Abstract
Chimeric antigen receptor (CAR) T-cell therapy is an exciting innovation in the treatment of cancer. However, CAR T-cell therapies have been associated with unique adverse events (AEs), including cytokine release syndrome (CRS) and neurologic events (also known as CAR T-cell–related encephalopathy syndrome [CRES] or, most recently, immune effector cell–associated neurotoxicity syndrome [ICANS]). Cytopenias and infection have also been observed. These AEs are treatable and reversible with appropriate treatment strategies but can become severe if not managed early. Therefore, it is essential for the advanced practitioner caring for patients undergoing these therapies to have a thorough understanding of the associated AEs, in particular their grading and management. Cytokine release syndrome and neurologic events can range in severity from low-grade symptoms that require supportive care only to a high-grade syndrome that can become life-threatening. While several grading and management recommendations have been used in clinical trials, until recently, there were no consistent grading and management guidelines. Here we provide the most recent recommendations, which have the ultimate goal of maintaining the benefits of CAR T-cell therapy, while minimizing life-threatening AEs. Improved understanding and management of AEs associated with CAR T-cell therapy will provide broader access to this innovative and potentially curative technology.
Chimeric antigen receptor (CAR) T-cell therapy is an exciting innovation in the treatment of cancer. Two anti-CD19 CAR T-cell therapies are approved for adults with relapsed or refractory large B-cell lymphoma after greater than two lines of therapy and have demonstrated significant efficacy for some patients (Kite Pharma Inc., 2017; Novartis Pharmaceuticals Corporation, 2018). However, CAR T-cell therapy is associated with unique adverse events (AEs), distinct from traditional chemotherapies, monoclonal antibodies, and small molecule therapies.
The two most common acute AEs are cytokine release syndrome (CRS) and neurologic events (also known as CAR T-cell–related encephalopathy syndrome [CRES] or most recently, immune effector cell–associated neurotoxicity syndrome [ICANS]). These AEs are reversible and treatable with appropriate strategies, but can become severe or life-threatening if not managed early (Neelapu et al., 2018). Cytopenias and infection have also been observed. Therefore, it is essential for the advanced practitioner caring for patients undergoing these therapies to be able to recognize, grade, and manage CAR T-cell therapy–associated AEs. It will be important for the advanced practitioner to be familiar with these complications in the CAR T-cell therapy context, as it can be complicated to determine their etiology in this patient population. The ultimate goal of AE management should be to maintain the benefits of CAR T-cell therapy, while minimizing life-threatening AEs. The recommendations provided in this article represent expert opinion based on the experience at our institution. While experience may vary between institutions, these recommendations are in line with the latest published guidance and best practices in the field.
CYTOKINE RELEASE SYNDROME
Cytokine release syndrome is one of the most common AEs associated with CAR T-cell therapy. However, it is not unique to CAR T-cell therapy; it has also previously been observed with other T-cell receptor gene therapies and bispecific T-cell–engaging antibodies, including blinatumomab (Blincyto; Neelapu et al., 2018).
When CAR T-cells bind to their target antigen, they proliferate, make cytokines, and produce cytotoxic molecules that mediate the destruction of tumor cells. When cells are destroyed, they release cytokines and other immune effector cells into circulation (Breslin, 2007). Elevations in interferon-γ, granulocyte macrophage colony-stimulating factor, interleukin (IL)-10, and IL-6 have been observed following CAR T-cell infusions (Bonifant, Jackson, Brentjens, & Curran, 2016). The release of high concentrations of cytokines affects a range of organ systems. Increased vascular permeability and third-spacing of fluid have been observed following this “cytokine storm,” which can result in vasodilation, decreased cardiac output, and intravascular volume depletion (Jhaveri & Rosner, 2018). In rare cases, severe CRS can evolve into fulminant hemophagocytic lymphohistiocytosis (HLH; also known as macrophage activation syndrome [MAS], a life-threatening, pathologic hyperactivation of the immune system).
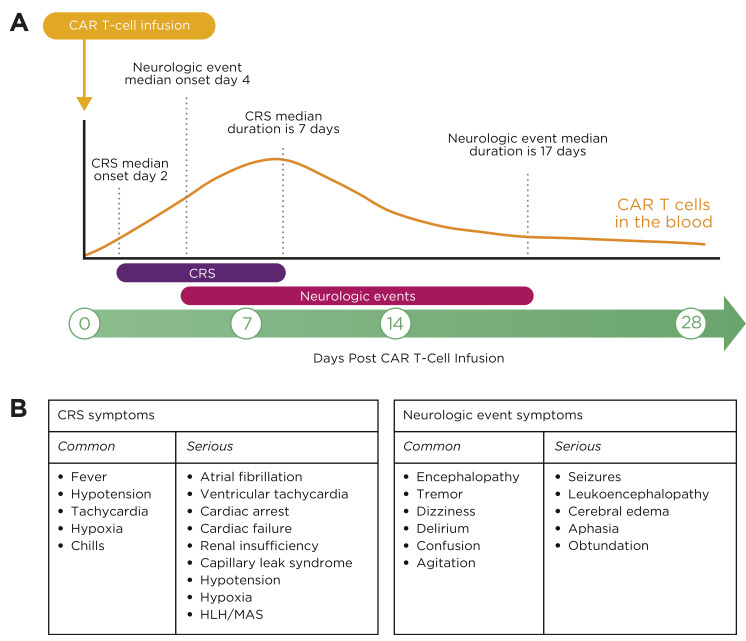
The most common symptoms of CRS are high fevers, hypotension, and hypoxia, and can include other organ toxicities (Figure 1; Neelapu et al., 2018). Fever is the hallmark symptom of CRS onset. Patients infused with CAR T cells in an outpatient setting should be instructed to return immediately to the hospital for further evaluation at the onset of fever, as other more serious symptoms may develop quickly. Cytokine release syndrome symptoms can also mimic sepsis, and, because patients are often neutropenic due to lymphodepleting chemotherapy, infection should always be in the differential (Neelapu et al., 2018). Fever workup should include cultures, chest x-ray, and lactic acid level. Empiric antibiotics should be started in patients with neutropenia with consideration of growth factors (although growth factors are contraindicated with some cell products for several days post infusion; Neelapu et al., 2018).
Figure 1.
CRS and neurologic events symptoms. (A) Onset and resolution of CRS and neurologic events in ZUMA-1. (B) Common and serious symptoms of CRS and neurologic events. CAR = chimeric antigen receptor; CRS = cytokine release syndrome; HLH = hemophagocytic lymphohistiocytosis; MAS = macrophage activation syndrome. Adapted from Kite Pharma Inc. (2017); Lee et al. (2014).
While there are differences between CAR T-cell products, the onset of CRS usually occurs within the first week after cell administration (Figure 1). Clinical trials of commercially available CAR T-cell therapies showed a median time of CRS onset of 2 to 3 days with a median duration of 7 to 8 days. The percentage of patients with grade ≥ 3 CRS was 13% in patients who received axicabtagene ciloleucel (Yescarta; Kite Pharma Inc., 2017) and 49% in patients who received tisagenlecleucel (Kymriah; Novartis Pharmaceuticals Corporation, 2018). It is important to note that CRS symptoms have occurred up to 3 weeks post CAR T-cell therapy. Educating patients and caregivers about symptoms to report post discharge is imperative for early intervention and positive outcomes. The Risk Evaluation and Mitigation Strategies (REMS) programs for the approved CAR T-cell therapies mandate that patients receive written information in the form of a wallet card regarding emergent symptoms and the need to stay within 2 hours of the treating facility for 4 weeks post cell therapy (Kite Pharma Inc., 2017; Novartis Pharmaceuticals Corporation, 2018).
Risk factors for the development of severe AEs have been identified, including high tumor burden and early onset of CRS (Neelapu et al., 2018). Predictive models for the development of severe CRS have been considered, and potential biomarkers continue to be studied to help identify patients at high risk of developing severe AEs (Teachey et al., 2016; Wang & Han, 2018). Based on clinical practice at The University of Texas MD Anderson Cancer Center, we have found that inflammatory markers such as C-reactive protein (CRP), ferritin, and various cytokine levels have been useful to trend recovery from AEs.
Cytokine Release Syndrome Grading
Several methods of grading AEs have been used in clinical trials, including the Penn grading scale (Porter, Frey, Wood, Weng, & Grupp, 2018), Lee grading scale (Lee et al., 2014), Common Terminology for Adverse Events (CTCAE; U.S. Department of Health and Human Services, 2017), and the CAR T-cell therapy–associated TOXicity (CARTOX) grading scale. To standardize grading across all institutions for both clinical trials and grading of toxicities in patients receiving commercial products, the American Society for Blood and Marrow Transplantation (ASBMT) has recently published consensus grading for CRS and neurologic toxicity associated with immune effector cells. The ASBMT CRS grading scale is based on three parameters: fever, hypotension, and hypoxia (Lee et al., 2018). Organ toxicity is no longer included in the grading of toxicities. If CRS is suspected, the grade should be evaluated at least twice a day and when the patient’s condition changes (Table 1).
Table 1. ASBMT Grading of Cytokine Release Syndromea.
| CRS parameter | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Feverb | ≥ 38°C | ≥ 38°C | ≥ 38°C | ≥ 38°C |
| With either: | ||||
| Hypotension | None | Not requiring vasopressors | Requiring one vasopressor with or without vasopressin | Requiring multiple vasopressors (excluding vasopressin) |
| And/or c: | ||||
| Hypoxia | None | Requiring low-flow nasal cannulad or blow-by | Requiring high-flow nasal cannula, face mask, non-rebreather mask, or Venturi mask | Requiring positive pressure (e.g., CPAP, BiPAP, intubation and mechanical ventilation) |
Note. Adapted from Lee et al. (2018). ASBMT = American Society for Blood and Marrow Transplantation; CRS = cytokine release syndrome; CPAP = continuous positive airway pressure; BiPAP = bilevel positive airway pressure.
aOrgan toxicities associated with CRS may be graded according to Common Terminology Criteria for Adverse Events, version 5.0, but they do not influence CRS grading.
bFever is defined as temperature ≥ 38°C not attributable to any other cause. In patients who have CRS and then receive antipyretics or anticytokine therapy such as tocilizumab or steroids, fever is no longer required to grade subsequent CRS severity. In that case, CRS grading is driven by hypotension and/or hypoxia.
cCytokine release syndrome grade is determined by the more severe event: hypotension or hypoxia not attributable to any other cause. For example, a patient with temperature of 39.5°C, hypotension requiring one vasopressor, and hypoxia requiring low-flow nasal cannula is classified as having grade 3 CRS.
dLow-flow nasal cannula is defined as oxygen delivered at ≤ 6 liters/minute. Low-flow also includes blow-by oxygen delivery, sometimes used in pediatrics. High-flow nasal cannula is defined as oxygen delivered at > 6 L/min.
Cytokine Release Syndrome Management
Management of CRS should be determined by the grade. Low-grade CRS can be managed mostly with supportive care. Tocilizumab (Actemra), an anti–IL-6 receptor antagonist, and/or corticosteroids may also be required for more severe events, such as persistent and refractory fever or hypotension refractory to fluid boluses (Neelapu et al., 2018). Tocilizumab, which was originally approved for the treatment of rheumatoid arthritis, is approved for the management of CRS that occurs after CAR T-cell therapy. Other agents, including siltuximab (Sylvant), infliximab (Remicade), etanercept, and anakinra, have also been used in clinical trials. Tocilizumab has not been shown to adversely affect the efficacy of CAR T-cell therapy (Neelapu et al., 2018).
Corticosteroids are also used in the management of CRS. Preliminary data from one clinical trial suggest that the use of steroids to treat CAR T-cell–related AEs has not been shown to affect objective and complete response rates, nor durability of responses in clinical trials, but the long-term effects of these drugs on CAR T-cell efficacy are still to be determined. Because corticosteroids are known to suppress and/or kill T cells, avoiding their use for other non–CAR T-cell–related AEs is prudent (Neelapu et al., 2018).
NEUROLOGIC EVENTS
Neurologic events are characterized by confusion, agitation, and delirium. In severe cases, they may also include receptive or expressive aphasia, obtundation, convulsive or nonconvulsive seizures, and cerebral edema (Figure 1). Early signs of neurologic events include disturbances in language and handwriting, and diminished attention (Neelapu et al., 2018). Educating patients and caregivers prior to CAR T-cell therapy and providing support, particularly to the caregivers, during the period of neurotoxicity cannot be emphasized enough, as it is a very frightening experience when their loved one cannot speak or recognize them.
The onset and severity of neurologic events also differ between CAR T-cell therapies. Median time to onset for commercially available CAR T-cell therapies is 4 to 6 days, with a median duration of 14 to 17 days (Figure 1; Kite Pharma Inc., 2017; Novartis Pharmaceuticals Corporation, 2018). Asking the patient to write a sentence every 8 to 12 hours or when the patient’s condition changes has helped to detect early neurologic impairment. The grade of the neurologic event also varies between therapies. Thirty-one percent of patients who received axicabtagene ciloleucel developed a grade 3 or higher neurologic event compared with 18% of patients who received tisagenlecleucel (Kite Pharma Inc., 2017; Novartis Pharmaceuticals Corporation, 2018).
Neurologic events may be biphasic, with onset of the first phase occurring simultaneously with CRS symptoms, usually within the first 5 days after CAR T-cell therapy (Neelapu et al., 2018). These early-phase neurologic events occurring with CRS tend to be shorter and milder and, in our experience, often respond to anti–IL-6 therapy. This may be due to greater blood-brain permeability allowing the therapeutic treatment to reach the central nervous system (CNS). High doses of tocilizumab may also exacerbate neurologic events, as once IL-6 receptors are saturated, IL-6 levels in the serum are transiently elevated and may diffuse into the CNS, contributing to the development of neurotoxicity (Nellan et al., 2018). Neurologic events that develop after the CRS symptoms have resolved do not respond to anti–IL-6 therapy (Neelapu et al., 2018). The underlying cause of the neurologic events is unknown. Theories include the passive leakage of cytokines through the blood-brain barrier and trafficking of T cells into the CNS (Neelapu et al., 2018).
Neurologic Event Grading
Neurologic symptoms associated with immune effector cell therapy have been termed ICANS per the new ASBMT guidelines. The grading of ICANS encompasses: (1) the immune effector cell–associated encephalopathy (ICE) score (which is very similar to the CARTOX-10 scoring system); (2) evaluation of level of consciousness; (3) seizure activity; (4) motor weakness/paraparesis; and (5) increased intracranial pressure/cerebral edema. The final ICANS score is determined by the most severe event (Table 2; Lee et al., 2018).
Table 2. Grading of Neurologic Events With the ASBMT ICANS Tool.
| Neurotoxicity domain | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| ICE scorea | 7–9 | 3–6 | 0–2 | 0 (patient is unarousable and unable to perform ICE) |
| Depressed level of consciousnessb | Awakens spontaneously | Awakens to voice | Awakens only to stimulus | Patient is unarousable or requires vigorous or repetitive tactile stimuli to arouse; stupor or coma |
| Seizures | NA | NA | Any clinical seizure, focal or generalized, that resolves rapidly; or nonconvulsive seizures on EEG that resolve with intervention | Life-threatening prolonged seizure (> 5 min); or repetitive clinical or electrical seizures without return to baseline in between |
| Motor findingsc | NA | NA | NA | Deep focal motor weakness such as hemiparesis or paraparesis |
| Raised intracranial pressure/cerebral edema | NA | NA | Focal/local edema on neuroimagingd | Diffuse cerebral edema on neuroimaging; decerebrate or decorticate posturing; or cranial nerve VI palsy; or papilledema; or Cushing’s triad |
Note. Adapted from Lee et al. (2018). ASBMT = American Society for Blood and Marrow Transplantation; ICANS = immune effector cell–associated neurotoxicity syndrome; ICE = immune effector cell-associated encephalopathy; EEG = electroencephalogram; NA = not applicable. ICANS grade is determined by the most severe event (ICE score, level of consciousness, seizure, motor findings, raised intracranial pressure/cerebral edema) not attributable to any other cause. For example, a patient with an ICE score of 3 who has a generalized seizure is classified as having grade 3 ICANS.
aA patient with an ICE score of 0 may be classified as having grade 3 ICANS if awake with global aphasia. But a patient with an ICE score of 0 may be classified as having grade 4 ICANS if unarousable.
bDepressed level of consciousness should be attributable to no other cause (e.g., no sedating medication).
cTremors and myoclonus associated with immune effector cell therapies may be graded according to CTCAE version 5.0 but they do not influence ICANS grading.
dIntracranial hemorrhage with or without associated edema is not considered a neurotoxicity feature and is excluded from ICANS grading. It may be graded according to Common Terminology Criteria for Adverse Events, version 5.0.
Neurologic Event Management
All patients with neurologic symptoms should undergo brain imaging. Magnetic resonance imaging of the brain is preferable over computed tomography (CT) but may not be feasible if the patient is unstable or agitated. Funduscopic exam and daily electroencephalography and comprehensive neurological exams should be continued until neurologic symptoms resolve.
Neurologic events management is based on the severity grade. Low-grade neurologic events can be managed primarily with supportive care. For patients with grade ≥ 1 neurologic events with concurrent CRS, tocilizumab is recommended. Neurologic events without concurrent CRS do not respond to anti–IL-6 therapy. Grade ≥ 2 neurologic events that are not associated with CRS can be treated with corticosteroids, with the dose dependent on the grade of the event. Grade 2 and 3 neurologic events can be treated with dexamethasone at 10 mg intravenously (IV) every 6 hours or methylprednisolone at 1 mg/kg IV every 12 hours. Treatment of grade 4 neurologic events requires high-dose steroids until improvement to grade 1 ICANS and then tapering, for example methylprednisolone at 1 g/day IV for 3 days, followed by a rapid taper at 250 mg every 12 hours for 2 days, 125 mg every 12 hours for 2 days, and 60 mg every 12 hours for 2 days (Kite Pharma Inc., 2017; Neelapu et al., 2018). Other corticosteroids, such as dexamethasone, may also be used according to protocols at other institutions.
Seizure activity and increased intracranial pressure should also be managed concurrent with the treatment of neurologic events per standard guidelines. Patients may be placed on prophylactic antiseizure medication on the day the CAR T-cells are infused and continued for 4 weeks post CAR T-cell therapy, but this recommendation varies between commercially available CAR T-cell therapies (Neelapu et al., 2018).
OTHER ADVERSE EVENTS
Long-term and late effects of CAR T-cell therapy may include both B-cell aplasia resulting in hypogammaglobulinemia and cytopenias, both of which can increase the risk of infection. Both malignant and normal B cells express CD19 on their surface, resulting in an “on target, off tumor” B-cell aplasia post anti-CD19 CAR T-cell therapy. B-cell aplasia can potentially last for months to years and may lead to frequent infections. Patients may require treatment with monthly immunoglobulin G (Brudno & Kochenderfer, 2016). For patients who had previous stem cell transplant and never received posttransplant vaccinations, it would be advisable to wait until the B-cells have recovered because they form antibodies. Consideration should be given to providing the seasonal influenza vaccine because a T-cell response has been reported in patients given the flu vaccine, which can provide some protection (Ljungman & Avetisyan, 2008). Cyclophosphamide and fludarabine are the most common chemotherapy agents used for lymphodepletion prior to CAR T-cell administration and have been shown to cause cytopenias for months after treatment when used in combination with rituximab (Strati et al., 2013).
Chimeric antigen receptor T-cell therapy can also induce immune-mediated pancytopenia. It is not uncommon to see grade 3/4 cytopenias in this patient population, and their blood counts should be followed closely in conjunction with transfusion support and growth factors as indicated (however, the commercial CAR T-cell package insert for tisagenlecleucel advises avoiding growth factors for 3 weeks after cell infusion; Novartis Pharmaceuticals Corporation, 2018). These patients frequently also have low CD4 counts, which puts them at risk for opportunistic infections, including Pneumocystis jiroveci pneumonia (PJP) and should be given PJP prophylaxis until the CD4 count is normal. Currently, there are no standardized guidelines for additional antimicrobial prophylaxis after CAR T-cell therapy; however, many institutions are incorporating guidelines similar to those used with cancer patients who are immunosuppressed (Taplitz et al., 2018).
CLINICAL CASE STUDY
Mr. H is a 49-year-old male with a history of diffuse large B-cell lymphoma diagnosed in 2015. He initially was treated with rituximab (Rituxan), cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (R-CHOP) and demonstrated a partial response. He received three more lines of therapy, including rituximab, gemcitabine, cisplatin, and dexamethasone (R-GDP), rituximab, ifosfamide, carboplatin, etoposide (R-ICE), and rituximab with lenalidomide (Revlimid) but did not demonstrate a response. He received anti-CD19 CAR T-cell therapy on March 25, 2017.
On day +2, Mr. H developed a fever of 39.5°C, which was treated unsuccessfully with acetaminophen and a cooling blanket. His oxygen saturation remained above 93% on room air. His heart rate was 120 beats per minute (bpm) and blood pressure was 80/50 mm Hg but improved to 100/60 mm Hg after a 500-cc 0.9% NaCl fluid bolus. Blood and urine cultures were obtained. A chest x-ray was negative for pneumonia but showed small bilateral pleural effusions.
Mr. H was then started on empiric IV antibiotics. His CRP and ferritin levels were trending up. His neurologic exam was normal, and his ICE score was 10/10. He was determined to have grade 2 CRS with fever and hypotension. He was given tocilizumab at 8 mg/kg × 1 and his fever resolved. The following day he maintained an oxygen saturation of 95% on room air. His blood pressure remained stable, but he developed a fever of 39.2°C, which improved intermittently with acetaminophen.
On day +5, Mr. H’s temperature was 37.5°C, heart rate 82 bpm, respiratory rate 12 breaths per minute, blood pressure 120/65 mm Hg, and oxygen saturation 96% on room air. His alanine aminotransferase, aspartate aminotransferase, total bilirubin, and creatinine levels all were within normal limits. C-reactive protein and ferritin levels continued to trend up. In the afternoon, Mr. H developed altered mental status. He was not able to state the year, month, or city he was in, and could not name the hospital or follow a simple command. He could correctly name two objects. His handwriting deteriorated (Figure 2).
Figure 2.
Handwriting sample from Mr. H depicting rapid deterioration.
An electroencephalogram was negative for epileptiform discharges and a CT of the brain showed no evidence of cerebral edema. The patient was not able to undergo lumbar puncture safely due to confusion and agitation. A neurology consultation determined that he did not have papilledema on funduscopic exam and no motor deficits were identified.
Mr. H. was determined to have a grade 3 neurologic event (ICE score 2). He was started on dexamethasone at 10 mg IV every 6 hours and transferred to the intensive care unit for closer monitoring.
The patient’s mental status improved on steroids, and 24 hours later the drugs were tapered; 2 days later they were discontinued. He was transferred back to the floor and transitioned to oral antibiotics as all cultures remained negative. C-reactive protein and ferritin levels were trending down and Mr. H was determined to be stable for discharge on day +10. He was followed with weekly laboratory tests, and a day +30 positron emission tomography-CT showed a complete response.
CONCLUSION
While CAR T-cell therapy has been associated with significant AEs, it has also demonstrated dramatic clinical benefit to many patients. Recommendations for AE grading and management are based on our current, ongoing experience with CAR T-cell therapy, and will continue to evolve as our experience broadens. As part of this evolution, efforts are continuing to further standardize the grading and management of CRS and neurologic events, which may help to address some of the current challenges. Improved understanding of potential AEs and their management will reduce barriers to use of this potentially curative technology, especially as we move toward more widespread access beyond specialized centers.
Acknowledgments
Medical writing support was provided by Katherine R. Nibouar, PhD, of Nexus Global Group Science LLC, sponsored by Kite, a Gilead Company.
Footnotes
This manuscript was sponsored by Kite, a Gilead Company. Ms. Adkins has served as a consultant for Kite, a Gilead Company, and Juno Therapeutics.
REFERENCES
- Bonifant C. L., Jackson H. J., Brentjens R. J., & Curran K. J. (2016). Toxicity and management in CAR T-cell therapy. Molecular Therapy Oncolytics, 3, 16011 10.1038/mto.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin S. (2007). Cytokine-release syndrome: Overview and nursing implications. Clinical Journal of Oncology Nursing, 11(1 suppl), 37–42. 10.1188/07.CJON.S1.37-42 [DOI] [PubMed] [Google Scholar]
- Brudno J. N., & Kochenderfer J. N. (2016). Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood, 127(26), 3321–3330. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri K. D., & Rosner M. H. (2018). Chimeric antigen receptor T cell therapy and the kidney. Clinical Journal of the American Society of Nephrology, 13(5), 796–798. 10.2215/CJN.12871117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kite Pharma Inc (2017). Yescarta (axicabtagene ciloleucel) package insert. Retrieved from https://www.yescarta.com/files/yescarta-pi.pdf
- Lee D. W., Gardner R., Porter D. L., Louis C. U., Ahmed N., Jensen M.,…Mackall C. L. (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood, 124(2), 188–195. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. W., Santomasso B. D., Locke F. L., Ghobadi A., Turtle C. J., Brudno J. N.,…Neelapu S. S. (2018). ASBMT consensus grading for cytokine release syndrome and neurological toxicity associated with immune effector cells. Biology of Blood and Marrow Transplantation, 25(4), 625–638. 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- Ljungman P., & Avetisyan G. (2008). Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant, 42(10), 637–641. 10.1038/bmt.2008.264 [DOI] [PubMed] [Google Scholar]
- Neelapu S. S., Tummala S., Kebriaei P., Wierda W. G., Gutierrez C., Locke F. L.,…Shpall E. J. (2018). Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nature Reviews Clinical Oncology, 15(1), 47–62. 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellan A., McCully C. M. L., Cruz Garcia R., Jayaprakash N., Widemann B. C., Lee D. W., & Warren K. E. (2018). Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood, 132(6), 662–666. 10.1182/blood-2018-05-846428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals Corporation (2018). Kymriah (tisagenlecleucel) package insert. Retrieved from https://www.yescarta.com/files/yescarta-pi.pdf
- Porter D., Frey N., Wood P. A., Weng Y., & Grupp S. A. (2018). Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. Journal of Hematology & Oncology, 11(1), 35 10.1186/s13045-018-0571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati P., Wierda W., Burger J., Ferrajoli A., Tam C., Lerner S.,…O’Brien S. (2013). Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: Analysis of persistent and new-onset cytopenia. Cancer, 119(21), 3805–3811. 10.1002/cncr.28318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplitz R. A., Kennedy E. B., Bow E. J., Crews J., Gleason C., Hawley D. K.,…Flowers C. R. (2018). Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. Journal of Clinical Oncology, 36(30), 3043–3054. 10.1200/JCO.18.00374 [DOI] [PubMed] [Google Scholar]
- Teachey D. T., Lacey S. F., Shaw P. A., Melenhorst J. J., Maude S. L., Frey N.,…Grupp S. A. (2016). Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Cancer Discovery, 6(6), 664–679. 10.1158/2159-8290.CD-16-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2017). Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Retrieved from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- Wang Z., & Han W. (2018). Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomarker Research, 6, 4–4. 10.1186/s40364-018-0116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]