Abstract
OBJECTIVE:
To examine the survival outcomes in women with endometrial cancer who were taking low-dose aspirin (81–100 mg/d).
METHODS:
A multicenter retrospective study was conducted examining patients with stage I—IV endometrial cancer who underwent hysterectomy-based surgical staging between January 2000 and December 2013 (N=1,687). Patient demographics, medical comorbidities, medication types, tumor characteristics, and treatment patterns were correlated to survival outcomes. A Cox proportional hazard regression model was used to estimate adjusted hazard ratio for disease-free and disease-specific overall survival.
RESULTS:
One hundred fifty-eight patients (9.4%, 95% confidence interval [CI] 8.8–11.9) were taking low-dose aspirin. Median follow-up time for the study cohort was 31.5 months. One hundred twenty-seven patients (7.5%) died of endometrial cancer. Low-dose aspirin use was significantly correlated with concurrent obesity, hypertension, diabetes mellitus, and hypercholesterolemia (all P<.001). Low-dose aspirin users were more likely to take other antihypertensive, antiglycemic, and anticholesterol agents (all P<.05). Low-dose aspirin use was not associated with histologic subtype, tumor grade, nodal metastasis, or cancer stage (all P>.05). On multivariable analysis, low-dose aspirin use remained an independent prognostic factor associated with an improved 5-year disease-free survival rate (90.6% compared with 80.9%, adjusted hazard ratio 0.46, 95% CI 0.25–0.86, P=.014) and disease-specific overall survival rate (96.4% compared with 87.3%, adjusted hazard ratio 0.23, 95% CI 0.08–0.64, P=.005). The increased survival effect noted with low-dose aspirin use was greatest in patients whose age was younger than 60 years (5-year disease-free survival rates, 93.9% compared with 84.0%, P=.013), body mass index was 30 or greater (92.2% compared with 81.4%, P=.027), who had type I cancer (96.5% compared with 88.6%, P=.029), and who received postoperative whole pelvic radiotherapy (88.2% compared with 61.5%, P=.014). These four factors remained significant for disease-specific overall survival (all P<.05).
CONCLUSION:
Our results suggest that low-dose aspirin use is associated with improved survival outcomes in women with endometrial cancer, especially in those who are young, obese, with low-grade disease, and who receive postoperative radiotherapy.
Endometrial cancer continues to be the most common gynecologic malignancy in the United States with more than 60,000 newly diagnosed cases projected for 2016.1 Incidence of endometrial cancer has steadily increased over the past decade in parallel with the pandemic increase of obesity in the United States.2 Obesity is a well-known risk factor for the development of endometrial cancer. The unopposed estrogen theory has been the mainstay of the oncogenic process for endometrial cancer in obese women.3 However, recent studies have proposed that chronic inflammation related to obesity is an important alternative mechanism of oncogenesis in various types of cancer, including in endometrial cancer.4,5 According to this theory, obesity stimulates inflammatory pathways, which promote tumor development predominantly by releasing proinflammatory cytokines, enhancing angiogenesis, inducing cell proliferation, suppressing the immune system, and generating reactive oxygen species, which damage DNA.6-9
Anti-inflammatory agents, including low-dose aspirin, have been identified as potential agents to decrease the risk of endometrial cancer. It has been suggested that aspirin use is more effective in obese women in preventing endometrial cancer development.7,10-13 However, the observed effects of aspirin on survival in endometrial cancer remain inconsistent. Although one study demonstrated survival benefits in type II cancer, other studies have shown either no association or adverse effects on survival14,15 (Brasky TM, Felix AS, Cohn DE, McMeekin DS, Mutch D, Walker JL, et al. Non-steroidal anti-inflammatory drugs and endometrial cancer mortality in the NRG Oncology/Gynecologic Oncology Group 210 trial. Annual Meeting of American Society of Preventive Oncology, March 12–15, 2016, Columbus, Ohio.). The objective of this study was to examine the survival outcomes of women with endometrial cancer who were taking low-dose aspirin and characterize the subgroup of patients who benefit from low-dose aspirin.
MATERIALS AND METHODS
A multicenter retrospective study was conducted from four institutions (University of Southern California, Osaka University, Tokai University, and Niigata University). Institutional review board approval was obtained at each participating institution. Eligibility criteria for the study were consecutive patients with endometrial cancer who underwent hysterectomy-based surgical staging between January 1, 2000, and December 31, 2013. Patients with uterine carcinosarcoma, endometrial hyperplasia, and metastatic cancer to the endometrium as well as patients without surgical staging were excluded from the study. Among the eligible patients, information for patient demographics, tumor characteristics, treatment pattern, and survival outcome were abstracted from archived medical records. The STrengthening the Reporting of OBservational studies in Epidemiology guideline for retrospective study was consulted.16 A proportion of patients (76.7%) was included within the context of our previous studies.17
Patient demographics including age, ethnicity, body mass index (BMI, calculated as weight [kg]/ [height (m)]2), medical comorbidity (hypertension, diabetes mellitus, hypercholesterolemia, and cigarette use), and medication type were collected at the time of the initial endometrial cancer diagnosis. Medication history was verified through multiple sources. For tumor characteristics, histologic subtypes, grade, stage, depth of myometrial invasion, presence of lymphovascular space invasion, lymph node metastasis (pelvic, paraaortic, or both), and hormonal receptor status (estrogen receptor [ER] and progesterone receptor) were collected from records for hysterectomy-based surgical staging. For treatment pattern, postoperative chemotherapy type (carboplatin and paclitaxel compared with others) and postoperative radiotherapy type (whole pelvic radiotherapy compared with intracavitary brachytherapy) were recorded. For survival outcome, disease-free survival and disease-specific overall survival were obtained from institutional medical records. Data entry into a deidentified data sheet was performed by coinvestigators in each participating institution, and the principal investigator reviewed all the data for accuracy and consistency.
Obesity was defined as a BMI of 30 or greater. Low-dose aspirin refers to an 81–100 mg oral daily dose. Nonsteroidal anti-inflammatory drugs (NSAIDs) other than aspirin were classified as other NSAIDs. Tumor grade was based on the International Federation of Gynecology and Obstetrics: 5% or less solid component for grade 1, 6–50% solid component for grade 2, and greater than 50% solid component for grade 3.18 Cancer stage was reclassified based on the 2009 International Federation of Gynecology and Obstetrics staging system.19 Type I cancer was defined as grade 1–2 endometrioid histology, and type II cancer was defined as grade 3 endometrioid, serous, clear cell, mixed, and other histology.20-22 Deep myometrial invasion was defined as greater than 50% tumor invasion of the myometrium. Medication types were grouped as antihypertensive, antiglycemic, anticholesterol, analgesia, antacid, psychiatric, and other class agents. Disease-free survival was defined as the time interval between the date of hysterectomy-based surgical staging and the date of the first recurrence of endometrial cancer or the last follow-up date if there was no recurrence. Disease-specific overall survival was defined as the time interval between the date of hysterectomy-based surgical staging and the date of death resulting from endometrial cancer. Cases were censored if the patient was alive at the last follow-up date or if the patient died from other causes. Causes of death other than endometrial cancer were also recorded.
The primary aim of analysis was to determine the effects of low-dose aspirin use on the survival outcome of women with endometrial cancer. The secondary interest of analysis was to examine the subgroup of patients who benefit from low-dose aspirin use. Sample size estimation was performed by using α=0.05 and β=0.20. Based on prior studies, a hazard ratio (HR) for disease-free survival of 0.75 and a 10% low-dose aspirin use rate were approximated for our study population.14 By this computation, 140 cases of low-dose aspirin use and 1,440 cases of nonuse were estimated.
Continuous variables were expressed with mean±standard deviation or median (range) based on the normality examined by Kolmogorov-Smirnov test. Statistical significances of continuous variables between the two groups were examined by Student t test or Mann-Whitney U test, as appropriate. Spearman’s correlation coefficient was determined among continuous variables. Kruskal-Wallis test was used for the comparison of median in more than two groups. Statistical significance of ordinal and categorical variables was examined by χ2 test or Fisher exact test as appropriate.
Log-rank test for univariate analysis and a Cox proportional hazard regression model for multivariable analysis were used for survival analysis. Covariates entered in the initial multivariable model were the statistically significant variables in univariate analysis with the cutoff value being P<.05. These included age (younger than 60 compared with 60 years or older), ethnicity (Caucasian, African American, Hispanic, and Asian), histologic subtype (endometrioid compared with nonendometrioid), grade (1–2 compared with 3), stage (I–II compared with III–IV), deep myometrial invasion (no compared with yes), lymphovascular space invasion (no compared with yes), postoperative chemotherapy (no compared with yes), postoperative radiotherapy (none, whole pelvic radiotherapy±intracavitary brachytherapy and intracavitary brachytherapy alone), low-dose aspirin (no compared with yes), acetaminophen (no compared with yes), other NSAIDs (no compared with yes), and metformin (no compared with yes) that were grouped in an a priori manner.19,22,23 Conditional backward method was then used to determine the independent prognostic factor for disease-free and disease-specific overall survival until all covariates became statistically significant in the final model, and magnitude of statistical significance was expressed with HR and 95% confidence interval (CI). Kaplan-Meier method was used to construct survival curves. All statistical tests were two-tailed, and a P<.05 was considered statistically significant. SPSS 12.0 was used for the analyses.
RESULTS
There were 1,687 women with endometrial cancer who underwent surgical staging and were included in this study (Table 1). For the entire cohort, mean age was 56.0 years, and the majority (53.8%) were Asian. Obesity was seen in 42.6%. Younger patients were more likely to be obese (r=−0.23, P<.001). Medical comorbidities were prevalent in this study population. The majority of cancers were obesity-related tumors (Table 2). Across the drug classes examined in the study (Table 3), the most common type of medication were statins (14.9%) followed by angiotensin-converting enzyme inhibitors (13.5%), calcium channel blockers (13.3%), and metformin (12.3%).
Table 1.
Demographics for Patients With Endometrial Cancer
| Characteristic | All (N=1,687) | Low-Dose ASA (n=158) |
No Low-Dose ASA (n=1,529) |
P |
|---|---|---|---|---|
| Age (y) | 56.0±11.4 | 56.5±10.1 | 56.0±11.6 | .57 |
| Younger than 60 | 1,053 (62.4) | 104 (65.8) | 949 (62.1) | |
| 60 or older | 634 (37.6) | 54 (34.2) | 580 (37.9) | |
| Ethnicity | <.001 | |||
| Caucasian | 176 (10.4) | 19 (12.0) | 157 (10.3) | |
| African American | 38 (2.3) | 3 (1.9) | 35 (2.3) | |
| Hispanic | 566 (33.6) | 101 (63.9) | 465 (30.4) | |
| Asian | 907 (53.8) | 35 (22.2) | 872 (57.0) | |
| BMI (kg/m2) | 30.2±10.0 | 36.7±10.3 | 29.6±9.7 | <.001 |
| Less than 30 | 963 (57.4) | 45 (28.8) | 918 (60.4) | |
| 30 or greater | 714 (42.6) | 111 (71.2) | 603 (39.6) | |
| Hypertension | <.001 | |||
| No | 1,006 (59.6) | 28 (17.7) | 978 (64.0) | |
| Yes | 681 (40.4) | 130 (82.3) | 551 (36.0) | |
| Diabetes mellitus | <.001 | |||
| No | 1,319 (78.2) | 69 (43.7) | 1,250 (81.8) | |
| Yes | 368 (21.8) | 89 (56.3) | 279 (18.2) | |
| Hypercholesterolemia | <.001 | |||
| No | 1,344 (79.7) | 73 (46.2) | 1,271 (83.1) | |
| Yes | 343 (20.3) | 85 (53.8) | 258 (16.9) | |
| Cigarette use | .001 | |||
| No | 1,249 (76.9) | 136 (87.2) | 1,113 (75.8) | |
| Yes | 376 (23.1) | 20 (12.8) | 356 (24.2) |
ASA, aspirin; BMI, body mass index.
Data are mean±standard deviation or n (%) unless otherwise specified.
Chi square test or Fisher exact test for P values.
Bold indicates significant P value.
There were 10 missing values for BMI and 62 for cigarette use.
Table 2.
Tumor Characteristics and Treatment Patterns for Endometrial Cancer
| Characteristic | All (N=1,687) | Low-Dose ASA (n=158) | No Low-Dose ASA (n=1,529) | P |
|---|---|---|---|---|
| Histology | .22 | |||
| Endometrioid | 1,421 (84.2) | 128 (81.0) | 1,293 (84.6) | |
| Serous | 93 (5.5) | 8 (5.1) | 85 (5.6) | |
| Clear cell | 38 (2.3) | 2 (1.3) | 36 (2.4) | |
| Mixed | 115 (6.8) | 17 (10.8) | 98 (6.4) | |
| Other | 20 (1.2) | 3 (1.9) | 17 (1.1) | |
| Grade | .97 | |||
| 1 | 967 (57.3) | 92 (58.3) | 875 (57.2) | |
| 2 | 358 (21.2) | 33 (20.9) | 325 (21.3) | |
| 3 | 362 (21.5) | 33 (20.9) | 329 (21.5) | |
| Stage | .17 | |||
| I | 1,209 (71.7) | 123 (77.8) | 1,086 (71.1) | |
| II | 123 (7.3) | 12 (7.6) | 111 (7.3) | |
| III | 245 (14.5) | 18 (11.4) | 227 (14.8) | |
| IV | 109 (6.5) | 5 (3.2) | 104 (6.8) | |
| Cancer type | .70 | |||
| I | 1,250 (74.1) | 115 (72.8) | 1,135 (74.2) | .70 |
| II | 437 (25.9) | 43 (27.2) | 394 (25.8) | |
| Myometrial invasion (%) | .13 | |||
| 50 or less | 1,217 (73.7) | 124 (79.0) | 1,093 (73.1) | |
| Greater than 50 | 435 (26.3) | 33 (21.0) | 402 (26.9) | |
| LVSI | .07 | |||
| No | 1,216 (73.3) | 125 (79.5) | 1,091 (72.7) | |
| Yes | 442 (26.7) | 32 (20.4) | 410 (27.3) | |
| Pelvic nodal metastasis* | .71 | |||
| No | 973 (86.7) | 56 (88.9) | 917 (86.6) | |
| Yes | 149 (13.3) | 7 (11.1) | 142 (13.4) | |
| Aortic nodal metastasis† | 1.0 | |||
| No | 269 (73.1) | 14 (73.7) | 255 (73.1) | |
| Yes | 99 (26.9) | 5 (26.3) | 94 (26.9) | |
| Postoperative chemotherapy | <.001 | |||
| No | 1,079 (64.0) | 123 (78.3) | 956 (62.5) | |
| Yes‡ | 608 (36.0) | 35 (21.7) | 573 (37.5) | |
| Postoperative radiotherapy | .001 | |||
| None | 1,390 (82.4) | 113 (71.5) | 1,277 (83.5) | |
| WPRT±ICBT | 182 (10.8) | 30 (19.0) | 152 (9.9) | |
| ICBT alone | 115 (6.8) | 15 (9.5) | 100 (6.5) |
ASA, aspirin;LVSI, lymphovascular space invasion;WPRT, whole pelvic radiotherapy;ICBT, intracavitary brachytherapy.
Data are n (%) unless otherwise specified.
Chi square test or Fisher exact test for P values.
Bold indicates significant P value.
Among 1,122 patients who underwent lymphadenectomy.
Among 368 patients who underwent lymphadenectomy. Thirty-five were missing information for depth of myometrial invasion, 29 for LVSI, and one for stage.
Three most common regimens included carboplatin and paclitaxel (69.9%);carboplatin, paclitaxel, and epirubicin (11.3%); and carboplatin and docetaxel (10.3%).
Table 3.
Medication Types and Low-Dose Aspirin in Endometrial Cancer
| Medication Type | All (N=1,687) | Low-Dose ASA (n=158) | No Low-Dose ASA (n=1,529) | P |
|---|---|---|---|---|
| Antihypertensive | ||||
| ACE inhibitor | 227 (13.5) | 67 (42.4) | 160 (10.5) | <.001 |
| Calcium channel blocker | 225 (13.3) | 44 (27.8) | 181 (11.8) | <.001 |
| ARB | 153 (9.1) | 29 (18.4) | 124 (8.1) | <.001 |
| β1 blocker | 153 (9.1) | 44 (27.8) | 106 (7.1) | <.001 |
| Hydrochlorothiazide | 139 (8.2) | 35 (22.2) | 104 (6.8) | <.001 |
| β1–2 blocker | 35 (2.1) | 7 (4.4) | 27 (1.8) | .039 |
| Loop diuretic | 33 (2.0) | 11 (7.0) | 22 (1.4) | <.001 |
| Aldosterone | 19 (1.1) | 5 (3.2) | 14 (0.9) | .027 |
| Clonidine | 9 (0.5) | 3 (1.9) | 6 (0.4) | .044 |
| Hydralazine | 4 (0.2) | 2 (1.3) | 2 (0.1) | .046 |
| Antiglycemic | ||||
| Metformin | 208 (12.3) | 66 (41.8) | 142 (9.3) | <.001 |
| Sulfonylurea | 145 (8.6) | 47 (29.7) | 98 (6.4) | <.001 |
| Insulin | 84 (5.0) | 28 (17.7) | 56 (3.7) | <.001 |
| Pioglitazone | 28 (1.7) | 9 (5.7) | 19 (1.2) | .001 |
| DPP4 inhibitor | 26 (1.5) | 0 | 26 (1.7) | .17 |
| Glucosidase inhibitor | 12 (0.7) | 1 (0.6) | 11 (0.7) | 1.0 |
| Anticholesterol | ||||
| Statin | 251 (14.9) | 64 (40.5) | 187 (12.2) | <.001 |
| Gemfibrozil | 19 (1.1) | 11 (7.0) | 8 (0.5) | <.001 |
| Analgesia | ||||
| NSAID | 101 (6.0) | 11 (5.9) | 90 (7.0) | .60 |
| Acetaminophen | 25 (1.5) | 6 (3.8) | 19 (1.2) | .024 |
| Opioid | 22 (1.3) | 3 (1.9) | 19 (1.2) | .45 |
| Antacid | ||||
| PPI | 68 (4.0) | 17 (10.8) | 51 (3.3) | <.001 |
| H2 blocker | 36 (2.1) | 9 (5.7) | 27 (1.8) | .005 |
| Psychiatric | ||||
| Benzodiazepine | 67 (4.0) | 4 (2.5) | 63 (4.1) | .52 |
| SSRI | 42 (2.5) | 7 (4.4) | 35 (2.3) | .11 |
| Gabapentin | 19 (1.1) | 5 (3.2) | 14 (0.9) | .027 |
| Carbamazepine | 10 (0.6) | 0 | 10 (0.7) | .61 |
| Phenytoin | 4 (0.2) | 1 (0.6) | 3 (0.2) | .33 |
| SNRI | 3 (0.2) | 1 (0.6) | 2 (0.1) | .26 |
| Other classes | ||||
| Synthroid | 68 (4.0) | 19 (12.0) | 49 (3.2) | <.001 |
| Steroid | 39 (2.3) | 0 | 39 (2.6) | .045 |
| Antihistamine | 30 (1.8) | 4 (2.5) | 26 (1.7) | .52 |
| Albuterol | 25 (1.5) | 4 (2.5) | 21 (1.4) | .29 |
| Warfarin | 18 (1.1) | 1 (0.6) | 17 (1.1) | 1.0 |
| Bisphosphonate | 12 (0.7) | 1 (0.6) | 10 (0.7) | 1.0 |
| Allopurinol | 10 (0.6) | 5 (3.2) | 5 (0.3) | .001 |
| OCP | 9 (0.5) | 1 (0.6) | 8 (0.5) | .59 |
| Nitroglycerin | 6 (0.4) | 4 (2.5) | 2 (0.1) | .001 |
ASA, aspirin; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; DPP4, dipeptidyl peptidase 4; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; OCP, oral contraceptive pill.
Data are n (%) unless otherwise specified.
Chi square test for P values.
Bold indicates significant P value.
Median follow-up time for the entire cohort was 31.5 months. There were 226 (13.4%) cases of endometrial cancer recurrence, and 127 (7.5%) women died of the disease. There were six (0.4%) women who died of other causes (respiratory failure n=3, other malignancy n=2, and sepsis n=1). There was no statistical difference in disease-free survival (P=.57) or disease-specific overall survival (P=.42) across the institutions.
Low-dose aspirin use was noted in 158 (9.4%, 95% CI 8.8–11.9) patients in the study population. Low-dose aspirin users were more likely to be obese, hypertensive, diabetic, and dyslipidemic compared with nonaspirin users (all P<.001; Table 1). Tumor characteristics were similar between low-dose aspirin users and nonusers (all P>.05; Table 2). Low-dose aspirin users were more likely to receive postoperative whole pelvic radiotherapy (19.0% compared with 9.9%, P<.001) and less likely to receive postoperative chemotherapy (21.7% compared with 37.5%, P<.001); these findings are likely the result of the difference in practice patterns across the institutions (Appendix 1, available online at http://links.lww.com/AOG/A816).
Correlation between low-dose aspirin use and other medication types was examined (Table 3). Low-dose aspirin users were more likely to take antihypertensive (the most common agent, angiotensin-converting enzyme inhibitor 42.4%) and anticholesterol agents (the most common agent, statin 40.5%) (all P<.05). Low-dose aspirin users were also more likely to take the majority of antiglycemic agents (the most common agent, metformin 41.8%).
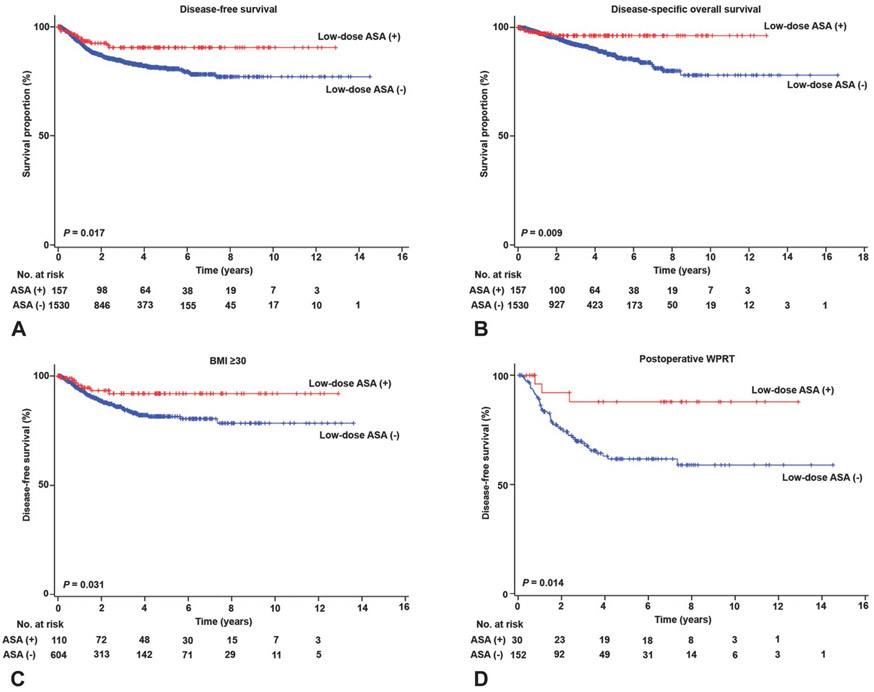
Next, we carried out survival analyses based on low-dose aspirin use (Table 4). Median follow-up time for low-dose aspirin users was significantly longer compared with nonusers (36.7 compared with 30.9 months, P=.016). On univariate analysis, low-dose aspirin use was statistically significantly associated with improved disease-free survival compared with nonaspirin use (5-year rates, 90.5% compared with 80.9%, P=.015; Fig. 1A). Medical comorbidities examined in this study were not associated with survival outcomes. On multivariable analysis, low-dose aspirin use remained an independent prognostic factor associated with improved disease-free survival (adjusted HR 0.46, 95% CI 0.25–0.86, P=.014). Similarly, low-dose aspirin use was associated with improved disease-specific overall survival (5-year rates, 96.3% compared with 87.3%, P=.008; Fig. 1B). On multivariable analysis, low-dose aspirin use remained an independent prognostic factor associated with improved disease-specific overall survival (adjusted HR 0.23, 95% CI 0.08–0.64, P=.005; Table 4).
Table 4.
Survival Outcome of Endometrial Cancer (N=1,687)
| Characteristic | Disease-Free Survival | ||||
|---|---|---|---|---|---|
| 5-y Survival Rate (%) |
Univariate | Multivariable | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (y) | <.001 | .005 | |||
| Younger than 60 | 85.2 | 1 | 1 | ||
| 60 or older | 76.2 | 1.79 (1.37–2.32) | 1.50 (1.13–2.00) | ||
| Ethnicity | .001 | ||||
| Caucasian | 70.5 | 1 | |||
| African American | 53.8 | 1.87 (0.91–3.87) | |||
| Hispanic | 83.5 | 0.62 (0.40–0.97) | |||
| Asian | 83.5 | 0.65 (0.43–0.98) | |||
| Histology | <.001 | <.001 | |||
| Endometrioid | 86.6 | 1 | 1 | ||
| Nonendometrioid | 56.0 | 4.42 (3.38–5.77) | 2.02 (1.45–2.81) | ||
| Grade | <.001 | <.001 | |||
| 1–2 | 88.6 | 1 | 1 | ||
| 3 | 58.3 | 4.99 (3.84–6.48) | 1.91 (1.38–2.65) | ||
| Stage | <.001 | <.001 | |||
| I–II | 91.1 | 1 | 1 | ||
| III–IV | 50.5 | 8.06 (6.14–10.6) | 4.89 (3.59–6.67) | ||
| Myometrial invasion (%) | <.001 | <.001 | |||
| 50 or less | 89.6 | 1 | 1 | ||
| Greater than 50 | 64.4 | 3.90 (2.97–5.11) | 1.80 (1.34–2.41) | ||
| LVSI | <.001 | ||||
| No | 89.6 | 1 | |||
| Yes | 63.9 | 4.12 (3.15–5.39) | |||
| Chemotherapy | <.001 | ||||
| No | 90.8 | 1 | |||
| Yes | 67.9 | 4.30 (3.23–5.72) | |||
| Radiotherapy | <.001 | ||||
| None | 85.2 | 1 | |||
| WPRT±ICBT | 66.3 | 2.25 (1.64–3.08) | |||
| ICBT alone | 74.9 | 1.50 (0.93–2.41) | |||
| Low-dose ASA | .015 | .014 | |||
| No | 80.9 | 1 | 1 | ||
| Yes | 90.6 | 0.49 (0.28–0.88) | 0.46 (0.25–0.86) | ||
| Acetaminophen | <.001 | .006 | |||
| No | 82.3 | 1 | 1 | ||
| Yes | 60.0 | 3.22 (1.59–6.52) | 2.72 (1.33–5.58) | ||
| Other NSAIDs | .003 | .009 | |||
| No | 82.7 | 1 | 1 | ||
| Yes | 70.0 | 1.94 (1.24–3.04) | 1.92 (1.18–3.13) | ||
| Metformin | |||||
| No | |||||
| Yes | |||||
| Disease-Specific Overall Survival | |||||
| 5-y Survival Rate (%) | Univariate | Multivariable | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| <.001 | .011 | ||||
| 91.9 | 1 | 1 | |||
| 81.5 | 2.28 (1.61–3.25) | 1.65 (1.12–2.42) | |||
| .048 | |||||
| 85.1 | 1 | ||||
| 72.7 | 2.36 (0.89–6.29) | ||||
| 89.9 | 0.75 (0.40–1.43) | ||||
| 88.0 | 0.83 (0.45–1.53) | ||||
| <.001 | <.001 | ||||
| 92.5 | 1 | 1 | |||
| 65.3 | 5.83 (4.11–8.26) | 2.81 (1.83–4.30) | |||
| <.001 | .011 | ||||
| 93.6 | 1 | 1 | |||
| 69.7 | 5.86 (4.11–8.35) | 1.71 (1.11–2.62) | |||
| <.001 | <.001 | ||||
| 94.3 | 1 | 1 | |||
| 68.5 | 9.65 (6.56–14.2) | 5.00 (3.19–7.84) | |||
| <.001 | .016 | ||||
| 94.3 | 1 | 1 | |||
| 76.0 | 4.65 (3.20–6.77) | 1.73 (1.11–2.72) | |||
| <.001 | .034 | ||||
| 93.8 | 1 | 1 | |||
| 76.3 | 4.62 (3.19–6.69) | 1.67 (1.04–2.69) | |||
| <.001 | |||||
| 94.3 | 1 | ||||
| 79.1 | 5.56 (3.69–8.37) | ||||
| <.001 | |||||
| 90.3 | 1 | ||||
| 80.3 | 2.21 (1.48–3.31) | ||||
| 81.1 | 1.33 (0.67–2.65) | ||||
| .008 | .005 | ||||
| 87.3 | 1 | 1 | |||
| 96.4 | 0.32 (0.13–0.78) | 0.23 (0.08–0.64) | |||
| <.001 | <.001 | ||||
| 88.8 | 1 | 1 | |||
| 57.2 | 5.17 (2.41–11.1) | 7.60 (3.44–16.8) | |||
| .017 | |||||
| 88.7 | 1 | ||||
| 87.0 | 1.87 (1.03–3.39) | ||||
| .036 | |||||
| 87.8 | 1 | ||||
| 91.9 | 0.51 (0.27–0.97) | ||||
HR, hazard ratio; CI, confidence interval; LVSI, lymphovascular space invasion; WPRT, whole pelvic radiotherapy; ICBT, intracavitary brachytherapy; ASA, aspirin; NSAIDs, nonsteroidal anti-inflammatory drugs.
Log-rank test and Cox proportional hazard regression model (conditional backward method) for P values.
Bold indicates significant P value.
Fig. 1.
Survival curves of endometrial cancer based on low-dose aspirin (ASA) use. Log-rank test for P values. Survival curves were constructed for (A) disease-free survival, (B) disease-specific overall survival, (C) body mass index (BMI), and (D) postoperative whole pelvic radiotherapy (WPRT).
All the collected variables were stratified by low-dose aspirin use and differences in survival outcome between users and nonusers were examined. This analysis found four conditions associated with maximum benefit from low-dose aspirin on survival outcome (Table 5). These included younger age, obesity, type I cancer, and postoperative whole pelvic radiotherapy. For age, low-dose aspirin use in women younger than 60 years of age was associated with improved disease-free survival (HR 0.34, P=.013); there was no statistical significance for women 60 years of age or older (P=.57). Low-dose aspirin use was beneficial among patients with BMIs 30 or greater (HR 0.43, P=.027; Fig. 1C) but not in patients with BMIs less than 30 (P=.44). Type I cancer was associated with improved disease-free survival with low-dose aspirin use (HR 0.30, P=.029), but type II cancer was not (P=.14). Low-dose aspirin use was significantly associated with improved disease-free survival among patients who received postoperative whole pelvic radiotherapy with the largest magnitude of statistical significance (HR 0.26, P=.014; Fig. 1D), but the benefit of low-dose aspirin was not seen in patients who did not receive whole pelvic radiotherapy (P=.10). These four factors remained significant for disease-specific overall survival (all P<.05; Table 5).
Table 5.
Contributing Factors Maximizing Low-Dose Aspirin Effects
| Characteristic | n | Disease-Free Survival | Disease-Specific Overall Survival | ||||
|---|---|---|---|---|---|---|---|
| 5-y Survival Rate (%) |
HR (95% CI) | P | 5-y Survival Rate (%) |
HR (95% CI) | P | ||
| Age factor | |||||||
| Age younger than 60 y | .013 | .006 | |||||
| No low-dose ASA | 949 | 84.0 | 1 | 90.0 | 1 | ||
| Low-dose ASA | 104 | 93.9 | 0.34 (0.14–0.83) | 98.8 | 0.11 (0.02–0.76) | ||
| Age 60 y or older | .57 | .57 | |||||
| No low-dose ASA | 580 | 75.5 | 1 | 80.6 | 1 | ||
| Low-dose ASA | 54 | 83.9 | 0.80 (0.37–1.72) | 91.7 | 0.75 (0.27-2.06) | ||
| Body habitus | |||||||
| BMI (kg/m2) less than 30 | .44 | .58 | |||||
| No low-dose ASA | 926 | 80.6 | 1 | 86.5 | 1 | ||
| Low-dose ASA | 47 | 86.4 | 0.71 (0.29–1.72) | 93.1 | 0.72 (0.23–2.30) | ||
| BMI 30 or greater | .027 | .007 | |||||
| No low-dose ASA | 603 | 81.3 | 1 | 88.7 | 1 | ||
| Low-dose ASA | 111 | 92.3 | 0.43 (0.20–0.93) | 97.8 | 0.16 (0.04–0.73) | ||
| Cancer type | |||||||
| Type I cancer | .029 | .012 | |||||
| No low-dose ASA | 1,135 | 88.6 | 1 | 93.3 | 1 | ||
| Low-dose ASA | 115 | 96.5 | 0.30 (0.09–0.95) | 100.0 | 0.04 (0.01–2.09) | ||
| Type II cancer | .14 | .15 | |||||
| No low-dose ASA | 394 | 59.6 | 1 | 71.0 | 1 | ||
| Low-dose ASA | 43 | 74.4 | 0.61 (0.31–1.19) | 86.5 | 0.52 (0.21–1.29) | ||
| Postoperative radiotherapy | |||||||
| No WPRT | .10 | .17 | |||||
| No low-dose ASA | 1,377 | 83.4 | 1 | 89.0 | 1 | ||
| Low-dose ASA | 128 | 91.4 | 0.56 (0.29–1.10) | 95.5 | 0.54 (0.22-1.33) | ||
| WPRT | .014 | .002 | |||||
| No low-dose ASA | 152 | 61.5 | 1 | 76.2 | 1 | ||
| Low-dose ASA | 30 | 88.2 | 0.26 (0.08–0.83) | 100.0 | 0.03 (0.01–1.18) | ||
Estrogen receptor and progesterone receptor status were examined in 459 (27.2%) and 453 (26.9%) patients, respectively. Positivity of ER and progesterone receptor were seen in 80% and 76.6% of examined patients, respectively. Among patients in whom tumors expressed ER (n=367), low-dose aspirin users had a higher 5-year disease-free survival rate compared with nonusers although this did not reach statistical significance (92.1% compared with 78.6%, P=.07; Appendix 2, available online at http://links.lww.com/AOG/A816) and 5-year disease-specific overall survival rate (97.6% compared with 87.2%, P=.08). Among patients whose tumors did not express ER, there was no difference in 5-year disease-free survival rates between aspirin users and nonusers (50.0% compared with 40.9%, P=.98).
Survival outcomes were examined based on combination patterns of low-dose aspirin and other medications. When compared with β-blocker alone, a combination of β-blocker and low-dose aspirin did not show a difference in 5-year disease-free survival rate (80.4% compared with 87.8%, P=.38). Similarly, there was no difference in 5-year disease-free survival rate between metformin alone and metformin with low-dose aspirin (86.6% compared with 88.7%, P=.49) or statin alone and statin with low-dose aspirin (81.0% compared with 85.3%, P=.44).
Patient demographics and tumor characteristics for other NSAIDs and acetaminophen use were examined (Appendices 3-6, available online at http://links.lww.com/AOG/A816). Patients taking other NSAIDs had greater risk of pelvic lymph node metastasis (22.4% compared with 12.8%, odds ratio [OR] 1.97, 95% CI 1.04–3.75, P=.045) and advanced-stage disease (stage IV disease, 11.9% compared with 6.1%, P=.04). Similarly, patients taking acetaminophen had a greater risk of advanced-stage disease (28.0% compared with 6.1%, P<.001).
DISCUSSION
This study demonstrated that low-dose aspirin use was associated with improved survival outcomes in women with endometrial cancer. This effect of low-dose aspirin on survival was particularly prominent in young and obese women as well as in those with low-grade disease. Our study also showed that low-dose aspirin users had higher survival rate compared with nonusers among women who received postoperative radiotherapy.
Our results differ from some prior studies that have investigated the association between aspirin use and survival in endometrial cancer14,15 (Brasky et al, 2016) Our study showed that aspirin users had favorable outcomes in type I cancer, whereas other studies showed favorable outcomes in type II cancer, showed no benefit, or showed decreased endometrial cancer survival. These four studies had different patient and tumor characteristics that likely contributed to the different effects of aspirin on survival (Appendix 7, available online at http://links.lww.com/AOG/A816). Specifically, our study population was younger, had more endometrioid histology, and had less grade 3 tumors and stage IV disease among the four studies.
In addition, the definition of type I cancer was different across the studies. That is, we defined grade 1–2 endometrioid type, whereas ther others examined all endometrioid types. Because grade 3 endometrioid type is clinically and biologically similar to serous and clear cell types, a different definition of type I cancer may result in different results.21 The most important difference across the four studies is that our study is the only one that specifically investigated low-dose aspirin, whereas the others did not specifically assess aspirin dose.
Our study showed that acetaminophen and other NSAIDs were associated with decreased survival outcome. This result was similar to a prior study demonstrating that nonaspirin NSAID use was associated with increased cancer-specific mortality (Brasky et al, 2016). One of the likely reasons for this is that these patients were more likely to have advanced-stage disease and, as a result, were more likely to have pain requiring these analgesic agents. However, further studies are warranted to determine whether biological effects and tumor progression differ across the various NSAID types.
A weakness of the study is that this is a retrospective study that may have missed possible confounders. For instance, the indication and frequency of low-dose aspirin use could not be abstracted from medical records. Another potential limitation of this study is that the majority of the study population was either Hispanic or Asian and as such may not be representative of a general population. In addition, low-dose aspirin users were different from nonusers in a greater number of ways that cannot be completely taken into account using statistical tests. Lastly, the relatively short follow-up time in this study may affect the results for survival outcome resulting from lead time bias. The main strength of this study is in the large sample size. Power analysis was performed using α=0.05. With HRs for disease-free survival 0.46 and disease-specific overall survival 0.32 with a median follow-up of 31.5 months, where low-dose aspirin was used in 9.4%, statistical power of this study was greater than 99% for both disease-free and disease-specific overall survival.
Based on our findings, we hypothesize both direct and indirect antitumor effects of low-dose aspirin. Low-dose aspirin inhibits cyclooxygenase (COX)-1 in platelets and COX-2 in tumor cells resulting in prostaglandin E2 (PGE2) downregulation (Oates J, Massion P, Knollmann B, Smith J, Haddad E, Lammers P, et al. Low dose aspirin that reduces mortality from lung adenocarcinoma inhibits both platelet COX-1 and the biosynthesis of PGE2. 13th Annual Meeting for American Association for Cancer Research, September 27-October 1, 2014, New Orleans, Louisiana.). Prostaglandin E2 induces cell proliferation, survival, and angiogenesis. Therefore, it is speculated that low-dose aspirin can reduce endometrial cancer recurrence through inhibition of the COX-2-PGE2 pathway. In colon cancer, aspirin use was associated with decreased COX-2-positive colon cancer risk.24 For indirect effects, low-dose aspirin inhibits the COX-2–PGE2 pathway in macrophages in excess adipose tissue to suppress proinflammatory and prooncogenic cytokines.
Aspirin may reduce estrogen action in endometrial cancer. Prior studies have shown that PGE2 regulates aromatase activity.25 Therefore, aspirin use inhibits the COX-2-PGE2 pathway resulting in reduced estrogen biosynthesis. A recent populationbased case–control study has shown that aspirin use reduces the risk of ER-positive breast cancer.26 Because the majority of obesity-driven endometrial cancer is type I and expresses ER,27 this may explain our finding of the association between low-dose aspirin use and improved survival in women with ER-positive endometrial cancer (Appendix 2, http://links.lww.com/AOG/A816).
Clinical implications of our study include possible chemoprevention of endometrial cancer recurrence by taking low-dose aspirin after initial treatment. Because radiotherapy remains a commonly used adjuvant therapy in selected cases of endometrial cancer with risk factors,28 it is of interest to examine whether concurrent low-dose aspirin use during postoperative pelvic radiotherapy will maximize treatment effects.
Supplementary Material
Acknowledgments
Supported by Ensign Endowment for Gynecologic Cancer Research (Koji Matsuo) and the Frank McGraw Memorial Chair in Cancer Research (Anil K. Sood).
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37:889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol 1989;129:1120–31. [DOI] [PubMed] [Google Scholar]
- 4.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancerrelated inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- 5.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 2005;14:2840–7. [DOI] [PubMed] [Google Scholar]
- 6.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013;1831:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM, et al. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case-control study, systematic review and meta-analysis. Int J Cancer 2013;132:1146–55. [DOI] [PubMed] [Google Scholar]
- 8.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008;57:3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008;371:771–83. [DOI] [PubMed] [Google Scholar]
- 10.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev 2009;18:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodelon C, Doherty JA, Chen C, Rossing MA, Weiss NS. Use of nonsteroidal antiinflammatory drugs and risk of endometrial cancer. Am J Epidemiol 2009;170:1512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res 2008;68:2507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moysich KB, Baker JA, Rodabaugh KJ, Villella JA. Regular analgesic use and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev 2005;14:2923–8. [DOI] [PubMed] [Google Scholar]
- 14.Nevadunsky NS, Van Arsdale A, Strickler HD, Spoozak LA, Moadel A, Kaur G, et al. Association between statin use and endometrial cancer survival. Obstet Gynecol 2015;126:144–50. [DOI] [PubMed] [Google Scholar]
- 15.Pierce SR, Doll KM, Davidson B, Lee C, Ko EM, Snavely AC, et al. Endometrial cancer outcomes in diabetic women treated with metformin, statins, and aspirin. Gynecol Oncol 2014; 133:43.24444820 [Google Scholar]
- 16.STROBE guideline Available at: http://www.strobe-statement.org/. Retrieved April 1, 2015. [Google Scholar]
- 17.Matsuo K, Moeini A, Machida H, Scannell CA, Casabar JK, Kakuda M, et al. Tumor characteristics and survival outcome of endometrial cancer arising in adenomyosis: an exploratory analysis. Ann Surg Oncol 2016;23:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose PG. Endometrial carcinoma. N Engl J Med 1996;335: 640–9. [DOI] [PubMed] [Google Scholar]
- 19.Pecorelli S Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105: 103–4. [DOI] [PubMed] [Google Scholar]
- 20.Doll A, Abal M, Rigau M, Monge M, Gonzalez M, Demajo S, et al. Novel molecular profiles of endometrial cancer–new light through old windows. J Steroid Biochem Mol Biol 2008;108: 221–9. [DOI] [PubMed] [Google Scholar]
- 21.Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer–a clinical and pathological evaluation. Gynecol Oncol 2012;124:15–20. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol 2015;125:424–33. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K, Garcia-Sayre J, Medeiros F, Casabar JK, Machida H, Moeini A, et al. Impact of depth and extent of lymphovascular space invasion on lymph node metastasis and recurrence patterns in endometrial cancer. J Surg Oncol 2015;112:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 2007;356:2131–42. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 1996;137:5739–42. [DOI] [PubMed] [Google Scholar]
- 26.Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA 2004;291:2433–40. [DOI] [PubMed] [Google Scholar]
- 27.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet 2012;379:1352–60. [DOI] [PubMed] [Google Scholar]
- 28.Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ, et al. Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2015; 33:2908–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.