Abstract
Background:
The American Heart Association recommends women with congenital heart defects (CHD) receive contraceptive counseling early in their reproductive years, but little is known about contraceptive method use among women with CHD. We describe recent female sterilization and reversible prescription contraceptive method use by presence of CHD and CHD severity in 2014.
Methods:
Using IBM MarketScan Commercial Databases, we included women aged 15–44 years with prescription drug coverage in 2014 who were enrolled ≥11 months annually in employer-sponsored health plans between 2011–2014. CHD, CHD severity, contraceptive methods, and obstetric-gynecology and cardiology provider encounters were identified using billing codes. We used log-binomial regression to calculate adjusted prevalence ratios (aPRs) and 95% confidence intervals (CIs) to compare contraceptive method use overall and by effectiveness tier by CHD presence and, for women with CHD, severity.
Results:
Recent sterilization or current reversible prescription contraceptive method use varied slightly among women with (39.2%) and without (37.3%) CHD, aPR=1.04, 95% CI [1.01–1.07]. Women with CHD were more likely to use any Tier I method (12.9%) than women without CHD (9.3%), aPR=1.41, 95% CI [1.33–1.50]. Women with severe, compared to non-severe, CHD were less likely to use any method, aPR=0.85, 95% CI [0.78–0.92], or Tier I method, aPR=0.84, 95% CI [0.70–0.99]. Approximately 60% of women with obstetric-gynecology and <40% with cardiology encounters used any included method.
Conclusions:
There may be missed opportunities for providers to improve uptake of safe, effective contraceptive methods for women with CHD who wish to avoid pregnancy.
Keywords: administrative data, congenital heart defects, contraception
Introduction
For women with congenital heart defects (CHD), pregnancy can be associated with an increased risk of morbidity and mortality;1 in addition, approximately one in 10 women of reproductive age with CHD may be using a Food and Drug Administration D or X cardiac-related medication.2 Thus, the American Heart Association (AHA) recommends all women with CHD receive counseling early in their reproductive years about the importance of pregnancy planning, including safe and effective contraceptive methods.1
Despite the importance of pregnancy planning for women with CHD, rates of unintended pregnancy among these women are comparable to the general population (45–54%)3,4 and few of these women receive AHA-recommended preconception care.2 Although literature is limited, estimates suggest only 20–36% of women with CHD report use of the most highly effective contraceptive methods (long-acting reversible contraception (LARC), or, if desired, female sterilization).4,5 Available studies have small samples, lack comparison groups of women without CHD, and include only women with CHD seeking care from specialized cardiac care providers,3–8 which limits the generalizability of findings to the broader population of women with CHD. It is unknown how many women with CHD, especially those with severe CHD, use highly effective contraceptive methods. In the present analysis, we improved upon methods used in the current literature by using a large U.S.-based insurance claims database, which includes women with and without CHD, to describe the use of recent female sterilization and reversible prescription contraceptive methods in women with and without CHD, and whether methods differed by presence of CHD and CHD severity.
Methods
Analytic Sample Selection
The IBM MarketScan Commercial Database contains individual-level healthcare claims information from a large convenience sample of persons with employer-sponsored insurance and their dependents in the United States. In our analytic years of interest, data were obtained about 35–53 million persons annually from more than 100 unique insurance carriers, with representation from all U.S. Census regions. Demographic data are available for all persons enrolled at any point during a given year, regardless of whether a claim is filed, and are linkable to submitted inpatient service, outpatient service, and filled outpatient prescription drug claims. Data are de-identified, administrative data and not considered human subjects research by the Centers for Disease Control and Prevention. No extramural funding was used in support of this work, and the authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Women were included in the analysis if they were aged 15–44 years in 2014, had prescription drug coverage included in the database in 2014, and were enrolled for ≥11 months per year annually during 2011–2014. We excluded women with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis code for Down Syndrome (758.0) because a disproportionate proportion of women with CHD have Down Syndrome, and women with Down Syndrome may have different contraceptive patterns than other women with CHD. As has been done previously,9,10 we excluded women with ICD-9-CM diagnosis or procedure or a Current Procedural Terminology (CPT) code for a hysterectomy (2011–2014) and women who were pregnant in 2014.
Identification of CHD
Women with CHD were identified using ICD-9-CM codes11 from inpatient and outpatient claims. A woman was considered to have CHD if, in 2011–2014, she had CHD codes on ≥2 outpatient claims separated by >30 days or ≥1 inpatient claim. Women with a CHD code not meeting this algorithm were excluded. We classified women with CHD into severe or non-severe CHD based on hemodynamic severity and basic anatomy, as has been done previously.11,12 Women with no CHD codes during 2011–2014 comprised the comparison group.
Identification of Contraceptive Use
Female sterilization and reversible prescription contraceptive methods were identified using ICD-9-CM diagnosis and procedure codes, Healthcare Common Procedure Coding System (HCPCS) supply codes, CPT codes, and National Drug (NDC) codes in inpatient service, outpatient service, and/or outpatient drug claims data, as in a previous publication.10 We considered claims to be a proxy for use and, hereafter, refer to claims as “use”. For all included methods, we considered women to have used the method in 2014 if it was used at any point in the year, regardless of use duration. We considered women to have undergone recent sterilization if they had a female sterilization code during 2011–2014. We considered women to be using LARC (i.e., IUD or implant) if they had (1) an insertion, HCPCS, or NDC code for an IUD or implant in 2011–2014 with no removal code before January 1, 2014 or (2) a removal code for an IUD or implant only, but removal date after January 1, 2014. Other shorter-acting reversible prescription methods (i.e., injectables, oral contraception, patches, and rings) were examined using data from 2014 only. We considered women to use injectable methods if they had a (1) HCPCS or NDC code for depot medroxyprogesterone acetate or (2) ICD-9-CM diagnosis code for family planning and a ICD-9-CM procedure or CPT code for a generic injection in the same claim.10 Women were considered to use oral contraception (combined oral contraception (COCs) and progestin-only pills (POPs)), patches, or rings if HCPCS or NDC codes for these methods were identified. Women without these codes were considered not to be using any included contraceptive method. Women could use two or more different methods; each individual contraceptive method type used in 2014 was counted separately. Contraceptive method use was also categorized by effectiveness.13 Tier I methods (typical use failure rate <1%) included IUDs, implants, and female sterilization. Tier II methods (typical use failure rate 6–12%) included injectables, oral contraception, patches, and rings. If a woman used Tier I and Tier II methods in 2014, she was categorized as using Tier I in all analyses that examined tier level.
Identification of Demographic Factors and Healthcare Encounters
Other variables of interest included age (five-year increments), U.S. Census region of residence, and presence of a healthcare encounter with an obstetric-gynecology (OB-GYN) provider or a cardiology provider. An encounter with an OB-GYN or cardiology provider was defined as having any inpatient or outpatient claim in 2014 that included a CPT code for a new or existing patient encounter (99201–5; 99211–15) with a provider code for an OB-GYN or a cardiology practice, respectively.
Primary Statistical Analysis
We first generated descriptive statistics for the demographic characteristics (age, geographic region) by presence of CHD and CHD severity. We then estimated the prevalence and 95% confidence intervals (CIs) of three outcomes related to contraceptive use: 1) any female sterilization or reversible prescription method (yes/no); 2) Tier I method (yes/no); and 3) Tier II method (yes/no), by presence of CHD and CHD severity. We used log-binomial regression to calculate age-adjusted (by 5-year age category) prevalence ratios (aPRs) and corresponding 95% CIs comparing women with CHD to those without CHD, and, among women with CHD, comparing women with severe CHD to those with non-severe CHD. For the Tier II method log-binomial regression models, women who used Tier I methods were excluded. Next, we calculated the prevalence of the specific types of contraceptive methods used by CHD presence, and among women with CHD, CHD severity. Differences in specific types of methods used by women with and without CHD, and women with severe and non-severe CHD, were examined using chi-squared tests. Finally, among women with CHD, stratified by severity, we examined the prevalence of any included method, Tier I method, and Tier II method use by age, geographic region, and 2014 healthcare encounter(s) with an OB-GYN or cardiology provider; differences between women with severe and non-severe CHD for each characteristic were examined using chi-square tests.
Sensitivity Analysis
In our primary analysis, we identified conditions or procedures of interest from 2011–2014. However, some women might have had codes for conditions or reproductive procedures prior to 2011, and therefore be misclassified. We conducted an extended year sensitivity analysis to determine the proportion of women who may have been ineligible for the primary analysis due to earlier codes for Down Syndrome or hysterectomy, or who had conditions or procedures of interest (i.e., CHD, sterilization, or IUD (as IUDs may be effective for up to 8–10 years14)) from 2007–2014, allowing us to examine the proportion that were misclassified (i.e., identified only from 2007–2010) in our primary analysis. Other aspects of the extended year sensitivity analysis were the same, including the continuous enrollment requirement from 2011–2014. We also conducted a CHD sensitivity analysis that excluded women with only the ICD-9-CM code 745.5 for CHD, given many adults with this code may be misclassified as having CHD when they are not true CHD cases.15 Analyses were run in SAS 9.4.
Results
Among the 2,067,554 eligible women, 6,561 (0.3%) were identified as having CHD, of which 1,163 (17.7%) had severe CHD and 5,398 (82.3%) had non-severe CHD (Figure I). Regardless of the presence of CHD, the largest numbers of women resided in the South and were aged 15–19 or 40–44 years. Compared to women without CHD, a higher proportion of women with CHD were aged 15–19 years. There were higher proportions of women with severe CHD in the younger age ranges than women with non-severe CHD (Table I).
Figure I.
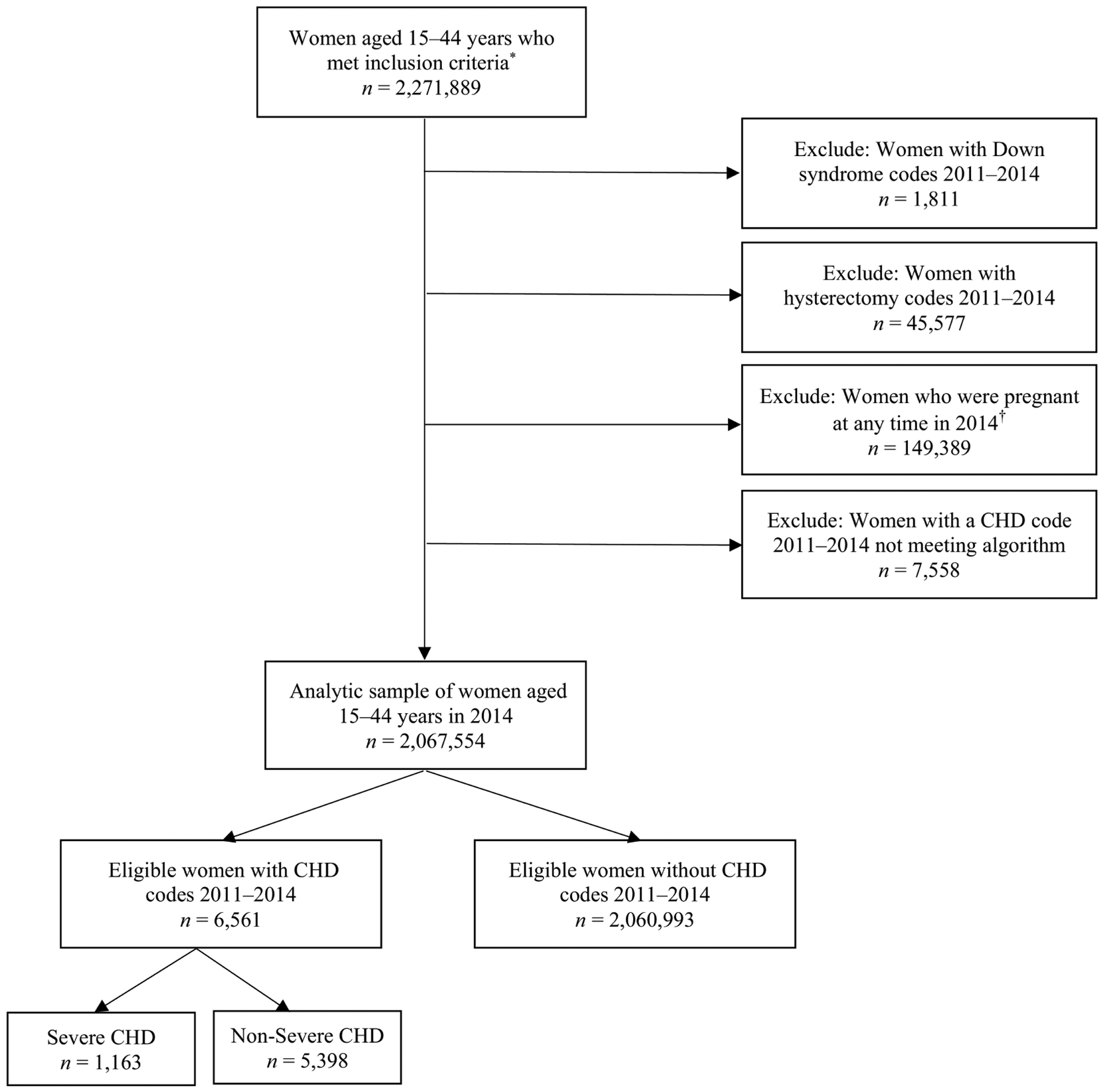
Sample selection for eligible privately-insured women aged 15–44 years with and without congenital heart defects (CHD), and CHD severity
*Women who were aged 15–44 years in 2014, had prescription drug coverage included in the database in 2014, and were enrolled for ≥11 months per year annually during 2011–2014. †Pregnancy was defined using end of pregnancy billing codes using an algorithm published previously.9
TABLE I.
Demographic characteristics of women with employer-sponsored insurance aged 15–44 years with and without congenital heart defects (CHD), by presence of any CHD and CHD severity, United States –2014
| No CHD | ||||
|---|---|---|---|---|
| Number of eligible women | 6,561 | 1,163 | 5,398 | 2,060,993 |
| Age (in years) | ||||
| 15–19 | 1,792 (27.3%) | 362 (31.1%) | 1,430 (26.5%) | 451,091 (21.9%) |
| 20–24 | 1,067 (16.3%) | 280 (24.1%) | 787 (14.6%) | 382,483 (18.6%) |
| 25–29 | 461 (7.0%) | 99 (8.5%) | 362 (6.7%) | 133,772 (6.5%) |
| 30–34 | 934 (14.2%) | 156 (13.4%) | 778 (14.4%) | 260,714 (12.7%) |
| 35–39 | 1,102 (16.8%) | 151 (13.0%) | 951 (17.6%) | 364,459 (17.7%) |
| 40–44 | 1,205 (18.4%) | 115 (9.9%) | 1,090 (20.2%) | 468,474 (22.7%) |
| Geographic Region† | ||||
| Northeast | 1,210 (19.0%) | 203 (18.0%) | 1,007 (19.2%) | 308,595 (15.4%) |
| Midwest‡ | 1,570 (24.7%) | 285 (25.3%) | 1,285 (24.6%) | 487,598 (24.4%) |
| South | 2,339 (36.8%) | 426 (37.9%) | 1,913 (36.6%) | 843,388 (42.2%) |
| West | 1,239 (19.5%) | 211 (18.8%) | 1,028 (19.6%) | 361,158 (18.1%) |
CHD = congenital heart defect. Data are N= 2,067,554 (100%).
Women with ≥1 inpatient codes for a CHD or ≥2 outpatient codes for a CHD separated by >30 days identified in the inpatient or outpatient services claims from 2011–2014.
Numbers and percentages may not sum to the expected N or 100% due to missing data on U.S. region;
In the IBM MarketScan Commercial Database, this region is named North Central; all states included in the North Central region in the MarketScan Database map to the U.S. Census Midwest region.
Based on presence and severity of CHD, 36.8–39.8% of women had undergone sterilization during 2011–2014 or used any reversible prescription method in 2014; 9.3–13.4% used Tier I methods and 26.2–28.0% used Tier II methods (Figure II). Women with CHD were only slightly more likely to use any included method compared to women without CHD, aPR=1.04, 95% CI [1.01–1.07]. Women with CHD were more likely to use any Tier I method compared to women without CHD, aPR=1.41, 95% CI [1.33–1.50]. Excluding women using a Tier I method, women with CHD were slightly less likely to use any Tier II method compared to women without CHD, aPR=0.96, 95% CI [0.93–0.99]. When considering only women with CHD, women with severe CHD were less likely to use any included method, aPR=0.85, 95% CI [0.78–0.92], Tier I methods, aPR=0.84, 95% CI [0.70–0.99], or Tier II methods, aPR=0.83, 95% CI [0.75–0.92], than women with non-severe CHD.
Figure II.
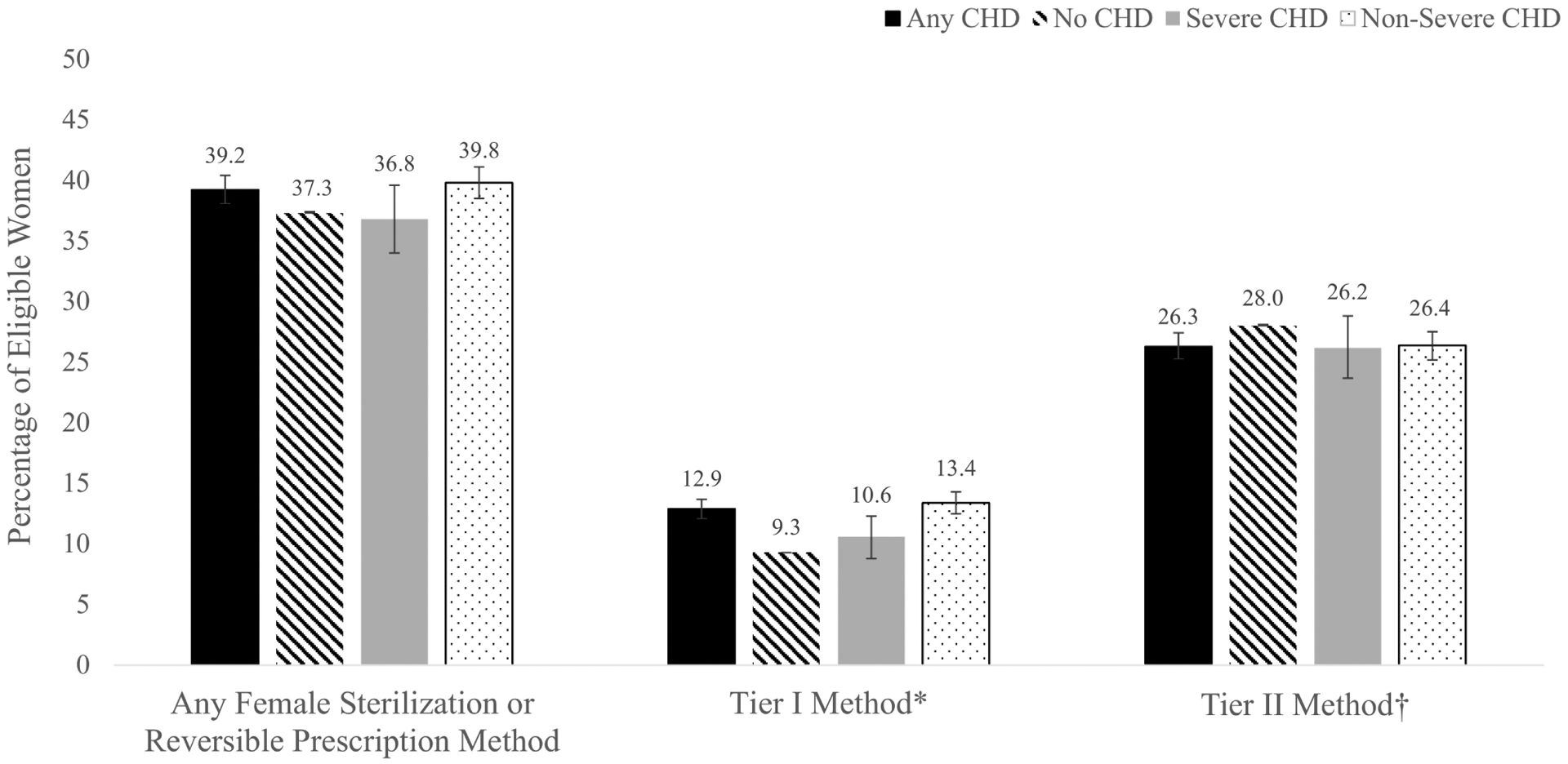
Frequency of female sterilization or reversible prescription contraceptive method use overall and by typical effectiveness tier for privately-insured women aged 15–44 years with and without congenital heart defects (CHD), by presence of any CHD and by CHD severity, United States – 2014
Confidence intervals for each estimate are depicted in the interval bars. *Includes female sterilization, intrauterine devices or implants; †Includes depot medroxyprogesterone acetate, oral contraception (combined or progestin-only), patches, or rings. 1.4% of women in the sample utilized a method from both effectiveness tiers in 2014; these women were assigned their highest use tier – Tier I – such that the Tier I and Tier II columns are mutually exclusive.
Among all women aged 15–44, 2.4% used ≥2 individual contraceptive method types during 2014. Oral contraception was the most common contraceptive method type used, although women with CHD were less likely to use oral contraception than women without CHD (Table II). Compared to women without CHD, women with CHD were more likely to use POPs and less likely to use COCs. However, COCs were the most common contraceptive method used among women with CHD, with 22.9% of women with CHD using COCs and 2.2% using POPs. Women with CHD, compared to those without, were more likely to have undergone sterilization or use IUDs, injectable methods, and patches, but less likely to use rings, than women without CHD (p < .05 for all). Few significant differences were found by CHD severity in the individual contraceptive methods used (Table II). However, women with severe CHD, compared to non-severe CHD, were significantly less likely to have undergone sterilization and more likely to have used POPs (p < .05 for all). Only 3.5% of women with severe CHD used POPs.
TABLE II.
Frequency of female sterilization and reversible prescription contraceptive method use among privately-insured women aged 15–44 years with and without congenital heart defects (CHD), by presence of any CHD and by CHD severity, United States – 2014
| Severity of CHD* | |||||
|---|---|---|---|---|---|
| Non-Severe | |||||
| Tier I | Female sterilization§, || | 247 (3.8%) | 48,189 (2.3%) | 25 (2.2%) | 222 (4.1%) |
| IUD§ | 537 (8.2%) | 124,451 (6.0%) | 83 (7.1%) | 454 (8.4%) | |
| Implant | 87 (1.3%) | 23,605 (1.2%) | 17 (1.5%) | 70 (1.3%) | |
| Tier II | DMPA injectable§ | 184 (2.8%) | 39,584 (1.9%) | 39 (3.4%) | 145 (2.7%) |
| Any oral contraception‡, § | 1,614 (24.6%) | 537,734 (26.1%) | 284 (24.4%) | 1,330 (24.6%) | |
| Progestin-only pill§, || | 144 (2.2%) | 20,770 (1.0%) | 41 (3.5%) | 103 (1.9%) | |
| Combined oral contraception§ | 1,504 (22.9%) | 523,006 (25.4%) | 249 (21.4%) | 1,255 (23.3%) | |
| Patch§ | 34 (0.5%) | 7,470 (0.4%) | 8 (0.7%) | 26 (0.5%) | |
| Ring§ | 82 (1.3%) | 35,413 (1.7%) | 10 (0.9%) | 72 (1.3%) | |
CHD = congenital heart defect; IUD = intrauterine device; DMPA = depot medroxyprogesterone acetate. Data are N= 2,067,554 (100%).
Women with ≥1 inpatient codes for a CHD or ≥2 outpatient codes for a CHD separated by >30 days identified in the inpatient or outpatient services claims from 2011–2014.
For each contraceptive method, the number and percentage of women displayed indicates use of the individual type of contraceptive method. Women are counted as users for each method for which they had a claim during the specified algorithm period for each contraceptive method (female sterilization, IUD, implant: 2011– 2014; injectables, oral contraception, patches, ring: 2014);
Includes codes for a progestin-only pill, combined oral contraception, and oral contraception, type not otherwise specified;
Chi-square test for the use of the individual type of contraceptive method by presence of any CHD was statistically significant at p < .05.
Chi-square test for the use of the individual type of contraceptive method by CHD severity was statistically significant at p < .05.
Among all women with CHD, the proportion of women that used an included method was highest for women aged 20–34 years. Among women who used any included method, Tier I, or Tier II method, there were significant differences in who used these methods by age for women with severe versus non-severe CHD (p < .05 for all; Table III). For women with severe and non-severe CHD, Tier I method use was highest among women aged 25–39 years (severe: 14.7–18.5%; non-severe: 19.4–22.2%), though 12.9% of women with non-severe CHD aged 40–44 also used Tier I methods. Region of residence did not significantly differ between women with severe and non-severe CHD, except among women who used Tier I methods. Women in the West with severe CHD had higher proportions of Tier I method use than the other regions; this difference was not mirrored in women with non-severe CHD.
TABLE III.
Proportion of privately-insured women aged 15–44 years with congenital heart defects (CHD)* who underwent recent sterilization or used reversible prescription contraceptive methods by demographic characteristics and healthcare encounters, by CHD severity, United States – 2014
| Tier II§ | ||||||
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (in years)¶,#,** | ||||||
| 15–19 | 104 (28.7%) | 19 (5.3%) | 85 (23.5%) | 551 (38.5%) | 65 (4.6%) | 486 (34.0%) |
| 20–24 | 125 (44.6%) | 27 (9.6%) | 98 (35.0%) | 427 (54.3%) | 83 (10.6%) | 344 (43.7%) |
| 25–29 | 44 (44.4%) | 16 (16.2%) | 28 (28.3%) | 213 (58.8%) | 77 (21.3%) | 136 (37.6%) |
| 30–34 | 69 (44.2%) | 23 (14.7%) | 46 (29.5%) | 358 (46.0%) | 173 (22.2%) | 185 (23.8%) |
| 35–39 | 56 (37.1%) | 28 (18.5%) | 28 (18.5%) | 342 (36.0%) | 184 (19.4%) | 158 (16.6%) |
| 40–44 | 30 (26.1%) | 10 (8.7%) | 20 (17.4%) | 255 (23.4%) | 141 (12.9%) | 114 (10.5%) |
| Geographic Region# | ||||||
| Northeast | 74 (36.5%) | 18 (8.9%) | 56 (27.6%) | 411 (40.8%) | 146 (14.5%) | 265 (26.3%) |
| Midwest‖ | 96 (33.7%) | 24 (8.4%) | 72 (25.3%) | 513 (39.9%) | 141 (11.0%) | 372 (29.0%) |
| South | 154 (36.2%) | 42 (9.9%) | 112 (26.3%) | 784 (41.0%) | 268 (14.0%) | 516 (27.0%) |
| West | 88 (41.7%) | 38 (18.0%) | 50 (23.7%) | 364 (35.4%) | 140 (13.6%) | 224 (21.8%) |
| Healthcare Encounters | ||||||
| OB-GYN encounter in 2014 | ||||||
| Yes | 125 (63.8%) | 41 (20.9%) | 84 (42.9%) | 605 (57.0%) | 239 (22.5%) | 366 (34.5%) |
| No/Not Known | 303 (31.3%) | 82 (8.5%) | 221 (22.9%) | 1,541 (35.5%) | 484 (11.2%) | 1,057 (24.4%) |
| Cardiologist encounter in 2014¶,** | ||||||
| Yes | 211 (37.6%) | 46 (8.2%) | 165 (29.4%) | 709 (38.6%) | 226 (12.3%) | 483 (26.3%) |
| No/Not Known | 217 (36.1%) | 77 (12.8%) | 140 (23.3%) | 1,437 (40.4%) | 497 (14.0%) | 940 (26.4%) |
CHD = congenital heart defect; OB-GYN = obstetric and gynecology provider. Data are N = 6,561 (0.3%).
Women with ≥1 inpatient codes for a CHD or ≥2 outpatient codes for a CHD separated by >30 days identified in the inpatient or outpatient services claims from 2011–2014.
Includes female sterilization, intrauterine devices, implants, depot medroxyprogesterone acetate, oral contraception (combined or progestin-only), patches, or rings.
Includes female sterilization, intrauterine devices or implants;
Includes depot medroxyprogesterone acetate, oral contraception (combined or progestin-only), patches, or rings. 1.4% of women in the sample utilized a method from both effectiveness tiers in 2014; these women were assigned their highest use tier – Tier I – such that the Tier I and Tier II columns are mutually exclusive.
In the IBM MarketScan Commercial Database, this region is named North Central; all states included in the North Central region in the MarketScan Database map to the U.S. Census Midwest region.
Among women who had undergone sterilization or used any reversible prescription method, chi-square test for the difference between women with severe and non-severe CHD by each characteristic was significant at p < .05.
Among women who used any Tier I method, chi-square test for the difference between women with severe and non-severe CHD by each characteristic was significant at p < .05.
Among women who used any Tier II method, chi-square test for the difference between women with severe and non-severe CHD by each characteristic was significant at p < .05.
Among women with CHD, those with OB-GYN encounters in 2014 had higher proportions of any included contraceptive, Tier I, or Tier II method use than women without these encounters (Table III). Among women with CHD who had an OB-GYN encounter, 63.8% of women with severe and 57.0% with non-severe CHD used any included permanent or reversible prescription contraceptive method; only 20.9% of women with severe and 22.5% of women with non-severe CHD used Tier I methods (Table III). Among all women with CHD, regardless of severity, women with cardiology provider encounters in 2014 had similar or lower proportions of any included permanent or reversible prescription contraceptive method or Tier I method use than those without cardiology encounters.
The extended year sensitivity analysis expanded the billing code search window to 2007–2014 for specific conditions and procedures. Based on codes identified solely between 2007–2010, an additional <0.1% of women would have been excluded from the primary analysis due to possibly having Down syndrome and 0.9% for having a hysterectomy code. An additional 0.1% of women would have met CHD criteria. An additional 1.4% of women would have been identified as having undergone sterilization, and 1.0% would have been identified as using an IUD; these percentages were similar for women with and without CHD. Results from the CHD sensitivity analysis that excluded women who were solely identified by ICD-9-CM code 745.5 were largely similar to that of the primary analysis. The only exceptions were that, in the CHD sensitivity analysis, there was not a statistically significant difference in the use of any included contraceptive method between women with CHD (25.5%) and without CHD (26.1%) (p > .05), and women with severe CHD (21.4%) were less likely to use COCs than women with non-severe CHD (24.4%) (p < .05).
Discussion
In our analysis, less than half of the insured women aged 15–44 with CHD had undergone recent sterilization or were using reversible prescription methods in 2014. Although women with CHD were more likely than women without CHD to use Tier I methods, less than 10% of women with CHD used reversible Tier I methods. A lower proportion of women with severe CHD used Tier I methods than women with non-severe CHD, although adverse pregnancy outcomes are more common among women with severe CHD, and unintended pregnancy may be of greater concern.1,16 For women with and without CHD, COCs were the most common method used. This was true even among women with severe CHD, despite that, for some women with certain types of severe CHD (e.g., Fontan palliation; CHD with pulmonary hypertension; women with poor systemic ventricular function), COCs may increase the risk of adverse events to an unacceptable level.1,16,17 Women with CHD with OB-GYN encounters more frequently had undergone recent sterilization or used reversible prescription methods than those without these encounters. However, there were similar proportions of highly effective contraceptive method use for women with CHD with or without cardiology encounters, indicating encounters with cardiology providers may not facilitate increased access to contraception for female CHD patients, despite AHA recommendations.1 Our findings suggest, even among women with cardiology and/or OB-GYN provider visits, there may be missed opportunities to increase safe, effective contraceptive use among women with CHD who wish to avoid pregnancy.
Recent AHA1 recommendations advise that women with CHD be counseled about risks associated with pregnancy and safe, effective contraceptive options as soon as possible post-menarche, as women with CHD are at higher risk of adverse pregnancy outcomes and pregnancy may exacerbate pre-existing heart conditions.1,16,18,19 In addition, among a similar population of privately-insured women with CHD, recent research indicates that, in the year before pregnancy, less than 1% of women with CHD received all AHA-recommended preconception care and nearly one in 10 of these women filled a prescription for a FDA category D or X cardiac-related medication.2 Yet, studies suggest many women with CHD do not receive counseling about safe, effective contraception or the risk of adverse pregnancy outcomes related to their heart condition.3,6 In agreement with our results, other studies have indicated that most women with CHD underutilize contraception, especially Tier I methods.3,5,6 Most women aged 15–45 with CHD (83–94%) report being sexually active;4,7 one U.K. study indicated that 15% of women with CHD at an adult CHD clinic initiated sexual intercourse at ≤15 years of age.7 Research on sexual activity among women with CHD–combined with our results–suggests many women with CHD are likely sexually active, but underutilizing highly effective reversible contraceptive methods if they wish to avoid pregnancy.
There are limitations of our analysis which must be considered. Data are from administrative claims, which are subject to misclassification of CHD and contraceptive use. Our extended year sensitivity analysis (data from 2007–2014) suggested that an additional 1.0% of women were using IUDs in 2014. An additional 1.4% of women were identified as having undergone sterilization in the sensitivity analysis; this may be an underestimate given only 30% of women in the primary sample were continuously enrolled in their health plan from 2007–2010. It is also possible that women may have accessed contraception via other sources, paid out-of-pocket, or undergone permanent procedures prior to any included time period. Women’s complete contraceptive history was not available. We considered claims to be a proxy for use, but were unable to verify that user-dependent reversible methods were used as prescribed. We did not have information on male sterilization or Tier III methods (e.g. condoms), current sexual activity, contraceptive counseling, or future pregnancy intentions, and were therefore unable to examine the use of different methods in the context of sexual activity or desire for reversible versus permanent contraception. We did not have clinical detail about the CHD and could not examine whether women were using methods that were unsafe in the context of their CHD. We used data from prior to and in 2014, which was based on ICD-9-CM coding; it is unclear how the transition to ICD-10-CM in 2015 might impact our findings. Results are not generalizable to all U.S. women with CHD as we used a large convenience sample of women aged 15–44 years with employer-sponsored insurance; however, this analysis substantially improved upon selected, small samples used in previous research.
This analysis addresses critical gaps in our understanding of the prevalence of recent female sterilization and reversible prescription contraceptive methods among women aged 15–44 with and without CHD. Only approximately 40% of women with CHD in our sample were using any permanent or reversible prescription contraceptive methods. These findings further support AHA recommendations to coordinate efforts between cardiologists and OB-GYN providers to improve knowledge, access, and utilization of effective contraceptive methods early and often for women with CHD who wish to avoid pregnancy.1
Source of funding:
The authors received no financial support for this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors report no conflict of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018. AHA/ACC guideline for the management of adults with congenital heart disease. Circulation 2018: e698–e800. [DOI] [PubMed] [Google Scholar]
- 2.Farr SL, Downing KF, Ailes EC, et al. Receipt of American Heart Association-recommended health care among privately insured women with congenital heart defects, 2007–2013. J Am Heart Assoc 2019;8:3013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinze A, Kutty S, Sayles H, Sandene EK, Meza J, Kugler JD. Reproductive and contraceptive counseling received by adult women with congenital heart disease: a risk-based analysis. Congenit Heart Dis 2013;8:20–31. [DOI] [PubMed] [Google Scholar]
- 4.Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol 2015;126:363–9. [DOI] [PubMed] [Google Scholar]
- 5.Koerten MA, Szatmari A, Niwa K, et al. Evaluation of contraceptive methods in women with congenital heart disease in Germany, Hungary and Japan. Int J Cardiol 2016;206;13–8. [DOI] [PubMed] [Google Scholar]
- 6.Vigl M, Kaemmerer M, Med C, et al. Contraception in women with congenital heart disease. Am J Cardiol 2010;106:1317–21. [DOI] [PubMed] [Google Scholar]
- 7.Rogers P, Mansour D, Mattinson A, O’Sullivan JJ. A collaborative clinic between contraception and sexual health services and an adult congenital heart disease clinic. J Fam Plann Reprod Health Care 2007;33:17–21 [DOI] [PubMed] [Google Scholar]
- 8.Miner PD, Canobbio MM, Pearson DD, et al. Contraceptive practices of women with complex congenital heart disease. Am J Cardiol 2017;119:911–915. [DOI] [PubMed] [Google Scholar]
- 9.Ailes EC, Simeone RM, Dawson AL, Petersen EE, Gilboa SM. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res A 2016;106:927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Champaloux SW, Tepper NK, Curtis KM, et al. Contraceptive use among women with medical conditions in a nationwide privately insured population. Obstet Gynecol 2015;126:1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glidewell J, Book W, Raskind-Hood C, et al. Population-based surveillance of congenital heart defects among adolescents and adults: surveillance methodology. Birth Defects Res 2018;110:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation 2007;115:163–172. [DOI] [PubMed] [Google Scholar]
- 13.Contraceptive Technology. Contraceptive efficacy. Available at: http://www.contraceptivetechnology.org/the-book/take-a-peek/contraceptive-efficacy/. Retrieved November 16, 2018.
- 14.Centers for Disease Control and Prevention. Contraception. Available at: https://www.cdc.gov/reproductivehealth/contraception/index.htm. Retrieved October 25, 2018.
- 15.Rodriguez FH, Ephrem G, Gerardin JF, Raskind-Hood, Hogue C, Book W. The 745.5 issue in code-based, adult congenital heart disease population studies: Relevance to current and future ICD-9-CM and ICD-10-CM studies. Congenit Heart Dis 2018;13:59–64. [DOI] [PubMed] [Google Scholar]
- 16.Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e50–e87. [DOI] [PubMed] [Google Scholar]
- 17.Abarbanell G, Tepper NK, Farr SL. Safety of contraceptive use among women with congenital heart disease: a systematic review. Congenit Heart Dis 2019. 10.1111/chd.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karamlou T, Diggs BS, McCrindle BW, Welke KF. A growing problem: maternal death and peripartum complications are higher in women with grown-up congenital heart disease. Ann Thorac Surg 2011;92:2193–9. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetric outcomes among pregnant women with congenital heart disease. Obstet Gynecol 2015;126:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


