Abstract
USA and China are two leading countries engaged in nanotechnology research and development. They compete with each other for fruits in this innovative area in a parallel and compatible manner. Understanding the status and developmental prospects of nanotechnology in USA and China is important for policy-makers to decide nanotechnology priorities and funding, and to explore new ways for global cooperation on key issues. We here present the nanoscience and nanomedicine research and the related productivity measured by publications, and patent applications, governmental funding, policies and regulations, institutional translational research, industrial and enterprise growth in nanotechnology-related fields across China and USA. The comparison reveals some marked asymmetries of nanotechnology development in China and USA, which may be helpful for future directions to strengthen nanotechnology collaboration for both countries, and for the world as a whole.
Keywords: Nanotechnology, Nanomedicine, National nanotechnology initiative
Nanotechnology is a transformative technology revolutionizing many areas including energy, security, information technology, agriculture, environmental protection, and healthcare. It is creating an international gold rush. Currently there are more than 60 countries that have already launched national nanotechnology programs.1 Among those countries, the United States took a lead from the start in the early 90’s. In 2000, the United States started the world’s first national nanotechnology program, the U.S. National Nanotechnology Initiative (NNI), and Congress passed the legislation to create NNI into law in 2003.2 From 2001 to 2014, about $19.4 billion was invested from the federal government in nanoscale engineering, science, and technology through NNI. In 2015, $1.5 billion has been proposed by President Obama for NNI funding.3 The continuously increasing investment in nanotechnology made the United States a global leader in the field.
Besides the European Union which is the second major funding source for nanotechnology in general, the Chinese government prioritizes nanotechnology by increasing funding for nanotechnology to face the global competition. For instance, in 2009 alone the Chinese government invested $1.6 billion in the field.4 Nanotechnology was one of the four major basic research areas supported by the Medium and Long Term Development Plan 2006–2020 (MLP) approved by the Chinese government (www.most.gov.cn/kjgh). As the two major countries with substantial and sustained investments in nanotechnology by governments and companies, the China–USA relationship is as compelling as it is complex. In today’s global economy, the two great countries compete with each other for various political, economic, and scientific issues in a parallel and compatible manner.
Here we review the race in nanotechnology between the two countries, including the race in nanotechnology research, productivity measured by publications and patent applications, government supports, policies and regulations, institutional translational development and R&D efforts, industrial and enterprise growth in nanotechnology-related fields across China and USA. The advantages and Achilles’ heel of China in nanotechnology are also analyzed through comparison between the two countries. We will certainly applaud each and every effort to strengthen the relationships between the two countries, and to explore new ways to cooperate on issues of worldwide concern.
Nanotechnology publication output
The annual totals of nano-related publications in the period 2001–2014 were collected using Thomson Science Citation Index’s (SCI) Web of Science database with Topic of “nano* NOT nanomolar” to show the recent achievements of the two countries (Figure 1, A). Both China and USA demonstrated a steady growth curve over the last 14 years. China lagged behind USA in the period 2001–2009. Until 2011, China surpassed the USA in the numbers of nano-related papers. The increased publication production in China is mostly benefited from the National Natural Science Foundation of China (NSFC) funding with the introduction of two major programs: the Nanoscience Basic Research in 2002 with a total funding of $12 M and Nanotechnology Manufacturing in 2008 with a total funding of $25 M (www.nsfc.gov.cn). The number of publications has increased significantly from just over 3100 papers in 2008 to 15,558 in 2011 with NSFC funding, which is an increase from 17.5% to 54.2% of all nanotechnology publications in China in just three years.5 The contribution of the returned overseas Chinese scholars might be another reason for the increased publication production in China.6 In 2008, China announced the one-thousand-talents plan (or program; www.1000plan.org) to attract its overseas researchers to return to China. Until 2014, about 2000 researchers were recruited to serve for China through this program, and most of them are trained in the USA. Analysis of author institutes of those nanotechnology-related publications revealed that the authorship in China is mostly concentrated in some prestigious academy and universities, while in USA the authorship contribution is less concentrated.7 Taking the total publication output in 2005 as an example (Table 1), in China, one fourth of the publications were from the most prolific Chinese Academy of Sciences (CAS), which consists of 115 institutes throughout China. While in the USA, three percent of the total publications were from the most prolific Universities of Illinois which is a multi-campus state university system.
Figure 1.
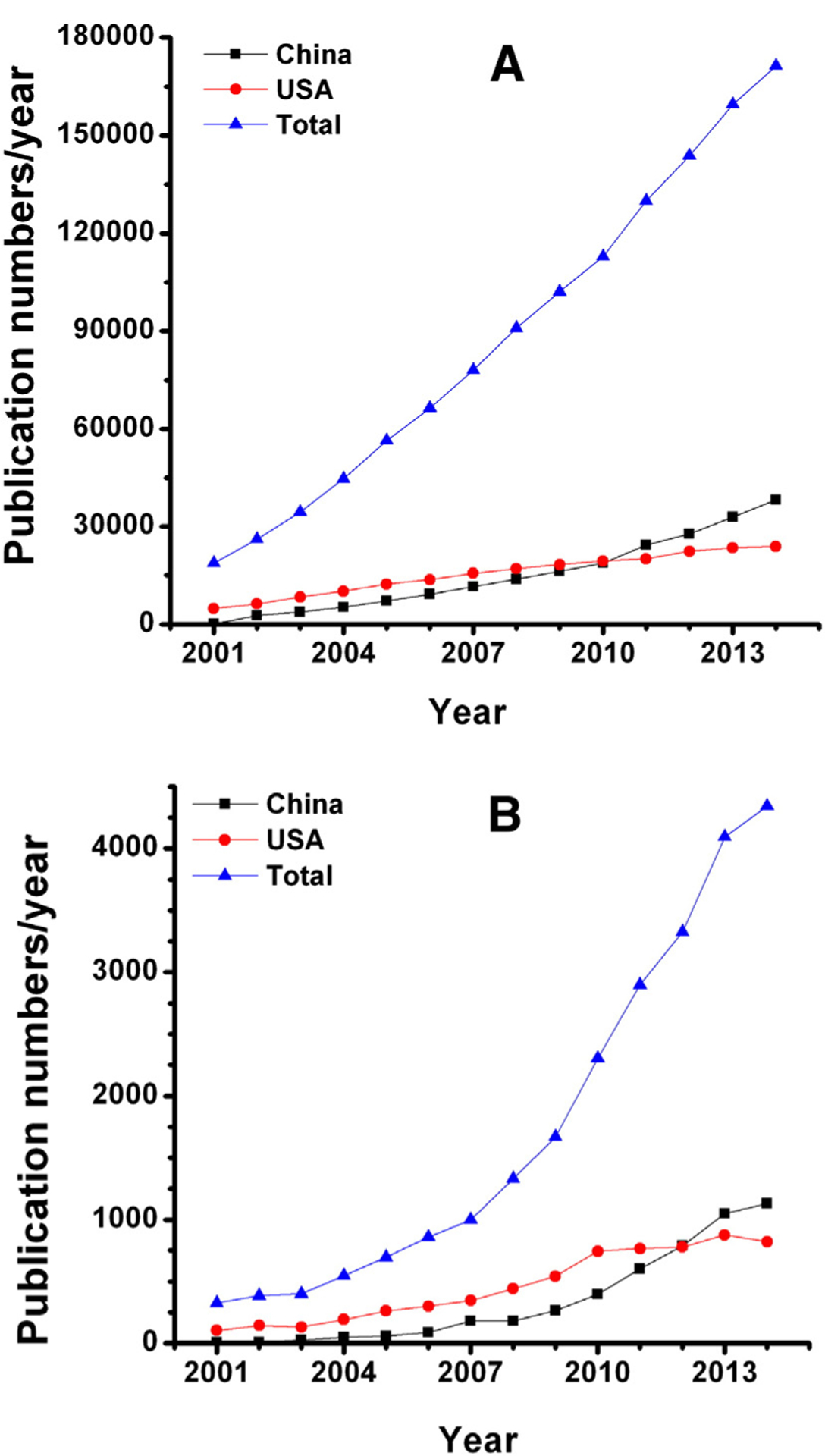
Numbers of the annual nano-related publications from China, USA and the world as a whole (A) and numbers of annual publications in the field of cancer bionanotechnology (B) from 2001 to 2014.
Table 1.
Comparison of institutions in China and USA that produced the most nanotechnology-related papers in 2005.7
| Institutions | Country | Total |
|---|---|---|
| Chinese Academy of Sciences | China | 2916 |
| Tsing Hua University | China | 749 |
| Nanjing University | China | 534 |
| Zhejiang University | China | 528 |
| University of Science &Technology of China | China | 482 |
| University of Illinois | USA | 461 |
| University of California at Berkeley | USA | 472 |
| University of Texas | USA | 419 |
| Peking University | China | 400 |
| Jilin University | China | 378 |
| Shanghai Jiaotong University | China | 367 |
| MIT | USA | 364 |
Cancer is a major public health burden both in China and in the USA. Nanotechnology has witnessed significant progress and its effect is now widespread in cancer research.8–14 The National Cancer Institute (NCI) recognized early the potential of nanotechnology and nanomaterials for cancer treatment and established and funded the NCI Alliance for Nanotechnology in Cancer in 2004 (http://nano.cancer.gov/). Funded by the NCI Alliance, highly creative ideas and innovative solutions are being pursued by researchers centered around the issues of cancer detection and/or therapy.15 In 2005, the US National Institutes of Health (NIH) set up a network of eight Nanomedicine Development Centers to form the NIH Nanomedicine Roadmap (now Common Fund) Initiative. Till now, four centers remained in the second half of the program in which studies could be applied directly to medical applications. In China, programs in nanomedicine and bionanotechnology are also funded by the nationwide key basic research project (the 973 Program) and by the National High Technology Plan (the 863 Program). Because both governments emphasize cancer nanotechnology research, articles in oncology-related nanotechnology have been increasing year by year, especially in recent years.
To analyze the publications in cancer nanomedicine, we performed literature search using PubMed search engines with MeSH terms (all)—(nanotechnology, nanomedicines, nanoparticle, nanocapsules, micellar systems, liposomes, nanotube, or nano-structure) and (oncology, cancer, or neoplasms). We collected 24,174 total articles published from 2001 to 2014, and then used Gopubmed.com to evaluate the cancer bionanotechnology-related publications that originated from either USA or China in comparison with the totals from all the countries worldwide (Figure 1, B). The search results show that the global cancer bionanotechnology publications increased linearly from 2001. The USA accounts for half of all articles before 2005, and thereafter, for about one-fourth of the global total outputs. China follows USA closely and the gap between the two countries has narrowed significantly since 2011. Since 2012, China has the highest number of cancer bionanotechnology publications, and continues to race with the USA in the field shoulder-by-shoulder (Figure 1, B). Although China’s progress is exciting in terms of the number of paper output, it is still far behind the USA in citations.16 There is a severe mismatch between China’s high publication productivity and low citation numbers.17
The application of nanotechnology to cancer research is an interesting and promising area for a China–USA collaboration.18 The joint support for cancer nanotechnology research by NIH and NSFC started in 2010, and continues today. The speedy trend of co-authorship between the USA and China demonstrates the reciprocal interests to each other for cooperation, providing a friendly platform for creating additional partnerships in competitive research areas. The US–China Symposium on Nanobiology and Nanomedicine held every two years since 2008 provides a unique opportunity for experienced scientists to exchange ideas and establish collaborations. It helps to increase China–USA cancer bionanotechnology partnerships include promoting new programs for reciprocal training and exchange, co-sponsoring workshops focused on specific cancer bionanotechnology topics of high priority to both countries, and joint financial assistance of collaborative research projects by China and USA funders.
Nanotechnology-related patent applications
The State Intellectual Property Office of China (SIPO, www.sipo.gov.cn) and the US Patent and Trademark Office (USPTO, www.uspto.gov) published the largest number of nanotechnology patent applications, and both experienced large increases.19 The 21st Century Nanotechnology Research and Development Act of 2003 could be a strong impetus to nanotechnology research in the USA as well as in the world. The number of applications has gone from 105 in 2000, to 5030 in 2008 in China.19 In comparison, the number of application in USA has gone from 405 in 2000 to 3729 in 2008 (Table 2). According to the China Patent Abstract Database managed by the SIPO, there were 30,863 nanotechnology patent applications from 1985 to 2009, and most of them were published after 2003.20 The quick increase in patent applications within a short period forced the governmental patent offices to hire more patent examiners during the period in order to timely process all heavily piled-up applications. Since China’s accession to the WTO, intellectual property protection has become very important. The rapid increase in the number of applications in China is closely related to the vigorous implementation of the intellectual property strategy by the Chinese government.
Table 2.
Numbers of nanotechnology-related patent applications to either USA or China patent offices during 1991–2008, and in the year 2000 and 2008, respectively. Also listed are the top five applicant countries and institutions in USA or China that originated the most patent applications to the patent offices.19
| Country (Number of Applications) | Rank | Applicant country | Numbers of nanotechnology patent applications from the applicant country (1991–2008) | Applicant institution | Numbers of nanotechnology patent applications from the applicant institution (1991–2008) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Totals | 2000 | 2008 | Totals | 2000 | 2008 | ||||
| USA (19,665) | 1 | USA | 12,606 | 285 | 2288 | IBM | 277 | 11 | 54 |
| 2 | Japan | 1866 | 42 | 308 | Univ California | 209 | 11 | 29 | |
| 3 | South Korea | 1272 | 6 | 343 | Samsung Electronics Co. Ltd. | 172 | 0 | 69 | |
| 4 | Germany | 1048 | 23 | 168 | Hon Hai Prec Ind Co. Ltd. | 157 | 0 | 54 | |
| 5 | Taiwan region | 839 | 7 | 175 | Ind Tech Res Inst | 106 | 3 | 15 | |
| China (18,438) | 1 | China | 16,348 | 85 | 4409 | Chinese Academy of Sciences | 1155 | 14 | 312 |
| 2 | USA | 805 | 3 | 260 | Zhejiang Univ | 464 | 3 | 129 | |
| 3 | South Korea | 327 | 5 | 80 | Tsing Hua Univ | 461 | 2 | 91 | |
| 4 | Japan | 301 | 2 | 64 | Shanghai Jiaotong Univ | 409 | 3 | 75 | |
| 5 | Germany | 145 | 3 | 43 | Fudan Univ | 317 | 3 | 81 | |
In most repositories, the largest numbers of nanotechnology patent applications have originally been submitted from the applicants’ own countries (typically in China), indicating that applicants know well about the patent application policy and procedures of their motherland (Table 2). The largest numbers of nanotechnology-related patent applications in the USA are from the industrial sector, whereas in China those are from the academic sector (Table 2).19 For example, in the USA, IBM, University of California, and Samsung Electronics were the top three patent applicants during 1991–2008, whereas, in China, the Chinese Academy of Science, Zhejiang University and Tsinghua University were the top three patent applicants.
Central government support
As a global power, the United States has invested heavily in nanotechnology and the Chinese government has certainly spared no effort to support nanotechnology. In 2004, government support for nanotechnology was $989 M in USA and $90 M in China, respectively.21 In 2012, USA corporate and government spending in the field was nearly $2.2 billion, while in China the number grew to $1.3 billion.22 From the NNI launched by former President Bill Clinton to the Advanced Manufacturing Partnership (AMP) launched by President Obama in 2011, the federal government in the USA has demonstrated a successive investment in nanotechnology innovation to promote its development. As for China, the investment in science and technology has increased every year and is expected to reach 2.5% of GDP by 2020.4 Besides the MLP, nanotechnology is emphasized by the 973 Program, the 863 Program, the CAS, and the NSFC. Figure 2 shows the amount of funds and number of nanotechnology-related projects approved by the NSFC as a whole, or by its Department of Medical Sciences from 2000 to 2014. In the past two years, NSFC greatly increased support for nanotechnology, and approved more than 1700 projects with more than one billion RMB (about $0.6 billion) per year (www.nsfc.gov.cn).
Figure 2.
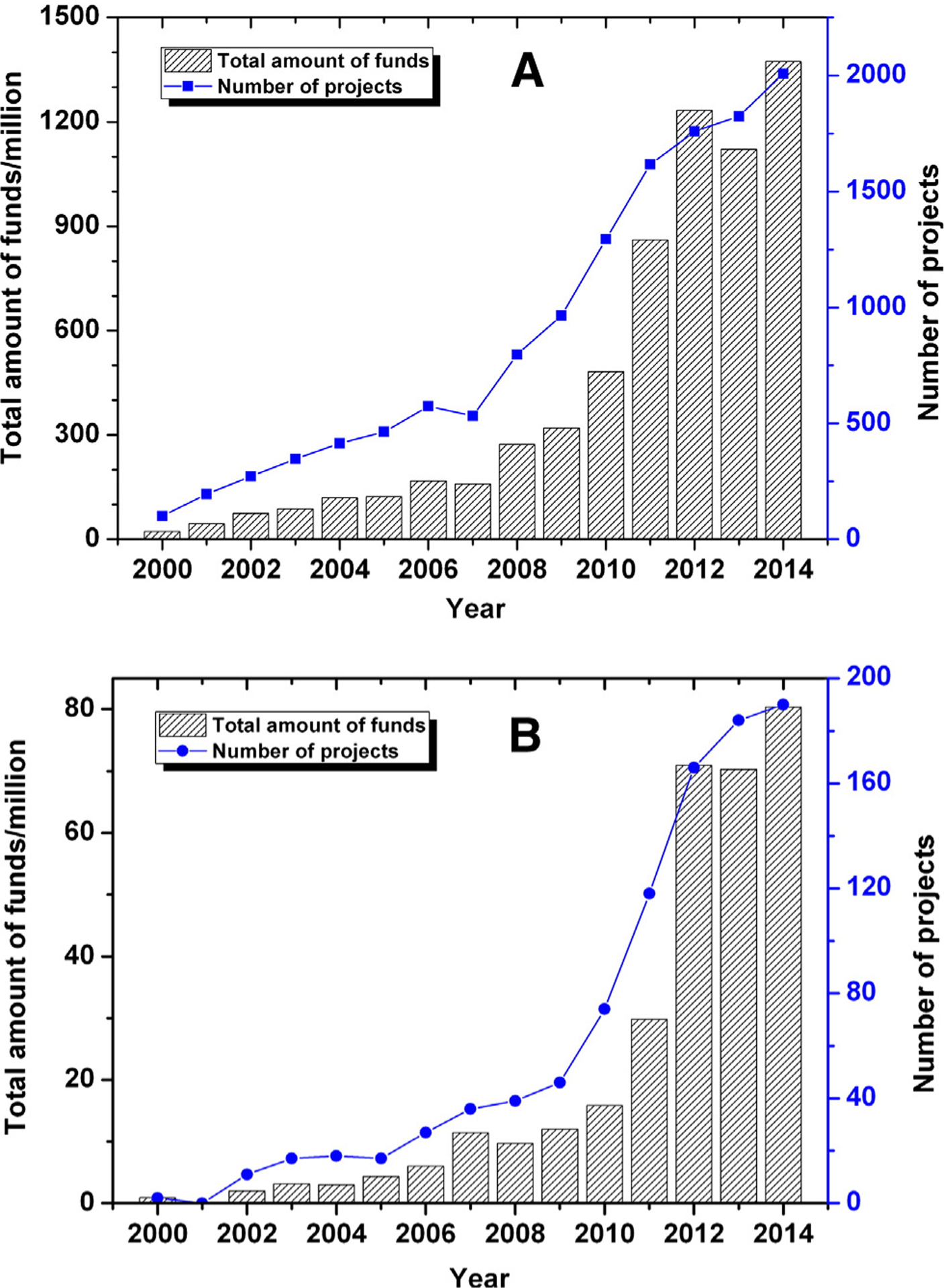
The amount of funds in millions of RMB (¥) and number of nanotechnology-related projects approved by the committee of the NSFC (A), or by the Department of Medical Sciences of the NSFC (B) from 2000 to 2014.
Continuous increase of investments in research and development in nanotechnology by the central government of China could be a successful response to challenging international economic competition. These large investments as well as the political support could facilitate creation of national laboratories, research facilities and universities, development of education and training programs, and creation of the links between industry and research institutes as well as universities. In addition, the government could offer tax incentives for R&D investments and create a legal framework that fosters nanotechnology innovation. All these could help to grow productivity and innovative capacity for increased competition and accelerated economic growth.
Local governmental investment
Within the United States, many states have supports from the private sector to promote nanotechnology innovation. Oregon, New York, Ohio, and Illinois are the four major states that provided research funding focused on nanotechnology development. The Oregon state government established the Oregon Nanoscience and Microtechnologies Institute (ONAMI) as Oregon’s first Signature Research Center to award $40 million each year in nanotechnology and microtechnology research grants to members. An integrated investment strategy was employed by the New York state government to nurture all of the components needed to spur innovation in nanotechnology. To date, New York state’s investment totals $1 billion. In Ohio, the Ohio Third Frontier Program has committed $1.6 billion over ten years to support a range of high-tech sectors. Venture capitalists, attorneys, and public-relations firms in Illinois support the development of nanotechnology23 to make Illinois as one of the nation’s largest nanotechnology clusters.
Local government support in China has been overwhelmingly concentrated in Beijing and Shanghai.24 Other than the primary support from NSFC, Beijing supports local nanotechnology R&D through Beijing Municipal Science and Technology Commission with an annual budget of about $14.7 M. The Shanghai Nanotechnology Promotion Center is one of the principal supporters to support local nanotechnology R&D with an annual budget of about $14.7 M. Tianjin is also a city obtaining intense investment from local government for nanotechnology R&D. Recently, local governments in Jiangsu and Zhejiang provinces also increased funding for nanotechnology R&D. The two dominant nanotechnology clusters formed in China include the Shanghai–Jiangsu–Zhejiang region and Beijing–Tianjin region.
Regulatory authorities
With the vigorous promotion and increasing commercial applications of nanotechnology as a part of people’s daily life, the importance of nanotechnology regarding its effects on the environment, health and safety issues is rising in the USA. There is consensus about the potential risks of nanotechnology among the experts.25,26 Policy issues have been raised regarding standard setting and risk assessment of nanomaterials.27
China has created related standards for quality assessment of nano-products. In April 2005, China established the National Nanotechnology Standardization Technical Committee, and the Technical Committee 279, a nanomaterial specific sub-committee under the Standardization Administration of China (SAC/TC279). Later, the standardization of nano-materials and technical commission and another four standardization working groups related to nano-measuring and processing were established.28 As for the safety assessment of nano-products, China has made a big effort. The Laboratory for Bio-Environmental Effects of Nanomaterials and Nano-safety was established in 2003, with the aim to detect the biological, environmental and toxicological effects of nanoparticles in vivo, and to establish protocols for the safe use of nanomaterials for medical and consumer use.29–31 In March 2010, the subordinate standardization working group of the SAC/TC279 was set up to draft the regulations on nanomaterial-related issues about public safety, environment, and health. Although the concerns about nanomaterial risks have been raised early in China, no governmental rules have been implemented to regulate this issue yet.
Compared to China, there are many regulatory authorities existing in the USA that are broad enough to cover every aspect of nanotechnology. The Food, Drug, and Cosmetic Act regulates nanotechnology applications to drugs, medical devices, biologics, cosmetics and food. The Occupational Safety and Health Act regulates the environmental effects of nanomaterials. The Toxic Substances Control Act is usually considered the primary Act for regulating nanotechnology safety. From 2005, NNI began to pay attention to the risk of nanotechnology and gave financial support for the related research. From December 2008, NNI increased the funding for safety study of nanomaterials, and the research funding was increased to $105.4 M in 2012, representing 5.7% of the total annual NNI investment. Under Federal support, the National Institute of Standards and Technology is actively involved in nanotechnology standardization. In 2012, FDA issued two guidance drafts related to nanotechnology applications in cosmetics and food substances.32
Institutional development and R&D efforts
The establishment of new institutions for basic research is one of the major elements of a national innovation system. Most of the nanotechnology research and commercialization activities were launched in the USA. As the world’s first national nanotechnology program, NNI was involved in the nanotechnology-related activities of 27 Federal agencies. To ensure the cooperation of investigators from different research areas, NNI created some of the most sophisticated nanoscience laboratories in the world, such as the Institute for Soldier Nanotechnologies at the Massachusetts Institute of Technology, the Nanotechnology Characterization Laboratory (NCL), and several other Nanomedicine Development Centers organized by NIH. Other than the efforts paid by NNI, a large number of universities and institutes are intensively engaged in nanotechnology research within the USA. For example, ONAMI shepherds nanoscience and microtechnology innovations from basic research to commercialization by generating research revenue and providing gap funding.23 Many universities established new nano-related institutes or centers which are dedicated to nanotechnology research.
In China, basic research in nanotechnology is also actively pursued. Since 2001, a number of new institutions and nanotechnology-related programs have been created at the national and regional levels in China. There are four national centers involved in nanotechnology R&D: (1) National Center for Nanoscience and Technology in Beijing, cofounded by the CAS, Tsinghua University, and Peking University in 2004; (2) the National Engineering Research Center for Nanotechnology in Shanghai; (3) National Nano-Commercialization Base in Tianjin; and (4) National Engineering Research Center for Nanomedicine in Hubei. Several institutions including the Tsinghua-Foxconn Nanotechnology Research Center were founded to enhance university–industry collaboration. Some institutions were established to promote commercialization including the Tianjin Nanotech Industrialization Base, Nanopolis Suzhou, and the National Engineering Research Center for Nanotechnology in Shanghai. Several international cooperation institutions were built to enhance international nanotechnology exchange and cooperation. For example, the Zhejiang California International Nanosystems Institute, which was co-founded by Zhejiang Provincial Government, Zhejiang University and the California NanoSystems Institute, is the only national joint international research center in China.
Industrial and enterprise growth
The US and China started at almost the same time to invest heavily in nanotechnology development. However, the increase in number of nanotechnology companies and patenting activity through business alliances and university–industry links in the USA and China is entirely different.20 It is estimated that over 350 industry enterprises have engaged in nanoscience and nanotechnology R&D and 32 companies directly participate in nanotechnology R&D in China. The listed companies are the examples of commercial entities: Anson Nano Biotechnology Company, Ltd., Beijing ChamgoNanotech Co., Ltd., Allrun, the Shanghai Nanotechnology Promotion Center, the Shenzhen Nanotech Port Co., Ltd., and Kunming Hande Nanobiotechnology Co., Ltd. By analysis of these companies in China, it was found that most of the foreign-invested companies have strong emphasis on nanodevices with high internal R&D and innovation ability. However, the Chinese companies demonstrated generally low internal R&D and innovation ability. They are strong in nanomaterials, but weaker in nanoelectronics, nanodevices, and nano-bimedicine.27
The USA has the most companies involved in nanotechnology research, manufacturing or applications. Several companies, like IBM and Intel, do research with nanotechnology to enhance their core products. As for patent activity, US firms were granted five times more patents than US universities and research institutions from 1990 to 2006. However, the majority of Chinese nanotechnology patents were produced by universities and research institutions rather than by industry.20 The poor mechanisms of technology transfer and the weak university–industry links including lack of communication channels might be the primary reason hampering commercialization of nanotechnology in China.5 “Research is high and the market is far away” might be a true portrayal of Chinese commercialization of nanotechnology.33
Future perspectives
From the above comparisons in the nanotechnology-related fields between China and USA, we found that the two countries have their own advantages and disadvantages in nanotechnology R&D. USA obtained more knowledge and experiences in nanotechnology development, while China is still a new competitor in this field and has a long way to go to develop nanotechnology. There are several issues that China needs to take action: (1) The mismatch between China’s high number of publications and their low citations, which may be partially due to the impatient research environment where people pursuit quantity and impact factor instead of the quality of publications. (2) Imperfect university–industry links. The lack of interest in acquiring frontier knowledge in many industries and the lack of communication channels between academic institutions and industry hinder the commercialization of nanotechnology-derived products.33 This can be overcome by enhancing industry–university collaborations. These partnerships could allow industry to access to research services at low costs and to potential future employees. The partnerships also could allow universities to get research grants from industry and drive students to solve practical problems. (3) The weak cross-regional collaborations. In general, average annual growth of cross-regional collaborations in nanoscale R&D by publication output between the top 25 most producing Chinese regions was only 1.3% in 2001–2011.5 The integrated collaboration across various sectors should be strengthened to avoid unnecessary waste of resources. Government agencies and the private sector could build partnerships to cooperatively develop nanotechnology products and establish new businesses. (4) The incomprehensive laws and regulations for standardization of nanotechnology. Compared with the USA, China still needs to develop or improve related laws and regulations for quality control and safety assessment of nanomaterials. There is a need of intensive institutional reform to develop nanomaterial-related regulations. China also lacks a robust system and laws to audit the utility of public grants and safeguard the resources for public health.34 In order to restore confidence in research fields it is necessary to investigate allegations of possible research misconducts as well.
Acknowledgments
This research was supported by China MOST grant 2015CB931804 (LJ), and NSFC grant 81571802 (YG), J1103303 (YG), and 81273548 (LJ); US National Institutes of Health grants (PJS) R01 AI117776 (NIAID/NIH), R37 AI051214 (NIAID/NIH), R01 CA155061 (NCI/NIH), U54 AR055073 (NIAMS/NIH); and the Science and Technology Development Foundation of Fuzhou University (2013-XQ-8 and 2014-XY-7).
References
- 1.Liu X, Zhang P, Li X, Chen H, Dang Y, Larson C, et al. Trends for nanotechnology development in China, Russia, and India. J Nanopart Res 2009;11:1845–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelps R, Fisher E. Legislating the laboratory? Promotion and precaution in a nanomaterials company. Methods Mol Biol 2011;726:339–58. [DOI] [PubMed] [Google Scholar]
- 3.Sargent JF. The National Nanotechnology Initiative: overview, reauthorization, and appropriations issues. Congressional research service reports; 2014 [RL34401; http://fas.org/sgp/crs/misc/RL34401.pdf]. [Google Scholar]
- 4.Jia L, Zhao Y, Liang X. Fast evolving nanotechnology and relevant programs and entities in China. Nanotoday 2011;6:6–11. [Google Scholar]
- 5.Klochikhin EA, Shapira P. Engineering small worlds in a big society: assessing the early impacts of nanotechnology in China. Rev Policy Res 2012;29:752–74. [Google Scholar]
- 6.Qiu J China targets top talent from overseas. Nature 2009;457:522. [DOI] [PubMed] [Google Scholar]
- 7.Kostoff RN, Koytcheff RG, Lau CGY. Technical structure of the global nanoscience and nanotechnology literature. J Nanopart Res 2007;9:701–24. [Google Scholar]
- 8.Dong X, Qiu XC, Liu Q, Jia J. Bibliometric analysis of nanotechnology applied in oncology from 2002 to 2011. Tumour Biol 2013;34:3273–8. [DOI] [PubMed] [Google Scholar]
- 9.Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, Kamal MA. Nanotechnology-based approaches in anticancer research. Int J Nanomedicine 2012;7:4391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia L, Lu Y, Shao J, Liang XJ, Xu Y. Nanoproteomics: a new sprout from emerging links between nanotechnology and proteomics. Trends Biotechnol 2013;31:99–107. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Xie J, Chen H, Gu S, Zhao R, Shao J, et al. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv 2014;32:761–77. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Chen Y, Ji XF, He XY, Yin Q, Zhang ZW, et al. Controlled intracellular release of doxorubicin in multidrug-resistant cancer cells by tuning the shell-pore sizes of mesoporous silica nanoparticles. ACS Nano 2011;5:9788–98. [DOI] [PubMed] [Google Scholar]
- 13.Shen JN, He QJ, Gao Y, Shi JL, Li YP. Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: performance and mechanism. Nanoscale 2011;3:4314–22. [DOI] [PubMed] [Google Scholar]
- 14.He QJ, Gao Y, Zhang LX, Zhang ZW, Gao F, Ji XF, et al. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials 2011;32:7711–20. [DOI] [PubMed] [Google Scholar]
- 15.Hull LC, Farrell D, Grodzinski P. Highlights of recent developments and trends in cancer nanotechnology research—view from NCI Alliance for Nanotechnology in Cancer. Biotechnol Adv 2014;32:666–78. [DOI] [PubMed] [Google Scholar]
- 16.Lenoir T, Herron P. Tracking the current rise of chinese pharmaceutical bionanotechnology. J Biomed Discov Collab 2009;4:1–38 [8]. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P, Loet L. The emergence of China as a leading nation in science, research policy. Res Policy 2006;35:83–104. [Google Scholar]
- 18.Schneider JA, Grodzinski P, Liang XJ. Cancer nanotechnology research in the United States and China: cooperation to promote innovation. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011;3:441–8. [DOI] [PubMed] [Google Scholar]
- 19.Dang Y, Zhang Y, Fan L, Chen H, Roco MC. Trends in worldwide nanotechnology patent applications: 1991 to 2008. J Nanopart Res 2010;12:687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C State-led technological development: a case of China’s nanotechnology development. World Dev 2012;40:970–82. [Google Scholar]
- 21.Jia L Global governmental investment in nanotechnologies. Curr Nanosci 2005;1:263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strange KL, Baucher MA. Challenges for governments in evaluating return on investment from nanotechnology and its broader economic impact OECD/NNI International Symposium on Assessing the Economic Impact of Nanotechnology; 2012. [Washington, DC, USA: ]. [Google Scholar]
- 23.Fetters E 2012 Illinois nanotechnology report: a road map for economic development. Nanotechnol Mag IEEE 2012;6:14–7. [Google Scholar]
- 24.Motoyama Y, Cao C, Appelbaum R. Observing regional divergence of Chinese nanotechnology centers. Tech Forecast Soc Change 2014;81:11–21. [Google Scholar]
- 25.Beaudrie CE, Satterfield T, Kandlikar M, Harthorn BH. Expert views on regulatory preparedness for managing the risks of nanotechnologies. PLoS One 2013;8:1–9 [e80250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahapatra I, Clark J, Dobson PJ, Owen R, Lead JR. Potential environmental implications of nano-enabled medical applications: critical review. Environ Sci Process Impacts 2013;15:123–44. [DOI] [PubMed] [Google Scholar]
- 27.Malloy TF. Nanotechnology regulation: a study in claims making. ACS Nano 2011;5:5–12. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis DSL, Richmond N. Regulation and governance of nanotechnology in China: regulatory challenges and effectiveness. Eur J Law Technol 2011;2:1–11. [Google Scholar]
- 29.Zhao Y, Xing G, Chai Z. Nanotoxicology: are carbon nanotubes safe? Nat Nanotechnol 2008;3:191–2. [DOI] [PubMed] [Google Scholar]
- 30.Qiu J Nano-safety studies urged in China. Nature 2012;489:350. [DOI] [PubMed] [Google Scholar]
- 31.Bai Y, Zhang Y, Zhang J, Mu Q, Zhang W, Butch ER, et al. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat Nanotechnol 2010;5:683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamburg MA. Science and regulation. FDA’s approach to regulation of products of nanotechnology. Science 2012;336:299–300. [DOI] [PubMed] [Google Scholar]
- 33.Cao C, Appelbaum RP, Parker R. “Research is high and the market is far away”: commercialization of nanotechnology in China. Technol Soc 2013;35:55–64. [Google Scholar]
- 34.Jia H Frequent cases force China to face up to scientific fraud. Nat Med 2006;12:867. [DOI] [PubMed] [Google Scholar]


