Abstract
Introduction
Previous studies have suggested that metformin use may enhance the therapeutic effect of progestin therapy for endometrial hyperplasia or malignancy. However, it is not known how the impact of concurrent metformin may be altered by route of progestin therapy, either locally via an intrauterine device or systemically. This study examined the effectiveness of concurrent metformin use and progestin therapy for women with complex atypical hyperplasia stratified by progestin route (systemic vs local).
Methods
This single-institution retrospective study examined consecutive women with complex atypical hyperplasia who received progestin therapy from 2003 to 2018. Time-dependent analyses for complete response rate were performed comparing concurrent metformin users versus non-users in the oral progestin group and in the levonorgestrel-releasing intrauterine device group.
Results
Across the study cohort (n=245), there were 137 (55.9%) women who responded to progestin therapy. In the oral progestin group (n=176), the median age and body mass index were 36 years and 37.7 kg/m2, respectively. 36 (20.5%) of women on oral progestins also took metformin. After controlling for diabetes status, women taking both oral progestins and metformin had a complete response rate similar to those not taking metformin (6 month cumulative rates, 23.1% vs 27.8%, adjusted hazard ratio (aHR) 0.71, 95% confidence interval (95% CI) 0.36 to 1.41). In the levonorgestrel-releasing intrauterine device group (n=69), the median age and body mass index were 35 years and 39.9 kg/m2, respectively. There were 15 (21.7%) women who took metformin in addition to the levonorgestrel-releasing intrauterine device. After controlling for diabetes status, women who had the levonorgestrel-releasing intrauterine device and took metformin had a significantly higher complete response rate compared with those not taking metformin (6 month cumulative rates, 86.7% vs 58.9%, aHR 2.31, 95% CI 1.09 to 4.89).
Conclusion
In a predominantly obese population, concurrent metformin may possibly offer treatment benefit when used with the levonorgestrel-releasing intrauterine device.
INTRODUCTION
Complex atypical hyperplasia is a pathological pre-malignant lesion in the endometrium and is a precursor of endometrial cancer.1,2 Nearly 30% of women with complex atypical hyperplasia have progression to endometrial cancer, and up to 40% of women diagnosed with complex atypical hyperplasia are found to have an occult endometrial cancer at the time of hysterectomy.1-3 Total hysterectomy is the standard definitive treatment for complex atypical hyperplasia1; however, reproductive-aged women who desire future fertility or those who are poor surgical candidates may undergo medical treatment with progestin therapy.1 Progestin therapy for complex atypical hyperplasia can be highly effective; nearly two thirds of women show complete regression.4,5 The remaining one third who have treatment failure with progestin therapy require additional or alternative treatment, even hysterectomy. One such strategy to overcome progestin-resistant complex atypical hyperplasia may be the adjunctive use of metformin concurrently with progestin therapy.
Metformin is an insulin-sensitizing biguanide commonly used in the treatment of type 2 diabetes mellitus.6 Pre-clinical studies have shown anti-tumor activity via the mammalian target of rapamycin pathway in endometrial cancer.7,8 A prior clinical study also suggested that metformin may enhance the therapeutic effect of oral progestin therapy for women with atypical endometrial hyperplasia or malignancy (>80% complete regression rate).9 However, this trial lacked an active comparator, making their results difficult to interpret. Another trial with similar results had a control group but was fairly limited due to sample size (n=16).10 Moreover, a 2017 systematic review remarked that there remains an insufficient body of evidence to support the pharmaco-adjunctive use of metformin with oral progestin in endometrial hyperplasia.11
Recently, several studies have suggested that the effectiveness of progestin therapy for complex atypical hyperplasia may differ by treatment route, and local progestin therapy may be more effective than systemic therapy, particularly in obese individuals.12,13 Based on available data, we hypothesized that the effectiveness of concurrent metformin may differ depending on route of progestin therapy (systemic vs local). The objective of the current study was to examine the effectiveness of concurrent metformin use and progestin therapy for complex atypical hyperplasia in a predominantly obese population, stratified by progestin route (systemic vs local).
METHODS
Data Source and Eligibility
This is a secondary exploratory analysis of a previously conducted retrospective study examining consecutive women with complex atypical hyperplasia who received treatment at the Los Angeles County Medical Center from 2003 to 2018.13,14 Institutional Review Board approval at the University of Southern California was properly obtained (HS-11–00131). The study population comprised 245 women who were diagnosed with complex atypical hyperplasia and who received medical treatment with progestins . These women had received at least 1 month of progestin therapy (either oral or local) following the complex atypical hyperplasia diagnosis. Women with non-atypical or simple hyperplasia or endometrial cancer were excluded. Additionally, those who received multiple progestins or progestin therapy before the complex atypical hyperplasia diagnosis were excluded. Finally, women who did not have a follow-up biopsy performed at least 1 month following treatment initiation were excluded from the study.
Clinical Information
Variables recorded in the database included patient demographics, medical comorbidities, medication types, treatment response, and follow-up. Patient demographics at the initial complex atypical hyperplasia diagnosis included age, year, race/ethnicity, gravidity, parity, and body mass index. Endometrial thickness on ultrasonographic imaging at the time of complex atypical hyperplasia diagnosis was also abstracted. Medical comorbidities included diabetes mellitus, hypertension, hyperlipidemia, polycystic ovary syndrome, and reported infertility. Medication types included metformin, aspirin, statins, and β-blockers at the time of complex atypical hyperplasia diagnosis.
Progestin route of administration was grouped as systemic versus local based on the first-line agent used after the initial diagnosis of complex atypical hyperplasia. Systemic progestins included oral medroxyprogesterone acetate, megestrol acetate, norethindrone, or intramuscular depo-medroxyprogesterone acetate (termed oral progestin group). Local therapy was defined as treatment with the levonorgestrel-releasing intrauterine device.
Treatment Follow-Up
In our practice, re-sampling of the endometrium is usually performed every 3 to 6 months in those who elect for medical management to assess treatment response. In those who achieve complete response, follow-up is generally continued in the same fashion but can be personalized, depending on specific patient risk factors or clinical considerations. Date of treatment initiation and last follow-up date were recorded. The chronologic sequence of complex atypical hyperplasia diagnosis, progestin therapy initiation, and treatment outcomes on follow-up endometrial biopsies were then ascertained from this information.
Study Definition
Body mass index was classified as: overweight (25–29.9 kg/m2), class I obesity (30–34.9 kg/m2), class II obesity (35–39.9 kg/m2), and class III obesity (≤40 kg/m2).15 Histopathology diagnoses of endometrial hyperplasia were based on the 1994 World Health Organization criteria as: simple hyperplasia without atypia, simple hyperplasia with atypia, complex hyperplasia without atypia, and complex hyperplasia with atypia.2
Response for progestin therapy was categorized as: complete response, defined as no residual hyperplasia identified on subsequent biopsies; partial response, defined as regression of complex atypical hyperplasia to simple or non-atypical hyperplasia; persistent disease; and progression to cancer. Overall response refers to complete response and partial response. Time to treatment response was defined as the time interval between initiation of progestin therapy and the first follow-up biopsy demonstrating treatment response. The last follow-up was defined as the time interval between progestin therapy initiation and the last endometrial biopsy. Cases were censored at the last follow-up if an outcome event was absent. Women who changed progestin therapy route, changed to a different progestin agent, initiated combination progestin therapy, or underwent hysterectomy were censored at those time points.
Statistical Consideration
The primary objective of the analysis was to examine the effectiveness of concurrent metformin use during progestin therapy stratified by route of progestin administration (systemic vs local). The study cohort was grouped as oral progestin versus levonorgestrel- releasing intrauterine device. In each cohort, women were divided based on metformin use (users vs non-users), and treatment response was assessed. The secondary aim was to identify the characteristics associated with metformin use.
Differences in baseline demographics between the two groups were assessed with the Mann-Whitney U test, Fisher exact test, or χ2 test as appropriate. A binary logistic regression model was fitted to identify the independent characteristics associated with metformin use. The conditional backward method was used to retain only covariates with a p<0.05 level in the final model due to limited sample size. The effect size of statistical significance was expressed with odds ratio and 95% confidence interval (OR, 95% CI).
As treatment response to progestin therapy depends on follow-up duration, a time-dependent analysis was performed to assess progestin treatment response. Cumulative response curves were plotted with the Kaplan-Meier method, and differences in curves were assessed with the log-rank test. A Cox proportional hazard regression model was then used to estimate the effect size of metformin use on progestin therapy route and treatment response (complete response and overall response). A parsimonious adjustment model was fitted for this multivariable analysis, and the association between metformin use and treatment response was adjusted for independent factors associated with metformin use in each cohort. In an additional adjustment, the association was controlled for body mass index. This was based on the post-hoc assessment of the study population that showed different body habitus per treatment allocations. The effect size of statistical significance was expressed with adjusted hazard ratio (aHR) and 95% CI.
All statistical analyses were based on two-sided hypotheses, and p<0.05 was considered statistically significant. Statistical Package for Social Sciences (SPSS, version 25.0, Armonk, NY, USA) was used for all analyses. The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines were utilized to outline this observational cohort study.16
RESULTS
Systemic Progestin Cohort
Across the study cohort (n=245), there were 137 (55.9%) women who responded to progestin therapy. There were 176 women who received systemic progestin treatment for complex atypical hyperplasia. Patient demographic characteristics are shown in Table 1. The median age and body mass index were 36 years and 37.7 kg/m2, respectively. The majority of women were obese (n=147, 83.5%), and nearly half were nulligravid (n=90, 51.1%). Diabetic women represented nearly one third of this group (n=56, 31.8%).
Table 1.
Patient demographics (systemic progestin group)
| Characteristics | All | Metformin (−) | Metformin (+) | P value | OR (95% CI) | P value* |
|---|---|---|---|---|---|---|
| Number | n=176 | n=140 | n=36 | |||
| Age (years) | 36 (30–43) | 36 (30–43) | 35 (27–41) | 0.204 | ||
| <30 | 40 (22.7%) | 28 (20.0%) | 12 (33.3%) | |||
| 30–39 | 74 (42.0%) | 62 (44.3%) | 12 (33.3%) | |||
| 40–49 | 43 (24.4%) | 34 (24.3%) | 9 (25.0%) | |||
| ≥50 | 19 (10.8%) | 16 (11.4%) | 3 (8.3%) | |||
| Year | 0.099 | |||||
| 2003–2008 | 75 (42.6%) | 65 (46.4%) | 10 (27.8%) | |||
| 2009–2013 | 75 (42.6%) | 57 (40.7%) | 18 (50.0%) | |||
| 2014–2018 | 26 (14.8%) | 18 (12.9%) | 8 (22.2%) | |||
| Race/ethnicity | 0.072 | |||||
| White | 111 (63.1%) | 83 (59.3%) | 28 (77.8%) | |||
| Black | 12 (6.8%) | 8 (5.7%) | 4 (11.1%) | |||
| Hispanic | 3 (1.7%) | 3 (2.1%) | 0 | |||
| Asian | 6 (3.4%) | 6 (4.3%) | 0 | |||
| Other/unknown | 44 (25.0%) | 40 (28.6%) | 4 (11.1%) | |||
| BMI (kg/m2) | 37.7 (31.9–45.0) | 38.3 (31.2–45.0) | 37.1 (32.7–46.3) | 0.905 | ||
| <25 | 9 (5.1%) | 9 (6.4%) | 0 | |||
| 25–29.9 | 20 (11.4%) | 16 (11.4%) | 4 (11.1%) | |||
| 30–34.9 | 39 (22.2%) | 28 (20.0%) | 11 (30.6%) | |||
| 35–39.9 | 32 (18.2%) | 23 (16.4%) | 9 (25.0%) | |||
| ≥40 | 72 (40.9%) | 61 (43.6%) | 11 (30.6%) | |||
| Unknown | 4 (2.3%) | 3 (2.1%) | 1 (2.8%) | |||
| Gravidity | 0.286 | |||||
| 0 | 90 (51.1%) | 71 (50.7%) | 19 (52.8%) | |||
| 1 | 30 (17.0%) | 27 (19.3%) | 3 (8.3%) | |||
| 2 | 22 (12.5%) | 18 (12.9%) | 4 (11.1%) | |||
| ≥3 | 34 (19.3%) | 24 (17.1%) | 10 (27.8%) | |||
| Parity | 0.887 | |||||
| 0 | 104 (59.1%) | 81 (57.9%) | 23 (63.9%) | |||
| 1 | 32 (18.2%) | 27 (19.3%) | 5 (13.9%) | |||
| 2 | 15 (8.5%) | 12 (8.6%) | 3 (8.3%) | |||
| ≥3 | 25 (14.2%) | 20 (14.3%) | 5 (13.9%) | |||
| Hypertension | 0.833 | |||||
| No | 130 (72.2%) | 104 (74.3%) | 26 (72.2%) | |||
| Yes | 46 (26.1%) | 36 (25.7%) | 10 (27.8%) | |||
| Diabetes | <0.001 | |||||
| No | 120 (68.2%) | 112 (80.0%) | 8 (22.2%) | 1 | ||
| Yes | 56 (31.8%) | 28 (20.0%) | 28 (77.8%) | 12.5 (4.21 to 37.4) | <0.001 | |
| Hyperlipidemia | <0.001 | |||||
| No | 138 (78.4%) | 124 (88.6%) | 14 (38.9%) | |||
| Yes | 38 (21.6%) | 16 (11.4%) | 22 (61.1%) | 6.29 (2.33 to 16.9) | <0.001 | |
| PCOS | 0.014 | |||||
| No | 144 (81.8%) | 120 (85.7%) | 24 (66.7%) | 1 | ||
| Yes | 32 (18.2%) | 20 (14.3%) | 12 (33.3%) | 6.20 (1.83 to 21.1) | 0.003 | |
| Infertility | 0.339 | |||||
| No | 107 (60.8%) | 88 (62.9%) | 19 (52.8%) | |||
| Yes | 69 (39.2%) | 52 (37.1%) | 17 (47.2%) | |||
| Aspirin | 0.269 | |||||
| No | 164 (93.2%) | 132 (94.3%) | 32 (88.9%) | |||
| Yes | 12 (6.8%) | 8 (5.7%) | 4 (11.1%) | |||
| Statin | 0.002 | |||||
| No | 162 (92.0%) | 134 (95.7%) | 28 (77.8%) | |||
| Yes | 14 (8.0%) | 6 (4.3%) | 8 (22.2%) | |||
| β-blocker | 0.465 | |||||
| No | 165 (93.8%) | 130 (92.9%) | 35 (97.2%) | |||
| Yes | 11 (6.3%) | 10 (7.1%) | 1 (2.8%) | |||
| Endometrial echo complex (mm) | 0.450 | |||||
| 0–4 | 8 (4.5%) | 7 (5.0%) | 1 (2.8%) | |||
| 5–9 | 40 (22.7%) | 32 (22.9%) | 8 (22.2%) | |||
| 10–14 | 54 (30.7%) | 38 (27.1%) | 16 (44.4%) | |||
| 15–19 | 30 (17.0%) | 25 (17.9%) | 5 (13.9%) | |||
| ≥20 | 32 (18.2%) | 28 (20.0%) | 4 (11.1%) | |||
| Not measured | 12 (6.8%) | 10 (7.1%) | 2 (5.6%) | |||
Median (IQR) or number (percentage per column) is shown. Mann-Whitney U test, Fisher exact test, or χ2 test for p value on univariable analysis.
Significant p values are in bold.
P value for multivariable analysis with a binary logistic regression model.
BMI, body mass index; PCOS, polycystic ovary syndrome.
In the systemic progestin cohort, 36 (20.5%) women used concurrent metformin and the remaining 140 (79.5%) women received progestin therapy alone. In univariable analysis (Table 1), women who received metformin were more likely to have diagnoses of diabetes (77.8% vs 20.0%), dyslipidemia (61.1% vs 11.4%), and/ or polycystic ovary syndrome (33.3% vs 14.3%), and were more likely to also use a statin (22.2% vs 4.3%), compared with those who did not use metformin (all, p<0.05). On multivariable analysis (Table 1), diabetes mellitus (adjusted OR (aOR) 12.5, 95% CI 4.21 to 37.4), hyperlipidemia (aOR 6.29, 95% CI 2.33 to 16.9), and polycystic ovary syndrome (aOR 6.20, 95% CI 1.83 to 21.1) remained independent factors associated with metformin use (all, p<0.05).
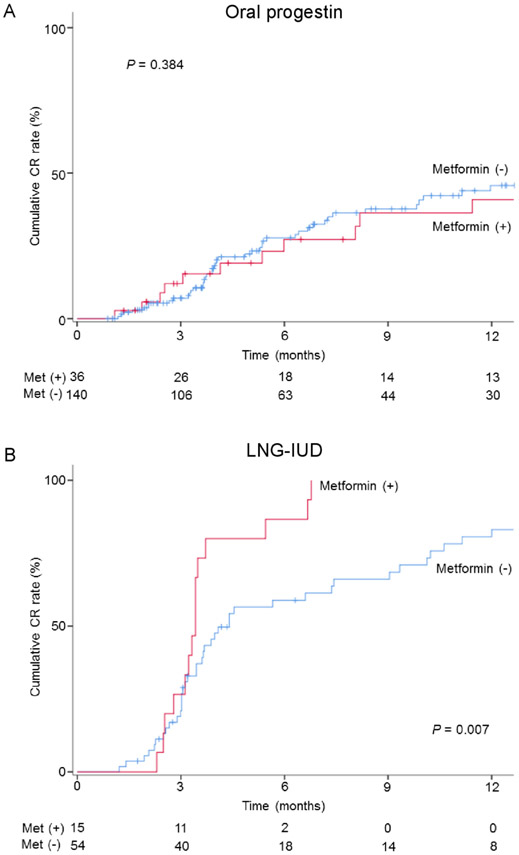
The median follow-up time was 9.0 months (IQR 3.5–31.1), and there were 60 (34.1%) women who had a complete response and 18 (10.2%) women who had a partial response (overall response, n=78, 44.3%). On univariable analysis (Figure 1A), metformin users had a cumulative complete response rate similar to those of non-users (6 month cumulative rates, 23.1% vs 27.8%, and 1 year cumulative rates, 40.8% vs 45.8%, p=0.384). After controlling for diabetes status (Figure 2), metformin use was not associated with complete response (aHR 0.71, 95% CI 0.36 to 1.41; p=0.326). When controlling for body mass index, concurrent metformin was not associated with complete response (aHR 0.74, 95% CI 0.38 to 1.46; p=0.390). Similarly, metformin use was not associated with overall response (online supplementary figure S1A and S2).
Figure 1.
Cumulative complete response (CR) rate per metformin use. Log-rank test for p values. Cumulative rate of CR is compared with metformin users and non-users in (A) oral progestin therapy and (B) levonorgestrel-releasing intrauterine device (LNG-IUD) therapy.
Figure 2.
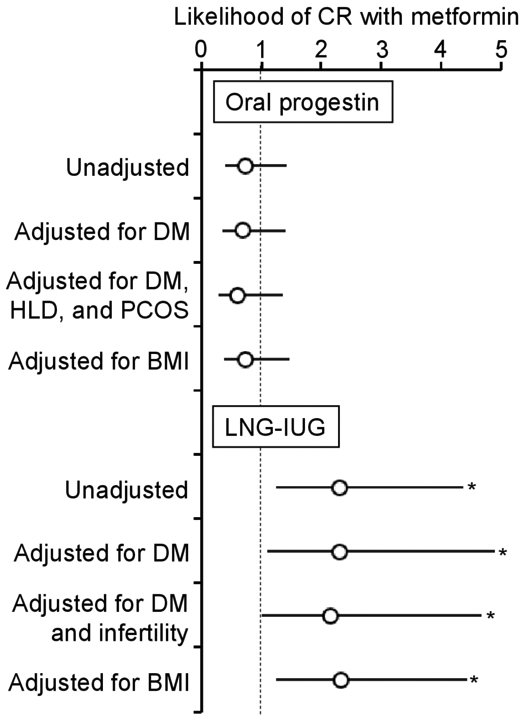
Adjustment models for association between metformin use and complete response (CR) (systemic progestin and levonorgestrel-releasing intrauterine device (LNG-IUD) cohorts). Cox proportional hazard regression test for adjustment models. Circles represent hazard ratio for the likelihood of CR or partial response (PR) with metformin compared with non-metformin. Bars represent 95% confidence interval. *P<0.05. BMI, body mass index; DM, diabetes mellitus; HLD, hyperlipidemia, PCOS, polycystic ovarian syndrome.
Local Progestin Cohort
There were 69 women who received the levonorgestrel-releasing intrauterine device for the treatment of complex atypical hyperplasia. Patient demographic characteristics are shown in Table 2. The median age and body mass index were 35 years and 39.9 kg/m2, respectively. Nearly half of women in this group were morbidly obese (class III obesity, n=34, 49.3%) and nearly half were nulligravid (n=37, 53.6%). Nearly one quarter of the group was diabetic (n=18, 26.1%).
Table 2.
Patient demographics (levonorgestrel-releasing intrauterine device group)
| Characteristics | All | Metformin (−) | Metformin (+) | P value | OR (95% CI) | P value* |
|---|---|---|---|---|---|---|
| Number | n=69 | n=54 | n=15 | |||
| Age (years) | 35 (29.5–42) | 36 (29.8–44.3) | 34.0 (29–39) | 0.423 | ||
| <30 | 17 (24.6%) | 13 (24.1%) | 4 (26.7%) | |||
| 30–39 | 33 (47.8%) | 24 (44.4%) | 9 (60.0%) | |||
| 0–49 | 12 (17.4%) | 11 (20.4%) | 1 (6.7%) | |||
| 50 | 7 (10.1%) | 6 (11.1%) | 1 (6.7%) | |||
| Year | 0.720 | |||||
| 2003–2008 | 7 (10.1%) | 6 (11.1%) | 1 (6.7%) | |||
| 2009–2013 | 18 (26.1%) | 13 (24.1%) | 5 (33.3%) | |||
| 2014–2018 | 44 (63.8%) | 35 (64.8%) | 9 (60.0%) | |||
| Race/ethnicity | 0.446 | |||||
| White | 49 (71.0%) | 36 (66.7%) | 13 (86.7%) | |||
| Black | 9 (13.0%) | 7 (13.0%) | 2 (13.3%) | |||
| Hispanic | 4 (5.8%) | 4 (7.4%) | 0 | |||
| Asian | 5 (7.2%) | 5 (9.3%) | 0 | |||
| Other/unknown | 2 (2.9%) | 2 (3.7%) | 0 | |||
| BMI (kg/m2) | 39.9 (34.0–46.9) | 39.9 (32.2–46.3) | 42.0 (38.0–60.5) | 0.150 | ||
| <25 | 1 (1.4%) | 1 (1.9%) | 0 | |||
| 25–29.9 | 6 (8.7%) | 6 (11.1%) | 0 | |||
| 30–34.9 | 11 (15.9%) | 11 (20.4%) | 0 | |||
| 35–39.9 | 17 (24.6%) | 10 (18.5%) | 7 (46.7%) | |||
| ≥40 | 34 (49.3%) | 26 (48.1%) | 8 (53.3%) | |||
| Gravidity | 0.578 | |||||
| 0 | 37 (53.6%) | 30 (55.6%) | 7 (46.7%) | |||
| 1 | 11 (15.9%) | 7 (13.0%) | 4 (26.7%) | |||
| 2 | 8 (11.6%) | 7 (13.0%) | 1 (6.7%) | |||
| ≥3 | 13 (18.8%) | 10 (18.5%) | 3 (20.0%) | |||
| Parity | 0.570 | |||||
| 0 | 42 (60.9%) | 34 (63.0%) | 8 (53.3%) | |||
| 1 | 11 (15.9%) | 7 (13.0%) | 4 (26.7%) | |||
| 2 | 8 (11.6%) | 6 (11.1%) | 2 (13.3%) | |||
| ≥3 | 8 (11.6%) | 7 (13.0%) | 1 (6.7%) | |||
| Hypertension | 0.999 | |||||
| No | 48 (69.6%) | 37 (68.5%) | 11 (73.3%) | |||
| Yes | 21 (30.4%) | 17 (31.5%) | 4 (26.7%) | |||
| Diabetes | <0.001 | |||||
| No | 51 (73.9%) | 47 (87.0%) | 4 (26.7%) | 1 | ||
| Yes | 18 (26.1%) | 7 (13.0%) | 11 (73.3%) | 20.5 (4.52 to 93.2) | <0.001 | |
| Hyperlipidemia | 0.020 | |||||
| No | 50 (72.5%) | 43 (79.6%) | 7 (46.7%) | |||
| Yes | 19 (27.5%) | 11 (20.4%) | 8 (53.3%) | |||
| PCOS | 0.232 | |||||
| No | 46 (66.7%) | 38 (70.4%) | 8 (53.3%) | |||
| Yes | 23 (33.3%) | 16 (29.6%) | 7 (46.7%) | |||
| Infertility | 0.031 | |||||
| No | 45 (65.2%) | 39 (72.2%) | 6 (40.0%) | 1 | ||
| Yes | 24 (34.8%) | 15 (27.8%) | 9 (60.0%) | 4.68 (1.03 to 21.3) | 0.046 | |
| Aspirin | 0.604 | |||||
| No | 63 (91.3%) | 50 (92.6%) | 13 (86.7%) | |||
| Yes | 6 (8.7%) | 4 (7.4%) | 2 (13.3%) | |||
| Statin | 0.641 | |||||
| No | 62 (89.9%) | 49 (90.7%) | 13 (86.7%) | |||
| Yes | 7 (10.1%) | 5 (9.3%) | 2 (13.3%) | |||
| β-blocker | 0.578 | |||||
| No | 64 (92.8%) | 49 (90.7%) | 15 (100%) | |||
| Yes | 5 (7.2%) | 5 (9.3%) | 0 | |||
| Endometrial echo complex (mm) | 0.715 | |||||
| 0–4 | 9 (13.0%) | 7 (13.0%) | 2 (13.3%) | |||
| 5–9 | 18 (26.1%) | 16 (29.6%) | 2 (13.3%) | |||
| 10–14 | 16 (23.2%) | 11 (20.4%) | 5 (33.3%) | |||
| 15–19 | 10 (14.5%) | 7 (13.0%) | 3 (20.0%) | |||
| ≥20 | 8 (11.6%) | 6 (11.1%) | 2 (13.3%) | |||
| Not measured | 8 (11.6%) | 7 (13.0%) | 1 (6.7%) | |||
Median (IQR) or number (percentage per column) is shown. Mann-Whitney U test, Fisher exact test, or χ2 test for p value on univariable analysis.
Significant p values are in bold.
P value for multivariable analysis with a binary logistic regression model.
BMI, body mass index; PCOS, polycystic ovary syndrome.
In the levonorgestrel-releasing intrauterine device group (n=69), there were 15 (21.7%) women who took metformin in addition to the levonorgestrel-releasing intrauterine device, and the remaining 54 (78.3%) women did not. On univariable analysis (Table 2), metformin users were more likely to have diabetes (73.3% vs 13.0%), dyslipidemia (53.3% vs 20.4%), and/or infertility (60.0% vs 27.8%) compared with non-users (all, p<0.05). On multivariable analysis (Table 2), diabetes mellitus (aOR 20.5, 95% CI 4.52 to 93.2) and infertility (aOR 4.68, 95% CI 1.03 to 21.3) remained independent factors associated with metformin use (both, p<0.05).
The median follow-up time in the levonorgestrel-releasing intrauterine device group was 9.8 months (IQR 4.1–23.3); there were 58 (84.1%) women who had a complete response and one (1.4%) woman who had a partial response (overall response, n=59, 85.5%). On univariable analysis (Figure 1B), metformin users had a significantly higher cumulative complete response rate compared with non-users (6 month cumulative rates, 86.7% vs 58.9%, and 1 year cumulative rates, 100% vs 80.7%, p=0.007). After controlling for diabetes status (Figure 2), metformin users had a twofold increased likelihood of achieving complete response (aHR 2.31, 95% CI 1.09 to 4.89; p=0.030). After controlling for body mass index, metformin use was significantly associated with increased likelihood of achieving complete response (aHR 2.35, 95% CI 1.24 to 4.43; p=0.008). Similar results were observed for overall response (online supplementary figure S1B and S2).
DISCUSSION
A key finding of the current study is that the benefit of concurrent metformin may differ depending on the route of progestin therapy for complex atypical hyperplasia, particularly in obese women. Among women who received systemic progestin treatment, concurrent metformin was not associated with improved treatment response, whereas in women using local progestin therapy, treatment response was improved with the addition of metformin. Possible direct and indirect associations between progestin and metformin merit further discussion.
These findings suggest that systemic progestins may possibly limit any added benefit of concurrent metformin therapy. Metformin is typically given orally; thus, there is a possibility of a direct drug–drug interaction with oral progestins in the systemic circulation. In general, metformin-related drug–drug interaction involves transporters in enterocytes, hepatocytes, and tubular cells via multidrug and toxin extrusion transporter, organic cation transporter, and plasma membrane monoamine transporter.17 Thus, if progestin interacts with these transporters, it is possible that the metabolism of metformin could potentially be altered. A prior in vitro study showed that organic cation transporter activity may increase with progestin exposure in endometrial cancer cell lines,18 suggesting that a drug–drug interaction between metformin and oral progestin may be present via the organic cation transporter pathway. To date, in a contemporary view of literature, direct drug–drug interaction between metformin and oral progestin has not been examined but should be further investigated.17
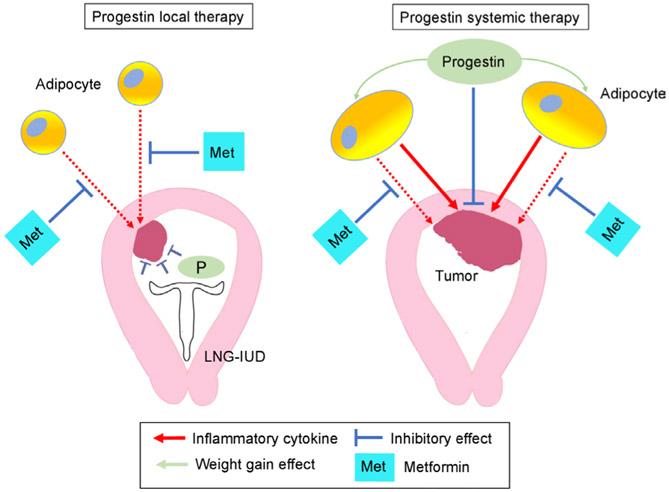
Decreased efficacy of metformin in the systemic progestin group may also be due to indirect interactions between metformin and oral progestins. One possibility for an indirect effect is the impact of oral progestins on patients’ weight (Figure 3). A recent study showed that oral progestin use is associated with significant weight gain compared with local progestin therapy among women with complex atypical hyperplasia and endometrial cancer (2.95 kg vs 0.05 kg weight gain in 1 year).19 Therefore, increased adiposity due to weight gain from oral progestin therapy may result in an increase in inflammatory cytokines from excess adipocytes that can stimulate oncogenesis in the endometrium.20 Given the fact that metformin’s anti-tumor activity seems to be via the inhibition of circulating pro-inflammatory mediators rather than a direct anti-tumor effect, as demonstrated in a recent prospective study,21 weight gain may counteract the anti-inflammatory mechanism by which metformin exerts benefit in the setting of complex atypical hyperplasia.22
Figure 3.
Hypothesis-generating schema for the interaction between metformin and systemic progestin. Possible pathways for the interaction of progestin and metformin in obesity are shown. Inflammatory cytokines from excess adiposity stimulate endometrial cells for oncogenesis. Metformin inhibits inflammatory cytokines from the adipocytes. Systemic progestin may cause weight gain, resulting in increases in inflammatory cytokines and decreases in the relative effectiveness of metformin. LNG-IUD, levonorgestrel-releasing intrauterine device.
Therefore, local progestin therapy with the levonorgestrel- releasing intrauterine device may be an attractive approach to deliver progestin while maintaining the anti-inflammatory benefits of metformin in the treatment of women with complex atypical hyperplasia. The rationale for this hypothesis is based on the following: (1) our observation demonstrating higher treatment response with metformin in the levonorgestrel-releasing intrauterine device group but not in the systemic progestin group; (2) local therapy with levonorgestrel-releasing intrauterine device does not lead to weight gain19; (3) concurrent use of metformin during progestin therapy may be more effective in women with large body habitus23; and (4) large body habitus is associated with increased recurrence risk with progestin therapy.24 The latter two rationales suggest that the anti-inflammatory effects of metformin are responsible for its anti-tumor activity, particularly in obese women who have increased inflammation from adipose tissue. This effect may be evident with the levonorgestrel-releasing intrauterine device given the absence of weight gain, while weight gain from oral progestins may mask any positive effects of metformin. Further study is definitely warranted to support this hypothesis.
There are several limitations in this study. First, there is unmeasured bias in all retrospective observational studies. For example, we are unable to know details that contributed to the decision process for route of progestin therapy. The exact indication for, timing of, and compliance with metformin therapy were also not assessable. Similarly, patient compliance, adverse effects, and weight changes related to progestin treatment were not available but likely impacted the results. Second, information regarding glycemic control in diabetic or pre-diabetic patients was not included. Also, metformin dose, frequency, and duration of treatment were not assessed but may possibly interact with the analysis. Third, the majority of this patient population was Hispanic and obese, and generalizability to patients of different race/ethnicity or body habitus is unknown. The relatively lower response rate in this study compared with other studies may be secondary to the unique characteristics of our population with a high rate of morbid obesity, historically low rates of treatment compliance, and short follow-up intervals as described previously.13,14 Fourth, while we speculated based on prior evidence that the mechanism of metformin’s anti-tumor effect is secondary to anti-inflammatory pathways in excess adipocytes, this study does not provide actual data to support this theory. Lastly, a central pathology review was not performed for the diagnostic confirmation of complex atypical hyperplasia in the study. It is possible that the relatively low response rate to progestin therapy in our study may be due to misclassification of complex atypical hyperplasia and low-grade endometrioid endometrial cancer, as distinguishing the two diagnoses can often be challenging.25,26
In summary, our study suggests that concurrent use of metformin during progestin therapy may be more effective when the progestin is given with the levonorgestrel-releasing intrauterine device. As this recommendation is based on a single study of retrospective observation, prospective studies are surely necessary to confirm this finding. There is an ongoing randomized controlled trial comparing the complete response rates of the levonorgestrel- releasing intrauterine device with and without metformin for obese women with complex atypical hyperplasia or grade 1 endometrioid adenocarcinoma of the endometrium (NCT01686126).27 The study team used an estimated complete response rate of 68% (95% CI 45% to 86%) with levonorgestrel-releasing intrauterine device treatment and assumed a 60% increase in the estimated likelihood of complete response with metformin.28 Based on their sample size estimation, 45 patients will be examined for each group. This trial will ultimately help answer questions regarding the effectiveness of metformin as an adjunct to progestin therapy in complex atypical hyperplasia.
Supplementary Material
HIGHLIGHTS.
In women taking oral progestins, concurrent metformin does not impact treatment response.
Concurrent metformin was associated with improved response in women using the levonorgestrel-releasing intrauterine device.
Effectiveness of concurrent metformin for complex atypical hyperplasia may depend on the progestin route.
Acknowledgments
Funding This study was funded by Ensign Endowment for Gynecologic Cancer Research (KM).
Footnotes
Competing interests Consultant, Quantgene (LDR); honorarium, Chugai, textbook editorial expense, Springer, and investigator meeting attendance expense, VBL therapeutics (KM); Research funding, MSD (SM).
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
REFERENCES
- 1.Trimble CL, Method M, Leitao M, et al. Management of endometrial precancers. Obstet Gynecol 2012;120:1160–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer 1985;56:403–12. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K, Ramzan AA, Gualtieri MR, et al. Prediction of concurrent endometrial carcinoma in women with endometrial hyperplasia. Gynecol Oncol 2015;139:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunderson CC, Fader AN, Carson KA, et al. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson CC, Dutta S, Fader AN, et al. Pathologic features associated with resolution of complex atypical hyperplasia and grade 1 endometrial adenocarcinoma after progestin therapy. Gynecol Oncol 2014;132:33–7. [DOI] [PubMed] [Google Scholar]
- 6.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574–9. [DOI] [PubMed] [Google Scholar]
- 7.Hanna RK, Zhou C, Malloy KM, et al. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol Oncol 2012;125:458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantrell LA, Zhou C, Mendivil A, et al. Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecol Oncol 2010;116:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsuhashi A, Sato Y, Kiyokawa T, et al. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol 2016;27:262–6. [DOI] [PubMed] [Google Scholar]
- 10.Shan W, Wang C, Zhang Z, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol 2014;25:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement NS, Oliver TR, Shiwani H, et al. Metformin for endometrial hyperplasia. Cochrane Database Syst Rev 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal N, Broaddus RR, Urbauer DL, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet Gynecol 2018;131:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel- releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol 2020. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccone MA, Whitman SA, Conturie CL, et al. Effectiveness of progestin-based therapy for morbidly obese women with complex atypical hyperplasia. Arch Gynecol Obstet 2019;299:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Defining Adult Overweight and Obesity, Overweight & Obesity. Available: https://www.cdc.gov/obesity/adult/defining.html [Accessed 13 Jan 2020].
- 16.von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stage TB, BrØsen K, Christensen MMH. A comprehensive review of drug-drug interactions with metformin. Clin Pharmacokinet 2015;54:811–24. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Ito K, Onogawa T, et al. Expression of organic cation transporter SLC22A16 in human endometria. Int J Gynecol Pathol 2007;26:53–60. [DOI] [PubMed] [Google Scholar]
- 19.Cholakian D, Hacker K, Fader AN, et al. Effect of oral versus intrauterine progestins on weight in women undergoing fertility preserving therapy for complex atypical hyperplasia or endometrial cancer. Gynecol Oncol 2016;140:234–8. [DOI] [PubMed] [Google Scholar]
- 20.Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol 2016;34:4225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitson SJ, Maskell Z, Sivalingam VN, et al. Pre-surgical metformin in uterine malignancy (PREMIUM): a multi-center, randomized double-blind, placebo-controlled phase III trial. Clin Cancer Res 2019;25:2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron AR, Morrison VL, Levin D, et al. Anti-Inflammatory effects of metformin irrespective of diabetes status. Circ Res 2016;119:652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuhashi A, Habu Y, Kobayashi T, et al. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J Gynecol Oncol 2019;30:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J-Y, Kim D-Y, Kim J-H, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur J Cancer 2013;49:868–74. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol 2000;13:309–27. [DOI] [PubMed] [Google Scholar]
- 26.Allison KH, Reed SD, Voigt LF, et al. Diagnosing endometrial hyperplasia: why is it so difficult to agree? Am J Surg Pathol 2008;32:691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Improving the treatment for women with early stage cancer of the uterus (feMMe). Available: https://clinicaltrials.gov/ct2/show/NCT01686126 [Accessed 14 Jan 2020].
- 28.Hawkes AL, Quinn M, Gebski V, et al. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device ± metformin ± weight loss in endometrial cancer (feMME) trial. Contemp Clin Trials 2014;39:14–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.