Abstract
Objective.
To examine survival of women with stage I–II endometrioid endometrial cancer whose peritoneal cytology showed malignant or atypical cells (abnormal peritoneal cytology).
Methods.
This is a multi-center retrospective study examining 1668 women with stage I–II endometrioid endometrial cancer who underwent primary hysterectomy with available peritoneal cytology results between 2000 and 2015. Abnormal peritoneal cytology was correlated to clinico-pathological characteristics and oncological outcome.
Results.
Malignant and atypical cells were seen in 125 (7.5%) and 58 (3.5%) cases, respectively. On multivariate analysis, non-obesity, non-diabetes mellitus, cigarette use, and lympho-vascular space invasion were independently associated with abnormal peritoneal cytology (all, P < 0.05). Abnormal peritoneal cytology was independently associated with decreased disease-free survival (hazard ratio 3.07, P < 0.001) and cause-specific survival (hazard ratio 3.42, P = 0.008) on multivariate analysis. Abnormal peritoneal cytology was significantly associated with increased risks of distant-recurrence (5-year rates: 8.8% versus 3.6%, P = 0.001) but not local-recurrence (5.2% versus 3.0%, P = 0.32) compared to negative cytology. Among women with stage I disease, abnormal peritoneal cytology was significantly associated with an increased risk of distant-recurrence in the low risk group (5-year rates: 5.5% versus 1.0%, P < 0.001) but not in the high-intermediate risk group (13.3% versus 10.8% P = 0.60). Among 183 women who had abnormal peritoneal cytology, postoperative chemotherapy significantly reduced the rate of peritoneal recurrence (5-year rates: 1.3% versus 9.2%, P = 0.039) whereas postoperative radiotherapy did not (7.1% versus 5.5%, P = 0.63).
Conclusion.
Our study suggests that abnormal peritoneal cytology may be a prognostic factor for decreased survival in women with stage I–II endometrioid endometrial cancer, particularly for low-risk group.
Keywords: Endometrial cancer, Peritoneal cytology, Recurrence, Low risk, Survival
1. Introduction
In 2009 the International Federation of Gynecology and Obstetrics (FIGO) revised the endometrial cancer staging system; abnormal peritoneal cytology was no longer included in the FIGO staging system [1]. The exclusion of abnormal peritoneal cytology from the current staging system is likely due to lack of evidence regarding the prognostic impact of abnormal peritoneal cytology in endometrial cancer [2-7].
Contrary, there has been mounting evidence for decreased survival in women with endometrial cancer who have abnormal peritoneal cytology [8-19]. Because these studies were conducted in heterogeneous populations across various stages and histology types with relatively limited sample size (median, n = 292), the exact population in which evaluation of peritoneal cytology would be beneficial in the management of endometrial cancer remains undetermined [2-19].
Theoretically, abnormal peritoneal cytology will be most likely impactful in women with early-stage endometrioid endometrial cancer. This is based on the rationale that women with non-endometrioid endometrial cancer (any stages) and with advanced-stage endometrioid endometrial cancer receive postoperative chemotherapy regardless of peritoneal cytology results per the current treatment guidelines [20].
In early-stage endometrioid endometrial cancer, the vast majority of women usually do not receive adjuvant therapy, or receive radiotherapy if indicated [20,21]. Therefore, if abnormal peritoneal cytology indeed alters recurrence patterns in this population, particularly for distant-recurrence, consideration of adjuvant chemotherapy would be reasonable because chemotherapy as opposed to radiotherapy seems to be a more applicable treatment approach for abnormal peritoneal cytology [14]. The objective of the study was to examine associations of abnormal peritoneal cytology and survival in stage I–II endometrioid endometrial cancer.
2. Materials and methods
2.1. Study population
This is a multicenter retrospective observational study conducted in two United States institutions and four Japanese institutions. Institutional Review Board approval was obtained at each site. Eligibility criteria were consecutive women with stage I–II, grade 1–3 endometrioid adenocarcinoma of the endometrium who underwent primary hysterectomy-based surgical treatment with available peritoneal cytology results between January 1, 2000 and December 31, 2015. Exclusion criteria were absence of hysterectomy, use of neoadjuvant therapy, stage III-IV disease, non-endometrioid histology, synchronous malignancy at endometrial cancer diagnosis, and lack of peritoneal cytology results. Some of the patients were included within the context of our previous study [22-24].
2.2. Clinical information
Among eligible cases, patient demographics, treatment type, pathology results, and survival outcomes were abstracted. Patient demographics included age, race, body mass index (BMI, kg/m2), pregnancy history, medical comorbidities (hypertension, diabetes mellitus, hypercholesterolemia), cigarette use, and surgical history (tubal sterilization). Treatment type included route of hysterectomy (abdominal versus minimally-invasive), use of lymphadenectomy (pelvic and paraaortic), and type of adjuvant therapy (radiotherapy, chemotherapy, or none).
For pathology results, tumor grade (1, 2, or 3), cervical stromal tumor invasion (yes versus no), depth of myometrial tumor invasion (<50% versus ≥50%), lympho-vascular space invasion (LVSI; yes versus no), and peritoneal cytology test results (malignant cells, atypical cells, or negative) were collected from records for hysterectomy-based surgical staging. In our institutions, peritoneal cytology is generally collected at the beginning of surgery.
For survival outcome, disease-free survival (DFS) and cause-specific survival (CSS) were recorded. Among recurrent cases, anatomical locations were collected (local- versus distant-recurrence). Data entry into a de-identified data sheet was performed by co-investigators in each participating institution, and the principal investigator reviewed all the data for accuracy and consistency.
2.3. Study definition
Cutoffs for age (<60 versus ≥60 years) and BMI (<30 versus ≥30 kg/m2) were based on previous studies [22-24]. Cancer stage was re-classified based on the 2009 FIGO staging system [1]: stage IA refers disease with myometrial tumor invasion of <50% whereas stage IB refers disease with myometrial tumor invasion of ≥50%. Stage II disease refers tumors with cervical stromal invasion. Tumor grade was based on the FIGO classification: ≤5% solid component for grade 1, 6–50% solid component for grade 2, and >50% solid component for grade 3 [25]. Presence of malignant or atypical cells on peritoneal cytology was defined as abnormal peritoneal cytology in this study whereas absence of malignant or atypical cells was defined as negative cytology.
DFS was defined as the time interval between the date of hysterectomy and the date of the first recurrence of endometrial cancer or the last follow-up date if there was no recurrence. CSS was defined as the time interval between the date of hysterectomy and the date of death due to endometrial cancer, and the alive status at last follow-up or death from other causes were censored. Local-recurrence was defined as recurrence in the vaginal cuff or pelvis. Distant-recurrence was defined as recurrence other than local-recurrence, grouped into peritoneal, lymphatic, or hematogenous recurrence.
Among stage I endometrial cancer, the high-intermediate risk group was defined either by the ESMO-ESGO-ESRTO criteria (stage IA grade 3 endometrioid tumor, stage IA-B grade 1–2 endometrioid tumor with LVSI) [26], the PORTEC criteria for unstaged cases (≥2 out of the following 3 risk factors: age ≥ 60 years, grade 3 tumors, and myometrial invasion ≥50%) [27], or the GOG-099 criteria for staged cases (3 risk factors for age < 50 years, ≥2 risk factors for age 50–69 years, and ≥1 risk factors for age ≥ 70 years; LVSI, grade 2–3 tumors, and myometrial invasion ≥50% for risk factors) [28]. We used 50% for the cutoff of myometrial invasion instead of >66% in this study.
Low-intermediate risk group was defined per GOG-099 criteria for staged cases: stage IB cases that did not meet high-intermediate risk group. Intermediate risk group was defined per the ESMO-ESGO-ESTRO criteria (stage IB grade 1–2 endometrioid tumor without LVSI) [26]. Low risk group was defined per the modified NCCN criteria (stage IA grade 1–2 endometrioid tumors) or the ESMO-ESGO-ESTRO criteria (stage IA grade 1–2 endometrioid tumors without LVSI) [20,26].
2.4. Statistical consideration
The primary interest of analysis was to identify the clinico-pathological factors associated with abnormal peritoneal cytology in women with stage I–II endometrioid endometrial cancer. The secondary interests of analysis were to examine the association of abnormal peritoneal cytology with recurrence pattern and survival in this study population. We also examined the utilization of peritoneal cytology during the study period.
Normality of continuous variables was examined with the Kolmogorov-Smirnov test, expressed with mean (±standard deviation) or median (interquartile range) as appropriate. Statistical significance of continuous variables in more than two groups was examined by the one-way ANOVA test or the Kruskal-Wallis H test as appropriate. Statistical significance of ordinal and categorical variables was examined by the chi-square test.
A binary logistic regression model was used to identify the independent clinico-pathological factors for abnormal peritoneal cytology (malignant or atypical cells versus negative). In this model, all the patient and tumor factors with P < 0.05 on univariate analysis were entered in the initial model, and only the significant covariates with P < 0.05 in the final model were kept in the final model (conditional backward). Magnitude of statistical significance was expressed with odds ratios (OR) and 95% confidence intervals (CI). The Hosmer-Lemeshow test was used to assess goodness-of-fit in the final model, and P > 0.05 was interpreted as a good-fit model.
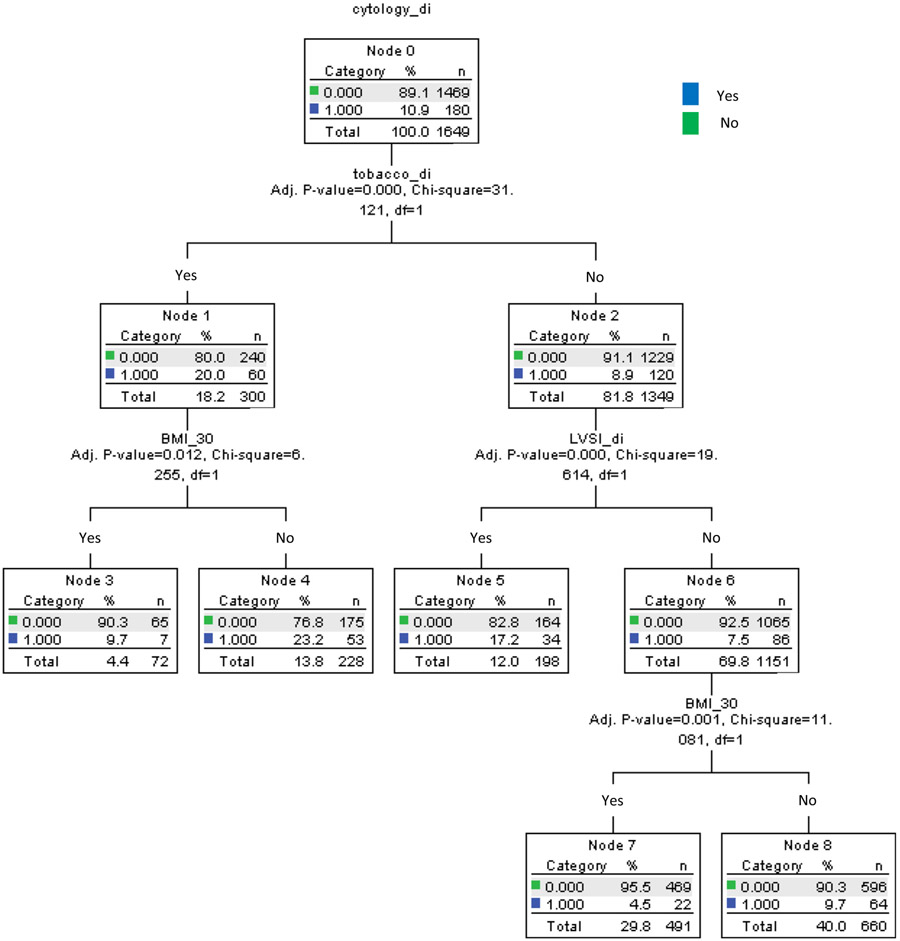
In order to predict a subgroup of women with increased risk of abnormal peritoneal cytology, a recursive partitioning analysis was performed to construct a regression-tree model for abnormal peritoneal cytology [29]. All independent risk factors of abnormal peritoneal cytology were entered in the analysis, and the chi-square automatic interaction detector method was used for the model. Among the determined nodes in this analysis, incidences of abnormal peritoneal cytology were evaluated.
For survival analysis, a log-rank test for univariate analysis and a Cox proportional hazard regression model for multivariate analysis were utilized. The Kaplan-Meier method was used to construct survival curves. For multivariate analysis, covariates entered in the initial model were the statistically significant variables in univariate analysis (cutoff, P < 0.05). Conditional backward method was then used to determine the independent prognostic factor for survival. Magnitude of statistical significance was expressed with hazard ratios (HR) and 95% CI.
Among stage I disease, various sensitivity analyses were performed to examine the association of abnormal peritoneal cytology and survival or recurrence pattern. These included women with high-intermediate risk, low-intermediate risk, intermediate risk, and low risk groups defined as above. All statistical tests were two-tailed, and a P < 0.05 was considered statistically significant. Statistical Package for the Social Sciences (IBM SPSS, version 24.0, Armonk, NY, USA) was used for the analyses. The STROBE guidelines were used to outline the results of retrospective observational study [30].
3. Results
The patient selection schema is shown in Fig. 1. Among 2679 women, there were 2420 women who underwent primary hysterectomy. Of those, women with stage III-IV disease (n = 398), non-endometrioid histology (n = 171), and synchronous tumors (n = 128) were excluded. The remaining 1723 women with stage I–II endometrioid endometrial cancer who underwent primary hysterectomy were examined for peritoneal cytology results. After 55 (3.2%) women who did not undergo peritoneal cytology evaluation were excluded, a total of 1668 women were available for analysis.
Fig. 1.
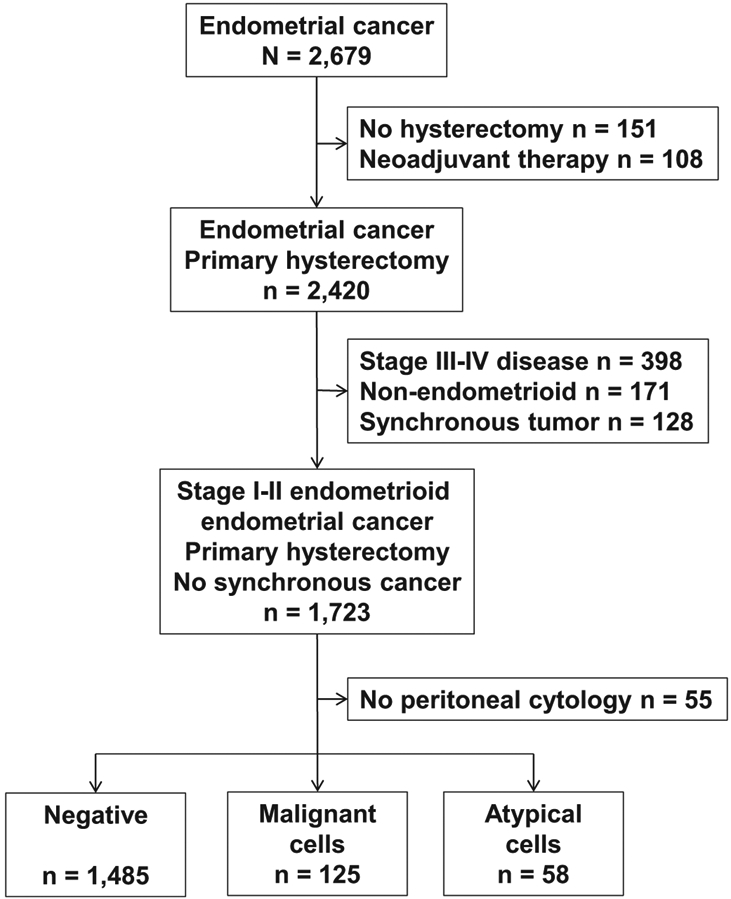
Study selection schema.
When utilization of peritoneal cytology was examined among 1723 women with stage I–II endometrioid endometrial cancer who underwent primary hysterectomy-based surgical treatment, there was a significant decrease in the proportion of surgery with peritoneal cytology evaluation during the study period (before 2010 versus 2010 or later, 99.2% versus 96.0%, P < 0.001).
There were 125 (7.5%, 95% CI 6.2–8.8) women with malignant cells and 58 (3.5%, 95% CI 2.6–4.4) women with atypical cells recorded in the peritoneal cytology results. Patient demographics are shown in Table 1. On univariate analysis, race, body habitus, hypertension, diabetes mellitus, cigarette use, hysterectomy mode, use of pelvic and paraaortic lymphadenectomy, LVSI, and postoperative chemotherapy use were all significantly associated with peritoneal cytology results (all, P < 0.05).
Table 1.
Patient demographics (N = 1668).
| Characteristic | Negative | Malignant cells | Atypical cells | P-values |
|---|---|---|---|---|
| Number | 1485 (89.0%) | 125 (7.5%) | 58 (3.5%) | |
| Age (years) | 57 (IQR 13) | 55 (IQR 13) | 54 (IQR 14) | 0.37 |
| <60 | 919 (88.2%) | 80 (7.7%) | 43 (4.1%) | |
| ≥60 | 565 (90.5%) | 45 (7.2%) | 14 (2.2%) | |
| Race | <0.001 | |||
| Caucasian | 110 (92.4%) | 1 (0.8%) | 8 (6.7%) | |
| African | 17 (94.4%) | 0 | 1 (5.6%) | |
| Hispanic | 412 (94.5%) | 12 (2.8%) | 12 (2.8%) | |
| Asian | 943 (86.4%) | 112 (10.3%) | 37 (3.4%) | |
| Pregnancy | ||||
| Gravidity | 2(2) | 2(3) | 2(2) | 0.30 |
| Parity | 2 (2) | 2 (2) | 2 (2) | 0.34 |
| BMI (kg/m2) | 27.4 (IQR 12.5) | 24.0 (IQR 7.2) | 27.6 (IQR 12.9) | <0.001 |
| <30 | 896 (86.3%) | 107 (10.3%) | 35 (3.4%) | |
| ≥30 | 587 (93.6%) | 18 (2.9%) | 22 (3.5%) | |
| Hypertension | 0.007 | |||
| No | 895 (87.9%) | 92 (9.0%) | 31 (3.0%) | |
| Yes | 590 (90.8%) | 33 (5.1%) | 27 (4.2%) | |
| Diabetes mellitus | <0.001 | |||
| No | 1166 (87.6%) | 118 (8.9%) | 47 (3.5%) | |
| Yes | 319 (94.7%) | 7 (2.1%) | 11 (3.3%) | |
| Hypercholesterolemia | 0.85 | |||
| No | 1196 (89.1%) | 101 (7.5%) | 45 (3.4%) | |
| Yes | 289 (88.7%) | 24 (7.4%) | 13 (4.0%) | |
| Cigarette use | <0.001 | |||
| No | 1243 (91.1%) | 80 (5.9%) | 42 (3.1%) | |
| Yes | 242 (79.9%) | 45 (14.9%) | 16 (5.3%) | |
| Tubal ligation | 0.14 | |||
| No | 1440 (88.8%) | 125 (7.7%) | 56 (3.5%) | |
| Yes | 45 (95.7%) | 0 | 2 (4.3%) | |
| Hysterectomy mode | 0.022 | |||
| Laparotomy | 1023 (87.9%) | 101 (8.7%) | 40 (3.4%) | |
| MIS | 459 (91.6%) | 24 (4.8%) | 18 (3.6%) | |
| Pelvic lymphadenectomy | <0.001 | |||
| Not performed | 763 (93.2%) | 29 (3.5%) | 27 (3.3%) | |
| Performed | 722 (85.0%) | 96 (11.3%) | 31 (3.7%) | |
| Para-aortic lymphadenectomy | 0.001 | |||
| Not performed | 1376 (89.8%) | 104 (6.8%) | 53 (3.5%) | |
| Performed | 109 (80.7%) | 21 (15.6%) | 5 (3.7%) | |
| Cervical stromal invasion | 0.40 | |||
| Absent | 1394(89.2%) | 116 (7.4%) | 52 (3.3%) | |
| Present | 91 (85.8%) | 9 (8.5%) | 6 (5.7%) | |
| Grade | 0.99 | |||
| 1 | 1084(89.2%) | 89 (7.3%) | 42 (3.5%) | |
| 2 | 275 (88.4%) | 25 (8.0%) | 11 (3.5%) | |
| 3 | 126 (88.7%) | 11 (7.7%) | 5 (3.5%) | |
| Myometrial invasion | 0.07 | |||
| Inner half | 1213 (89.9%) | 92 (6.8%) | 45 (3.3%) | |
| Outer half | 268 (85.6%) | 33 (10.5%) | 12 (3.8%) | |
| LVSI | <0.001 | |||
| Absent | 1261 (90.5%) | 95 (6.8%) | 38 (2.7%) | |
| Present | 213 (81.6%) | 29 (11.1%) | 19 (7.3%) | |
| Postop radiotherapy | 0.09 | |||
| No | 1353 (89.0%) | 118 (7.8%) | 49 (3.2%) | |
| Yes | 132 (89.2%) | 7 (4.7%) | 9 (6.1%) | |
| Postop chemotherapy | <0.001 | |||
| No | 1287 (92.5%) | 59 (4.2%) | 46 (3.3%) | |
| Yes | 198 (71.7%) | 66 (23.9%) | 12 (4.3%) | |
Number with percentage per row or median with IQR are shown. Chi-square test or Kruskal-Wallis H test for P-values. Significant P-values are emboldened. Abbreviations: IQR, interquartile range; BMI, body mass index; and LVSI, lympho-vascular space invasion.
On multivariate analysis (Table 2), obesity (adjusted-OR 0.57, 95% CI 0.39–0.84, P = 0.004) and diabetes mellitus (adjusted-OR 0.52, 95% CI 0.31–0.87, P = 0.013) were independently associated with reduced risk of abnormal peritoneal cytology. On the contrary, cigarette use (adjusted-OR 2.26, 95% CI 1.60–3.20, P < 0.001) and LVSI (adjusted-OR 1.89, 95% CI 1.31–2.74, P = 0.001) were independently associated with increased risk of abnormal peritoneal cytology.
Table 2.
Independent risk factors for abnormal peritoneal cytology.
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| BMI (kg/m2) | ||||
| < 30 | 1 | 1 | ||
| ≥ 30 | 0.26 (0.15–0.43) | <0.001 | 0.57 (0.39–0.84) | 0.004 |
| Diabetes mellitus | ||||
| No | 1 | 1 | ||
| Yes | 0.22 (0.10–0.47) | <0.001 | 0.52 (0.31–0.87) | 0.013 |
| Cigarette use | ||||
| No | 1 | 1 | ||
| Yes | 2.88 (1.95–4.26) | <0.001 | 2.26 (1.60–3.20) | <0.001 |
| LVSI | ||||
| Absent | 1 | 1 | ||
| Present | 1.78 (1.15–2.77) | 0.01 | 1.89 (1.31–2.74) | 0.001 |
A binary logistic regression model for multivariate analysis (malignant or atypical cells versus negative). All the patient and tumor factors with P < 0.05 on univariate analysis were entered in the initial model, and only the significant covariates with P < 0.05 in the final model are shown (conditional backward). Hosmer-Lemeshow test indicates goodness-of-fit in the final model (P = 0.72). Significant P-values are emboldened. Abbreviations: BMI, body mass index; LVSI, lympho-vascular space invasion; OR, odds ratio; and CI, confidence interval.
Based on these four independent risk factors for abnormal peritoneal cytology, a regression-tree model was constructed to identify a subgroup of women with increased risks of abnormal peritoneal cytology (Fig. 2). There were 1649 women who had information for all four risk factors. Of those women, non-obese smokers (n = 228, 13.8%) had the highest incidence of abnormal peritoneal cytology results (23.2%), followed by non-smokers whose tumor had LVSI (n = 198, 12.0%) with a 17.2% incidence of abnormal peritoneal cytology results. In contrast, obese non-smokers whose tumors had no LVSI (n = 491, 29.8%) had the lowest incidence of abnormal peritoneal cytology (4.5%).
Fig. 2.
Regression-tree model for abnormal peritoneal cytology. Abbreviations: BMI, body mass index; and LVSI, lympho-vascular space invasion.
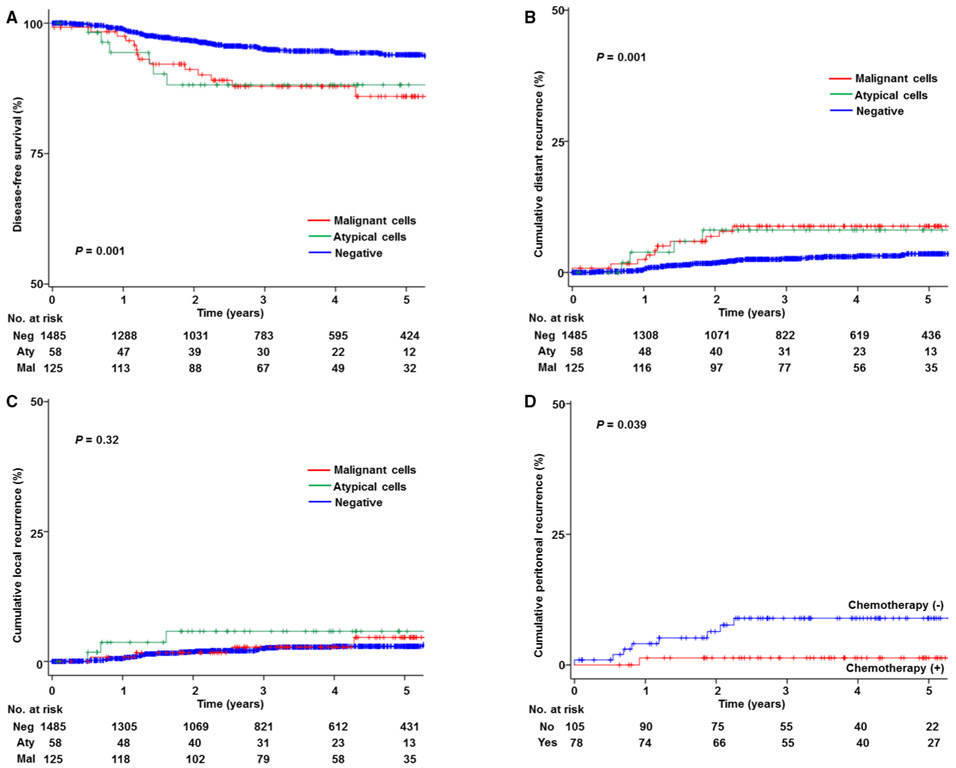
The median follow-up time of women who were censored at the last visit was 3.5 years. There were 86 women who had disease recurrence or death due to endometrial cancer during the follow-up in this study population. On univariate analysis, peritoneal cytology results were significantly associated with DFS (5-year rates: 85.4% for malignant cells, 88.1% for atypical cells, and 93.9% for negative cytology results, P = 0.001; Fig. 3A).
Fig. 3.
Kaplan-Meier curves. Log-rank test for P-values. Y-axes are truncated to 0–50% or 50–100%. Kaplan-Meier curves were constructed based on peritoneal cytology results for (A) disease-free survival, (B) cumulative incidence for distant-recurrence, (C) cumulative incidence for local-recurrence. Among cases with abnormal peritoneal cytology (D) cumulative incidence for peritoneal recurrence is shown based on postoperative chemotherapy use
On multivariate analysis (Table 3), abnormal peritoneal cytology was independently associated with decreased DFS compared to negative cytology (adjusted-HR 3.07, 95% CI 1.81–5.23, P < 0.001). Moreover, abnormal peritoneal cytology was independently associated with decreased CSS (adjusted-HR 3.42, 95% CI 1.39–8.42, P = 0.008). There was no difference in DFS between the malignant cells group and the atypical cells group on multivariate analysis (adjusted-P = 0.45; Supplemental Table S1).
Table 3.
Independent prognostic factors (multivariate analysis).
| Characteristic | Disease-free survival | Cause-specific survival | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| <60 | 1 | 1 | ||
| ≥60 | 2.41 (1.53–3.80) | <0.001 | 3.18 (1.34–7.57) | 0.009 |
| BMI (kg/m2) | ||||
| <30 | 1 | |||
| ≥30 | 0.22 (0.06–0.77) | 0.018 | ||
| Grade | ||||
| 1–2 | 1 | 1 | ||
| 3 | 2.39 (1.38–4.15) | 0.002 | 5.58 (2.45–12.7) | <0.001 |
| Myometrial invasion | ||||
| Inner half | 1 | 1 | ||
| Outer half | 1.79 (1.07–2.99) | 0.025 | 2.55 (1.05–6.20) | 0.038 |
| Cervical stromal invasion | ||||
| Absent | 1 | |||
| Present | 2.70 (1.48–4.95) | 0.001 | ||
| LVSI | ||||
| Absent | 1 | 1 | ||
| Present | 2.09 (1.25–3.50) | 0.005 | 3.43 (1.41–8.32) | 0.006 |
| Peritoneal cytology | ||||
| Negative cytology | 1 | 1 | ||
| Abnormal cytologya | 3.07 (1.81–5.23) | <0.001 | 3.42 (1.39–8.42) | 0.008 |
| Postop radiotherapy | ||||
| No | 1 | |||
| Yes | 1.88 (1.07–3.30) | 0.028 | ||
| Postop chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 0.52 (0.29–0.93) | 0.026 | 0.30 (0.13–0.72) | 0.007 |
Cox proportional hazard regression models for P-values. All the covariates with P < 0.05 on univariate analysis were entered in the initial model, and only the significant covariates with P < 0.05 in the final model are shown (conditional backward). Significant P-values are emboldened. Abbreviations: BMI, body mass index; LVSI, lympho-vascular space invasion; HR, hazard ratio; and CI, confidence interval.
Malignant or atypical cells on peritoneal cytology results.
Women who had abnormal peritoneal cytology had a significantly increased risk of distant-recurrence compared to those who had negative cytology (5-year rates: 8.8% versus 3.6%, HR 2.99, 95% CI 1.61–5.52, P = 0.001; Fig. 3B). On the contrary, women who had abnormal peritoneal cytology had a similar local-recurrence risk compared to those who had negative cytology (5-year rates: 5.2% versus 3.0%, HR 1.60, 95% CI 0.71–3.61, P = 0.32; Fig. 3C).
When anatomical sites of distant-recurrence were further stratified, abnormal peritoneal cytology was particularly associated with increased risk of peritoneal recurrence (HR 10.1, 95% CI 3.77–27.2, P < 0.001). Among 183 women who had abnormal peritoneal cytology, use of postoperative chemotherapy was significantly associated with decreased risk of peritoneal recurrence (5-year rates: 1.3% versus 9.2%, P = 0.039; Fig. 3D) whereas postoperative radiotherapy was not (7.1% versus 5.5%, P = 0.63). Similarly, among 125 women in the malignant cells group, use of postoperative chemotherapy was significantly associated with decreased risk of distant recurrence (HR 0.20, 95% CI 0.04–0.93, P = 0.04) while radiotherapy was not (P = 0.68).
Various sensitivity analyses were performed in stage I disease (Table 4). Among women in the high-intermediate risk group, abnormal peritoneal cytology was not associated with recurrence risk and recurrence pattern per the GOG-099 criteria (5-year rates: 13.3% versus 10.8%) and the ESMO-ESGO-ESTRO criteria (6.2% versus 9.9%) (all, P > 0.05). However among women in the low-risk group per the modified NCCN criteria, abnormal peritoneal cytology was significantly associated with increased risk of distant-recurrence (5-year rates: 5.8% versus 1.3%, HR 5.71, 95% CI 2.03–16.0, P = 0.001) but not local-recurrence (P = 0.18). The magnitude of statistical significance for distant-recurrence was even larger among the low risk group per the ESMO-ESGO-ESTRO criteria (5-year rates: 5.5% versus 1.0%, HR 7.46, 95% CI 2.28–24.4, P < 0.001).
Table 4.
Sensitivity analysis for stage I endometrial cancer.
| Characteristic | No. | Disease-free survival | Local-recurrence | Distant-recurrence | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| GOG-099 (high-intermediate risk)a | |||||||
| Negative cytology | 161 | 1 | 1 | 1 | |||
| Abnormal cytology | 24 | 1.49 (0.50–4.41) | 0.47 | 1.09 (0.13–9.05) | 0.94 | 1.39 (0.40–4.90) | 0.60 |
| GOG-099 (low-intermediate risk)b | |||||||
| Negative cytology | 56 | 1 | 1 | 1 | |||
| Abnormal cytology | 10 | 1.37 (0.16–12.0) | 0.78 | na | 0.60 | 1.99 (0.20–19.6) | 0.55 |
| Low riskc | |||||||
| Negative cytology | 1093 | 1 | 1 | 1 | |||
| Abnormal cytology | 122 | 3.27 (1.52–7.03) | 0.001 | 2.09 (0.70–6.26) | 0.18 | 5.71 (2.03–16.0) | 0.001 |
| PORTEC-1 (high-intermediate risk)d | |||||||
| Negative cytology | 55 | 1 | 1 | 1 | |||
| Abnormal cytology | 3 | na | na | na | na | na | na |
| ESMO (high-intermediate risk)e | |||||||
| Negative cytology | 208 | 1 | 1 | 1 | |||
| Abnormal cytology | 37 | 1.09 (0.37–3.16) | 0.88 | 1.47 (0.31–6.93) | 0.62 | 0.75 (0.17–3.30) | 0.71 |
| ESMO (intermediate)e | |||||||
| Negative cytology | 119 | 1 | 1 | 1 | |||
| Abnormal cytology | 15 | 1.18 (0.15–9.66) | 0.88 | na | 0.47 | 1.44(0.17–12.3) | 0.74 |
| ESMO (low risk)e | |||||||
| Negative cytology | 1016 | 1 | 1 | 1 | |||
| Abnormal cytology | 108 | 3.21 (1.36–7.60) | 0.005 | 1.74(0.50–6.01) | 0.38 | 7.46 (2.28–24.4) | <0.001 |
Unadjusted Cox proportional hazard regression model for analysis. Significant P-values are emboldened. Abbreviations: HR, hazard ratio; CI, confidence interval; and LVSI, lympho-vascular space invasion.
Analyzed stage cases for GOG-099 high-intermediate risk group (modified): 3 risk factors forage < 50 years, ≥2 risk factors forage 50–69 years, and ≥1 risk factors forage ≥ 70 years (LVSI, grade 2–3 tumors, and myometrial invasion ≥50%).
GOG-099 low-intermediate risk group (modified): stage IB cases that did not meet high-intermediate risk group
Low risk group (modified NCCN criteria): stage IA grade 1–2 endometrioid tumors (staged and unstaged).
Analyzed unstaged cases for PORTEC criteria for high-intermediate risk group: ≥2 out of the following 3 risk factors (age ≥ 60 years, grade 3 tumors, and myometrial invasion ≥50%).
Analyzed staged and unstaged cases for ESMO-ESGO-ESTRO criteria: low risk, stage IA grade 1–2 endometrioid tumors without LVSI; intermediate risk, stage IB grade 1–2 endometrioid tumor without LVSI; and high-intermediate risk, stage IA grade 3 endometrioid tumor or stage IA–B grade 1–2 endometrioid tumor with LVSI.
4. Discussion
Our study found that abnormal peritoneal cytology is a prognostic indicator for decreased survival in early-stage endometrioid endometrial cancer. Moreover, abnormal peritoneal cytology is associated with distant failure particularly in women with low risk endometrial cancer, and use of postoperative chemotherapy decreased peritoneal recurrence.
Findings of our study are similar to prior studies that demonstrated abnormal peritoneal cytology as a marker for increased disease recurrence and mortality [8-19]. However, given heterogeneous study populations in these prior reports, our study is more specific and clinically meaningful, as we solely examined endometrioid type early-stage disease utilizing a large and homogenous study population.
Prior studies, including ours, have been conflicting in demonstrating the significance of abnormal peritoneal cytology on endometrial cancer prognosis [2-19]. We interpreted that study size, stage distribution, and rate of abnormal peritoneal cytology were grossly similar between the studies demonstrating prognostic impact and those that did not (Supplemental Table S1). One possible explanation to elucidate the differences in outcome is that quality of peritoneal cytology evaluation may differ across the studies. Indeed, a prior study showed that quality of peritoneal cytology (scalloped clusters versus well-defined edges) correlates with recurrence risk [11]. No other studies, including ours, examined quality of peritoneal cytology, and this may be possibly the factor leading to differences in outcome between “positive” and “negative” studies.
Differences in outcome of women with abnormal peritoneal cytology were also seen even in a subset of stage I endometrial cancer. A prior study reported that abnormal peritoneal cytology is predictive of survival in intermediate but not in low risk disease [10]. Another study also failed to show that abnormal peritoneal cytology was a prognostic factor in low and intermediate risk groups [7]. In our study, opposite findings were observed in that abnormal peritoneal cytology correlated with increased distant-recurrence in the low risk group but not in the low-intermediate and high-intermediate risk groups. This inconsistency across studies may be due to differences in postoperative therapy patterns. In our patients, a fraction of patients received chemotherapy in the low risk group whereas in other studies this was not the case [7, 10].
Differences in recurrence patterns were also seen across studies. A prior study has shown that abnormal peritoneal cytology is associated with increased risk of both local- and distant-recurrences among women with clinical stage I endometrial cancer [8]. Our study is different from this other study in that they included extra-uterine disease and non-endometrioid histology. By limiting the study population to early-stage endometrioid disease, we found that abnormal peritoneal cytology only increases risk of distant- but not local-recurrence.
Unique aspects of our study include characterization of clinico-pathological factors associated with abnormal peritoneal cytology. We found that obese and diabetic women were less likely to have abnormal peritoneal cytology. These factors represent classic type I tumors that result in less-aggressive tumor behavior [31]. On the contrary, cigarette use was associated with an increased risk of abnormal peritoneal cytology. While exact causality remains unknown it indirectly points toward carcinogenic effects related to cigarette use.
Past years have witnessed a marked paradigm shift in the surgical treatment modality for endometrial cancer from laparotomy to minimally-invasive surgery (9.3% to 61.7% between 2006 and 2011) [32]. In our study, there was a relatively low rate of minimally-invasive hysterectomy (30.1%), likely due to the fact that total laparoscopic hysterectomy was only recently approved as a treatment modality in Japan.
While there may be a concern that minimally-invasive surgery may potentially miss omental or upper abdominal tumor implants, multiple studies have shown that a minimally-invasive approach has comparable effects on survival compared to the open approach [32,33]. Another theoretical concern may be an increased risk of atypical cells in peritoneal cytology with intra-uterine manipulator use [34]. In our study, however, a minimally-invasive approach was not associated with an increased risk of atypical cells (3.6% versus 3.4%). Moreover, our prior analyses, which demonstrated no association between use of an intra-uterine manipulator during minimally-invasive hysterectomy and peritoneal cytology or the likelihood of LVSI, suggest a negligible effect of intrauterine manipulator use on tumor dissemination [35,36]. Additionally, because the current study population was limited only to early-stage endometrioid disease, no conclusion can be made regarding other histologies. Collectively, this controversy merits further investigations.
Strengths of this study include rigorous inclusion and exclusion criteria and one of the largest sample sizes in the literature. Weaknesses of this study include that this is a retrospective study that may miss possible confounders. For instance, we do not have information on diagnostic modality prior to hysterectomy, including hysteroscopy use that may disseminate tumor cells into the peritoneal cavity [37]. Additionally, exact indications for postoperative therapy could not be abstracted. Therefore, we do not know if adjuvant therapy was given for abnormal peritoneal cytology versus other reasons. This is particularly concerning because this study was conducted in multiple institutions and countries with possible variable treatment approaches.
Limitations of the study include that central pathology review was not performed to confirm abnormal cytology results. We examined cases with atypical cells on peritoneal cytology and demonstrated that survival was similar between malignant and atypical cytology groups. While this can be an intriguing finding, it may be premature to conclude that significance of atypical cells on survival is the same as malignant cells unless detailed pathology review of archived slides is performed. Another limitation is the lack of molecular profiling. Mounting evidence has demonstrated that molecular signature plays a pivotal role in tumor progression and survival in endometrial cancer [38]. Thus, it remains unknown if abnormal peritoneal cytology is driven by certain oncogenic signaling factors versus clinico-pathological factors as shown in our study (Fig. 2).
A possible clinical implication of our study may be in the area of adjuvant therapy for low risk endometrial cancer. To date, the utility of adjuvant chemotherapy has not yet been determined in early-stage endometrioid disease with abnormal peritoneal cytology [31]. While our study suggested that chemotherapy use may possibly reduce peritoneal and distant recurrences among low risk women with abnormal peritoneal cytology, further studies are required before one can make this a definitive conclusion. This is particularly applicable when the peritoneal cytology result shows atypical cells. In such a case, additional evaluation of the cytology specimen would be necessary, including immunohistochemistry studies as well as expert review, to distinguish reactive change versus possible malignant cells. Further study in a prospective fashion is warranted to address this clinical dilemma.
Per the society’s current consensus for post-treatment surveillance in low risk endometrial cancer, follow-up evaluations are recommended every six months for the first two years followed by annual examination [39]. However, given our findings of considerably increased risks of recurrence in the presence of abnormal peritoneal cytology in the low risk group, it may be reasonable for gynecologic oncologists to follow these women per the high risk surveillance protocol (follow-up every three months for the first two years, every six months between years two and five, and annually thereafter). Moreover, we found that there is a significant decrease in the utilization of peritoneal cytology after the implementation of the new FIGO staging system. While we do not know if this decrease reflects the change in staging system, routine evaluation of peritoneal cytology may be considered until its value is fully determined [15].
Supplementary Material
HIGHLIGHTS.
Examined abnormal peritoneal cytology (APC) in early-stage endometrial cancer.
Incidence of APC was ~10% (malignant cells 7.5%, atypical cells 3.5%).
Non-obese smoker had the highest incidence of APC (>20%).
APC was independently associated with decreased survival.
In low risk stage I disease, APC was associated with distant recurrence.
Acknowledgments
Financial support: Ensign Endowment for Gynecologic Cancer Research (K.M.). Abstract of the study was presented at 49th Annual Meeting on Women’s Cancer, New Orleans, LA, March 24–27, 2018.
Footnotes
Disclosure statement
There is no conflict of interest in all authors.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps://doi.org/10.1016/j.ygyno.2018.02.012.
References
- [1].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet 105 (2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [2].Grimshaw RN, Tupper WC, Fraser RC, Tompkins MG, Jeffrey JF, Prognostic value of peritoneal cytology in endometrial carcinoma, Gynecol. Oncol 36 (1990) 97–100. [DOI] [PubMed] [Google Scholar]
- [3].Tebeu PM, Popowski GY, Verkooijen HM,Casals J, Ludicke F, Zeciri G, Usel M, Bouchardy C, Major AL, Impact of peritoneal cytology on survival of endometrial cancer patients treated with surgery and radiotherapy, Br. J. Cancer 89 (2003) 2023–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kasamatsu T, Onda T, Katsumata N, Sawada M, Yamada T, Tsunematsu R, Ohmi K, Sasajima Y, Matsuno Y, Prognostic significance of positive peritoneal cytology in endometrial carcinoma confined to the uterus, Br. J. Cancer 88 (2003) 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tebeu PM, Popowski Y, Verkooijen HM, Bouchardy C, Ludicke F, Usel M, Major AL, Positive peritoneal cytology in early-stage endometrial cancer does not influence prognosis, Br. J. Cancer 91 (4) (2004) 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fadare O, Mariappan MR, Hileeto D, Wang S,McAlpine JN, Rimm DL, Upstaging based solely on positive peritoneal washing does not affect outcome in endometrial cancer, Mod. Pathol 18 (2005. ) 673–680. [DOI] [PubMed] [Google Scholar]
- [7].Scott SA, van der Zanden C, Cai E, McGahan CE, Kwon JS, Prognostic significance of peritoneal cytology in low-intermediate risk endometrial cancer, Gynecol. Oncol 145 (2017) 262–268. [DOI] [PubMed] [Google Scholar]
- [8].Grigsby PW, Perez CA, Kuten A, Simpson JR, Garcia DM, Camel HM, Kao MS, Galakatos AE, Clinical stage I endometrial cancer: prognostic factors for local control and distant metastasis and implications of the new FIGO surgical staging system, Int. J. Radiat. Oncol. Biol. Phys 22 (1992) 905–911. [DOI] [PubMed] [Google Scholar]
- [9].Kashimura M, Sugihara K, Toki N, Matsuura Y, Kawagoe T, Kamura T, Kaku T, Tsuruchi N, Nakashima H, Sakai H, The significance of peritoneal cytology in uterine cervix and endometrial cancer, Gynecol. Oncol 67 (1997) 285–290. [DOI] [PubMed] [Google Scholar]
- [10].Takeshima N, Nishida H, Tabata T, Hirai Y, Hasumi K, Positive peritoneal cytology in endometrial cancer: enhancement of other prognostic indicators, Gynecol. Oncol 82 (2001)470–473. [DOI] [PubMed] [Google Scholar]
- [11].Yanoh K, Takeshima N, Hirai Y, Minami A, Tsuzuku M, Toyoda N, Hasumi K, Identification of a high-risk subgroup in cytology-positive stage IIIA endometrial cancer, Acta Cytol. 45 (2001) 691–696. [DOI] [PubMed] [Google Scholar]
- [12].Santala M, Talvensaari-Mattila A, Kauppila A, Peritoneal cytology and preoperative serum CA 125 level are important prognostic indicators of overall survival in advanced endometrial cancer, Anticancer Res. 23 (2003) 3097–3103. [PubMed] [Google Scholar]
- [13].Saga Y, Imai M, Jobo T, Kuramoto H, Takahashi K, Konno R, Ohwada M, Suzuki M, Is peritoneal cytology a prognostic factor of endometrial cancer confined to the uterus? Gynecol. Oncol 103 (2006) 277–280. [DOI] [PubMed] [Google Scholar]
- [14].Havrilesky LJ, Cragun JM, Calingaert B, Alvarez Secord A, Valea FA, Clarke-Pearson DL, Berchuck A, Soper JT, The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer, Gynecol. Oncol 104 (2007) 401–405. [DOI] [PubMed] [Google Scholar]
- [15].Garg G, Gao F, Wright JD, Hagemann AR, Mutch DG, Powell MA, Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer, Gynecol. Oncol 128 (2012) 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Milgrom SA, Kollmeier MA, Abu-Rustum NR, Makker V, Gardner GJ, Barakat RR, Alektiar KM, Positive peritoneal cytology is highly predictive of prognosis and relapse patterns in stage III (FIGO 2009) endometrial cancer, Gynecol. Oncol 130 (2013)49–53. [DOI] [PubMed] [Google Scholar]
- [17].Shiozaki T, Tabata T, Yamada T, Yamamoto Y, Yamawaki T, Ikeda T, Does positive peritoneal cytology not affect the prognosis for stage I uterine endometrial cancer?: the remaining controversy and review of the literature, Int. J. Gynecol. Cancer 24 (2014) 549–555. [DOI] [PubMed] [Google Scholar]
- [18].Cetinkaya K, Atalay F, Peritoneal cytology in endometrial cancer, Tumori 101 (2015) 697–700. [DOI] [PubMed] [Google Scholar]
- [19].Tanaka K, Kobayashi Y, Sugiyama J, Yamazaki T, Dozono K, Watanabe M, Shibuya H, Nishigaya Y, Momomura M, Matsumoto H, Umezawa S, Takamatsu K, Iwashita M, Histologic grade and peritoneal cytology as prognostic factors in type 1 endometrial cancer, Int. J. Clin. Oncol 22 (2017) 533–540. [DOI] [PubMed] [Google Scholar]
- [20].Uterine Neoplasms, NCCN clinical practice guidelines in oncology, https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf, Accessed date: 2 December 2017.
- [21].Klopp A, Smith BD, Alektiar K, Cabrera A, Damato AL, Erickson B, Fleming G, Gaffney D, Greven K, Lu K, Miller D, Moore D, Petereit D, Schefter T, Small W Jr., Yashar C, Viswanathan AN, The role of postoperative radiation therapy for endometrial cancer: executive summary of an American Society for Radiation Oncology evidence-based guideline, Pract. Radiat. Oncol 4 (2014) 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuo K, Cahoon SS, Yoshihara K, Shida M, Kakuda M, Adachi S, Moeini A, Machida H,Garcia-Sayre J, Ueda Y, Enomoto T, Mikami M, Roman LD, Sood AK, Association of low-dose aspirin and survival of women with endometrial cancer, Obstet. Gynecol 128 (2016) 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matsuo K, Moeini A, Machida H, Scannell CA, Casabar JK, Kakuda M, Adachi S, Garcia-Sayre J, Ueda Y, Roman LD, Tumor characteristics and survival outcome of endometrial cancer arising in adenomyosis: an exploratory analysis, Ann. Surg. Oncol. 23 (2016)959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsuo K, Yessaian AA, Lin YG, Pham HQ, Muderspach LI, Liebman HA, Morrow CP, Roman LD, Predictive model of venous thromboembolism in endometrial cancer, Gynecol. Oncol 128 (2013) 544–551. [DOI] [PubMed] [Google Scholar]
- [25].Rose PG,Endometrial carcinoma, N. Engl.J. Med 335 (1996) 640–649. [DOI] [PubMed] [Google Scholar]
- [26].Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A,Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C, ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up, Radiother. Oncol. 117 (2015) 559–581. [DOI] [PubMed] [Google Scholar]
- [27].Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M, Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Postoperative radiation therapy in endometrial carcinoma, Lancet 355 (2000) 1404–1411. [DOI] [PubMed] [Google Scholar]
- [28].Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG, A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study, Gynecol. Oncol 92 (2004) 744–751. [DOI] [PubMed] [Google Scholar]
- [29].Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R, Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials, Int. J. Radiat. Oncol. Biol. Phys 37 (1997) 745–751. [DOI] [PubMed] [Google Scholar]
- [30].von Elm E, Altman DG, Egger M, Pocock SJ, GØtzsche PC, Vandenbroucke JP, STROBE initiative. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, BmJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wethington SL, Barrena Medel NI,Wright JD, Herzog TJ, Prognostic significance and treatment implications of positive peritoneal cytology in endometrial adenocarcinoma: unraveling a mystery, Gynecol. Oncol 115 (2009) 18–25. [DOI] [PubMed] [Google Scholar]
- [32].Wright JD, Burke WM, Tergas AI, Hou JY, Huang Y, Hu JC, Hillyer GC, Ananth CV, Neugut AI, Hershman DL, Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer, J. Clin. Oncol 34 (2016) 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, Jobling TW, Land R, Manolitsas T, Nascimento M, Neesham D, Nicklin JL, Oehler MK, Otton G, Perrin L, Salfinger S, Hammond I, Leung Y, Sykes P, Ngan H, Garrett A, Laney M, Ng TY, Tam K, Chan K, Wrede CD, Pather S, Simcock B, Farrell R, Robertson G, Walker G, Armfield NR, Graves N, McCartney AJ, Obermair A, Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial, JAMA 317 (2017) 1224–1233. [DOI] [PubMed] [Google Scholar]
- [34].Zhang C, Havrilesky LJ, Broadwater G, Di Santo N, Ehrisman JA, Lee PS, Berchuck A, Alvarez Secord A, Bean S, Bentley RC, Valea FA, Relationship between minimally invasive hysterectomy, pelvic cytology, and lymph vascular space invasion: a single institution study of 458 patients, Gynecol. Oncol 133 (2014) 211–215. [DOI] [PubMed] [Google Scholar]
- [35].Machida H, Casey JP, Garcia-Sayre J, Jung CE, Casabar JK, Moeini A, Kato K, Roman LD, Matsuo K, Timing of intrauterine manipulator insertion during minimally invasive surgical staging and results of pelvic cytology in endometrial cancer, J. Minim. Invasive Gynecol 23 (2016) 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Machida H, Hom MS, Adams CL, Eckhardt SE, Garcia-Sayre J, Mikami M, Matsuo K, Intrauterine manipulator use during minimally invasive hysterectomy and risk of lymphovascular space invasion in endometrial cancer, Int. J. Gynecol. Cancer 28 (2018) 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chang YN, Zhang Y, Wang YJ, Wang LP, Duan H, Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis, Fertil. Steril. 96 (2011) 957–961. [DOI] [PubMed] [Google Scholar]
- [38].Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA, Integrated genomic characterization of endometrial carcinoma, Nature 497 (2013) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Salani R, Khanna N, Frimer M, Bristow RE, Chen LM, An update on post-treatment surveillance and diagnosis ofrecurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations, Gynecol. Oncol. 146 (2017) 3–10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.