Abstract
Chinese caterpillar fungus (Ophiocordyceps sinensis) is a precious traditional medicine which is mostly distributed on the Qinghai-Tibetan Plateau (QTP). Due to its medicinal values, it has become one of the most valuable biological commodities and widely traded in recent years worldwide. However, its habitat has changed profoundly in recent years under global warming as well as anthropogenic pressures, resulting in a sharp decline in its wild population in recent years. Based on the occurrence samples, this paper estimates the potential distribution of caterpillar fungus using MaxEnt model. The model simulates potential geographical distribution of the species under current climate conditions, and examine future distributions under different climatic change scenarios (i.e., RCP 2.6, RCP 4.5, RCP 6.0 and RCP 8.5 have been modelled in 2050s and 2070s, respectively). For examining the impacts of climate change in future, the integrated effects of climatic impact, trading, and overexploitation had been analyzed in detailed routes. The results show that: 1) The distribution patterns of caterpillar fungus under scenario RCP 2.6 have been predicted without obvious changes. However, range shift has been observed with significant shrinks across all classes of suitable areas in Tianshan, Kunlun Mountains, and the southwestern QTP in 2050s and 2070s under RCP 4.5, RCP 6.0 and RCP 8.5 scenarios, respectively. 2) The exports were decreasing drastically in recent years. Guangzhou and Hongkong are two international super import and consumption centres of caterpillar fungus in the world. 3) Both ecological and economic sustainable utilization of the caterpillar fungus has been threatened by the combined pressures of climate change and overexploitation. A strict but effective regulation and protection system, even a systematic management plan not just on the collectors but the whole explore process are urgently needed and has to be issued in the QTP.
Keywords: Ophiocordyceps sinensis, Climate change, Species distribution modelling, MaxEnt, Conservation, Qinghai-Tibetan Plateau
Graphical abstract
1. Introduction
Unsustainable wildlife trade is regarded as a major driver of biodiversity loss and ecosystem degradation (Alacs and Georges, 2008; Toledo et al., 2012; Shrestha and Bawa, 2013). There are ample scientific evidences that demonstrate the detrimental effect of world-wide climatic changes in recent decades (IPCC, 2014), on suitable habitats and even the survivals of certain species, especially the most precious natural medicinal resources (Thomas et al., 2004; Colwell et al., 2008; Jump et al., 2009; Shrestha, 2012; Yan et al., 2017). The Chinese caterpillar fungus (Ophiocordyceps sinensis) is a precious traditional Tibetan medicinal mushroom. In China it is known colloquially as chongcao, a shortened form of dongchong xiacao, which is itself a translation of the Tibetan name yartsa gunbu (“summer-grass, winter-worm”). It is a well-described remedy that has been used in traditional Chinese medicine for over 700 years (Zhong et al., 2009). The fungus is endemic to the Tibetan Plateau and its surroundings, including Tibet, Qinghai, Sichuan, Yunnan provinces in China and Himalayas such as Bhutan, India and Nepal (Li et al., 2011; Yan et al., 2017). The price of natural caterpillar fungus has sharply increased over recent years and is now sold at the price of gold and up to 4 or more times as much for high quality products (Shrestha and Bawa, 2013; Li et al., 2015; Cunningham et al., 2018). It makes this commodity one of the most valuable exports and generates a substantial proportion of regional gross domestic product. Its harvesting has become an important livelihood strategy for mountain communities of Nepal, Bhutan and Tibet in the Tibetan Plateau and its surroundings (Shrestha and Bawa, 2014; Shrestha et al., 2017; Hopping et al., 2018).
Caterpillar fungus possesses a plant-like fruiting body and originates from dead caterpillar that fill with mycelia (Fig. 1 ), it is called chongcao in Chinese due to its insect-shape appearance (Paterson, 2008; Lo et al., 2012). There are 2 critical stages in the growth of Caterpillar fungus. Firstly, Ophiocordyceps sinensis parasitizes underground dwelling larvae of moths (Lepidoptera), especially species of Thitarodes. Secondly, the body of the insect host is infected by the fungus as substrate to form the mycelium and finally converted into a sclerotium, leaving the exoskeleton intact (Paterson, 2008). After the stroma of the fungus grows from the sclerotium and emerges from the ground, it is collected with the sclerotium as a whole for medicine. The larvae of the host insect live underground for their entire larval stage of 3–4 years or longer, the longer growth, the larger size, feeding on roots and caudexes of plants. They usually die in winter after infected by the fungus. And the fungal stroma comes out in earlier April to later May of the following year (Winkler, 2008; Li et al., 2011; Lo et al., 2012). Caterpillar fungus is a slow-growing fungus that needs to be grown at relatively low temperature, i.e., above 2 °C, but the hyphal growth is restrained when the temperature reaches 25 °C and stopped below 0 °C (Li et al., 2011). Both growth rate and restricted habitat are crucial factors identifying it from other similar fungi and the distributions are restricted on the Qinghai-Tibetan Plateau (QTP) (Lo et al., 2012; Dai et al., 2019). As it is difficult to identify the authenticity, some similar substitutes, such as fermented Cordyceps and cultured C. militaris, are usually found in markets (Ikeda et al., 2008; Au et al., 2012).
Fig. 1.
Ophiocordyceps sinensis: (A) and (B) In the natural habitat; (C) Mature Ophiocordyceps sinensis exposed in hand, cleaned of the mycelial velum that usually encloses the larva; (D) Collected mature samples for trade. (The photos were taken by Yanqiang Wei.).
The caterpillar fungus is mostly used as a tonic for boosting the immune system in traditional Chinese medicine. Modern pharmacological studies have shown its therapeutic effects on a wide range of diseases and conditions, such as respiratory, renal, liver, nervous system, and cardiovascular diseases, as well as possible anti-tumor, anti-cancer, and anti-viral activity, immuno-modulating, cholesterol-reducing and anti-oxidant effects, and potential to increase stamina and libido (Winkler, 2008; Zhong et al., 2009; Li et al., 2011; Au et al., 2012; Yan et al., 2014; Hopping et al., 2018; Dai et al., 2019). During the outbreak of the Severe Acute Respiratory Syndrome (SARS) in China in 2003, there was a drastically increase in the use of Ophiocordyceps sinensis (Yan et al., 2014). In recent years, more and more reports manifested that the wild caterpillar fungus was decreasing and couldn't meet the surging demand. This is the direct cause of the dramatically increased price of caterpillar fungus in China, Nepal, India and Bhutan (Shrestha and Bawa, 2013; Shrestha and Bawa, 2014; Shrestha et al., 2017; He, 2018; Hopping et al., 2018; Laha et al., 2018; Pouliot et al., 2018; Wang et al., 2018; Gao et al., 2019). The natural resource of this fungus is limited not only due to its strict host-specificity on moth insects, confined geographical distribution but overexploitation to meet enormous global market demand in recent years, which has severely endangered its wild population and jeopardized the sustainability. It has been listed as an endangered species under the second class of state protection since 1999. Recently, it was furtherly upgraded as an endangered species on the China Biodiversity Red List 2018 (MEE, 2018). Therefore, a status assessment and evaluation of caterpillar fungus is urgently needed for its decreasing production and concerning preservation.
With a booming economy in Tibetan Autonomous Region and its surroundings, it has been of increasing importance to collect and sell economically valuable caterpillar fungus. Given caterpillar fungus is by far the most profitable mushroom on the QTP, its current geographic distribution remains incompletely understood. By examining 203 occurrence localities of caterpillar fungus, Li et al. (2011) suggested they were confined to the QTP and its surroundings, including Tibet, Gansu, Qinghai, Sichuan, and Yunnan in China and in certain areas of the southern flank of the Himalayas, i.e. Bhutan, India and Nepal, no lower than 3000 m a.s.l. According to the previous literatures, it is Winkler (2009) who made the first distribution map of caterpillar fungus with approximate boundaries. The distribution area and core distribution area had been distinguished. He suggested it was distributed in an altitude of 3000–5000 m a.s.l. grass- and shrub-lands that received a minimum of 350 mm average annual precipitation. The current potential distribution and the possible range shifts in response to climate change has been analyzed using an ensemble species distribution modelling, which demonstrated the distribution range of the fungus would decrease significantly, shifting upward in altitude and towards to the central part of the QTP (Yan et al., 2017). Moreover, by integrating local harvester knowledge of production trends with ecological modelling, Hopping et al. (2018) suggested the production had decreased due to the habitat degradation, climate change and especially overharvesting in the Himalayan regions. Up to now, however, the distribution of Ophiocordyceps sinensis is still unclear, and even without a high-resolution distribution map in its main growing place, China. Furthermore, it has been demonstrated much more prominent climatic warming effects on the QTP, especially on the cryosphere and alpine ecosystem (Yao et al., 2012; Wei and Fang, 2013; IPCC, 2014; Yang et al., 2019). In the past few decades, glaciers on the QTP have shrunk drastically, with a significant degradation of permafrost (Yao et al., 2012; Biskaborn et al., 2019; Yang et al., 2019). Subsequently, profound impacts of climate change on ecosystem functioning and services have also been recorded (Lamsal et al., 2017; Zhong et al., 2019). However, biophysical and community level changes of the habit, habitats, density, population and chemical characteristics of caterpillar fungus are still unclear. Meanwhile technological interventions for artificial cultivation of the species are still immature and have been largely unsuccessful (Yan et al., 2014; Pan, 2018). With the boom of caterpillar fungus in Tibet and its surroundings, overexploitation and excessive harvesting are key threats for the species (Winkler, 2009, Winkler, 2010; Shrestha and Bawa, 2014; Shrestha and Bawa, 2015; Shrestha et al., 2017; Cunningham et al., 2018; Hopping et al., 2018). There are widespread concerns about the demand-stimulated economy and the sustainability of the current harvest rates of this species in the future, but the quantitative trends in harvest, trade, supply and demand are not well known. In this study, we conducted extensive sampling across the QTP with the aim of mapping the current distribution of caterpillar fungus in China. Furthermore, the study aimed at evaluating the impacts of climate change, trading and overexploitation on caterpillar fungus, and explore the future changes of it in China.
2. Materials and methods
2.1. Data description
2.1.1. Species occurrence data
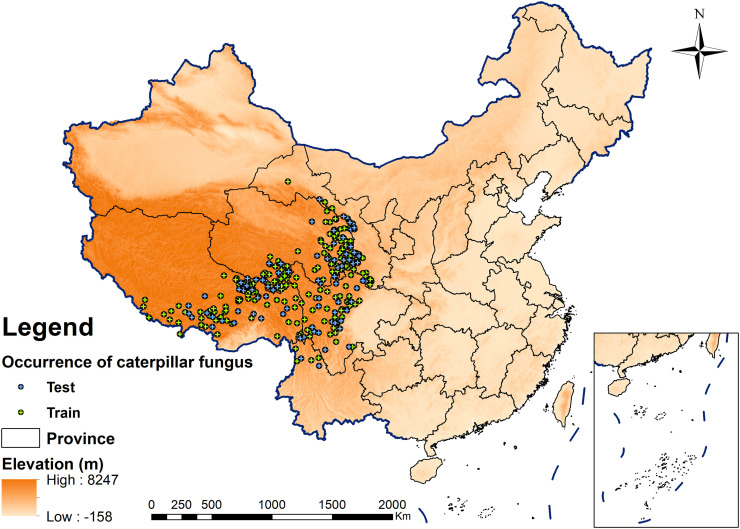
Precise geographic coordinates of sample Ophiocordyceps sinensis individuals are the precondition of suitability modelling and predicting. In this paper, the samples were obtained from field survey reports from the last 10 years, mainly in the specimen libraries, e.g. Global Biodiversity Information Facility (https://www.gbif.org/), Chinese Virtual Herbarium (http://www.cvh.ac.cn/), China National Specimen Information Infrastructure (http://www.naii.org.cn), and the reports in literatures. Reasonable sampling sites were selected based on the following principles: First, only one record was retained for replicated site data and the distance between two sampling points was more than 10 km. Second, the sampling sites must have precise latitude and longitude information to ensure geographic accuracy. Third, the sampling sites were selected based on different environmental conditions, e.g., slope, aspect, elevation, vegetation type, and distributed evenly to ensure independence of the species observations. These standards profoundly minimize the spatial autocorrelation of sampling points and reduce its errors in the model's results. Finally, 301 records of species occurrence data were obtained for analysis (Fig. 2 , they are available by contacting the corresponding author).
Fig. 2.
Geographic locations of caterpillar fungus occurrences in China.
2.1.2. Environmental data
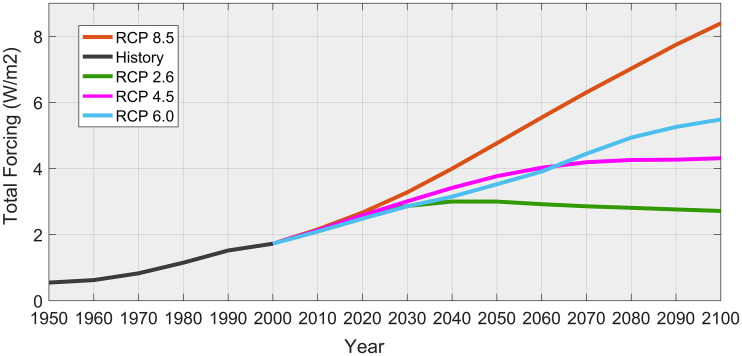
The 18 soil and 3 terrain indicators came from China Soil Map Based Harmonized World Soil Database (HWSD v1.1, https://data.tpdc.ac.cn/zh-hans/). The 18 soil datasets are 30 arc-second (1 km) raster databases with GRID formats. The DEM (Digital Elevation Model) data with cell size 90 m * 90 m were downloaded from the WIST geo-database of NASA (http://srtm.csi.cgiar.org/), was used to generate the slope, aspect and elevation data layers. The 19 bioclimatic indicators were selected from WorldClim-Global Climate Database (WorldClim 1.4, http://worldclim.org/). We resampled all of the variables at a 30″ (approximately 1 km2) spatial resolution (Table 1 ). These variables are the most widely used climatic variables in species potentially distribution models. Future bioclimatic variables for the 2050s and 2070s were from 4 IPCC-CMIP5 representative concentration pathways (RCPs). RCP 2.6, RCP 4.5, RCP 6.0 and RCP 8.5 represent the full possible range of total radiative forcing values +2.6, +4.5, +6.0 and + 8.5 W/m2, respectively, in the year 2100 relative to pre-industrial values (Fig. 3 ). The climate scenario data were provided by the International Center for Tropical Agriculture (http://ccafs-climate.org). They were derived from three global climate models (GCMs: CCSM4, BCC–CSM1–1, and MIROC5). CCSM4 is an efficient global climate tool for the simulation of future climatic conditions, which covers daily mean surface temperature, maximum temperature, minimum temperature, precipitation, etc. and has been thoroughly evaluated in China and successfully applied to predict the influence of future climate changes on the distribution of plant species in similar environments (Abdelaal et al., 2019; Li et al., 2020).
Table 1.
Environmental indicators used in this paper to model the potential distribution of Ophiocordyceps sinensis. There are 18 soil and 3 terrain indicators, and 19 bioclimatic indicators.
| Index | Name | Mean | Unit |
|---|---|---|---|
| Bioclimatic variables | Bio 1 | Annual mean temperature | °C |
| Bio2 | Mean diurnal range | °C | |
| Bio3 | Isothermality (BIO2/BIO7) (* 100) | % | |
| Bio4 | Temperature seasonality (standard deviation *100) | °C | |
| Bio5 | Max temperature of warmest month | °C | |
| Bio6 | Min temperature of coldest month | °C | |
| Bio7 | Temperature annual range (BIO5-BIO6) | °C | |
| Bio8 | Mean temperature of wettest quarter | °C | |
| Bio9 | Mean temperature of driest quarter | °C | |
| Bio10 | Mean temperature of warmest quarter | °C | |
| Bio11 | Mean temperature of coldest quarter | °C | |
| Bio12 | Annual precipitation | mm | |
| Bio13 | Precipitation of wettest month | mm | |
| Bio14 | Precipitation of driest month | mm | |
| Bio15 | Precipitation seasonality (coefficient of variation) | 1 | |
| Bio16 | Precipitation of wettest quarter | mm | |
| Bio17 | Precipitation of driest quarter | mm | |
| Bio18 | Precipitation of warmest quarter | mm | |
| Bio19 | Precipitation of coldest quarter | mm | |
| Terrain variables | ASL | Elevation | m |
| SLOP | Slope | ° | |
| ASPE | Aspect | ° | |
| Top soil variables | AWC_CLASS | AWC range | |
| T_GRAVEL | Topsoil gravel content | %vol | |
| T_SAND | Topsoil sand fraction | % wt | |
| T_SILT | Topsoil silt fraction | % wt | |
| T_CLAY | Topsoil clay fraction | % wt | |
| T_USDA_TEX_CLASS | Topsoil USDA texture classification | name | |
| T_REF_BULK_DENSITY | Topsoil reference bulk density | kg/dm3 | |
| T_ BULK_DENSITY | Topsoil bulk density | kg/dm3 | |
| T_OC | Topsoil organic carbon | % weight | |
| T_PH_H2O | Topsoil pH (H2O) | -log(H+) | |
| T_CEC_CLAY | Topsoil CEC (clay) | cmol/kg | |
| T_CEC_SOIL | Topsoil CEC (soil) | cmol/kg | |
| T_BS | Topsoil base saturation | % | |
| T_TEB | Topsoil TEB | cmol/kg | |
| T_CACO3 | Topsoil calcium carbonate | % weight | |
| T_CASO4 | Topsoil gypsum | % weight | |
| T_ESP | Topsoil sodicity (ESP) | % | |
| T_ECE | Topsoil salinity (Elco) | dS/m |
Fig. 3.
Global total radiative forcing scenarios of every representative concentration pathways (RCPs: RCP 2.6 W/m2, RCP 4.5 W/m2, RCP 6.0 W/m2 and RCP 8.5 W/m2).
2.1.3. Socio-economic data
The products and socio-economic statistical data of every county were obtained from the annual statistic yearbooks (1983–2018) of the Tibet (http://www.stats.gov.cn) and the annual China county (city) social economic statistic yearbooks (Department of Rural Socioeconomic Investigation, 1983-2019) of the Tibet. The export amounts of China were from China General Administration of Customs (http://www.customs.gov.cn/). The prices were based on the annual average prices on the website of Chinese herbal medicine information (https://www.zyctd.com/). Although caterpillar fungus is mainly distributed in the QTP, its harvest and trade are controlled by local natives. It is impossible to collect the production and trade volumes year by year in each county of QTP. Nagqu prefecture is a main production area of caterpillar fungus in Tibet. As a representative production area in QTP, we take it as an example for manifesting the fortunes of other most precious natural medicinal resources in QTP. The national basic geoinformatics for mapping, e.g., administrative boundaries, were obtained from the National Platform for Common Geospatial Information Services with the scale of 1:1 000 000 (http://bzdt.ch.mnr.gov.cn/).
2.2. Model process
2.2.1. Model settings
Maximum entropy (MaxEnt) modelling is one of the most currently popular distribution models (Phillips et al., 2004; Phillips, 2011). It is based on freely available and user-friendly MaxEnt.jar software V3.4.1 (https://www.cs.princeton.edu/schapire). MaxEnt can alternatively be explained as a machine-learning method (Elith et al., 2011; Phillips, 2011), by principles of Bayesian estimation (Elith et al., 2011; Merow et al., 2013), and as a maximum likelihood estimation method (Halvorsen et al., 2015). The algorithm evaluate the most probable potential geographic distribution of a species based on the relationship between the geographical data and the known distribution of the target species (Merow et al., 2013; Halvorsen et al., 2015; Halvorsen et al., 2016). It has a relatively high modelling robustness as compared to other distribution models, in situations with small number of occurrence samples and unclear correlations among bioclimatic variables (Phillips, 2011; Halvorsen et al., 2016).
As a unique growth forms of fungal species, Ophiocordyceps sinensis do not grow in the absence of suitable vegetation and soil conditions even if the climatic and topographical environment are favourable. Two distribution models have been utilized in this paper. One is for simulating the migration trend of the Ophiocordyceps sinensis distribution under climate change scenarios and the other is for comprehensive prediction of the potential current geographic distribution considering limiting ecological factors. Comprehensive prediction model adopts all of the 18 soil and 3 terrain indicators and together with 19 bioclimatic indicators (Table 1) for predicting the current potential suitable habitats distribution of Ophiocordyceps sinensis. However, only 10 indicators (Table 2 ) which were selected by PCA analysis have been adopted in the prediction model for simulating the migration trend of the Ophiocordyceps sinensis distribution under climate change scenarios. There are 301 longitude-latitude geoinformation samples in this study. Randomly 75% of the occurrence records were selected as the training data in MaxEnt model, and the other 25% of the samples were used as the test set (Fig. 2). The regularization multiplier was set at “2” to reduce overfitting and the maximum iterations as 1000 to allow more time for convergence (threshold 0.00001). To minimize the uncertainty of the random sampling in MaxEnt and reduce the errors in the results, the process was repeated 10 times to generate an averaged result for subsequent analyses. Other parameter settings for the MaxEnt model were set according to the MaxEnt software Tutorial (Phillips, 2011).
Table 2.
The contribution proportions and cumulative proportion contributions of the top 10 indicators in principle component analysis (PCA).
| Variable | Meaning | Percentage of variance (%) | Cumulative percentage (%) |
|---|---|---|---|
| ASL | Elevation (m) | 60.5 | 60.5 |
| Bio18 | Precipitation of warmest quarter (mm) | 16.6 | 77.1 |
| Bio11 | Mean temperature of coldest quarter (°C) | 3.9 | 81.0 |
| T_TEB | Topsoil TEB (cmol/kg) | 3.4 | 84.4 |
| Bio12 | Annual precipitation (mm) | 3.3 | 87.7 |
| Bio6 | Min temperature of coldest month (°C) | 2.3 | 90.0 |
| T_CASO4 | Topsoil gypsum (% weight) | 1.2 | 91.2 |
| T_CEC_CLAY | Topsoil CEC (clay) (cmol/kg) | 1.1 | 92.3 |
| Bio14 | Precipitation of driest month (mm) | 0.8 | 93.1 |
| AWC_CLASS | AWC range | 0.8 | 93.9 |
2.2.2. Model evaluation
Previous studies have demonstrated that a serious multicollinearity problem exists among the bioclimatic variables (Yang et al., 2013; Guisan et al., 2017; Guo et al., 2019). In this paper, we applied principle component analysis (PCA) to select a subset of the environmental variables (Guo et al., 2019). We finally selected 10 most representative indicators, i.e., Elevation (gloelev), Precipitation of Warmest Quarter (bio_18), Mean Temperature of Coldest Quarter (bio_11), Topsoil TEB (t_teb), Annual Precipitation (bio_12), Min Temperature of Coldest Month (bio_06), Topsoil Gypsum (t_caso4), Topsoil CEC (clay) (t_cec_clay), Precipitation of Driest Month (bio_14) and AWC Range (awc_class) as the modelling factors (Table 2). The accumulated contribution of the top 10 indicators to the model was up to 93.9%. The Pearson correlation coefficient (r) was used to examine the cross-correlation of these 10 variables, and the results manifested that all of the correlation coefficients of these variables were less than 0.7.
The accuracy of each model prediction was quantified by calculating the area under the Receiver Operating Characteristic (ROC) curve (AUC) (Fielding and Bell, 1997; Lobo et al., 2008). The ROC curve has been recommended because it summarises model performance over all conditions a model could operate in (Swets, 1988), using all the information provided by the predictive model (Fielding and Bell, 1997). AUC is the area between ROC and x-axis and its value is [0.5, 1]. The higher the value, the higher accuracy of the model. Generally, AUC values below 0.7 were considered as poor, values between 0.7 and 0.9 were moderate and higher than 0.9 were considered as high accuracy (Marmion et al., 2009; Franklin, 2010; Halvorsen et al., 2015).
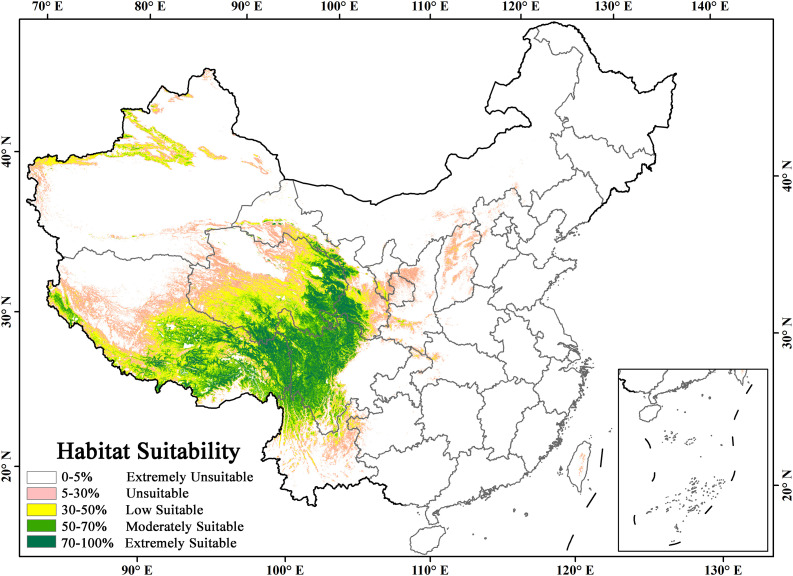
The MaxEnt results for Ophiocordyceps sinensis niche suitability index ranged from 0 to 100% (Fig. 4 ), and 0–5% was regarded as extremely unsuitable, 5–30% was regarded as unsuitable, 30–50% was regarded as low suitable, 50–70% was regarded as moderately suitable, 70–100% was regarded as extremely suitable (Guo et al., 2019; Liu et al., 2019a).
Fig. 4.
The potential and suitable habitats distribution of Ophiocordyceps sinensis in China.
3. Results and analyses
3.1. Model accuracy
The mean test AUC and mean training AUC obtained from the final model were 0.970 and 0.980 in current and future predictions respectively, while the random prediction AUC was 0.5, denoting the model had high accuracy and good performance for modelling the geographic distributions of Ophiocordyceps sinensis.
3.2. Critical environmental factors
Of the top 10 variables (Table 2) used for modelling, the most significant factors affecting the spatial distribution of Ophiocordyceps sinensis were elevation (60.5% of variation), precipitation of warmest quarter (Bio18, 16.6% of variation), and mean temperature of coldest quarter (Bio11, 3.9% of variation). The cumulative contribution of the top 3 factors were up to 81.0%. Elevation was the most critical factor determining the distribution of Ophiocordyceps sinensis. The following factors were bioclimatic precipitation of warmest quarter and mean temperature of coldest quarter. It manifested Ophiocordyceps sinensis was a high climate sensitive fungal species and much dominated by the climate regimes especially the summer precipitation and winter mean temperature. Of the top 10 factors, high contributions of annual precipitation (Bio12, 3.4% of variation) and minimum temperature of coldest month (Bio6, 2.3% of variation) demonstrated the importance of precipitation and winter temperature again. Another bioclimatic factor was precipitation of driest month (Bio14, 0.8% of variation). These climatic factors demonstrated Ophiocordyceps sinensis was a high precipitation and temperature sensitive fungal species, climate was its dominating niche factor. For the top soil conditions, only 4 variables were on the list of top 10 indicators. Additionally, all of them had low contribution. The cumulative contributions of Topsoil TEB (T_TEB, 3.4%), Topsoil Gypsum (T_CASO4, 1.2%), Topsoil CEC (T_CEC_CLAY, 1.1%) and AWC Range (AWC_CLASS, 0.8%) were only 6.5% of the total variation. The results manifested that topsoil conditions had very limited influences on the spatial distribution of Ophiocordyceps sinensis.
3.3. The current potential distribution of Ophiocordyceps sinensis
Based on the suitability classification, the Ophiocordyceps sinensis habitats distribution was reclassified into 6 grades. The potential and suitable habitats distribution of Ophiocordyceps sinensis is illustrated in Fig. 4. The areas with habitats suitability above 30% are mainly located on the Qinghai-Tibetan plateau (QTP). We took suitability index 30% as the suitable habitat threshold. The area of suitable habitats is 0.692 × 106 km2, and the area of the moderately and extremely suitable habitats is 0.285 × 106 km2. They are mainly located in Qilian mountains in the northeastern QTP, Gannan prefecture in Gansu province, Ganzi and Aba prefectures in Sichuan province, northwestern Yunnan province, Yushu and Guoluo prefectures in Qinghai province, Qamdo, Nyingchi and Nagqu in central and eastern Tibet. Interestingly, we found that a few habitats of moderate and extreme suitability are distributed sporadically in Tianshan mountains in Xinjiang province. These results indicate that the mountainous regions with high elevations are the main distribution areas for Ophiocordyceps sinensis. We found the moderately and extremely suitable habitats were mainly located in the southeast of the line of Gannan-Guoluo-Yushu-Nagqu, which is the isotherm of 400 mm annual mean precipitation on QTP. They converge on high precipitation and humidity areas on the QTP.
According to the distributions, the statistical threshold values (suitability index, >0.3) and optimal ranges (suitability index >0.5) for each critical environmental variable were calculated (Table 3 ). The threshold elevation is 857 m to 6076 m, the optimal elevation ranges from 979 m to 6043 m and the average is 3865 m. The precipitation of warmest quarter ranges from 16 mm to 1205, the optimal ranges are 16 mm to 900 mm and its average is 338 mm. The annual precipitation ranges from 21 mm to 1969 mm, the optimal ranges are 21 mm to 1542 mm and its average is 579 mm. However, the precipitation of driest month ranges from 0 mm to 20 mm and the average is only 3 mm. There are only 2 temperature related indicators. Of which, the mean temperature of coldest quarter has a high contribution to the model. It ranges from −25.2 °C to 10.3 °C, the optimum ranges are −22.8 °C to 8.6 °C and the average is −7.1 °C. From the above, we could conclude that high elevations with high precipitation and low temperature are much favourable for Ophiocordyceps sinensis. The soil statistical results demonstrate that the optimal topsoil total exchangeable bases (T_TEB, stand for the sum of exchangeable cations in a soil: sodium (Na), calcium (Ca), magnesium (Mg) and Potassium (K)) range from 1.6 cmol/kg to 68.2 cmol/kg, the average is 12.1 cmol/kg. Topsoil Gypsum (T_CASO4) ranges from 0 to 1.8%, its average is 0.02%. They both manifest a low content of organic nutrition. However, the cation exchange capacity in topsoil (T_CEC_CLAY) ranges from 8 cmol/kg to 109 cmol/kg, its average is 57.4 cmol/kg. It ranks in class 5 with high nutrient fixing capacity of the topsoil. The mode of topsoil available water capacity classes (AWC_CLASS) is 5, accordingly, the optimal available water capacity is 50 mm/m (Table 3). It manifests a relatively moderate water capacity of the topsoil. As the dominated soil types of Southeastern QTP is alpine meadow leptosols (about 45%) and cryosols (about 25%) (FAO, 2012), all of these soil features indicate that the unique characteristics of it is favourable for Ophiocordyceps sinensis on the QTP and its surroundings.
Table 3.
The threshold values (suitability index >30%) and optimal ranges (suitability index >50%) for each critical environmental variable.
| Variable | Meaning | Threshold values (SI > 30%) |
Optimal ranges (SI > 50%) |
||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | ||
| ASL | Elevation (m) | 857 | 6076 | 3977 | 979 | 6043 | 3865 |
| Bio18 | Precipitation of warmest quarter (mm) | 16 | 1205 | 344 | 16 | 900 | 338 |
| Bio11 | Mean temperature of coldest quarter (°C) | −25.2 | 10.3 | −7.4 | −22.8 | 8.6 | −7.1 |
| T_TEB | Topsoil TEB (cmol/kg) | 1.6 | 68.2 | 11.5 | 1.6 | 68.2 | 12.1 |
| Bio12 | Annual precipitation (mm) | 21 | 1969 | 582 | 21 | 1542 | 579 |
| Bio6 | Min temperature of coldest month (°C) | −30.5 | 2.4 | −16.9 | −28.2 | 0.7 | −16.8 |
| T_CASO4 | Topsoil gypsum (% weight) | 0 | 1.8 | 0.03 | 0 | 1.8 | 0.02 |
| T_CEC_CLAY | Topsoil CEC (clay) (cmol/kg) | 8 | 109 | 50.3 | 8 | 109 | 57.4 |
| Bio14 | Precipitation of driest month (mm) | 0 | 20 | 3 | 0 | 19 | 3 |
| AWC_CLASS | AWC range | 1 | 7 | 5 | 1 | 6 | 5 |
3.4. Potential distributions under global warming scenarios
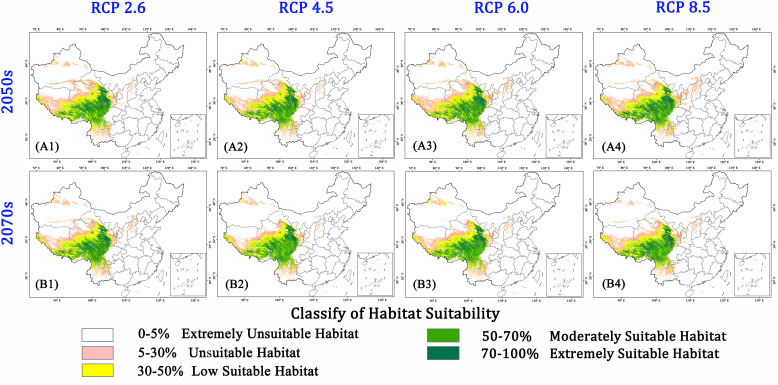
To understand the implications of climate change on Ophiocordyceps sinensis habitats, we used 8 scenarios (4 RCPs each for 2050s and 2070s) to model habitats suitability. The results showed that the distribution range of Ophiocordyceps sinensis had a significant reduction in the future (Fig. 5 ). The most similar spatial distribution patterns to present are the RCP 2.6 scenarios. Both of the distributions in 2050s and 2070s have very slight changes comparing to present (Fig. 5A1, B1). As RCP 2.6 is the least emission scenario leading to very low greenhouse gas concentration levels, global warming is not significant in this situation (Fig. 3). Model results suggest that the distribution patterns of RCP 2.6 have not obvious changes. However, significant range shrinks could be observed in parts of Tianshan, Kunlun Mountains, and southwestern QTP in 2070s in RCP 4.5 and RCP 6.0 (Fig. 5A2, B2, A3, B3). All classes of suitable area of Ophiocordyceps sinensis would significantly reduce, especially the moderately and extremely suitable habitats. While comparing the two scenarios, range shrink would be lesser in the RCP 8.5 scenarios. Few regions would experience a contraction in range in the 2070s under the RCP 8.5 scenarios. The area of unsuitable habitat was higher in the 2070s in RCP 4.5 and RCP 6.0 scenarios (Fig. 5A4, B4). There would be no occupancy in Tianshan, Kunlun Mountains, and southwestern QTP. Whereas the east and southeast of QTP would be a suitable habitat for Ophiocordyceps sinensis in every scenario. RCP 4.5 and RCP 6.0 are two similar moderate stabilization scenarios where total radiative forcing is slowly increasing and stabilized after 2100. RCP 8.5 is characterized by continuing increasing greenhouse gas emissions overtime in the literature leading to the highest concentration levels (Fig. 3). Unexceptionally, all of the areas of predicted suitable habitats under these scenarios demonstrate a shrink trend. Although the area contraction in 2070s under the RCP 8.5 scenarios is the least, the shrinking trends demonstrate the future climate conditions are not favourable for Ophiocordyceps sinensis. The future climatic environment will jeopardize the suitable habitats of Ophiocordyceps sinensis. Undoubtedly, there are many uncertainties in these scenarios. Further analyses are highly needed for these contractions.
Fig. 5.
The habitat suitability of caterpillar fungus populations in China under 4 climate scenarios (RCP 2.6, RCP 4.5, RCP 6.0 and RCP 8.5) in the year 2050s (A1-A4) and 2070s (B1-B4).
4. Discussions
During the whole growth of caterpillar fungus, the most critical stage is that the body of the insect host is infected by the fungus as substrate to form the mycelium and finally converted into a sclerotium. However, until now, we have very limited knowledge about this process and that is the quite reason why caterpillar fungus cultivations in laboratory are always failed and it is difficult to infect the body of the insect host artificially by the fungus as substrate to form the mycelium (Paterson, 2008; Zhong et al., 2009; Lo et al., 2012; Yan et al., 2014). Therefore, most part of caterpillar fungus grows naturally and is collected in wild field by native residents. In this paper, we only discussed the potential and suitable habitats distribution of Ophiocordyceps sinensis in China by MaxEnt modelling. All of the samples only come from our field investigation and literature reports of caterpillar fungus. As MaxEnt is good at the modelling of plant and fungus, the validations of distribution modelling of animal and moth are very limited (Elith et al., 2011; Phillips, 2011; Merow et al., 2013). Additionally, we have very limited knowledges and field investigated data about moths (Lepidoptera), especially species of Thitarodes (Paterson, 2008; Zhong et al., 2009; Au et al., 2012; Lo et al., 2012; Zhang et al., 2012; Yan et al., 2014). It is difficult to model the distribution of Thitarodes Larvae and examine its sensitivity to climate change and its influence on caterpillar fungus. This is one of the first studies to investigate the impact of climate change on the potential distribution of caterpillar fungus in China. Based on the results, three issues are discussed in the paper.
4.1. Distribution trend
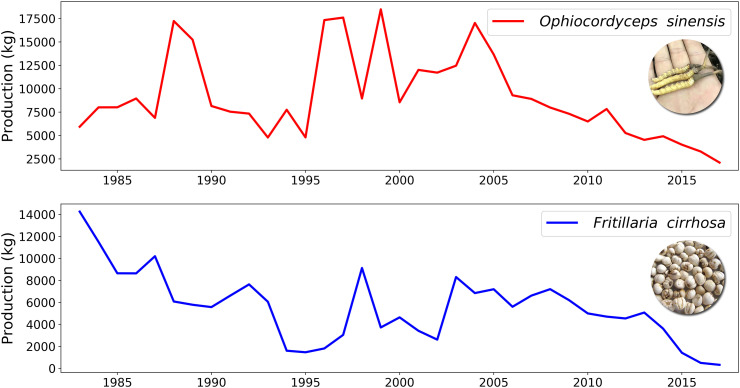
The QTP is the main distribution area of Ophiocordyceps sinensis in China. Our results suggest that suitable habitats for O. sinensis are likely to shrink in the coming decades under the climate change scenarios RCP 4.5, RCP 6.0 and RCP 8.5. Future climate conditions would jeopardize the suitable habitats of caterpillar fungus. Actually, the real distribution area and production of caterpillar fungus has been reducing sharply in recent years (He, 2018; Hopping et al., 2018). Similarly, several other rare traditional Tibetan and Chinese herbal medicines face similar threats due to overexploitation and habitat loss (Shrestha and Bawa, 2013; Cunningham et al., 2018; Gao et al., 2019). Fig. 6 illustrates the productions of Ophiocordyceps sinensis and Fritillaria cirrhosa in Nagqu prefecture, a representative main production area of caterpillar fungus in Tibet, during 1983 to 2017. The production of caterpillar fungus was 18,490.55 kg in 1999, which was peak production and the tipping point during the last decades. Prior to 1999 production of caterpillar fungus was increasing drastically. However, post 1999 production values have shown a steady and sharp decline year by year and had never reached the record in history after 1999. In 2017 total production reached an all time low of 2105.9 kg, which was merely 11.39% of the production in 1999, and broke the lowest record in history (Fig. 6). Similar losses in net production has been observed in another famous Chinese herbal medicine Fritillaria cirrhosa. Unlike caterpillar fungus, the production of Fritillaria cirrhosa was continually diminishing during 1983 to 2017. The production in 1983 was 14,260.9 kg, however, the production in 2017 was only 325.7 kg, 2.28% of the record in history (Fig. 6). Until now, there have been no signs of potential increase in natural production of these medical plants, with several reports suggesting that these two species are at high risk of extinction. If the diminishing trend continued, there would be no more wild caterpillar fungus and Fritillaria cirrhosa in the coming decades in China (Cunningham et al., 2018; Hopping et al., 2018; Gao et al., 2019). Therefore, many studies promote prohibiting the harvesting of wild natural caterpillar fungus and protecting and encouraging artificial cultivation (Pan, 2018; Wang et al., 2018; Liu et al., 2019b). Our results showed the ranges of suitable habitats would shrink significantly in scenarios of RCP 4.5, RCP 6.0 and RCP 8.0 in 2050s and 2070s, respectively. The vulnerability and risk for caterpillar fungus is likely to increase in the coming decades. Although there are lots of uncertainties in these scenarios, the influencing aspects should be analyzed closely.
Fig. 6.
Total productions of caterpillar fungus (upper) and Fritillaria cirrhosa (lower) in the main production area Nagqu Prefecture in Tibet, China from 1983 to 2017.
4.2. Climate changes
The PCA results showed there were 5 climate related indicators in the top 10 critical environmental factors, i.e., precipitation of warmest quarter (Bio18, 16.6% of variation), mean temperature of coldest quarter (Bio11, 3.9% of variation), annual precipitation (Bio12, 3.4% of variation), minimum temperature of coldest month (Bio6, 2.3% of variation), and precipitation of driest month (Bio14, 0.8% of variation). The cumulative contribution of them were 37%. Therefore, climate change should be considered as a primary and critical factor influencing the distribution of caterpillar fungus in future. The model results manifested a moderate elevation (979 m to 6043 m, the mean is 3865 m) with high humidity (the annual precipitation is 21 mm to 1542 mm, the mean is 579 mm) and low temperature (the mean temperature of coldest quarter is −22.8 °C to 8.6 °C, the mean is −7.1 °C) were much favourable for Ophiocordyceps sinensis. These climatic conditions restrict caterpillar fungus to high altitudes of the Himalayas and Tibet. However, the Himalaya and High Asian regions, especially at the “third pole”, with the high vulnerability to climate change, are the most sensitive and representative areas of global warming. At present, global warming is accelerating and a continual temperature increase is forecasted (IPCC, 2014). To examine the trend of climate change, we took the gridded 30″ × 30″ (approximately 1 km2) spatial resolution CCSM4 climate scenarios to analyze the spatial variation of climate change. A moderate greenhouse gas scenario RCP 4.5 had been selected. Two time periods 2050 (average for 2041–2060) and 2070 (average for 2061–2080) were analyzed in this paper.
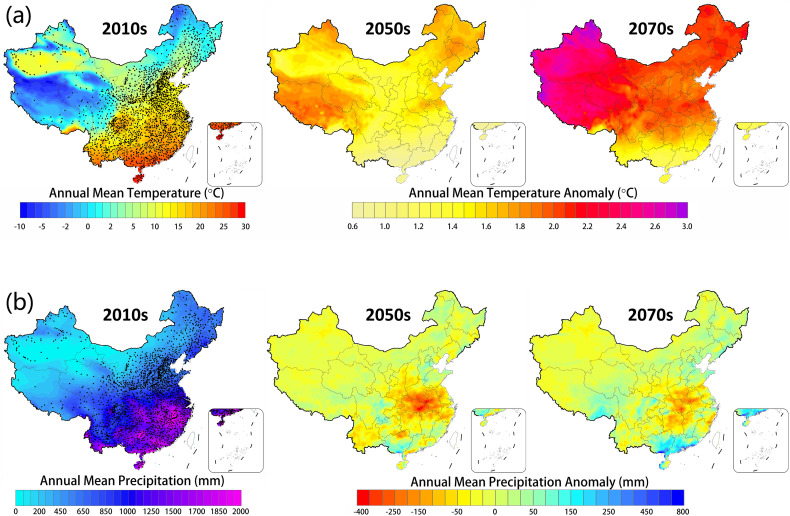
From the spatial mean temperature change map (Fig. 7a), we observe that entire China will undergo significant warming in the future. By 2050s, the annual mean temperature of China will increase about 0.6 °C to 2.0 °C with reference to present mean level. The QTP is likely to witness most significant warming in China, where annual mean temperature anomaly will to be 1.6 °C to 2.0 °C with reference to present; markedly higher than its surroundings and any other regions of China (Fig. 7a). By 2070s, the annual mean temperature of China will increase 1.2 °C to 3.0 °C in reference to present. Most places will show a temperature increase of 2.0 °C or more, while the most prominent changes would be observed in the western China, especially the QTP and its surroundings. The annual mean temperature anomaly of QTP will reach up to 2.2 °C to 3.0 °C in 2070s and the thoroughly warming trend is very significant and robust (Fig. 7a). However, there is no significant trend of annual mean precipitation in 2050s and 2070s (Fig. 7b). The annual mean precipitation of QTP will show a minor increase by 0 to 30 mm in 2050s, and 0 to 50 mm in 2070s with reference to present. Although there are considerable uncertainties in these scenarios, the simulated climate conditions in 2050s and 2070s demonstrate that the entire QTP will undergo significant warming in the coming decades. Considering the trend of precipitation in future, we can expect that in future the QTP will become warmer and drier. Our results manifest a humid and cold environment is more favourable for Ophiocordyceps sinensis, therefore, the warmer and drier climate will aggravate the deteriorating trends of habitats and increase its survival risk. This is a big threat to Ophiocordyceps sinensis in the coming decades.
Fig. 7.
(a) The annual mean temperature in 2010s and its anomalies in 2050s (average for 2041-2060) and 2070s (average for 2061-2080), respectively. (b) The annual mean total precipitation in 2010s and its anomalies in 2050s and 2070s, respectively. Note that the greenhouse gas scenario in this figure is RCP 4.5, the dots are meteorological stations.
Many studies suggested global warming is a big challenge for the species in alpine mountains. It is one of the main reasons of species redistribution, suitable habitat loss and species extinction (Thomas et al., 2004; Lewis, 2005; He and Hubbell, 2011; Ahmadi et al., 2019; Wang et al., 2019). Hopping et al. (2018) report that caterpillar fungus was more productive under colder conditions and growing in close proximity to permafrost. With significant warming, the populations had been negatively affected by climate change. An investigation demonstrated 95.1% of the harvesters perceived that caterpillar fungus was less abundant than before in Dolpa region of Nepal, a warmer-drier climate (annual average temperature increase rate is 0.04 °C per year, annual precipitation decrease rate is 3.3 mm per year during 1961 to 2007) and overharvesting were considered as the main reasons (Shrestha and Bawa, 2015). Although there were many relevant studies that mentioned the influence of climate change on caterpillar fungus, very few of them had any definite numerical analyses (Paterson, 2008; Winkler, 2008; Weckerle et al., 2010; Winkler, 2010; Shrestha and Bawa, 2013; Shrestha et al., 2017; Pan, 2018; Pouliot et al., 2018; Wang et al., 2018; Dai et al., 2019). For adapting to the warming environment, an interesting consensus is that, species range always shifts poleward or towards higher elevations (Colwell et al., 2008; Jump et al., 2009; Hughes, 2011; Diez et al., 2020). Therefore, high altitude mountainous areas are always refuges for species sensitive to global warming (Beardall et al., 2009; Harley and Paine, 2009; Diez et al., 2020). Yan et al. (2017) suggested the distribution range of caterpillar fungus would shrink significantly, shifting to high altitudes and concentrated on the central part of the QTP. However, this kind of study is very rare. In this study we predict that future climate on the QTP would be warmer and drier in the coming decades, which is likely to exacerbate the vulnerability of caterpillar fungus in response to the new climate regime. A strategy for adapting to global warming is to migrate towards higher elevations. However, the suitable habitat areas will be narrowed accordingly with altitudes increase. That is the main reason why the suitable habitat areas of caterpillar fungus are shrinking continually either in the past or in the future climate scenarios. If the altitude is very limited, there would be a very narrow space for them to immigrate. This would be a major challenge for species that prefer cold climate, in future climate change scenarios. An example is the Australian continent, a remarkably flat region with 99% of the land area is less than 1000 m asl., and few summits exceed 2000 m asl., with the highest peak, Mt. Kosciuszko, at only 2228 m asl. The lack of topographic relief limits the ability of many alpine species to shift to higher elevations as temperatures increase (Hughes, 2011). However, in order to ascertain the distribution of a species in future it is pertinent to evaluate potential uncertainties in future climate scenarios, and the influence of other non-bioclimatic factors on species distribution.
4.3. Anthropogenic influence and trading
Although climatic influence is a critical factor in determining species distribution, in recent decades anthropogenic activities have played a more and more critical role in caterpillar fungus degeneration. Caterpillar fungus is one of precious Chinese traditional medicines. Its high nutritious and medicinal functions stimulate the boom of overharvesting, commercially trading, and even illegally smuggling behind its high profits. An investigation demonstrated harvesting of caterpillar fungus had become an important livelihood strategy for mountain communities of Nepal. The income was the second largest contributor to the total household income after farm income with 21.1% contribution to the total household income and 53.3% to the total cash income (Shrestha and Bawa, 2014). The earnings from caterpillar fungus contributed 60–78% to the annual household income of collectors, with noncollectors earning 15–55% less than collectors (Laha et al., 2018). On average, 40% of the rural cash income in Tibet was derived from collection of caterpillar fungus (Winkler, 2008, Winkler, 2010). With increasing market demand, the price has surged accordingly, with a 350% increase in the price paid to pickers between 1997 and 2004 (Winkler, 2008). Shrestha and Bawa (2013) reported that the price of caterpillar fungus in local market of Nepal have risen up to 2300% between 2001 and 2011. Many studies report drastic decrease of caterpillar fungus due to extensive overexploitation. By using a multiple-evidence based approach that makes use of complementarities between local knowledge and ecological modelling, Hopping et al. (2018) found caterpillar fungus production had decreased due to habitat degradation, climate change, and especially overexploitation. Another report revealed that caterpillar fungus abundance in the Himalayas was dwindling, the average harvest per collector dropped by around half between 2006 and 2010 (Shrestha, 2012). After legalization of trade in Nepal in 2001, trade volume increased persistently, 95.1% of the harvesters believed that availability of the caterpillar fungus in the pastures was declining, and 67% considered current harvesting practices to be unsustainable (Shrestha and Bawa, 2013). Besides that, an increase in the number of harvesters has led to an observed decline in individual harvests (Laha et al., 2018).
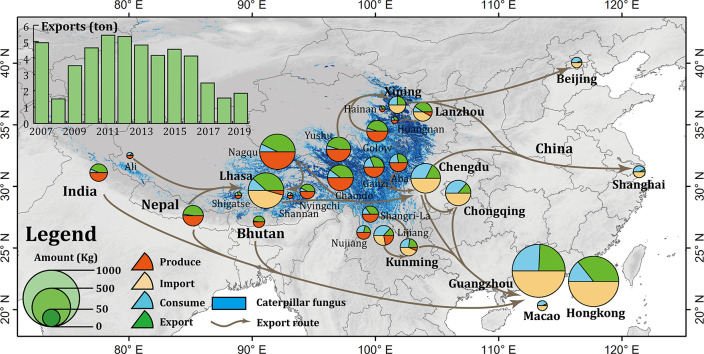
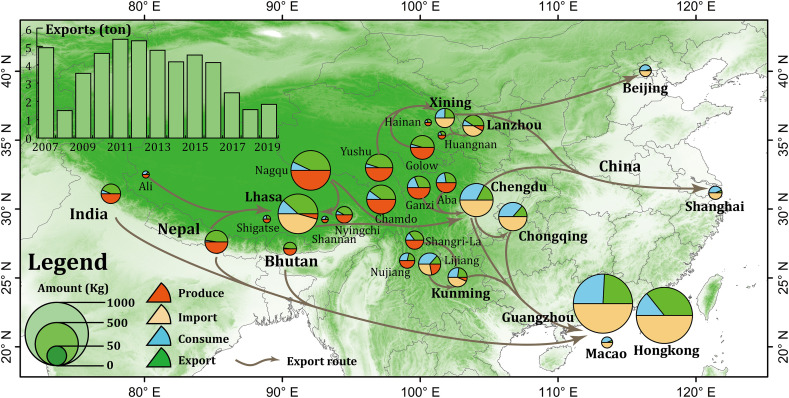
China is the largest caterpillar fungus producing and exporting country in the world. Most of this production is concentrated in Tibet and Qinghai Province, which together account for more than 80% of total caterpillar fungus production in China. Fig. 8 illustrates the produce and export amounts and the main export routes in China. It reveals the most productive areas in China are Nagqu and Chamdo in Tibet, Yushu and Golow in Qinghai province, Ganzi and Aba in Sichuan province, Shangri-La, Nujiang and Lijiang in Yunnan province. These places are also the main export regions of China. Interestingly, the local consumption in these areas are relatively low, most of them are less than 20% of the production. The most parts of them are exported to central cities and abroad for foreign cash. The statistics of China General Administration of Customs (http://www.customs.gov.cn/) show that, during 2007 to 2019, the caterpillar fungus export of China manifested a decrease trend on the whole. The export in 2007 was 4.93 tons, however, the export amount was only 1.84 tons in 2019, almost hit the least record in history (Fig. 8). The statistics of Chinese herbal medicine information website show that the average price soared from 8780 USD/kg in 2007 to 24,640 USD/kg in 2019, increased by 2.81 times (https://www.zyctd.com/). From the map of main export routes, we could find the main inland destinations are regional central cities, e.g., Lhasa, Chengdu, Chongqing, Xining, Lanzhou and Yunnan, and some international economic hubs, e.g., Guangzhou, Hongkong, Macao, Shanghai and Beijing. These cities have no native products of caterpillar fungus, they are the main export destinations and redistributing centres for consumption and abroad export. Especially Guangzhou and Hongkong, they are the main export and consumption centres in China, taking a high proportion of the total consumption. An interesting phenomenon is that the most products of caterpillar fungus in other regions around Himalayas, i.e. India, Nepal and Bhutan, are also exported to Guangzhou and Hongkong. It demonstrates these two cities are the international super consumption centres of caterpillar fungus in the world.
Fig. 8.
The average produce, consume, import, and export volume and export routes of caterpillar fungus in China during 2007 to 2019.
Given its high nutritious and medicinal value, there is a huge demand market for caterpillar fungus. That is the main reason why caterpillar fungus is continually decreasing either native produce or export to abroad in recent years. Additionally, there is no strict or effective regulation and protection system, even a systematic management plan on the collectors' overexploitations and for avoiding competition over caterpillar mushroom collection in QTP (Winkler, 2008; Weckerle et al., 2010; Winkler, 2010; Shrestha and Bawa, 2015; He, 2018; Hopping et al., 2018; Laha et al., 2018). Despite the influence of climate change, overharvest and intensively exploitation are regarded as the main reasons for caterpillar fungus shrinkage. Human influence has become increasingly significant as compared to climate change in QTP. Human impact in association with climate change, poses major threats to the habit and sustainable survival of caterpillar fungus in the coming decades.
5. Conclusions
In this study we modelled the current geographical distributions of caterpillar fungus in China and examined critical niche indicators that influence species distribution. Potential changes in suitable habitat under future climate change scenarios (4 RCP scenarios in 2050s and 2070s, respectively) were also modelled. Additionally, the study carefully analyses climatic and anthropogenic influences on the distribution of caterpillar fungus in China. Some of the key conclusions are:
-
(1)
The suitable habitats of caterpillar fungus are mainly located in Qilian mountains in the northeastern QTP, Gannan prefecture in Gansu, Ganzi and Aba prefectures in Sichuan, northwestern Yunnan, Yushu and Guoluo prefectures in Qinghai, Qamdo, Nyingchi and Nagqu in central and eastern Tibet. The mountainous regions with high elevations are the main distribution areas. The moderately and extremely suitable habitats are mainly located in the southeast of the line of Gannan-Guoluo-Yushu-Nagqu, the isotherm of 400 mm annual mean precipitation on QTP.
-
(2)
The most critical influencing factors are elevation (979–6043 m, average is 3865 m), precipitation of warmest quarter (16–900 mm, average is 338 mm), annual precipitation (21–1542 mm, average is 579 mm), and precipitation of driest month (0–20 mm, average is 3 mm). The favourite soil type is Southeastern QTP alpine meadow soil.
-
(3)
The forecasted distribution patterns of RCP 2.6 do not highlight any obvious changes. However, significant range shrinks could be observed in all classes of suitable areas in Tianshan, Kunlun Mountains, and southwestern QTP in 2050s and 2070s in RCP 4.5, RCP 6.0 and RCP 8.5 scenarios, respectively.
-
(4)
The export quantity has shown a drastic decline in recent years. Guangzhou and Hongkong are two international super import and consumption centres of caterpillar fungus in the world.
-
(5)
The sustainability of the caterpillar fungus ecology and economy is threatened by the combined pressures of climate change and overexploitation for traditional medicine. A strict but effective regulation and protection system, even a systematic management plan for collectors are highly needed in QTP.
CRediT authorship contribution statement
Yanqiang Wei: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Liang Zhang: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Jinniu Wang: Conceptualization, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Wenwen Wang: Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Naudiyal Niyati: Writing - original draft, Writing - review & editing. Yanlong Guo: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Xufeng Wang: Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This research was jointly supported by the Strategic Priority Research Program of Chinese Academy of Sciences (grant no. XDA19040500) and National Science Foundation of China (grant no. 41701505, 41661144045).
Data availability statement
The sample data are available at Global Biodiversity Information Facility (https://www.gbif.org/), Chinese Virtual Herbarium (http://www.cvh.ac.cn/) and China National Specimen Information Infrastructure (http://www.naii.org.cn). The Harmonized World Soil Database (HWSD v1.1) are available at http://westdc.westgis.ac.cn/. The DEM (Digital Elevation Model) data are available at http://srtm.csi.cgiar.org/. The 301 species occurrence records used in the study are available by contacting the corresponding author. The 19 bioclimatic indicators are available at WorldClim-Global Climate Database (http://worldclim.org/). The climate scenario data are available at http://ccafs-climate.org. The prices are available at https://www.zyctd.com/. The national basic geoinformatics are available at http://bzdt.ch.mnr.gov.cn/.
Editor: Ouyang Wei
References
- Abdelaal M., Fois M., Fenu G., Bacchetta G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019;50(1):68–75. doi: 10.1016/j.ecoinf.2019.01.003. [DOI] [Google Scholar]
- Ahmadi M., Hemami M.-R., Kaboli M., Malekian M., Zimmermann N.E. Extinction risks of a Mediterranean neo-endemism complex of mountain vipers triggered by climate change. Sci. Rep. 2019;9(1):6332. doi: 10.1038/s41598-019-42792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alacs E., Georges A. Wildlife across our borders: a review of the illegal trade in Australia. Aust. J. Forensic Sci. 2008;40(2):147–160. doi: 10.1080/00450610802491382. [DOI] [Google Scholar]
- Au D., Wang L.J., Yang D.J., Mok D.K.W., Chan A.S.C., Xu H.X. Application of microscopy in authentication of valuable Chinese medicine i—Cordyceps sinensis, its counterfeits, and related products. Microsc. Res. Tech. 2012;75(1):54–64. doi: 10.1002/jemt.21024. [DOI] [PubMed] [Google Scholar]
- Beardall J., Stojkovic S., Larsen S. Living in a high CO2 world: impacts of global climate change on marine phytoplankton. Plant Ecol. Divers. 2009;2(2):191–205. http://www.informaworld.com/10.1080/17550870903271363 [Google Scholar]
- Biskaborn B.K., Smith S.L., Noetzli J., Matthes H., Vieira G., Streletskiy D.A., Schoeneich P., Romanovsky V.E., Lewkowicz A.G., Abramov A., et al. Permafrost is warming at a global scale. Nat. Commun. 2019;10(1):264. doi: 10.1038/s41467-018-08240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R.K., Brehm G., Cardelús C.L., Gilman A.C., Longino J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322(5899):258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- Cunningham A.B., Brinckmann J.A., Pei S.J., Luo P., Schippmann U., Long X., Bi Y.F. High altitude species, high profits: can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J. Ethnopharmacol. 2018;223:142–151. doi: 10.1016/j.jep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Dai Y.D., Wu C.K., Yuan F., Wang Y.B., Huang L.D., Chen Z.H., Zeng W.B., Wang Y., Yang Z.L., Zeng P.S., et al. Evolutionary biogeography on Ophiocordyceps sinensis: an indicator of molecular phylogeny to geochronological and ecological exchanges. Geosci. Front. 2019 doi: 10.1016/j.gsf.2019.09.001. [DOI] [Google Scholar]
- Department of Rural Socioeconomic Investigation, N.B.S. China Statistics Press; Beijing: 1983-2019. China County (City) Social Economic Statistic Yearbooks. [Google Scholar]
- Diez J., Kauserud H., Andrew C., Heegaard E., Krisai-Greilhuber I., Senn-Irlet B., Høiland K., Egli S., Büntgen U. Altitudinal upwards shifts in fungal fruiting in the Alps. Proc. R. Soc. B Biol. Sci. 2020;287(1919):20192348. doi: 10.1098/rspb.2019.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J., Phillips S.J., Hastie T., Dudík M., Chee Y.E., Yates C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011;17(1):43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- FAO, 2012. FAO/IIASA/ISRIC/ISSCAS/JRC: Harmonized World Soil Database (version 1.2). FAO, Rome, Italy and IIASA, Laxenburg, Austria.
- Fielding A.H., Bell J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997;24(1):38–49. doi: 10.1017/S0376892997000088. [DOI] [Google Scholar]
- Franklin J. Cambridge University Press, Cambridge; Biodiversity and Conservation: 2010. Mapping Species Distributions: Spatial Inference and Prediction. [Google Scholar]
- Gao R.R., Hu Y.T., Dan Y., Hao L.J., Liu X., Song J.Y. Chinese herbal medicine resources: where we stand. Chinese Herbal Medicines. 2019 doi: 10.1016/j.chmed.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A., Thuiller W., Zimmermann N.E. Cambridge University Press; Cambridge: 2017. Habitat Suitability and Distribution Models: With Applications in R. [Google Scholar]
- Guo Y.L., Li X., Zhao Z.F., Nawaz Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019;648:1–11. doi: 10.1016/j.scitotenv.2018.07.465. [DOI] [PubMed] [Google Scholar]
- Halvorsen R., Mazzoni S., Bryn A., Bakkestuen V. Opportunities for improved distribution modelling practice via a strict maximum likelihood interpretation of MaxEnt. Ecography. 2015;38(2):172–183. doi: 10.1111/ecog.00565. [DOI] [Google Scholar]
- Halvorsen R., Mazzoni S., Dirksen J.W., Næsset E., Gobakken T., Ohlson M. How important are choice of model selection method and spatial autocorrelation of presence data for distribution modelling by MaxEnt? Ecol. Model. 2016;328:108–118. doi: 10.1016/j.ecolmodel.2016.02.021. [DOI] [Google Scholar]
- Harley C.D.G., Paine R.T. Contingencies and compounded rare perturbations dictate sudden distributional shifts during periods of gradual climate change. Proc. Natl. Acad. Sci. 2009;106(27):11172–11176. doi: 10.1073/pnas.0904946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. Harvest and trade of caterpillar mushroom (Ophiocordyceps sinensis) and the implications for sustainable use in the Tibet Region of Southwest China. J. Ethnopharmacol. 2018;221(1):86–90. doi: 10.1016/j.jep.2018.04.022. [DOI] [PubMed] [Google Scholar]
- He F.L., Hubbell S.P. Species–area relationships always overestimate extinction rates from habitat loss. Nature. 2011;473(7347):368–371. doi: 10.1038/nature09985. [DOI] [PubMed] [Google Scholar]
- Hopping K.A., Chignell S.M., Lambin E.F. The demise of caterpillar fungus in the Himalayan region due to climate change and overharvesting. Proc. Natl. Acad. Sci. 2018;115(45):11489–11494. doi: 10.1073/pnas.1811591115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L. Climate change and Australia: key vulnerable regions. Reg. Environ. Chang. 2011;11(1):189–195. doi: 10.1007/s10113-010-0158-9. [DOI] [Google Scholar]
- Ikeda R., Nishimura M., Sun Y., Wada M., Nakashima K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomed. Chromatogr. 2008;22(6):630–636. doi: 10.1002/bmc.980. [DOI] [PubMed] [Google Scholar]
- IPCC . II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC, Geneva, Switzerland: 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I. [Google Scholar]
- Jump A.S., Mátyás C., Peñuelas J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009;24(12):694–701. doi: 10.1016/j.tree.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Laha A., Badola R., Hussain S.A. Earning a livelihood from Himalayan Caterpillar fungus in Kumaon Himalaya: opportunities, uncertainties, and implications. Mt. Res. Dev. 2018;38(4):323–331. doi: 10.1659/MRD-JOURNAL-D-17-00063.1. 329. [DOI] [Google Scholar]
- Lamsal P., Kumar L., Shabani F., Atreya K. The greening of the Himalayas and Tibetan Plateau under climate change. Glob. Planet. Chang. 2017;159:77–92. doi: 10.1016/j.gloplacha.2017.09.010. [DOI] [Google Scholar]
- Lewis O.T. Climate change, species–area curves and the extinction crisis. Phil. Trans. R. Soc. B. 2005;361 doi: 10.1098/rstb.2005.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang X.L., Jiao L., Jiang Y., Li H., Jiang S.P., Lhosumtseiring N., Fu S.Z., Dong C.H., Zhan Y., et al. A survey of the geographic distribution of Ophiocordyceps sinensis. J. Microbiol. 2011;49:913–919. doi: 10.1007/s12275-011-1193-z. [DOI] [PubMed] [Google Scholar]
- Li Y., Hu X.D., Yang R.H., Hsiang T., Wang K., Liang D.Q., Liang F., Cao D.M., Zhou F., Wen G., et al. Complete mitochondrial genome of the medicinal fungus Ophiocordyceps sinensis. Sci. Rep. 2015;5(1):13892. doi: 10.1038/srep13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.J., Fan G., He Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020;698:134141. doi: 10.1016/j.scitotenv.2019.134141. [DOI] [PubMed] [Google Scholar]
- Liu G.Q., Han R.C., Cao L. Artificial cultivation of the Chinese cordyceps from injected ghost moth larvae. Environ. Entomol. 2019;48(5):1088–1094. doi: 10.1093/ee/nvz099. [DOI] [PubMed] [Google Scholar]
- Liu Y., Huang P., Lin F., Yang W., Gaisberger H., Christopher K., Zheng Y. MaxEnt modelling for predicting the potential distribution of a near threatened rosewood species (Dalbergia cultrata Graham ex Benth) Ecol. Eng. 2019;141:105612. doi: 10.1016/j.ecoleng.2019.105612. [DOI] [Google Scholar]
- Lo H.C., Hsieh C.Y., Lin F.Y., Hsu T.H. A systematic review of the mysterious caterpillar fungus Ophiocordyceps sinensis in DongChongXiaCao (冬蟲夏草 Dōng Chóng Xià Cǎo) and related bioactive ingredients. J. Tradit. Complement. Med. 2012;3(1):16–32. doi: 10.1016/S2225-4110(16)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo J.M., Jiménez-Valverde A., Real R. AUC: a misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008;17(2):145–151. doi: 10.1111/j.1466-8238.2007.00358.x. [DOI] [Google Scholar]
- Marmion M., Luoto M., Heikkinen R.K., Thuiller W. The performance of state-of-the-art modelling techniques depends on geographical distribution of species. Ecol. Model. 2009;220(24):3512–3520. doi: 10.1016/j.ecolmodel.2008.10.019. [DOI] [Google Scholar]
- MEE Redlist of China’s biodiversity — macrofungi. Ministry of Ecology and Environment, PRC, Chinese Academy of Sciences. 2018;85 http://www.mee.gov.cn/gkml/sthjbgw/sthjbgg/201805/t20180524_441393.htm [Google Scholar]
- Merow C., Smith M.J., Silander J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography. 2013;36(10):1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x. [DOI] [Google Scholar]
- Pan Z.G. In: Trends in Insect Molecular Biology and Biotechnology. Kumar D., Gong C., editors. Springer International Publishing; Cham: 2018. Research advancement of insect origin fungus cordyceps; pp. 253–282. [DOI] [Google Scholar]
- Paterson R.R.M. Cordyceps – a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69(7):1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S.J. AT&T Research; Princeton, NJ: 2011. A Brief Tutorial on Maxent. [Google Scholar]
- Phillips S.J., Dudík M., Schapire R.E. Association for Computing Machinery; Banff, Alberta, Canada: 2004. A Maximum Entropy Approach to Species Distribution Modeling. In: Proceedings of the Twenty-First International Conference on Machine Learning; p. 83. [Google Scholar]
- Pouliot M., Pyakurel D., Smith-Hall C. High altitude organic gold: the production network for Ophiocordyceps sinensis from far-western Nepal. J. Ethnopharmacol. 2018;218:59–68. doi: 10.1016/j.jep.2018.02.028. [DOI] [PubMed] [Google Scholar]
- Shrestha U.B. Asian medicine: a fungus in decline. Nature. 2012;482(7383):35. doi: 10.1038/482035b. [DOI] [PubMed] [Google Scholar]
- Shrestha U.B., Bawa K.S. Trade, harvest, and conservation of caterpillar fungus (Ophiocordyceps sinensis) in the Himalayas. Biol. Conserv. 2013;159:514–520. doi: 10.1016/j.biocon.2012.10.032. [DOI] [Google Scholar]
- Shrestha U.B., Bawa K.S. Economic contribution of Chinese caterpillar fungus to the livelihoods of mountain communities in Nepal. Biol. Conserv. 2014;177:194–202. doi: 10.1016/j.biocon.2014.06.019. [DOI] [Google Scholar]
- Shrestha U.B., Bawa K.S. Harvesters’ perceptions of population status and conservation of Chinese caterpillar fungus in the Dolpa region of Nepal. Reg. Environ. Chang. 2015;15(8):1731–1741. doi: 10.1007/s10113-014-0732-7. [DOI] [Google Scholar]
- Shrestha U.B., Dhital K.R., Gautam A.P. Economic dependence of mountain communities on Chinese caterpillar fungus Ophiocordyceps sinensis (yarsagumba): a case from western Nepal. Oryx. 2017;53(2):256–264. doi: 10.1017/S0030605317000461. [DOI] [Google Scholar]
- Swets J.A. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Thomas C.D., Cameron A., Green R.E., Bakkenes M., Beaumont L.J., Collingham Y.C., Erasmus B.F.N., de Siqueira M.F., Grainger A., Hannah L., et al. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Toledo L.F., Asmüssen M.V., Rodríguez J.P. Track illegal trade in wildlife. Nature. 2012;483(7387):36. doi: 10.1038/483036e. [DOI] [PubMed] [Google Scholar]
- Wang C.G., Tang Z., Nan Z.B. The caterpillar fungus boom on the Tibetan plateau: curse or blessing? China Econ. Rev. 2018;47:65–76. doi: 10.1016/j.chieco.2017.12.003. [DOI] [Google Scholar]
- Wang W.J., Thompson Iii F.R., He H.S., Fraser J.S., Dijak W.D., Jones-Farrand T. Climate change and tree harvest interact to affect future tree species distribution changes. J. Ecol. 2019;107(4):1901–1917. doi: 10.1111/1365-2745.13144. [DOI] [Google Scholar]
- Weckerle C.S., Yang Y.P., Huber F.K., Li Q.H. People, money, and protected areas: the collection of the caterpillar mushroom Ophiocordyceps sinensis in the Baima Xueshan Nature Reserve, Southwest China. Biodivers. Conserv. 2010;19(9):2685–2698. doi: 10.1007/s10531-010-9867-0. [DOI] [Google Scholar]
- Wei Y.Q., Fang Y.P. Spatio-temporal characteristics of global warming in the Tibetan plateau during the last 50 years based on a generalised temperature zone - elevation model. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler D. Yartsa Gunbu (Cordyceps sinensis) and the fungal commodification of Tibet’s rural economy. Econ. Bot. 2008;62(3):291–305. doi: 10.1007/s12231-008-9038-3. [DOI] [Google Scholar]
- Winkler D. Caterpillar fungus (Ophiocordyceps sinensis) production and sustainability on the Tibetan plateau and in the Himalayas. 2009;5(2):291. doi: 10.1163/157342109X568829. [DOI] [Google Scholar]
- Winkler D. CORDYCEPS SINENSIS: a precious parasitic fungus infecting Tibet. Field Mycology. 2010;11(2):60–67. doi: 10.1016/j.fldmyc.2010.04.009. [DOI] [Google Scholar]
- Yan J.K., Wang W.Q., Wu J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: a review. J. Funct. Foods. 2014;6:33–47. doi: 10.1016/j.jff.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Li Y., Wang W.-J., He J.-S., Yang R.-H., Wu H.-J., Wang X.-L., Jiao L., Tang Z., Yao Y.-J. Range shifts in response to climate change of Ophiocordyceps sinensis, a fungus endemic to the Tibetan Plateau. Biol. Conserv. 2017;206:143–150. doi: 10.1016/j.biocon.2016.12.023. [DOI] [Google Scholar]
- Yang X.Q., Kushwaha S.P.S., Saran S., Xu J.C., Roy P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013;51:83–87. doi: 10.1016/j.ecoleng.2012.12.004. [DOI] [Google Scholar]
- Yang M., Wang X., Pang G., Wan G., Liu Z. The Tibetan Plateau cryosphere: observations and model simulations for current status and recent changes. Earth Sci. Rev. 2019;190:353–369. doi: 10.1016/j.earscirev.2018.12.018. [DOI] [Google Scholar]
- Yao T.D., Thompson L., Yang W., Yu W., Gao Y., Guo X., Yang X., Duan K., Zhao H., Xu B., et al. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat. Clim. Chang. 2012;2:663. doi: 10.1038/nclimate1580. [DOI] [Google Scholar]
- Zhang Y.J., Bai F.R., Zhang S., Liu X.Z. Determining novel molecular markers in the Chinese caterpillar fungus Ophiocordyceps sinensis by screening a shotgun genomic library. Appl. Microbiol. Biotechnol. 2012;95(5):1243–1251. doi: 10.1007/s00253-012-4028-x. [DOI] [PubMed] [Google Scholar]
- Zhong S., Pan H.J., Fan L.F., Lv G.Y., Wu Y.Z., Binod P., Ashok P., Carlos R.S. Advances in research of polysaccharides in Cordyceps species. Food Technol. Biotechnol. 2009;47(3):304–312. https://hrcak.srce.hr/file/62488 [Google Scholar]
- Zhong L., Ma Y.M., Xue Y.K., Piao S.L. Climate change trends and impacts on vegetation greening over the Tibetan plateau. Journal of Geophysical Research: Atmospheres. 2019;124(14):7540–7552. doi: 10.1029/2019jd030481. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sample data are available at Global Biodiversity Information Facility (https://www.gbif.org/), Chinese Virtual Herbarium (http://www.cvh.ac.cn/) and China National Specimen Information Infrastructure (http://www.naii.org.cn). The Harmonized World Soil Database (HWSD v1.1) are available at http://westdc.westgis.ac.cn/. The DEM (Digital Elevation Model) data are available at http://srtm.csi.cgiar.org/. The 301 species occurrence records used in the study are available by contacting the corresponding author. The 19 bioclimatic indicators are available at WorldClim-Global Climate Database (http://worldclim.org/). The climate scenario data are available at http://ccafs-climate.org. The prices are available at https://www.zyctd.com/. The national basic geoinformatics are available at http://bzdt.ch.mnr.gov.cn/.