Abstract
Loss of central vision can be partially compensated by increased use of peripheral vision. For example, patients experiencing central vision loss due to disease (macular degeneration) or healthy participants trained with simulated central vision loss, tend to develop eccentric fixation spots for reading or other visual tasks. In both patients and in simulated conditions, there are substantial individual variations in the effective use of the periphery. The factors driving these individual differences are still unclear. Although early approaches have described some dimensions of these strategies, the field is still in its initial stages and important elements are often conflated when examining gaze patterns. Here, we propose a systematic approach to characterize oculomotor strategies in cases of central vision loss that distinguishes different components: saccadic re-referencing, saccadic precision, first saccade landing dispersion, fixation stability, latency of target acquisition, and percentage of trials that are useful. We tested this approach in healthy individuals trained with a gaze-contingent display obstructing the central 10 degrees of the visual field. The use of simulated scotoma helps overcome known challenges in clinical research, from recruitment and compliance to the diverse extent and nature of the visual loss. Importantly, this approach offers the ability to examine oculomotor strategies as they develop in controlled settings where viewing conditions are similar across participants. Results show substantial differences in characteristics of peripheral looking strategies, both across trials and individuals. This more complete characterization of peripheral looking strategies can help us understand individual differences in rehabilitation after central vision loss.
Keywords: eye movement, central vision loss, macular degeneration, simulated scotoma
Introduction
Characterizing eye-movement strategies is a critical step in understanding and developing therapies for individuals suffering from visual field loss. For example, in the case of macular degeneration (MD), a leading cause of visual impairment in western countries (Wong, Su, Li, Cheung, Klein, Cheng, & Wong, 2014), central vision loss negatively impacts daily tasks, such as reading, navigation, and face recognition, and thus quality of life. Patients in the late stages of MD develop a central retinal scotoma, and therefore must adopt compensatory strategies that involve the use of the peripheral retina. In many cases, patients develop a preferred retinal locus (PRL) (Cummings, Whittaker, Watson, & Budd, 1985; Fletcher & Schuchard, 1997; Timberlake, Mainster, Peli, Augliere, Essock, & Arend, 1986; Von Noorden & Mackensen, 1962; Schuchard & Fletcher, 1994) that is used for fixation and oculomotor reference (Crossland, Engel, & Legge, 2011). The development of a PRL is a complex process, currently not completely understood, and there is evidence of patients developing multiple PRLs (Duret, Issenhuth, & Safran, 1999; Safran, Duret, Issenhuth, & Mermoud, 1999). Sometimes, the eye movements are initially directed to the PRL rather than the fovea (a phenomenon called “re-referencing”; Schuchard & Raasch, 1992; White & Bedell, 1990). Subjectively, many patients who experience re-referencing report that they are looking straight ahead when fixating with their PRL (White & Bedell, 1990; Whittaker & Cummings, 1990). However, the effective use of the periphery differs across individuals with central vision loss and not all patients show peripheral re-referencing and/or effective peripheral viewing strategies (Crossland, Culham, Kabanarou, & Rubin, 2005; Fletcher and Schuchard, 1997). Precise characterization of strategies is required for understanding the mechanisms underlying compensatory oculomotor strategies and clarifying individual differences in effective peripheral vision.
Development of oculomotor strategies in the context of central vision loss can be studied in the laboratory using a controlled model that simulates a central scotoma in normally sighted participants (Aguilar & Castet, 2011; Barraza-Bernal, Ivanov, Nill, Rifai, Trauzettel-Klosinski, & Wahl, 2017; Kwon, Nandy, & Tjan, 2013; Liu & Kwon, 2016; Walsh & Liu, 2014). This increasingly popular approach uses a gaze-contingent computer display to generate an artificial scotoma, and allows study of PRL development, testing potential rehabilitative strategies to counteract central vision loss, without the constraints of clinical research. Simulating pathologies that affect central vision present a number of advantages, in particular concerning recruitment, comorbidity, and compliance, common drawbacks of low vision research (Maniglia, Cottereau, Soler, & Trotter, 2016). Notably, simulated scotomas have a number of differences from MD-induced scotomas, and the time course of PRL development is slower in patients with MD (Crossland et al., 2005) than with simulated scotoma (Kwon et al., 2013). This may be due to the visibility of the simulated scotoma boundaries, which may be used as an oculomotor reference to redirect saccades (Van der Stigchel, Bethlehem, Klein, Berendschot, Nijboer, & Dumoulin, 2013; Walsh & Liu, 2014). A strength of the approach is that a simulated scotoma allows the same degree of vision change to be applied to every participant, however, this also means that the simulations can differ very dramatically from the experience of patients, especially because simulations cannot perfectly capture the natural progression of the disease. Further, participants experiencing simulated scotomas go back to normal vision between sessions when they leave the laboratory. Still, the additional control offered by simulating central vision loss provides opportunity to help the field understand oculomotor mechanisms underlying the peripheral looking strategies following loss of central vision and have potential to be translated to patients to verify their informativeness to understanding individual differences arising from real world central vision loss.
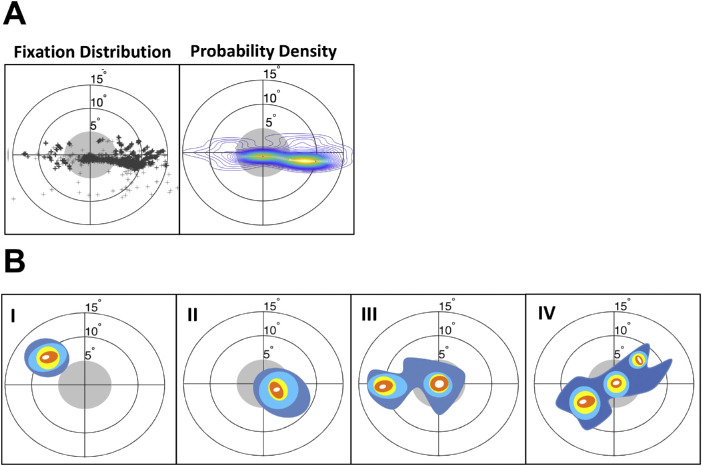
Current approaches to understand peripheral viewing strategies after central vision loss mostly focus on analyzing fixation distributions to estimate fixation stability and the location of the PRL (Castet & Crossland, 2011; Crossland et al., 2005; Fujii et al., 2002; Kwon et al., 2013; Midena & Pilotto, 2017; Walsh & Liu, 2014). In a typical study, fixation stability is computed by either quantifying the percentage of fixations within a 2 or 4 degree radius circular region (Fujii et al., 2002), or, more commonly in research studies, by plotting the coordinates of each fixation and calculating the bivariate contour ellipse area (BCEA) encompassing a given percentage of fixations (Steinman, 1965), as shown in Figure 1A. Crossland et al., 2004 proposed a probability density analysis that uses a Kernel Density Estimator (KDE) to visually represent clusters of high density of fixations. Although these approaches provide an overview of participants’ gaze patterns over time, they are also limited in that they do not dissociate how gaze patterns differ between trials.
Figure 1.
Standard representations of eye movement behavior during a visual task. (A) Left side shows dots at the location of every fixation made during the task. Data are for one participant with simulated scotoma. A standard representation of this distribution of fixations is shown on the right as a probability density map (measured with a Kernel Density Estimator [KDE], e.g., Crossland et al., 2004; Kwon et al., 2013). Peaks of the density map (yellow/orange regions) indicate clusters of fixations. The position of the target is normalized to the center. The gray circle indicates the area within which a fixation would place the target inside the scotoma (i.e. fixations outside the gray circle indicate optimal fixations that place the target within a visible area outside the scotoma). (B) Examples of fixation distributions for hypothetical cases illustrating typical patterns of fixation. I: Fixation distribution showing consistent peripheral re-referencing (cluster outside the scotoma). II: Lack of peripheral re-referencing (participant still places target within the scotoma). III: Partial re-referencing showing a central and a peripheral cluster of fixation. IV: Inhomogeneous, complex fixation pattern.
Although for some individuals, peripheral looking strategies fall into easy-to-interpret categories where there is a single well-defined PRL (see Figure 1B panels I and II) that is well described with a conventional KDE approach, others develop more complex configurations (see Figure 1B panels III and IV). These more complex strategies are known to be especially prominent if the participant is engaged in an active visual task (e.g. visual search), rather than a clinical assessment of fixation stability (Fuji et al., 2002), or if the participant has only partially learned a peripheral looking strategy. In these hypotheticals, Figure 1B-I shows a fixation distribution exhibiting consistent peripheral re-referencing with the eye positioned so that the target appears outside of the scotoma within a single location (the PRL). In Figure 1B-II, the eye is mostly positioned with the target within the scotoma, thus obstructing and rendering the target partially invisible. This shows a lack of peripheral re-referencing because the participant is still placing the target near the fovea (persistent foveal reference) and would lead to poor performance on visual tasks. Figure 1B-III illustrates a case where there are both central and peripheral clusters of fixations, suggesting partial peripheral re-referencing. Figure 1B-IV illustrates an even more complex inhomogeneous, fixation pattern without a single clear strategy.
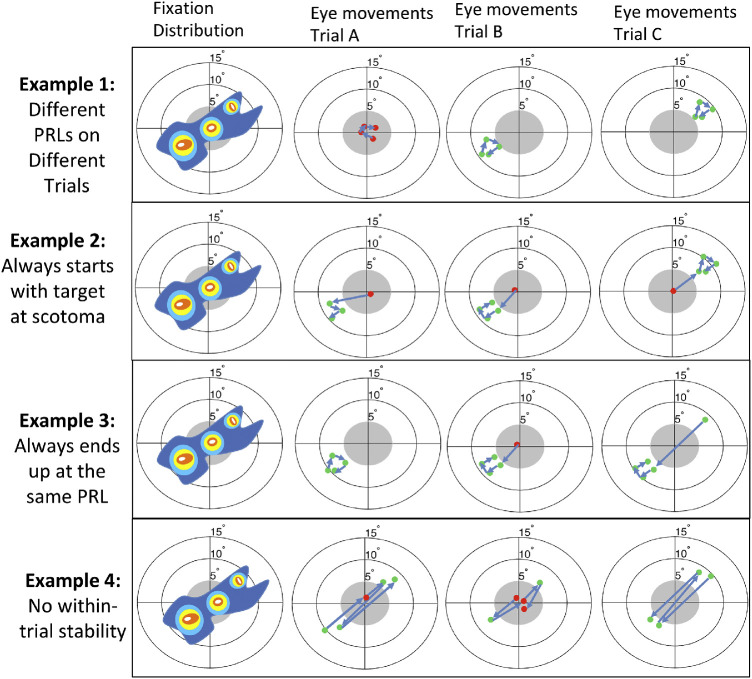
Notably, the pattern in Figure 1B-IV is ambiguous. It might reflect that in each trial participants move their eyes across each of the multiple fixation loci displayed, or alternatively that these complex patterns arise because different fixation loci are used in different trials. This is illustrated in Figure 2, showing how a given fixation probability density can potentially arise from combining a variety of different trial-by-trial behaviors ranging from: (1) two stable PRLs that are used on different trials that are “re-referenced” so that the first saccade always lands outside the scotoma, (2) two stable PRLs that are not re-referenced, (3) one stable PRL and one unstable PRL, or (4) highly unstable patterns of fixation on each trial. The main focus of the current paper is to propose and validate a more systematic approach to characterize compensatory eye movement strategies that distinguishes different oculomotor components that may help disambiguate between these different possibilities.
Figure 2.
Different within-trial behaviors can give rise to similar representations of eye movements when averaged across time: The fixation distribution displayed in the leftmost column can emerge from different behaviors, showing that simple fixation distribution analyses currently common in the field provide incomplete information about the underlying oculomotor strategies. In example 1, the overall distribution is due to the use of different PRLs between trials. In example 2, there is a lack of referencing and on each trial the first fixation is within the scotoma, but subsequent eye movements are made to one or the other PRL and are very stable. In example 3, there is a dominant PRL toward the left but on some trials initial eye movements are made to the center or upper-right. In example 4, there is poor within-trial stability and eye movements are scattered both within and across trials.
To help better understand these more complex peripheral looking strategies and in particular to help quantify the use of multiple PRLs (Duret, Issenhuth, & Safran, 1999; Lei & Schuchard, 1997; Safran, Duret, Issenhuth, & Mermoud, 1999), different approaches have been proposed. For example, the KDE method proposed by Crossland, Sims, Galbraith, & Rubin (2004), which identifies clusters of fixations, can be used to determine the number of PRLs across the visual field (see Figure 1A). Additionally, in the context of simulated central vision loss, some authors have looked at the landing dispersion of the first saccade in each trial as a measure of re-referencing (Kwon et al., 2013; Liu and Kwon, 2016). By looking at its location relative to the fovea, this analysis allows evaluation of whether participants developed a systematic strategy to plan saccades so that their PRL lands on the target immediately, indicating re-referencing of saccades toward this peripheral location.
Here, we build upon these approaches by characterizing different components of oculomotor behavior. These components look at: (1) whether the first saccade after target presentation places the target outside the scotoma (saccadic re-referencing), (2) whether the first eye movement placing the target outside the scotoma lands in a consistent location (saccadic precision), (3) the dispersion of the landing location of the first saccade after target presentation (first saccade landing dispersion), (4) whether the eyes keep this position stable within each trial (fixation stability), (5) how long it takes to bring the target to a location outside the scotoma (latency of target acquisition), and (6) the percentage of trials where some fixations occurred with the target outside the scotoma (percentage of trials that are useful).
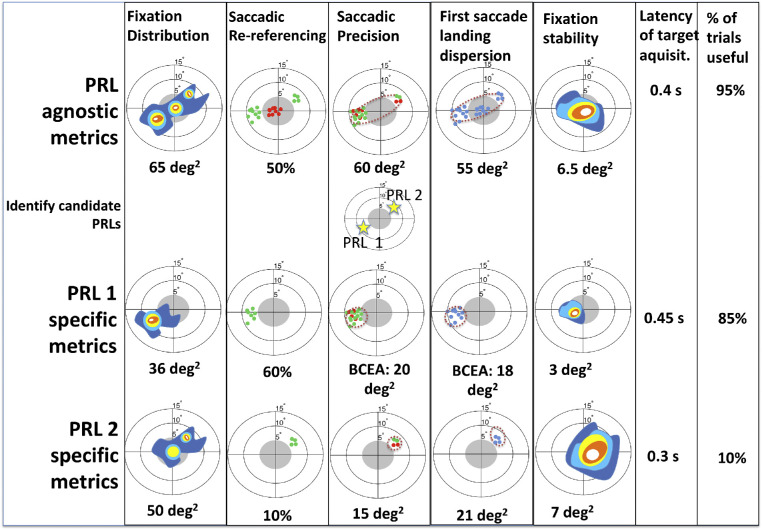
The use of these metrics is illustrated in Figure 3, where we describe the metrics as they might be applied to example IV from Figure 1B. The procedure involves two steps: first, to characterize peripheral viewing strategies at a global level, in which the analysis does not make assumptions about the development or number of PRLs (these are “PRL agnostic” metrics). This step identifies the location(s) of a PRL. In a second step, we perform a trial-based classification of within-trial behavior in participants with both single and multiple PRLs. For example, in Figure 3, we illustrate how the complex pattern from Figure 1B IV can be broken down into PRL agnostic and PRL specific analysis. To evaluate whether this approach is applicable to real data we trained healthy participants on a visual task with simulated scotoma and used these metrics to describe their varying peripheral looking strategies.
Figure 3.
Overview of the proposed metrics applied to the example pattern of fixation distribution from Figure 1B IV. Eye movement classification involves first analyzing the whole dataset (“PRL agnostic,” first row), and defining PRLs based on this analysis. In cases of multiple PRLs, metrics are calculated separately for trials where each PRL was used (“PRL specific,” bottom two rows). Metrics shown are (from left to right): probability density map of the fixation distribution. This is the “standard” approach described in Figure 1A. Saccadic re-referencing: proportion of trials where the landing point of the first saccade places the target outside scotoma. This tells us how often participants immediately place the target in a visible location. Saccadic precision: dispersion of the landing point of the first saccade that puts the target outside scotoma. This tells us whether the same retinal location is being used to observe the target, even if not with the first saccade. Identification of candidate PRLs is based on these landing points. First saccade landing dispersion: dispersion of the end point of the first saccade. This tells us how precisely saccades are planned. Fixation stability: dispersion of eye positions after a first saccade on a given trial (mean across trials). This tells us whether the eye tends to remain stable (e.g. PRL 1), or tends to move from place to place on a given trial (e.g. PRL 2). Latency of target acquisition: mean time until a saccade puts the target outside the scotoma. This tells us how long it takes for the participants to place the target in a visible location. Percentage of trials that are useful: this tells us how often participants eventually place the target outside of the scotoma (% of dots in Saccadic precision relative to total trials). In case of multiple PRLs, this tells us the proportion of trials in which participants used that location (i.e. that PRL) first.
Methods
Participants
Nineteen healthy participants (mean age: 20.4 years +/− 1.8 standard deviation, 12 women, 7 men) with normal or corrected-to-normal vision and no known visual pathologies, cognitive, or neurological impairments, were recruited at the University of California at Riverside to take part in the study. Experimental protocols were approved by the Human Research Review Board (HRRB) of the University of California at Riverside and all participants gave written informed consent prior to the experiment.
Stimuli and apparatus
Viewing was binocular. The Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) and the Eyelink toolbox (Cornelissen, Peters, & Palmer, 2002) were used to generate and control the visual stimuli. Participants’ eye movements were monitored (monocular tracking of the right eye) using an infrared video-based eye-tracker sampled at 500 Hz (EyeLink 1000 Plus Tower Mount; SR Research Ltd., Ontario, Canada). Although the maximum spatial resolution reported from the manufacturer is 0.01 degrees, it is likely that it was actually less accurate. In order to ensure sufficient precision for each participant, each session started with a nine-point calibration/validation sequence that was repeated until the validation error was smaller than 1 degree on average. We note that this was small relative to the 10 degrees of diameter scotoma used in the study. The gaze position error (i.e. the difference between the target position and the computed gaze position), was estimated during the nine-point validation procedure. The tower mount chin and forehead rests from the Eyelink system were used throughout the experiment to minimize head movements and trial-to-trial variability in the estimation of gaze position. Real-time gaze positions were sent to the display computer through a high-speed Ethernet link. The monitor was a Viewsonic PF817 professional series CRT with a resolution of 1280 × 1024 pixels and a refresh rate of 75 Hz. Each pixel subtended 0.032 degrees of visual angle at the viewing distance of 57 cm. The continuous gaze information was used to draw the artificial scotoma on the experimental monitor at a refresh rate of 75 Hz where the gaze position corresponded to the center of the scotoma. The Eyelink 1000 has a worst-case latency of 4 ms, and with the 75 Hz frame rate used and a CRT monitor with almost complete phosphor decay by 2 ms, we had ample time to recompute the stimuli between frames. To verify the system latency, we used the method described in Saunders and Woods (2014). High-frame video recording using a similar setup showed a screen update of about 28 ms (median value of 50 measurements; this value corresponds to 3 frames in the worst-case scenario), however. we were unable to test the exact system used for the study due to an on-going campus shut-down caused by the coronavirus disease 2019 (COVID-19) pandemic. Notably, subjective reports from both the research staff and participants using the experimental setup was that the artificial scotoma appeared to smoothly follow eye movements and that the target could not be made visible to foveal viewing even when making rapid eye movements.
Procedure
Across 12 sessions, a gaze-contingent display simulating a 10 degree (diameter) circular scotoma obstructing central vision was used to induce peripheral viewing strategies that were then evaluated in a 13th session. Sessions 1 and 2 consisted of visual search tasks and in sessions 3 to 12, participants took part in approximately 10 hours of contrast detection training where they judged the orientation of low-contrast Gabor patterns presented on the screen. In sessions 1, 2, and 13, participants performed a visual acuity task aimed at measuring the location of their PRL and oculomotor strategies (PRL test session). The central artificial scotoma was used in all sessions.
Given the focus of the current paper to characterize resulting eye movement strategies, eye-movement metrics are drawn from session 13. In this session, participants were presented with a Landolt C at a random location (anywhere on the screen where the full C could be rendered) and asked to report its orientation (C opens up, down, left, or right). This random placement was meant to avoid the contribution of any systematic location expectations that might bias estimates of participants’ looking strategy. The size of the letter C was adaptively adjusted using a 3:1 staircase (0.03 log unit steps, initial value 1 degree) and the small size of the C motivated participants to view the C as close to the fovea as possible given the constraint of the scotoma.
Analysis
Identification of candidate PRLs
To address the variety of complex eye movement patterns found across participants, including multiple PRLs or other complex distributions of fixations, some of our analyses examined effects in each of the candidate PRL separately (see PRL specific analysis in the Result section). To accomplish this, we first identified, for each trial, the eye position of the first fixation at least 100 ms after target presentation that placed the target outside of the scotoma area (the first “useful fixation”; i.e. the first fixation that places the target in a visible location). We then analyzed these eye positions through a KDE (Crossland et al., 2004), to test whether multiple clusters exist. If the KDE analysis resulted in multiple peaks (e.g. Figure 1B III shows two separate peaks, red regions), we then used a K-means analysis to spatially subdivide trials according to which PRL was used first in each trial.
Oculomotor metrics
As follows, we describe each of the metrics used to assess peripheral looking strategy. Further, Table 1 summarizes these and a comprehensive summary of statistics for each metric is presented in the Supplementary Material.
Table 1.
Summary of the proposed metrics to characterize oculomotor strategies after central vision loss simulation. The last 6 columns in Figure 3 illustrate each of these metrics.
| Metric | Description | Unit |
|---|---|---|
| Saccadic re-referencing | Percentage of first absolute saccades landing outside the scotoma. Useful to identify how reliably the participant re-references saccades. | % |
| Saccadic precision | Dispersion of the landing location of the first saccade that does not cover the target. Useful to identify how precise the PRL identification is, regardless of whether it's the target of the first eye movement. | BCEA (deg2) |
| First saccade landing dispersion | Dispersion of the landing locations of the first saccade after target appearance across trials. Useful to identify how precise re-referencing is. | BCEA (deg2) |
| Fixation stability | Eye position dispersion within trials. Useful because it indexes the stability of the target on the retina. | BCEA (deg2) |
| Latency of target acquisition | Time interval between the target appearance and the first eye movement that puts the target in a visible spot (i.e. outside of the scotoma). Useful because this measures time before a stimulus can be seen. | Seconds |
| Percentage of trials that are useful | Percentage of trials containing at least one saccade landing outside the scotoma. Useful because this measures what percent of trials will have a visible stimulus. | % |
Saccadic re-referencing. This analysis helps determine whether or not the foveal reference is still present by computing the percentage of first fixations per trial, which fall outside the scotoma (i.e. the percentage of first fixations that put the target in a visible location). In this context, fixations are defined as a period of eye stability (eye velocity < 10 deg /s) for at least 150 ms, and which happened at least 100 ms after target presentation. A value of 100% indicates that the fovea is never used for the first fixation to the target and 0% indicates that the first fixation is always to place the fovea on the target (and thus obscuring the target from view). Of note, this estimate is independent from the existence of defined PRL(s) outside the fovea.
Saccadic precision. This analysis addresses the consistency of PRLs across trials by calculating the distribution of locations of the trial's first fixation that lands outside the scotoma. This fixation could therefore be the first fixation, the second, or the third, etc., and represents the first fixation during which the target can be seen. The measure of saccadic precision is then represented as the size of the BCEA fitted on these fixation positions. The BCEA is calculated to encompass a given proportion (P) of the overall number of fixations. Following previous studies (Chung, 2013a; Crossland et al., 2004; Kwon et al., 2013), we chose p = 0.68. In plots of the individual saccade landing locations, we further visually distinguish “absolute” first fixations (i.e. first fixations outside the scotoma that happen to be the first fixation in the trial) with a different color from other fixations following initial fixations to the scotoma (see third column Figure 4).
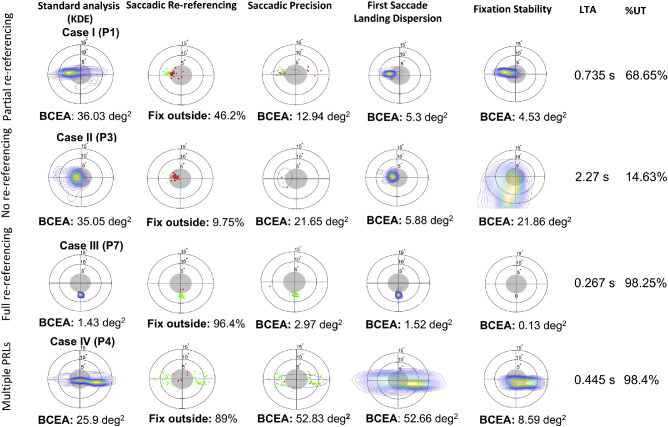
Figure 4.
Oculomotor metric analysis for four example participants showing different eye movement behaviors. The first column shows the standard representation of oculomotor behavior as a probability density map estimated on the fixation distribution (Crossland et al., 2004). The second column plots, for each trial, the location of the first fixation. Saccadic re-referencing is the percentage of trials in which this first fixation placed the target in a visible position (outside the scotoma). The third column plots, for each trial, the first fixation that does not cover the target. Saccadic precision is a measure of the dispersion of these locations. The fourth column plots the probability density of the first ‘absolute’ fixation of the trial, whether or not the target was visible. The first saccade landing dispersion is a measure of the dispersion of these fixation locations. The fifth column shows the distribution of all eye positions after the first saccade in each trial. The center of the image represents the location of the first saccade. Fixation stability measures the dispersion of these eye positions. The sixth column shows the latency of target acquisition, the time from target appearance until the target is in a visible position on the retina. The seventh column shows the percentage of trials that were useful (% UT), indicating the proportion of trials per session in which at least one fixation placed the target outside the scotoma.
First saccade landing dispersion. This analysis addresses the consistency of first saccade landing locations across trials by calculating the area of first saccade landing locations (whether or not the scotoma covered the target). The distribution of the saccade landing locations was quantified using a BCEA (Crossland et al., 2004). Both first saccade landing dispersion and saccadic re-referencing describe the shift in oculomotor reference away from the fovea, albeit in different ways. Although saccadic re-referencing measures how often the first saccade to the target is within or outside the scotoma, the first saccade landing dispersion allows us to understand the spatial distribution of these saccades, including whether this may include multiple PRLs.
Fixation stability. This analysis characterizes the dispersion of eye positions within a trial by controlling for differing fixation locations across trials when characterizing the average dispersion of eye positions within trials. This metric was computed by first identifying all eye positions after the first fixation of the trial (as defined in the saccadic re-referencing section). KDE was fit to these positions, and was weighted for duration (KDE/duration of the trial in frames). The location of each trial's KDE was then normalized to the estimated across-trial PRL location. This location was defined as the averaged center of the single-trial BCEAs normalized by the position of the first fixation, and centered on the averaged center of the single-trial BCEAs.
Latency of target acquisition. This is the time interval between the target appearance and the first fixation outside the scotoma (same fixation as used in the saccadic precision analysis), expressed in seconds.
Percentage of trials that are useful. This represents the portion of trials in which at least one saccade landed outside the scotoma (“useful” trials, as in trials in which the target was visible outside the scotoma). This value is expressed as a percentage of the overall number of trials.
Results
To understand how the proposed oculomotor metrics can be informative to different peripheral looking strategies, we discuss data from example participants exhibiting different strategies and compare these to standard fixation distribution analysis (Crossland et al., 2004; Kwon et al., 2013). We first characterize peripheral viewing strategies at a global level (e.g. without assumptions about the development or number of PRLs), and then demonstrate how the methods can be used to understand multiple candidate PRLs within an individual. In order to systematically present the eye movement components, and discuss their differences across subjects, we selected four case example participants whose data are presented in Figure 4. Data for the rest of the participants can be found in the Supplementary Material.
“Case I” (participant 1) is an example of partial re-referencing, showing a PRL on the left border of the scotoma with some additional gaze-points to the right of center. “Case II” (participant 3) shows lack of re-referencing, mostly retaining a foveal oculomotor reference (peak of the probability density map is within the border of the scotoma). “Case III” (participant 7) shows a complete peripheral re-referencing, with a well-formed, single PRL in the lower field outside of the scotoma. Finally, “Case IV” (participant 4) exhibits multiple PRLs, one to the left and a second one to the right of the scotoma. In the following paragraph, we discuss how each metric is informative for describing these cases.
Saccadic re-referencing characterizes changes in oculomotor reference in response to simulated visual loss. Figure 4 shows saccadic re-referencing for the four case examples: cases III and IV both show a large re-referencing (96.4% and 89%), case II shows a lack of re-referencing (9.75%), and case I is somewhere in between, suggesting partial re-referencing (46.2%). The large re-referencing for cases III and IV, despite using a different number of PRLs (one versus two) are consistent with evidence from clinical research that re-referencing in patients with MD can happen in the presence of multiple PRLs (Duret et al., 1999; Safran et al., 1999).
Saccadic precision addresses how precisely participants target specific retinal loci outside of the scotoma. We define saccadic precision as the distribution of locations of the first fixation of each trial that places the target outside the scotoma. Similar to a standard analysis of fixation distribution (e.g. Crossland et al., 2004), BCEA is used as a measure of the consistency of these landing points across trials. Case I shows first fixations on both sides of the scotoma, with a higher concentration on the left side that contains most of the first fixations that landed outside the scotoma (green dots). Case II shows a small number of fixations dispersed mostly toward the left of the scotoma. Case III shows a highly concentrated cluster of fixations in the lower hemifield outside the scotoma. Finally, case IV shows a pattern of fixations on both sides of the scotoma. Both case I and case II show a limited number of first fixations outside the scotoma (green dots), suggesting the presence of a stronger foveal reference than case III and case VI. Interestingly, case IV exhibits the worst saccadic precision out of the four examples participants (52.7 degrees2), clearly due to the assumption of this analysis that the fixations should fall into a unimodal distribution. Further analyses, which take into account specific PRLs, are described later in the paper and help deal with this issue.
First saccade landing dispersion identifies the existence and extent of a consistent region, whether inside or outside the scotoma, where the oculomotor system tends to orient the first saccade of each trial (with a smaller dispersion indicating a more precise location). Our example participants exhibit overall small dispersions (case I, case II, and case III with 5.3 degrees2, 5.88 degrees2, and 1.52 degees2, respectively), except for case IV (52.66 degrees2). This suggests that, other than case IV, these participants were relatively consistent, trial by trial, in their first eye movement and provides complementary information to the re-referencing statistic that indicates the percentage of these saccades that were outside of the foveal region.
Fixation stability is often used as one of the main indicators of vision quality in MD, and it is usually quantified through the use of microperimetry, either as a proportion of fixations within a certain region (e.g. Nidek MP-3: circular areas of 2 and 4 degrees radius) or a BCEA of a certain proportion (i.e. 68%; Crossland et al., 2004) of overall fixations over a certain period (i.e. 15–30 seconds; Bellmann et al., 2004; Gonzalez et al., 2011; Rosengarth et al., 2013) of recording. However, when examining BCEA over a longer period, including multiple trials and stimuli (as shown in Figure 4), fixation stability is confounded by saccadic precision and re-referencing (as shown in Figure 2). Therefore, in this study, we consider fixation stability as the ability to maintain steady fixation within a trial once the target is acquired. To do so, we compute fixation stability independently for each trial and then align these to the average centroid across trials (see Methods section for details). This means that if the fixations are centered in different locations on different trials (e.g. in the case of multiple PRLs), this method plots all the distributions at the same location, obscuring the location of the PRL. This is exemplified by case IV who exhibits two PRLs in the standard analysis but in the plot of fixation stability they show a single cluster near the center. The four example participants show various degrees of stability, from highly stable (case I and case III with 4.53 degrees2 and 0.13 degrees2, respectively), to medium (case IV with 8.59 degrees2) to low (case II with 21.66 degrees2).
Latency of target acquisition indicates the time interval between the target appearance and the time of the end point of the first saccade that places the target in a visible position outside the scotoma for each trial. Latency of target acquisition for the four example profiles show cases of both rapid (case III with 0.27 seconds and case IV with 0.45 seconds) and slow target acquisition (case II with 2.27 seconds). Case III shows a short latency (0.27 seconds) that, when combined with tight saccadic precision (2.97 degrees2 BCEA), indicates a functional adaptation to central vision loss where a target would be quickly and precisely placed in a visible region of the peripheral visual field. On the other hand, case II shows a long latency (2.27 seconds), and relatively looser saccadic precision (21.65 and 10.28 degrees2 BCAE), which suggests a less efficient or not fully developed compensatory strategy. Finally, case I shows first “useful fixations” on both sides of the scotoma, suggesting the possible presence of multiple PRLs. However, a closer look at the distribution of absolute first fixations in case I points toward the existence of a stronger PRL on the left side of the scotoma.
Percentage of trials that are useful addresses the proportion of trials in which at least one fixation put the target in a visible position outside the scotoma. All the example cases, except case II, show a high number of trials with at least one useful saccade (3 participants > 65% to 98%). Only one participant showed a small percentage of useful trials (case II with < 15%), indicating that participant was unable to view the stimulus using the periphery, thus suggesting ineffective adaptation to simulated central vision loss.
An advantage of using multiple metrics to define a profile of behavior, as opposed to combining into one general metric, is the possibility of combining scores to identify strategies. For example, by comparing saccadic re-referencing and first saccade landing location within the same participants, we can understand both the extent to which re-referencing occurs as well as the consistency of the new reference locations. Looking at our four cases, we can observe four different profiles: small first saccade landing dispersion and medium re-referencing (case I), small first saccade landing dispersion and low re-referencing (case II), small first saccade landing dispersion and high re-referencing (case III), and large first saccade landing dispersion and high re-referencing (case IV). This shows that when re-referencing occurs, it is sometimes, but not always to a consistent location.
Another way of looking at the metrics is by examining saccadic precision and latency of target acquisition together to describe different profiles of oculomotor behavior. For example, case III shows short latency and tight saccadic precision, suggesting a functional adaptation to central vision loss where a target would be quickly and precisely placed in a visible region of the peripheral visual field. On the other hand, case II shows a long latency and looser saccadic precision, which suggests a less efficient or not fully developed compensatory strategy.
Examining multiple metrics can disambiguate information from two cases that may initially look to be adopting similar strategies. Cases I and IV both show first “useful fixations” on both sides of the scotoma, suggesting the possible presence of multiple PRLs. However, a closer look at the distribution of absolute first fixations in case I points toward the existence of a stronger PRL on the left side of the scotoma. Case I may be moving toward developing a single PRL, whereas case IV seems to be moving toward adopting multiple PRLs.
Finally, while looking at saccadic re-referencing together with percentage of trials that are useful, participant 5 (Supplementary Material) shows yet another possible compensatory oculomotor strategy, that includes high percentage of trials that are useful (85%) despite a low level of re-referencing (16%) indicating a strategy that does not make use of re-referencing of the first saccade to a PRL. Instead, first saccades go to the fovea, and subsequent saccades are made to allow viewing of the target outside the scotoma. These different patterns of eye movements may reflect different points along a trajectory toward PRL development, or different strategies. Characterization of eye movements that allows this type of fine scale description is therefore essential for subsequent work on compensation after central vision loss.
PRL specific analysis
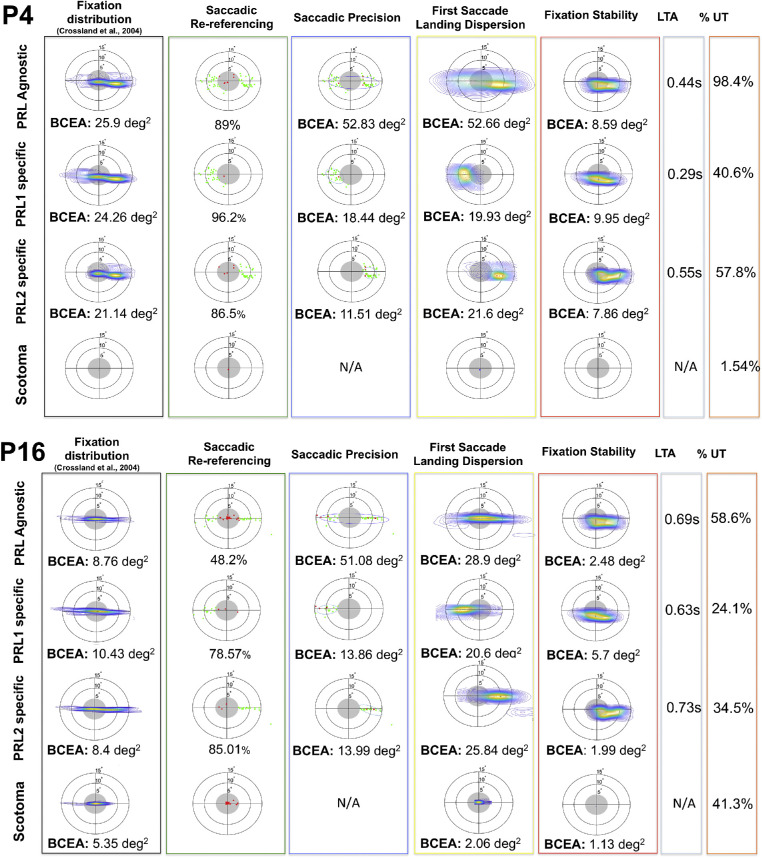
As discussed in the introduction, when examining eye movement patterns during a visual task, the traditional approach can conflate multiple, trial-specific behaviors into a single oculomotor profile, thus offering a potentially fallacious representation of the participant's strategy. To illustrate this point, Figure 5 uses two example participants to show PRL-agnostic and PRL-specific metrics in the case of multiple PRLs, to help disentangle trial-specific behavior.
Figure 5.
PRL-specific analysis for two participants (participant 4 and participant 16) exhibiting multiple PRLs. In each figure, the first row shows the metrics computed for the whole dataset, whereas the remaining rows show the analyses conducted on trials specific to a given PRL. The first column presents the data in the standard representation, whereas the remaining columns show the proposed metrics, following the template of Figure 3.
PRL-specific analysis allows observation of characteristics of the oculomotor strategies that would be conflated in the PRL-agnostic analysis. For example, participant 16's two PRLs, whereas exhibiting similar saccadic precision (see Figure 5, lower panel, third column), have different patterns of fixation stability (see Figure 5, lower panel, fifth column). Trials starting in the left PRL (PRL 1) have a much less stable fixation and show a tendency to eventually drift toward the right PRL (see Figure 5, lower panel, fixation stability column, second row). This (somewhat unexpected) strategy means that on trials where the participant initially goes to PRL 1, they often end up at PRL 2. However, on trials where they initially go to PRL 2, they tend to stay there. This strategy could possibly reflect a developing preference for PRL 2. This type of strategy can be contrasted with results from participant 4, shown in the top panel of Figure 5, who shows similar overall patterns, with two PRLs at the left and right sides. However, there does not seem to be such a late preference for one PRL over the other. Distinguishing these two types of strategies can be important for understanding how performance differs across trials, and across training, and this type of detail would be missed without PRL specific analyses.
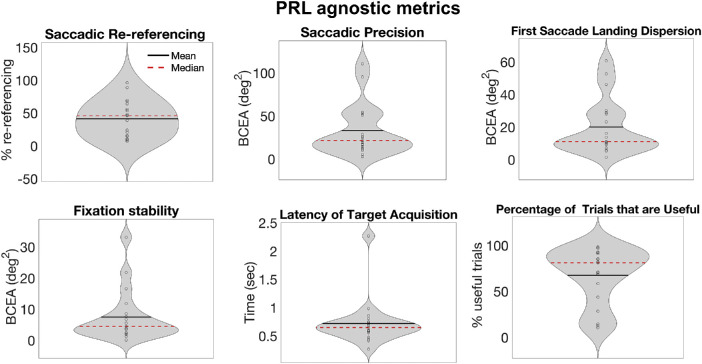
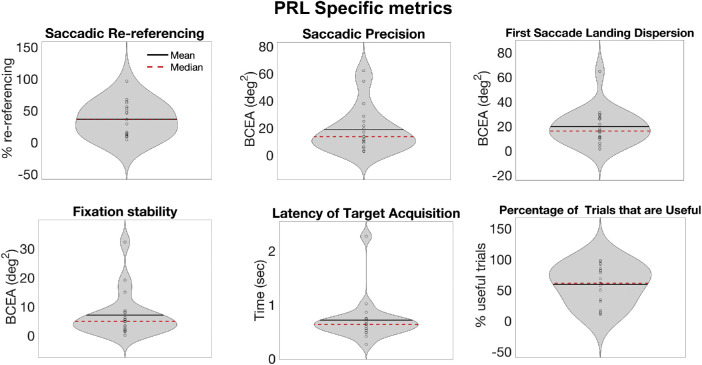
Group analysis
Although the goal of the paper is to present a new way of analyzing eye movements in the context of central vision loss, rather than present groupwise effects, Figure 6 reports group data for the six metrics as an indication of the diverse oculomotor strategies that can be observed in a sample of participants trained with artificial central scotoma. Interestingly, the average distribution of the first saccade landing location in our sample was 20.05 degrees2 +/− 17.04, similar to what was reported by Kwon and colleagues (2013) for healthy participants trained on a visual search task with a gaze-contingent simulated scotoma, indicating reproducibility of these metrics with training. In Figure 6, we show the metrics as assessed based on all trials together (a PRL-agnostic analysis). In Figure 7, we show metrics for only those trials on which participants used their most common PRL. The results are somewhat similar across these two analysis techniques. Of note, our participant sample showed diverse learning gain, suggesting that the overall amount of training (10 sessions of approximately 40 minutes) might not have been sufficient for some of the participants to fully develop effective strategies. Consequently, the interpretation of the group results should take this into account.
Figure 6.
Group average for the 6 metrics measured on the full dataset of 19 participants, expressed as violin plots. The width of the violin indicates the number of points with that value. The dark line is the mean, whereas the red dashed line is the median.
Figure 7.
Group-level PRL specific metrics. For each participant, the PRL with the larger number of fixations that were useful was chosen, and metrics for that PRL are shown.
Summary analysis of eye movement metrics
The goal of the present paper is to provide a method for the analysis of eye movement patterns that could shed light on the underlying behavioral profiles that participants might adopt when presented with simulated central vision loss. In order to provide a summary analysis of the results of these metrics, we conducted a principal component analysis (PCA) to illustrate how the distributions of the different metrics in the sample relate to one another. The six metrics for each participant's analysis for the PRL-agnostic data from Figure 6 were entered into a PCA. A second analysis was performed examining the PRL-specific data for participants’ primary PRL, as shown in Figure 7. The resulting PCA coefficients for component 1, component 2, and component 3 for each analysis are presented in Table 23. The variances explained for these top 3 components are 36.87%, 34.08%, and 13.23% for the PRL-specific analysis and 36.03%, 30.87%, and 17.2% for PRL-agnostic. All the metrics are strongly (> 0.5) associated with at least one component, suggesting that all the metrics are important in describing the participants’ behavior. Further, no component was driven exclusively by one metric, indicating that combinations of metrics provide useful information.
Table 2.
PCA coefficients of components 1, 2, and 3 for the PRL-agnostic analysis, and PRL-specific analysis, for the six metrics plus the standard analysis.
| Metric | C1 Agnostic | C2 Agnostic | C3 Agnostic | C1 Specific | C2 Specific | C3 Specific |
|---|---|---|---|---|---|---|
| Standard analysis (dispersion of eye positions) | 0.524 | 0.193 | ‒0.2127 | 0.418 | ‒0.339 | ‒0.44 |
| Saccadic re-referencing | ‒0.286 | 0.509 | 0.123 | 0.132 | 0.607 | ‒0.089 |
| Saccadic precision | 0.297 | 0.237 | 0.7838 | 0.424 | 0.053 | 0.603 |
| First saccade landing dispersion | 0.5197 | ‒0.145 | 0.252 | 0.447 | ‒2.03 | 0.493 |
| Fixation stability | 0.502 | 0.2829 | ‒0.3667 | 0.52 | ‒0.208 | ‒0.391 |
| Latency | 0.096 | 0.501 | ‒0.315 | 0.328 | 0.354 | ‒0.177 |
| % useful trials | ‒0.18 | 0.543 | 0.167 | 0.2215 | 0.55 | ‒0.084 |
Table 3.
Correlations between metrics and visual acuity for overall distribution and PRL-specific analyses. Crosses indicate results after outlier removal (>3SD, one outlier per analysis), asterisk indicates statistically significant correlation at alpha = 0.05.
| Metric | VA (overall) | VA (PRL specific) |
|---|---|---|
| Saccadic re-referencing |
r = ‒0.281 p = 0.24 |
r = ‒0.32 p = 0.18 |
| Saccadic precision |
r = 9.144 p = 0.554 |
r = 0.096 p = 0.69 |
| First saccade landing location |
r = 0.247 p = 0.306 |
r = 0.63+ p = 0.005+* |
| Fixation stability |
r = 0.08 p = 0.72+ |
r = 0.13+ p = 06+ |
| Latency of target acquisition |
r = 0.25+ p = 0.305+ |
r = 0.218+ p = 0.38+ |
| % of fixations that were useful |
r = ‒0.25 p = 0.29 |
r = ‒0.267 p = 0.27 |
Eye-movement metrics’ relationship to visual performance
A key question is to what extent different compensatory oculomotor strategies are related to visual performance. To address this, we next examine the extent to which eye movement metrics correlated with visual acuity performance. Visual acuity was measured during the PRL test as the threshold size of the Landolt C. This size was converted to logarithm of the minimum angle of resolution (logMAR) by log transforming the C's gap (one fifth the size of the letter) expressed in arcminutes.
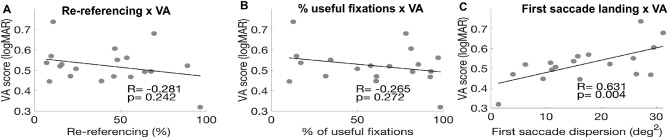
As a first analysis, we looked at the relationship between saccadic re-referencing and performance in the visual acuity task. Re-referencing, both in patients and in healthy participants trained with simulated scotoma, is one of the most frequently used metrics to judge how well a patient compensates for vision loss (Crossland, Culham, Kabanarou, & Rubin, 2005; Kwon et al., 2013; Liu and Kwon, 2016). However, re-referencing showed a weak and nonsignificant relationship to visual acuity (see Figure 8A). This suggests that the ability to place a first saccade outside the scotoma is not a strong predictor of acuity. Similarly, in Figure 8B, we show that there is a weak, nonsignificant relationship between acuity and percentage of trials that are useful. This suggests that the ability to get the target outside the scotoma at some point during the trial is not an essential driver of acuity.
Figure 8.
Correlations between oculomotor metrics and visual acuity (VA) performance. (A) VA thresholds as a function of the re-referencing metric (percentage of first fixations falling outside the scotoma). No significant relationship between these variables. (B) VA thresholds as a function of percentage of useful fixations (trials in which the participants had at least one fixation outside the scotoma. No significant relationship between these variables. (C) Visual Acuity thresholds as a function of the dispersion of the first saccade landing location for the trials in which the participants had at least one useful fixation. There is a strong, significant relationship between these variables so that visual acuity is better when the first saccade landing location is more precise (N = 18 because one participant had a first saccade landing dispersion score > 3 SD from the mean). Figure 2 in the Supplementary Material shows the graphs and correlations values without outlier correction.
On the other hand, Figure 8C shows a strong, significant relationship between acuity and saccade landing dispersion for the main PRL (see Figure 8C). In other words, people with better acuity had a relatively tight retinal location to which they planned saccades. Figure 8 and Table 2 as a whole, therefore, shows that while neither saccadic re-referencing nor percentage of fixations that are useful at the “best” PRL predict visual acuity, the precision of ballistic saccades to a PRL does predict performance. This suggests that it is not necessarily the “visibility” of the target that matters for acuity in this context, but something about the planning of the eye movements to the target.
We also examined correlations with other metrics that can be found in Supplementary Table S3 and Supplementary Figure S2. Except for PRL-specific first saccade landing dispersion (Figure 8C), no metrics showed a significant relationship to acuity. In general, however, better acuity related to more precise saccadic metrics, suggesting that acuity in peripheral vision relates to saccadic control.
Given the likelihood that metrics jointly predict task performance, we examined the extent to which principle components correlated with our measure of acuity. Table 4 shows the correlations to visual acuity of the three principle components described in Table 2. Although in the PRL agnostic analysis no component correlates significantly with visual acuity performance, in the PRL specific analysis, component 1 is significantly correlated with the visual acuity performance. This is consistent with the idea that, in cases of multiple PRLs, a trial-specific analysis might be more accurate in describing oculomotor strategies in conditions of (simulated) central vision loss. However, we note that further work will be required to more clearly relate the multiple metrics that we have described to visual task performance, including more complex visual tasks.
Table 4.
correlations between PCA scores and visual acuity for overall distribution and PRL-specific metrics.
| PCA scores | VA r | VA p |
|---|---|---|
| Component 1 (overall) | 0.284 | 0.239 |
| Component 2 (overall) | 0.372 | 0.117 |
| Component 3 (overall) | ‒0.043 | 0.86 |
| Component 1 (PRL specific) | 0.469 | 0.042* |
| Component 2 (PRL specific) | 0.083 | 0.733 |
| Component 3 (PRL specific) | ‒0.183 | 0.45 |
Discussion
In this paper, we describe a method for the classification of compensatory oculomotor strategies following simulated central vision loss. This method takes into account multiple aspects of oculomotor behaviors which are likely to have different influences on visual performance. We systematically examined whether the first saccade of each trial lands outside the simulated scotoma (saccadic re-referencing), where the first “useful” saccade lands (i.e. a saccade that allows the participant to place the target in a visible region outside the scotoma; saccadic precision), where the first saccade of each trial lands (first saccade landing location), whether the eye is stable after this first saccade (fixation stability), the length of time it takes to initiate a saccade that puts the target in a visible location (latency of target acquisition), and the percentage of trials containing at least one of these “useful” fixations (percentage of trials that are useful). Further, we show that these analyses can be applied to different subsets of trials to understand how each retinal locus may be used. Together, these provide a powerful set of analysis tools to better understand the diverse peripheral viewing strategies that may accompany central vision loss.
Although the proposed method can provide insights into the compensatory strategies adopted by participants undergoing training with simulated vision loss, its metrics and ability to disentangle specific strategies can, in principle, be applied to patients suffering from pathological central vision loss. Indeed, retinal diseases are becoming an increasingly common health concern in the Western World, leading, in their more serious forms, to loss of foveal vision. There is evidence that the brain and the oculomotor system exhibit spontaneous plastic changes to compensate for the lack of central vision by rewiring vision toward peripheral retinal areas (Baker, Dilks, Peli, & Kanwisher, 2008; Baker, Peli, Knouf, & Kanwisher, 2005; Dilks, Baker, Peli, & Kanwisher, 2009), often developing a new eccentric fixation spot called a PRL (Timberlake et al., 1986). However, despite longitudinal studies in clinical populations (Crossland et al., 2005) and more recent papers using simulated central vision loss in healthy participants (Kwon et al., 2013; Liu & Kwon, 2016), the mechanisms, extent, and time course of these compensatory processes are still unclear.
For example, current clinical examinations in patients with MD, such as microperimetry, present additional disadvantages in that they mostly focus on fixation location and stability, thus limiting eye movements by asking patients to keep steady fixation and respond to a stimulus appearing in a random position in the visual field. Because there is evidence that patients might use different portions of the residual retina to perform different tasks (Duret et al., 1999; Lei & Schuchard, 1997; Safran et al., 1999), the translational value of the microperimetry measurement into everyday life tasks might not be straightforward. Despite attempts at more accurate analyses that take into account the potentially multiple number of PRLs (Crossland et al., 2004) or studies investigating eye movements during more active tasks, such as visual search in participants with MD (Van der Stigchel et al., 2013) there is still a lack of a meaningful and systematic evaluation of the way the oculomotor system compensates for central vision loss.
To better characterize the development of a peripheral oculomotor reference to replace central vision, two of the metrics we used here address two different aspects of re-referencing, namely toward a PRL and away from the fovea. First saccade landing dispersion is a measure of spatial consistency for the first absolute fixation following target appearance. Previous studies used a similar measure as an index of re-referencing (Kwon et al., 2013; Liu & Kwon, 2016). On the other hand, saccadic re-referencing provides a measure of the persistence of foveal referencing, with a small percentage suggesting that participants are learning to use a different retinal location as the landing location of their first saccade. The utility of characterizing re-referencing through two measures is supported, in the context of simulated central vision loss, by the evidence of different degrees of consistency between the two (as shown in Figure 5, with some participants exhibiting low peripheral re-referencing and high consistency in first saccade landing dispersion (e.g. case II), others showing high peripheral re-referencing and low consistency in first saccade landing dispersion (e.g. case IV) and others showing mixed behaviors. Interestingly, despite never explicitly enforcing participants to use a specific portion of the visual field during our PRL test phase, we observed saccadic re-referencing in roughly half of the participants (see Figure 6, and Table 2, and Supplementary Material), and the average BCEA of the first saccade landing location was comparable with previous studies in which participants were explicitly required to use a specific peripheral location outside the simulated scotoma by blurring the residual visual field outside the assigned location (Kwon et al., 2013).
Another metric in our classification is saccadic precision, the distribution of the first fixation in each trial that places the target outside the scotoma. This analysis is complementary to saccadic re-referencing in that it addresses the possibility that, even in the absence of clear re-referencing, participants might still exhibit a specific spot outside the scotoma that they systematically use to fixate on the target after a first fixation within the scotoma. High saccadic precision might then be a precursor of re-referencing of first saccades. The metric percentage of trials that are useful allows us to quantify these trials with respect to the overall number of trials per session. A high score in this metric can be seen as an indication of a functional adaptation to central vision loss. Latency of target acquisition refers to the time interval between target appearance and the first “useful” fixation. A slow latency would suggest a suboptimal visual exploration strategy, with several fixations still falling so that the target is within the scotoma. In general, MD is associated with prolonged initiation latencies and low accuracy (White & Bedell, 1990; Whittaker, Cummings, & Swieson, 1991). It is important to note that no current clinical test is able to disentangle saccadic re-referencing from saccadic precision in patients with MD, thus making the proposed metrics potentially useful in understanding and characterizing PRL development in clinical populations.
With fixation stability, we measure the dispersion of eye positions after the first saccade of each trial, normalized by the location of the first saccade and the duration of the trial. This analysis expands on both saccadic re-referencing and saccadic precision in that it addresses the aforementioned possibility that compensatory strategies might be location-independent, so that participants would still be able to keep stable fixation despite not systematically using the same retinal spot in every trial.
Each of these metrics can be examined both in a global analysis combining all trials, but also in separate analyses that independently examine subsets of trials that use different PRLs. This approach allows identification of strategies that participants may adopt on only a subset of trials. Our data show that examining these PRL-specific metrics may lead to more sensitive relationships to behavior. In the examples here, only PRL-specific metrics predicted visual acuity scores. Breaking data down by PRL allows separation of performance on trials with “useful” fixation versus trials without. By doing so, we can better observe compensatory behaviors that might be masked by the conflation with trials without useful fixations. Additionally, for participants exhibiting multiple PRLs, this breakdown can help distinguish whether there exists a “primary” PRL and one or more “secondary” ones (such as for participant 4 described above). Further, because participants’ strategies may be reflected in combinations of eye movement metrics, we performed a factor analysis to examine these patterns across participants. Interestingly, the resulting factors did not load on one or two of these metrics, indicating that all the metrics contribute. When a factor analysis was conducted on the PRL-specific metrics, we found a significant correlation between the scores of the first principal component and the visual acuity thresholds, suggesting that a trial-specific analysis might be more appropriate for describing the oculomotor behavior of the participants. The contribution of the present paper is to provide a framework to break down eye-movement behavior after central vision loss into component behaviors, however, future research will be required to better understand how these metrics relate to the complexity of behaviors that are found ecologically.
Given its exploratory aim, this study presents some limitations: for one, we collected data on a group of young, healthy participants, which helped for obtaining clean eye tracking data, but means that further work will be needed to apply these techniques to low-vision populations. We do note that there are a number of challenges that need to be addressed to bring these techniques to patients with MD. It is well known that patients with MD tend to have unstable fixation, thus despite recent attempts at calibrating eye tracking devices used in vision research in this population (Harrar, Le Trung, Malienko, Khan, 2018), this might prove challenging. Additionally, simulated and pathological scotoma present a number of differences in terms of time course of development of compensatory strategies and overall fixation stability and might be qualitatively different (Ağaoğlu, Fung, Chung, 2019). Thus, the data reported here might not provide an indication of what we would observe in patients. However, the possibility of breaking down eye movement behaviors within each cohort of participants and being able to characterize the development of oculomotor strategies at different stages of a simulated training or a low-vision intervention contributes a valuable tool for better understanding visual system adaptation to simulated or pathological central vision loss.
Further, there is the possible concern that the eye-tracking technology may not have at all times presented the artificial scotoma properly, perhaps allowing participants to get foveal glimpses of the target. To address this concern, we ensured that we were able to get a good calibration on all of our participants. Each session started with a calibration and validation procedure, which only concluded if the validation showed error less than 1 degree of visual angle. All participants underwent this procedure, on every session, showing that calibration went reasonably well for all participants. However, even if eye tracking had been somewhat off for some participants, this would add noise to the data, and would not materially influence the interpretation.
To provide other groups the ability to examine the utility of these metrics, we are making the code publicly available. This will facilitate other groups to use these eye movement metrics to identify patterns within their own datasets. Importantly, this also allows comparison of these metrics across studies. It is our hope that by making these metrics available, the field will grow to use them to make detailed examinations, build upon them, and that this will lead to improvements in diagnoses and treatments for those with vision loss. The website for obtaining code is https://github.com/Visscher-Lab/OculomotorStrategyToolkit.
Taken together, the results presented here show the possibility of capturing a variety of compensatory strategies following central vision loss by extracting different metrics from the same dataset. In particular, the proposed analysis can help define different models of oculomotor behaviors that current analyses might overlook, such as the case of location-nonspecific re-referencing or multiple PRLs. As the clinical population suffering from central vision loss grows, there is a need to better understand the mechanisms underlying spontaneous compensatory strategies and possibly inform clinical interventions. The analysis scheme introduced here can help shed light on the large differences in functional compensatory behavior observed in the MD population and guide individually tailored rehabilitative interventions focused on specific aspects (e.g. fixation stability, re-referencing, etc.). Moreover, by applying these metrics at different time points during the training with the simulated scotoma or during longitudinal observations of patients with MD, this classification can help characterize the time course of the development of the compensatory strategies.
Supplementary Material
Acknowledgments
Commercial relationships: none.
Corresponding author: Marcello Maniglia.
Email: mmanig@ucr.edu.
Address: Department of Neurobiology, University of Alabama at Birmingham, 1720 2nd Avenue South, Birmingham, AL 35294, USA.
References
- Ağaoğlu M. N., Fung W. T., & Chung S. T. L. (2019). What does an “artificial scotoma” simulate? Investigative Ophthalmology & Visual Science, 60, 4379. [Google Scholar]
- Aguilar C., & Castet E. (2011). Gaze-contingent simulation of retinopathy: some potential pitfalls and remedies. Vision Research, 51, 997–1012. [DOI] [PubMed] [Google Scholar]
- Baker C. I., Dilks D. D., Peli E., & Kanwisher N. (2008). Reorganization of visual processing in macular degeneration: replication and clues about the role of foveal loss. Vision Research, 48, 1910–1919, 10.1016/j.visres.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. I., Peli E., Knouf N., & Kanwisher N. G. (2005). Reorganization of visual processing in macular degeneration. The Journal of Neuroscience, 25, 614–618, 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza-Bernal M. J., Ivanov I. V., Nill S., Rifai K., Trauzettel-Klosinski S., & Wahl S. (2017). Can positions in the visual field with high attentional capabilities be good candidates for a new preferred retinal locus? Vision Research, 140, 1–12, 10.1016/j.visres.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Barraza-Bernal M. J., Rifai K., & Wahl S. (2017). Transfer of an induced preferred retinal locus of fixation to everyday life visual tasks. Journal of Vision, 17(14), 2, 10.1167/17.14.2. [DOI] [PubMed] [Google Scholar]
- Bellmann C., Feely M., Crossland M. D., Kabanarou S. A., & Rubin G. S. (2004). Fixation stability using central and pericentral fixation targets in patients with age-related macular degeneration. Ophthalmology, 111, 2265–2270. [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436, 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Castet E., & Crossland M. (2011). Quantifying eye stability during a fixation task: A review of definitions and methods. Seeing and Perceiving, 25, 449–469, doi: 10.1163/187847611X620955. [DOI] [PubMed] [Google Scholar]
- Chung S. T. L. (2013a). Cortical reorganization after long-term adaptation to retinal lesions in humans. The Journal of Neuroscience, 33, 18080–18086, 10.1523/JNEUROSCI.2764-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen F. W., Peters E. M., & Palmer J. (2002). The Eyelink Toolbox: Eye tracking with MATLAB and the Psychophysics Toolbox. Behavior Research Methods, Instruments, & Computers : A Journal of the Psychonomic Society, Inc, 34, 613–617, 10.3758/BF03195489. [DOI] [PubMed] [Google Scholar]
- Crossland M D, Sims M., Galbraith R. F., & Rubin G. S. (2004). Evaluation of a new quantitative technique to assess the number and extent of preferred retinal loci in macular disease. Vision Research, 44, 1537–1546, 10.1016/j.visres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Crossland Michael D., Culham L. E., Kabanarou S. A., & Rubin G. S. (2005). Preferred retinal locus development in patients with macular disease. Ophthalmology, 112, 1579–1585, 10.1016/j.ophtha.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Crossland Michael D., Engel S. A., & Legge G. E. (2011). The preferred retinal locus in macular disease: toward a consensus definition. Retina, 31, 2109–2114, 10.1097/IAE.0b013e31820d3fba. [DOI] [PubMed] [Google Scholar]
- Cummings R. W., Whittaker S. G., Watson G. R., & Budd J. M. (1985). Scanning characters and reading with a central scotoma. Optometry and Vision Science, 62, 833–843, 10.1097/00006324-198512000-00004. [DOI] [PubMed] [Google Scholar]
- Dilks D. D., Baker C. I., Peli E., & Kanwisher N. (2009). Reorganization of visual processing in macular degeneration is not specific to the “preferred retinal locus”. The Journal of Neuroscience, 29, 2768–2773, 10.1523/JNEUROSCI.5258-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret F., Issenhuth M., & Safran A. B. (1999). Combined use of several preferred retinal loci in patients with macular disorders when reading single words. Vision Research, 39, 873–879, 10.1016/S0042-6989(98)00179-5. [DOI] [PubMed] [Google Scholar]
- Fletcher D. C., & Schuchard R. A. (1997). Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmology, 104, 632–638. [DOI] [PubMed] [Google Scholar]
- Fujii G. Y., de Juan E. Jr., Pieramici D. J., Humayun M. S., Phillips S., Reynolds S. M., et al. (2002). Inferior limited macular translocation for subfoveal choroidal neovascularization secondary to age-related macular degeneration: 1-year visual outcome and recurrence report. American Journal of Ophthalmology, 134, 69–74. [DOI] [PubMed] [Google Scholar]
- González E. G., Tarita-Nistor L., Mandelcorn E. D., Mandelcorn, M., Steinbach, M. J. (2011). Fixation control before and after treatment for neovascular age-related macular degeneration. Investigative Ophthalmology & Visual Science, 52(7), 4208–4213. [DOI] [PubMed] [Google Scholar]
- Harrar V., Le Trung V., Malienko A., & Khan A. Z. (2018). A nonvisual eye tracker calibration method for video-based tracking. Journal of Vision, 18(9), 13–13. [DOI] [PubMed] [Google Scholar]
- Kwon M., Nandy A. S., & Tjan B. S. (2013). Rapid and persistent adaptability of human oculomotor control in response to simulated central vision loss. Current Biology, 23, 1663–1669, 10.1016/j.cub.2013.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., & Schuchard R. A. (1997). Using two preferred retinal loci for different lighting conditions in patients with central scotomas. Investigative Ophthalmology & Visual Science, 38(9), 1812–1818. [PubMed] [Google Scholar]
- Liu R., & Kwon M. (2016). Integrating oculomotor and perceptual training to induce a pseudo fovea: A model system for studying central vision loss. Journal of Vision, 16(6), 10, 10.1167/16.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniglia M., Cottereau B. R., Soler V., & Trotter Y. (2016). Rehabilitation approaches in macular degeneration patients. Frontiers in Systems Neuroscience, 10, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midena E., & Pilotto E. (2017). Microperimetry in age: Related macular degeneration. Eye, 31, 985–994, 10.1038/eye.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10, 437–442, 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Rosengarth K., Keck I., Brandl‐Rühle S., Frolo J., Hufendiek K., Greenlee M. W., et al.. . (2013). Functional and structural brain modifications induced by oculomotor training in patients with age‐related macular degeneration. Frontiers in Psychology, 4, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safran A. B., Duret F., Issenhuth M., & Mermoud C. (1999). Full text reading with a central scotoma: pseudo regressions and pseudo line losses. The British Journal of Ophthalmology, 83, 1341–1347, 10.1136/bjo.83.12.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D., & Woods R. (2014). Direct measurement of the system latency of gaze-contingent displays. Behavior Research Methods, 46(2), 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchard R. A., & Fletcher D. C. (1994). Preferred retinal locus: A review with applications in low vision rehabilitation. Ophthalmology Clinics of North America, 7, 243–256. [Google Scholar]
- Schuchard R. A., & Raasch T. W. (1992). Retinal locus for fixation: Pericentral fixation targets. Clinical Vision Sciences, 7, 511–520. [Google Scholar]
- Steinman R. M. (1965). Effect of target size, luminance, and color on monocular fixation*. Journal of the Optical Society of America, 55, 1158, 10.1364/JOSA.55.001158. [DOI] [Google Scholar]
- Timberlake G. T., Mainster M. A., Peli E., Augliere R. A., Essock E. A., & Arend L. E. (1986). Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investigative Ophthalmology and Visual Science, 27, 1137–1147. [PubMed] [Google Scholar]
- Van der Stigchel S., Bethlehem R. A. I., Klein B. P., Berendschot T. T. J. M., Nijboer T. C. W., & Dumoulin S. O. (2013). Macular degeneration affects eye movement behavior during visual search. Frontiers in Psychology, 4, 579, 10.3389/fpsyg.2013.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Noorden G. K., & Mackensen G. (1962). Phenomenology of eccentric fixation. American Journal of Ophthalmology, 53, 642–660. [DOI] [PubMed] [Google Scholar]
- Walsh D. V., & Liu L. (2014). Adaptation to a simulated central scotoma during visual search training. Vision Research, 96, 75–86, 10.1016/j.visres.2014.01.005. [DOI] [PubMed] [Google Scholar]
- White J. M., & Bedell H. E. (1990). The oculomotor reference in humans with bilateral macular disease. Investigative Ophthalmology & Visual Science, 31, 1149–1161. [PubMed] [Google Scholar]
- Whittaker S. G., & Cummings R. W. (1990). Foveating saccades. Vision Research, 30, 1363–1366, 10.1016/0042-6989(90)90009-A. [DOI] [PubMed] [Google Scholar]
- Whittaker S. G., Cummings R. W., & Swieson L. R. (1991). Saccade control without a fovea. Vision Research, 31, 2209–2218. [DOI] [PubMed] [Google Scholar]
- Wong W. L., Su X., Li X., Cheung C. M. G., Klein R., Cheng C.-Y., et al. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. The Lancet Global Health, 2, e106–e116, 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.