interval.
Cases 3–4/week and 5–7/week were grouped together due to small number for 5–7/week.
Fig. 2.
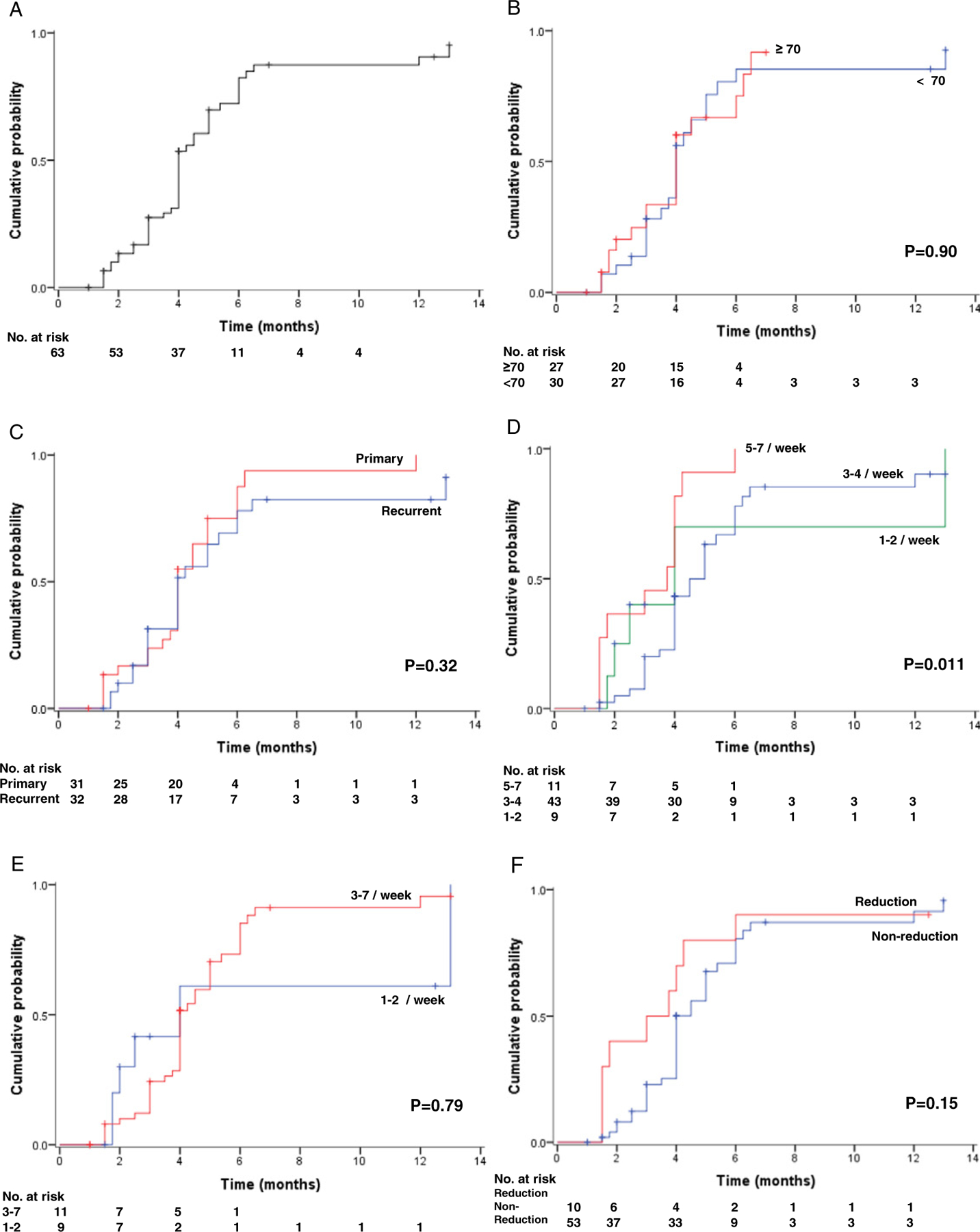
Cumulative complete response curves of imiquimod therapy for vulvar Paget’s disease. Log-rank test for p-values. Cumulative complete remission rate is shown based on (A) all cases, (B) age at diagnosis, (C) disease type, (D) initial treatment frequency, (E) final treatment frequency (cases 3–4/week and 5–7/week were grouped together due to small number for 5–7/week), and (F) dose reduction.
4. Discussion
The standard treatment for vulvar Paget’s disease has been, and still is surgical resection. Paget’s disease is characterized by multicentricity and is microscopically extended beyond the clinically visible margins. Unfortunately, disease recurrence is common regardless of surgical margin status [1,5]. Patients often require potentially disfiguring surgery multiple times, which can impact the quality of life. Therefore, developing alternative management options to surgery is desirable. The rationale of imiquimod therapy is to modulate cellular immunity against tumor cells. Imiquimod exerts its effect by eliciting a strong anti-tumoral immune response via activating toll-like receptor-7/8, which is expressed on monocytes and dendritic cells. Activated dendritic cells release cytokines such as INFα, TNFα, and IL-12, which induce and activate CD8(+) T cells [39]. Imiquimod therapy has been commonly used as a treatment for genital warts, and the effectiveness of imiquimod therapy in vulvar Paget’s disease has not been completely elucidated. Indeed, imiquimod use for vulvar Paget’s disease was only first reported in 2002 [40].
An outline of various treatment modalities for vulvar Paget’s disease is summarized in Table S1–2. Surgical resection remains the mainstay for the treatment of vulvar Paget’s disease. Previous studies have shown that the response rate of surgical resection for vulvar Paget’s disease ranges from 33–70% [4,5,41–43]. However, the rate of recurrence after surgical resection is high. A systematic review of the literature reviewing 529 cases of surgically treated vulvar Paget’s disease reported a recurrence rate of 58.0% (95% CI: 54.0–62.4) [9]. In women with negative margins, 18–38% experienced a local recurrence [3, 41–43], as opposed to 46–61% of the women who had positive surgical margins [3,42,43]. Performance of a complete vulvectomy to try to excise the disease is extremely disfiguring and often requires reconstructive procedures. Skin graft placement after vulvectomy has a 6.8% risk of postoperative complications such as physical or sexual dysfunction [44]. Also, due to the multifocal nature of this disease, recurrence can occur after complete vulvectomy.
Radiotherapy is an effective treatment in localized vulvar Paget’s disease with a reported response rate ranging from 62–100% [45–48]. The recurrence rate after radiotherapy is 0–35%. The downside of radiotherapy is that skin complications are common, and further treatment after recurrence in women who have received radiation can be challenging due to compromised healing.
Topical chemotherapy with fluorouracil and bleomycin was reported in a treatment for local disease [49,50]. According to their reports, response rate is 57–100% and the recurrence rate is 25%. Various adverse effects such as severe pain, moist desquamation, and allergic reaction have been reported. Summary for chemotherapy treatment for metastatic or invasive Paget’s disease but not localized disease is shown in Table S1–2.
Photodynamic therapy is a technique that uses a tumor-localizing photo-reactive drug such as 5-aminolevulinic acid with appropriate wavelengths to eliminate tumor cells [51]. The response rate of photodynamic therapy of vulvar Paget’s disease ranges from 14–50%, and the recurrence rate is similarly high at 38–56% [52–54]. Photosensitivity reaction and a burning sensation were reported as adverse effects for photodynamic therapy.
Laser therapy also is associated with high recurrence rate (67%). Because laser ablation only penetrates to the surface of the skin, there is a high chance of leaving a source of residual Paget’s disease as affected apocrine cells can occur deeper from the surface than the laser ablation can penetrate [55–57]. Both photodynamic therapy and laser therapy have the advantage of preserving vulvar anatomy, however the high recurrence rate after treatment is of concern, and laser ablation requires anesthesia.
The existing data of imiquimod therapy for vulvar Paget’s disease reports a response rate ranging from 52–80%, and 19% of women experienced a recurrence [9,10,13,38]. These studies are limited by small sample size (median, 21 cases) and study design (retrospective). Also the varied doses, regimens, and durations of imiquimod therapy utilized make the results difficult to adopt and interpret for a proper treatment application [10,13,34,36,38]. Our study, by combining the data, allows the analysis of a larger sample size. Based on our results, we recommend a starting dose of imiquimod therapy 3–4 times weekly with a treatment duration of 6 months. This is because response to imiquimod therapy seems to be a time-dependent event, and reduction of frequency is high in 5–7 times weekly regimen. If adverse events are noted, the number of treatments per week should be reduced until therapy is tolerated.
A strength of the study is that this is a systematic review of literature with a relatively large sample size for evaluation. Possible limitations of this study are that it was based on retrospective reviews of literature, some of which did not provide detailed information about current status with a decent period of follow-up. Publication bias may also exist. In addition, there were various treatment regimens identified in the literature that make statistical analysis difficult.
In summary, our study highlighted the potential effectiveness of imiquimod therapy as an alternative management for vulvar Paget’s disease, especially for patients with recurrence or multiple surgical resections, or who are poor surgical candidates. There is currently one phase II clinical trial examining the efficacy of imiquimod in vulvar Paget’s disease actively enrolling patients (www.clinicaltrial.gov, searched June 3, 2015). Such a prospective study for validation as well as a comparison to other treatment modalities would be beneficial to further out knowledge regarding the use of imiquimod for this disease.
Supplementary Material
HIGHLIGHTS.
Complete response rates at 2, 4, and 6-month after imiquimod treatment were 9.8%, 31.1%, and 71.6%, respectively.
Treatment frequency reduction due to adverse effects was 9.5% for imiquimod therapy for vulvar Paget’s disease.
Age, disease type (primary versus recurrence), and treatment frequency reduction were not associated with response rate to imiquimod therapy.
Acknowledgment
The study is supported by Ensign Endowment for Ovarian Cancer Research (KM and LDR). The authors thank Dr. Mikio Mikami, MD, PhD and Midori Maeda for their technical support and assistance for the study.
Footnotes
Disclosure
The authors declare that there is no conflict of interest for the study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2015.07.097.
References
- [1].Fanning J, Lambert HC, Hale TM, Morris PC, Schuerch C, Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision, Am. J. Obstet. Gynecol 180 (1999) 24–27. [DOI] [PubMed] [Google Scholar]
- [2].Lam C, Funaro D, Extramammary Paget’s disease: summary of current knowledge, Dermatol. Clin 28 (2010) 807–826. [DOI] [PubMed] [Google Scholar]
- [3].Shaco-Levy R, Bean SM, Vollmer RT, Papalas JA, Bentley RC, Selim MA, et al. , Paget disease of the vulva: a histologic study of 56 cases correlating pathologic features and disease course, Int. J. Gynecol. Pathol 29 (2010) 69–78. [DOI] [PubMed] [Google Scholar]
- [4].Parker LP, Parker JR, Bodurka-Bevers D, Deavers M, Bevers MW, Shen-Gunther J, et al. , Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis, Gynecol. Oncol 77 (2000) 183–189. [DOI] [PubMed] [Google Scholar]
- [5].Tebes S, Cardosi R, Hoffman M, Paget’s disease of the vulva, Am. J. Obstet. Gynecol 187 (2002) 281–283. [DOI] [PubMed] [Google Scholar]
- [6].Regauer S, Extramammary Paget’s disease—a proliferation of adnexal origin? Histopathology 48 (2006) 723–729. [DOI] [PubMed] [Google Scholar]
- [7].Grin A, Colgan T, Laframboise S, Shaw P, Ghazarian D, “Pagetoid” eccrine carcinoma of the vulva: report of an unusual case with review of the literature, J. Low. Genit. Tract Dis 12 (2008) 134–139. [DOI] [PubMed] [Google Scholar]
- [8].Belousova IE, Kazakov DV, Michal M, Suster S, Vulvar toker cells: the longawaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease, Am. J. Dermatopathol 28 (2006) 84–86. [DOI] [PubMed] [Google Scholar]
- [9].Edey KA, Allan E, Murdoch JB, Cooper S, Bryant A, Interventions for the treatment of Paget’s disease of the vulva, Cochrane Database Syst. Rev 10 (2013) CD009245. [DOI] [PubMed] [Google Scholar]
- [10].Luyten A, Sorgel P, Clad A, Gieseking F, Maass-Poppenhusen K, Lelle RJ, et al. , Treatment of extramammary Paget disease of the vulva with imiquimod: a retrospective, multicenter study by the German Colposcopy Network, J. Am. Acad. Dermatol 70 (2014) 644–650. [DOI] [PubMed] [Google Scholar]
- [11].Huang SW, Liu KT, Chang CC, Chen YJ, Wu CY, Tsai JJ, et al. , Imiquimod simultaneously induces autophagy and apoptosis in human basal cell carcinoma cells, Br. J. Dermatol 163 (2010) 310–320. [DOI] [PubMed] [Google Scholar]
- [12].Kemeny L, Nagy N, New perspective in immunotherapy: local imiquimod treatment, Orv. Hetil 151 (2010) 774–783. [DOI] [PubMed] [Google Scholar]
- [13].Iavazzo C, Ntziora F, Karachalios C, Evangelia Iavazzo P, Gkegkes ID, The clinical evidence and the role of imiquimod in the extramammary Paget disease, Acta Dermatovenerol. Croat 22 (2014) 103–109. [PubMed] [Google Scholar]
- [14].Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. , Metaanalysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group, JAMA 283 (2000) 2008–2012. [DOI] [PubMed] [Google Scholar]
- [15].Verkauf BS, Von Thron J, O’Brien WF, Clitoral size in normal women, Obstet. Gynecol 80 (1992) 41–44. [PubMed] [Google Scholar]
- [16].Wang LC, Blanchard A, Judge DE, Lorincz AA, Medenica MM, Busbey S, Successful treatment of recurrent extramammary Paget’s disease of the vulva with topical imiquimod 5% cream, J. Am. Acad. Dermatol 49 (2003) 769–772. [DOI] [PubMed] [Google Scholar]
- [17].Badgwell C, Rosen T, Treatment of limited extent extramammary Paget’s disease with 5 percent imiquimod cream, Dermatol. Online J 12 (2006) 22. [PubMed] [Google Scholar]
- [18].Mirer E, El Sayed F, Ammoury A, Lamant L, Messer L, Bazex J, Treatment of mammary and extramammary Paget’s skin disease with topical imiquimod, J. Dermatolog. Treat 17 (2006) 167–171. [DOI] [PubMed] [Google Scholar]
- [19].Hatch KD, Davis JR, Complete resolution of Paget disease of the vulva with imiquimod cream, J. Low. Genit. Tract Dis 12 (2008) 90–94. [DOI] [PubMed] [Google Scholar]
- [20].Geisler JP, Manahan KJ, Imiquimod in vulvar Paget’s disease: a case report, J. Reprod. Med 53 (2008) 811–812. [PubMed] [Google Scholar]
- [21].Bertozzi S, Londero AP, Fruscalzo A, Marchesoni D, Lelle RJ, Paget disease of the vulva: resolution after local treatment with imiquimod—report of a case and review of the literature, Gynakol. Geburtshilfliche Rundsch 49 (2009) 326–330. [DOI] [PubMed] [Google Scholar]
- [22].Challenor R, Hughes G, Fitton AR, Multidisciplinary treatment of vulval extramammary Paget’s disease to maintain sexual function: an imiquimod success story, J. Obstet. Gynaecol 29 (2009) 252–254. [DOI] [PubMed] [Google Scholar]
- [23].Sendagorta E, Herranz P, Feito M, Ramirez P, Floristan U, Feltes R, et al. , Successful treatment of three cases of primary extramammary Paget’s disease of the vulva with imiquimod—proposal of a therapeutic schedule, J. Eur. Acad. Dermatol. Venereol 24 (2009) 490–492. [DOI] [PubMed] [Google Scholar]
- [24].Dias Coelho J, Vale E, Viana I, Martins O, Treatment of primary extramammary Paget’s disease of the perineum with topical imiquimod 5% cream, Eur. J. Dermatol 20 (2010) 532–533. [DOI] [PubMed] [Google Scholar]
- [25].Cecchi R, Pavesi M, Bartoli L, Brunetti L, Rapicano V, Perineal extramammary Paget disease responsive to topical imiquimod, J. Dtsch. Dermatol. Ges 8 (2010) 38–40. [DOI] [PubMed] [Google Scholar]
- [26].Herranz P, Sendagorta E, Feito M, Gomez-Fernandez C, Sustained remission of extramammary Paget disease following treatment with imiquimod 5% cream, Actas Dermosifiliogr 103 (2012) 742–743. [DOI] [PubMed] [Google Scholar]
- [27].Ho SA, Aw DC, Extramammary Paget’s disease treated with topical imiquimod 5% cream, Dermatol. Ther 23 (2010) 423–427. [DOI] [PubMed] [Google Scholar]
- [28].Tonguc E, Gungor T, Var T, Ozat M, Sahin I, Sirvan L, Treatment of recurrent vulvar Paget disease with imiquimod cream: a case report and review of the literature, Arch. Gynecol. Obstet 283 (2010) 97–101. [DOI] [PubMed] [Google Scholar]
- [29].Wagner G, Heine M, Sachse MM, Extramammary Paget disease: successful therapy with imiquimod 5% cream, Hautarzt 63 (2011) 42–46. [DOI] [PubMed] [Google Scholar]
- [30].Hiraldo-Gamero A, Gomez-Moyano E, Segura-Palacios JM, Sanchez-Fajardo F, Sanz-Trelles A, Extramammary Paget disease treated with 5% imiquimod cream, Actas Dermosifiliogr 102 (2011) 554–556. [DOI] [PubMed] [Google Scholar]
- [31].Anton C, Luiz AV, Carvalho FM, Baracat EC, Carvalho JP, Clinical treatment of vulvar Paget’s disease: a case report, Clinics (Sao Paulo) 66 (2011) 1109–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matin RN, Gibbon K, Rizvi H, Harwood CA, Cerio R, Cutaneous mucinous carcinoma arising in extramammary Paget disease of the perineum, Am. J. Dermatopathol 33 (2011) 705–709. [DOI] [PubMed] [Google Scholar]
- [33].Baiocchi G, Begnami MD, Fukazawa EM, Surima WS, Badiglian-Filho L, Costa FD, et al. , Conservative management of extramammary Paget disease with imiquimod, J. Low. Genit. Tract Dis 16 (2011) 59–63. [DOI] [PubMed] [Google Scholar]
- [34].Feldmeyer L, Kerl K, Kamarashev J, de Viragh P, French LE, Treatment of vulvar Paget disease with topical imiquimod: a case report and review of the literature, J. Dermatol. Case Rep 5 (2011) 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Toledo F, Silvestre JF, Cuesta L, Ballester I, Latorre N, Monteagudo A, Sequential use with imiquimod and surgery in extramammary Paget’s disease, Dermatol. Ther 25 (2012) 82–85. [DOI] [PubMed] [Google Scholar]
- [36].Sanderson P, Innamaa A, Palmer J, Tidy J, Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative, J. Obstet. Gynaecol 33 (2013) 479–483. [DOI] [PubMed] [Google Scholar]
- [37].Frances L, Pascual JC, Leiva-Salinas M, Betlloch I, Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene, Dermatol. Ther 27 (2014) 19–20. [DOI] [PubMed] [Google Scholar]
- [38].Marchitelli C, Peremateu MS, Sluga MC, Berasategui MT, Lopez DG, Wernicke A, et al. , Treatment of primary vulvar Paget disease with 5% imiquimod cream, J. Low. Genit. Tract Dis 18 (2014) 347–350. [DOI] [PubMed] [Google Scholar]
- [39].Fehres CM, Bruijns SC, van Beelen AJ, Kalay H, Ambrosini M, Hooijberg E, et al. , Topical rather than intradermal application of the TLR7 ligand imiquimod leads to human dermal dendritic cell maturation and CD8 + T-cell cross-priming, Eur. J. Immunol 44 (2014) 2415–2424. [DOI] [PubMed] [Google Scholar]
- [40].Flowers F, Imiquimod in the treatment of actinic keratoses and other intraepithelial neoplasms, Int. J. Dermatol 41 (2002) 12–15. [DOI] [PubMed] [Google Scholar]
- [41].Hendi A, Brodland DG, Zitelli JA, Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery, J. Am. Acad. Dermatol 51 (2004) 767–773. [DOI] [PubMed] [Google Scholar]
- [42].Cai Y, Sheng W, Xiang L, Wu X, Yang H, Primary extramammary Paget’s disease of the vulva: the clinicopathological features and treatment outcomes in a series of 43 patients, Gynecol. Oncol 129 (2013) 412–416. [DOI] [PubMed] [Google Scholar]
- [43].Black D, Tornos C, Soslow RA, Awtrey CS, Barakat RR, Chi DS, The outcomes of patients with positive margins after excision for intraepithelial Paget’s disease of the vulva, Gynecol. Oncol 104 (2007) 547–550. [DOI] [PubMed] [Google Scholar]
- [44].Lavoue V, Lemarrec A, Bertheuil N, Henno S, Mesbah H, Watier E, et al. , Quality of life and female sexual function after skinning vulvectomy with split-thickness skin graft in women with vulvar intraepithelial neoplasia or vulvar Paget disease, Eur. J. Surg. Oncol 39 (2013) 1444–1450. [DOI] [PubMed] [Google Scholar]
- [45].Brown RS, Lankester KJ, McCormack M, Power DA, Spittle MF, Radiotherapy for perianal Paget’s disease, Clin. Oncol. (R. Coll. Radiol.) 14 (2002) 272–284. [DOI] [PubMed] [Google Scholar]
- [46].Yanagi T, Kato N, Yamane N, Osawa R, Radiotherapy for extramammary Paget’s disease: histopathological findings after radiotherapy, Clin. Exp. Dermatol 32 (2007) 506–508. [DOI] [PubMed] [Google Scholar]
- [47].Hata M, Koike I, Wada H, Miyagi E, Kasuya T, Kaizu H, et al. , Postoperative radiation therapy for extramammary Paget’s disease, Br. J. Dermatol 172 (2014) 1014–1020. [DOI] [PubMed] [Google Scholar]
- [48].Karam A, Dorigo O, Treatment outcomes in a large cohort of patients with invasive extramammary Paget’s disease, Gynecol. Oncol 125 (2012) 346–351. [DOI] [PubMed] [Google Scholar]
- [49].Del Castillo LF, Garcia C, Schoendorff C, Garcia JF, Torres LM, Garcia Almagro D, Spontaneous apparent clinical resolution with histologic persistence of a case of extramammary Paget’s disease: response to topical 5-fluorouracil, Cutis 65 (2000) 331–333. [PubMed] [Google Scholar]
- [50].Bewley AP, Bracka A, Staughton RC, Bunker CB, Extramammary Paget’s disease of the scrotum: treatment with topical 5-fluorouracil and plastic surgery, Br. J. Dermatol 131 (1994) 445–446. [DOI] [PubMed] [Google Scholar]
- [51].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. , Photodynamic therapy, J. Natl. Cancer Inst 90 (1998) 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fontanelli R, Papadia A, Martinelli F, Lorusso D, Grijuela B, Merola M, et al. , Photodynamic therapy with M-ALA as non surgical treatment option in patients with primary extramammary Paget’s disease, Gynecol. Oncol 130 (2013) 90–94. [DOI] [PubMed] [Google Scholar]
- [53].Al Yousef A, Boccara O, Moyal-Barracco M, Zimmermann U, Saiag P, Incomplete efficacy of 5-aminolevulinic acid (5 ALA) photodynamic therapy in the treatment of widespread extramammary Paget’s disease, Photodermatol. Photoimmunol. Photomed 28 (2012) 53–55. [DOI] [PubMed] [Google Scholar]
- [54].Shieh S, Dee AS, Cheney RT, Frawley NP, Zeitouni NC, Oseroff AR, Photodynamic therapy for the treatment of extramammary Paget’s disease, Br. J. Dermatol 146 (2002) 1000–1005. [DOI] [PubMed] [Google Scholar]
- [55].Louis-Sylvestre C, Haddad B, Paniel BJ, Paget’s disease of the vulva: results of different conservative treatments, Eur. J. Obstet. Gynecol. Reprod. Biol 99 (2001) 253–255. [DOI] [PubMed] [Google Scholar]
- [56].Valentine BH, Arena B, Green E, Laser ablation of recurrent Paget’s disease of vulva and perineum, J. Gynecol. Surg 8 (1992) 21–24. [DOI] [PubMed] [Google Scholar]
- [57].Becker-Wegerich PM, Fritsch C, Schulte KW, Megahed M, Neuse W, Goerz G, et al. , Carbon dioxide laser treatment of extramammary Paget’s disease guided by photodynamic diagnosis, Br. J. Dermatol 138 (1998) 169–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


