Abstract
In December 2019, a large number of coronavirus cases were emerged in Wuhan, Hubei Province, China and rapidly spread to different countries and territories around the world within four months. The World Health Organization (WHO) declared this outbreak as a global health emergency. The spread of COVID-19 over globe is highly contagious; they transmitted from person-to-person through small droplets of infected person. Many diagnosis and treatment methods have been implemented to reduce and control the outbreak. Efforts have been made to develop coronavirus vaccine against S protein or spike glycoprotein of coronavirus. COVID-19 outbreak will affect the Gross Domestic Product (GDP) of the world. At the time of preparing manuscript, total number of active cases reaches to more than 8.9 million and confirmed death reaches to approx. 4.6 lakh. This article highlights the ongoing research and advances in designing vaccine and therapeutics against COVID-19 and also focusing on the epidemiology, transmission, future direction and control the spread of infectious diseases.
Keywords: COVID-19, Epidemiology, Vaccines, Convalescent plasma transfusion, Economic effect
1. Introduction
Coronavirus is one of the most infectious pathogens that targets human respiratory system. In December 2019, a cluster of pneumonia patients was admitted in hospital, which were epidemiologically linked to a seafood market in Wuhan, Hubei Province, China.1 The disease was first named as 2019 novel coronavirus (2019-nCoV) on 7 January 2020 and later renamed Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV-2).2 Number of cases was reported from all over countries and then WHO declared this outbreak as a global health emergency on 30 January 2020 and retitled coronavirus disease (COVID-19).3 The most common symptoms of COVID-19 are fever, dry cough and tiredness.4 Patients have different symptoms like aches, nasal congestion, running nose, sore throat while some patients have no symptoms but they act as a carrier for the diseases. About 80% of infected people recover without any special treatment and approximately 1.0 out of 6.0 patients is seriously ill and facing breathing difficulty.5 People having weak immunity, diabetes, heart problem, and are infected with coronavirus, need extra attention and clinical care.6 COVID-19 spread from person to person through small droplets, when infected person cough, exhale or speaks. This is why it is important to keep distance of at least 3–6 feet from a person who is ill.5 On 11th March 2020, WHO declared COVID-19 as a pandemic disease.7 All the country are doing their best and implementing the appropriate control and preventive strategies. In the absence of specific medicines for treatment of COVID-19, drugs that might be effective include remdesivir, ritonavir, chloroquine (CQ) and hydroxychloroquine (HCQ) alone or in combination with convalescent plasma, monoclonal antibodies and Interferons.8, 9, 10, 11, 12 Many efforts have been made to develop vaccine against CoV infection but there are certain limiting factors such as degree of cross-protection and their sequence diversity which prevent the development of suitable and effective vaccine.13 According to WHO guideline, the infected patients must be provide with proper oxygen therapy, fluid therapy, medicine and also recommends proper isolation system.
2. Structure and origin of Coronavirus
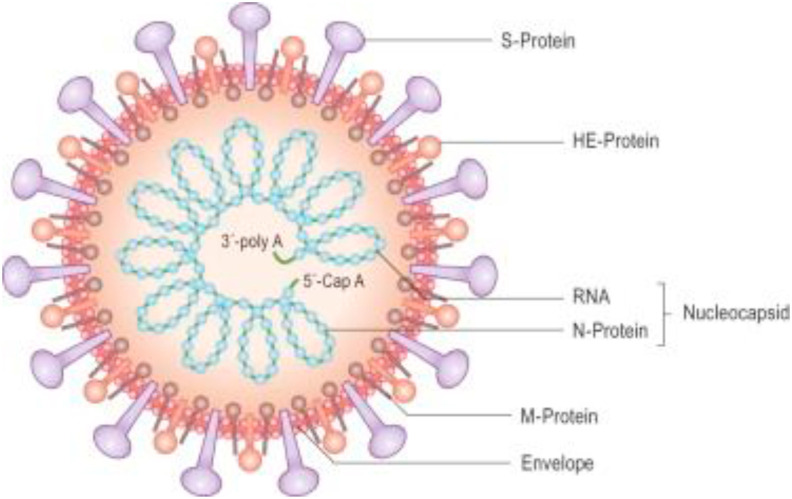
Coronavirus are enveloped virus, with single stranded, non-segmented, positive sense RNA belongs to the family Coronaviridae, subfamily Coronavirinae and order Nidovirales. Genome size of coronavirus is approximately 26–32 Kb and is the largest known genome of RNA virus.14 Its size ranges from 60 nm to 140 nm in diameter having club-shaped spike projections (Fig. 1 ). Under electron microscope, spike looks like crown and hence named coronavirus.15 Coronavirus have helically symmetrically nucleocapsids, which is very rare among positive sense RNA virus. On the basis of phylogeny, subfamily Coronavirinae consist of four genera: Alphacoronavirus (α-CoV), Betacoronavirus (β-CoV), Gammacoronavirus (γ-CoV) and Deltacoronavirus (δ-CoV). α -CoV and β -CoV usually causes respiratory problems in human, whereas γ -CoV and δ -CoV infect birds. In human two highly pathogenic viruses SARS-CoV and MERS-CoV, causes severe respiratory syndrome, whereas four human coronavirus HCoV-NL63, HCoV-OC43, HCoV-229E and HKU1 induced mild respiratory syndrome in immunocompetent person. According to current sequence database HCoV-OC43, HKU1 have originated from rodent and HCoV-NL63, HCoV-229E, SARS-CoV and MERS-CoV have originated from bats.14 , 16 Through sequencing, it was found that nCoV-2019 belongs to β –coronavirus.17
Fig. 1.
Skeleton of coronavirus; inside and outside morphology.19
In 2003, coronavirus of beta genera having bat origin transmit to human via civet cat as an intermediate host in the Guangdong province of china. This virus causes acute respiratory syndrome and nearly 8422 people were affected in China and Hong Kong. Another outbreak occur in 2012, Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in South Arabia in which 2494 people were affected and fatality rate was reported as 34%.18
3. Transmission
A novel β-coronavirus was first identified in December 2019, in Wuhan, Hubei province, China. This outbreak in China is the third epidemic in 21st century, which already surpassed SARS and MERS. Currently, large number of pneumonia patients were reported, who had exposed to seafood market, which is a hub for many species of live animal. In January 10, 2020, full genome sequences of COVID- 19 were released in public database, and were found that sequence has some similarity with SARS. 2019-nCoV was renamed as SARS-CoV-2 by International Committee on Taxonomy of Viruses. Genetic sequence of COVID-19 shows more than 80% similarity with SARS-CoV and 50% sequence identity with MERS-CoV.21 Extensive study from the phylogenetic analysis revealed that the COVID-19 belongs to betacoronavirus genus. Binding of receptor is the first step of viral infection followed by fusion with the cell. It is reported that COVID-19 binds to angiotensin-converting enzyme 2 (ACE2), as the sequence of receptor binding domain of coronavirus spikes is similar to that of SARS-CoV.22
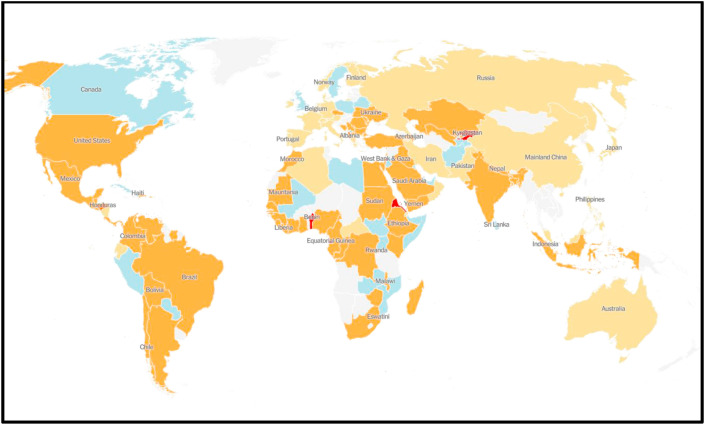
The number of coronavirus cases increases exponentially in Wuhan, China and first case was reported on November 17, 2019. The coronavirus spread rapidly from China to other countries that include Thailand, Nepal, Malaysia, Sri Lanka, Singapore, United Arab Emirates, United States, India, Australia, Finland, Germany, Combodia, Vietnam, Taiwan, Canada, France, The Philippines, Japan, Republic of Korea (Fig. 2 ).
Fig. 2.
Map of spread COVID-19 global outbreak as on June 28, 2020.20 Blue colour indicates decrease in coronavirus cases where as orange and red colour indicates the increasing number of coronavirus cases.
WHO declared novel Coronavirus outbreak as pandemic and reiterated the call for all countries to take immediate action to detect, treat and reduce the transmission to save people's lives. At the time of preparing manuscript 8.9 million coronavirus cases and approximately 4.6 lakh death cases were reported by WHO.23 Several reports suggest that person-to-person transmission via direct contact; through droplets by coughing or sneezing from infected person and indirect contact such as surface contamination are the routes for the transmission of COVID-19 infection. Other studies conducted on pregnant women, who were in third trimester of pregnancy and confirmed for COVID-19 infection, but the transmission from mother to child was not confirmed. Pregnant women are more susceptible to infection by respiratory pathogens.24
4. Symptoms and diagnosis
The common symptoms of this disease are fever, cough and tiredness.4 Some patients may have different symptoms like aches, nasal congestion, sputum production, haemoptysis, running nose, sore throat, diarrhoea, dyspnoea and lymphopenia.22 , 18 , 25 The symptoms appear after incubation of approximately 5.2 days.26 The total period from onset to death of coronavirus disease ranges from 6 to 41 days with a median of 14 days.27 The period of infection is dependent on patient's immunity and age. Infection period is shorter in patients with age >70 years than compared to those under the age of 70 years. Clinical features revealed by Chest CT scan presented as pneumonia, Acute Respiratory Distress Syndrome (ARDS), acute kidney injury, cardiac injury and even death may occur in severe cases.28 , 18 In some patients, multiple ground glass opacity observed in subpleural region of both the lungs which induced both localized and systemic immune response that leads to inflammation.29 Chest radiology of some patients shows an infiltrate in upper lobe of lungs which is associated with dyspnea and hypoxemia.30 Patients infected with COVID-19 also developed symptoms like diarrhoea, so faecal and urine sample test is important to include an alternative route of transmission of coronavirus.31
Patients infected with coronavirus shows increased level of pro-inflammatory cytokine, high leukocyte numbers, and abnormal respiratory function. The main pathogenesis of COVID-19 infection is severe pneumonia, incidence of ground glass opacities RNAaemia and acute cardiac injury. Blood sample of COVID-19 patients shows high level of cytokine and chemokine such as TNFα, IL7, IL8, IL9, IL10, VEGFA, GCSF, GMCSF, PGF2, etc.18
The respiratory samples (nasopharyngeal swab, sputum, throat swab, bronchoalveolar lavage, endotracheal aspirates) taken from the infected person both symptomatic and asymptomatic and sent to laboratory for diagnosis. The specimen were diagnosed by real-time reverse transcription polymerase chain reaction (RT-PCR) using the protocol published by WHO. As the numbers of patients were increasing on daily basis, it leads to shortage of laboratory based molecular testing capacity and reagents. Thus rapid and easy to use device were manufactured to test outside the laboratory settings in a couple of minutes. Antibody based testing kit is generally harder to get correct result, as the antibody present in the strip can detect antigens of virus other than COVID-19 which cause common cold. To overcome this problem antibody detecting rapid diagnosis test for patients care was developed. This rapid kit detect the antibody present in the blood of infected person. The strength of antibody response were depend on severity of infection, patients age, nutritional status, HIV patients having certain medication etc.32 The most widely used diagnostic kits were included in Table 1 .
Table 1.
| Product name | Manufacturer |
|---|---|
| cobas SARS-CoV-2 Qualitative assay for use on the cobas 6800/8800 Systems | Roche Molecular Systems, Inc. |
| Primerdesign Ltd COVID-19 genesig Real-Time PCR assay | Primerdesign Ltd |
| Abbott Realtime SARS-CoV-2 | Abbott Molecular Inc. |
| PerkinElmer® SARS-CoV-2 Real-time RT-PCR Assay | PerkinElmer Inc. |
| Real-time fluorescent RT-PCR kit for detecting 2019-nCoV | BGI Europe A/S |
| Detection Kit for 2019 Novel Coronavirus (2019-nCoV) RNA (PCR- Fluorescence Probing) | Da An Gene Co., Ltd. Of Sun Yat-sen University |
| RealStar SARS-CoV-2 RT-PCR kit 1.0 | Altona Diagnostics |
| Patho Detect | MY LAB |
| Allplex 2019-nCoV assay | Seegene |
| nCoV Real-Time Detection kit | SD Biosensor |
| TRUPCR SARS-CoV-2RT-qPCR kit version 2 | KILPEST (BLACKBIO) |
| Quantiplus CoV detection KIT Ver 2.0 | Huwel Lifesciences Pvt. Ltd. |
| TaqMan 2019-nCoV Control Kit v1 | ABI (Applied biosystems) |
| BIO COVID ID/COVID-19 qualitative PCR detection Kit version 2 | Biogenomics (India) |
| qSARS-CoV-2 IgG/IgM Rapid Test | Cellex.Inc |
| Quest SARS-CoV-2 rRT-PCR | Quest Diagnostics Infectious Disease, Inc. |
| Everlywell COVID-19 Test Home Collection Kit | Everlywell,Inc. |
| COVID-19 RT-PCR Test | Laboratory Corporation of America (LabCorp) |
| Panther Fusion SARS-CoV-2 Assay | Hologic, Inc. |
| TaqPath COVID-19 Combo Kit | Thermo Fisher Scientific,Inc. |
| Xpert Xpress SARS-CoV-2 test | Cepheid |
5. Therapeutics
Therapeutic options that can be used for COVID-19 includes siRNA, anti-sense RNA, monoclonal antibodies targeting host receptor, host cell protease inhibitors, targeting specific enzymes involved in viral replication and transcription, antiviral peptide targeting S2 and natural product.11 , 35 Neither any antiviral drugs nor vaccines are available for the treatment of COVID-19. Efforts have been made by scientist to develop vaccine against coronavirus, but a degree of cross-reactivity is the limiting factors.26 For developing a new vaccine, at least a year or 18 months will required said by Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, US. Johnson & Johnson said human test for experimental vaccine will begin by September 2020. So in the absence of specific medicines for treatment of COVID-19, many low-cost available drugs have been tried like chloroquine (CQ) and hydroxychloroquine (HCQ) which are used as antimalarial along with several other antiviral drugs such as remdesivir, ribavirin, oseltamivir, lopinavir, darunavir, cobicistat and favipiravir are in phase III trial for COVID-19.8 , 36 In vitro chloroquine and hydroxychloroquine (HCQ) have antiviral activity against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) and some other viruses such as influenza.37 As of April 2020, remdesivir (GS-5734) was comes into light as the most promising treatment for COVID-19 and included for evaluation of four stage treatments under European Discovery trial and international Solidarity trial.
S protein of coronavirus is considered as target for designing therapies such as RBD-ACE2 blocker, protease inhibitor, S protein inhibitor etc.38 All therapeutic strategies show potential activity in vitro and in vivo, but due to insufficient support of animal and human trial, they are not properly used against COVID-19. In order to develop pre- and post-prophylaxis against coronavirus diseases, there is urgent need for the establishment of animal model to replicate the severe disease observed in human. Scientists were continuously and rigorously working for developing potential vaccines to providing a better understanding of virus–host interaction.
6. Vaccine and plasma therapy
S-protein based strategies such as S1 receptor binding domain (S1-RBD), DNA, viral vector, full length S protein etc. have been used for developing vaccine against COVID-19.39, 40, 41 S1-RBD interact with angiotensin-converting enzyme 2 (ACE2) and S2 domain of S protein mediate fusion of virus and host cell membrane for releasing viral RNA into the cytoplasm. The S protein plays a major role for induction of immunity during COVID-19 infection, which elicits antibodies and T-cell response.38 Hence the S-protein based vaccine blocks not only viral binding receptor but also prevent viral genome uncoating.42 Therefore full length or may be the appropriate part of S protein are most promising candidate for vaccine production. The Coalition for Epidemic preparedness Innovation (CEPI) is continuously working with global health authorities for the development of vaccine against COVID-19. As on April 8, 2020, 115 vaccines were developed, of which 73 are at preclinical stage and most advanced vaccine such as mRNA-1273 from Moderna, INO-4800 from Inovio, pathogen specific aAPC (artificial antigen-presenting cell) from Shenzhen Gene-Immune Medical Institute, Ad5-nCoV from CanSino Biologicals moved to clinical stage (Table 2 ).
Table 2.
| Candidate | Vaccine Characterization | Lead Developer | Status |
|---|---|---|---|
| Ad5-nCoV | Adenovirus type 5 vector that expresses S protein |
CanSino Biologicals |
Phase I (NCT04313127) |
| mRNA-1273 | LNP- encapsulated mRNA vaccine encoding S protein | Moderna | Phase I (NCT04283461) |
| LV- SMENP- DC | DCs modified with lentiviral vectormexpressing synthetic minigene based on domains of selected viral proteins; administered with antigen- specific CTLs | Shenzhen Geno- Immune Medical Institute |
Phase I (NCT04276896) |
| Pathogen specific aAPC | aAPCs modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins | Shenzhen Geno- Immune Medical Institute |
Phase I (NCT04299724) |
| INO-4800 | DNA plasmid encoding S protein delivered by electroporation | Inovio Pharmaceuticals |
Phase I (NCT04336410) |
aAPC, artificial antigen-presenting cell; CTL, cytotoxic T lymphocyte; DC, dendritic cell; LNP, lipid nanoparticle; S protein, SARS- CoV-2 spike protein.
Convalescent plasma transfusion (CPT) is an immune based treatment against COVID-19. Plasma from the patients recovered from COVID-19 has been potent and last resort to increase the survival rate of COVID-19 patients.45 Several studies shows shorter hospital stay and decreased mortality in patients who are treated with plasma than those who are not treated with plasma. This technology was also previously used in case of MERS, 2009 pandemic influenza A H1N1 or in 2014 against Ebola virus disease.46 , 47 Recently it has been suggested by Food and Drug Administration that administration of CPT may provide an effective treatment against COVID-19 during public health emergency.48 According to WHO, COVID-19 management is primarily focus on preventing infection transmission, early detection and proper health care support system.
There are some limitation such as along with CPT, patients receive antiviral drug, thus there is a possibility that these antiviral drugs may also contribute in the recovery of patients. Some patients also administered with glucocorticoids, which might interfere with immune response. Duan et al49 concluded that CPT shows a potential effect with low risk against COVID-19 treatment. Single dose of CPT with high antibody concentration can rapidly reduce the virus load and improve patient's condition. A definitive conclusion cannot be drawn against CPT, as the optimal dose and treatment time point can be further investigated. Clinical studies are further needed to control this pandemic situation.
7. Prevention and control
-
•
Individual with respiratory symptoms, advised to meet medical health care for proper treatment.
-
•
Regular hand wash with disinfection or soap and use of alcohol (at least 70%) based sanitizer.
-
•
Use of face mask, and avoid direct contact with infected person.
-
•
Safe distance of at least 1 meter should be maintained.
-
•
Health care are recommended to use N95 mask and FFPE kit while handling suspected or confirmed cases of coronavirus.
-
•
Avoid touching nose, eye and mouth without proper hand sanitization.
-
•
Patients with pre-existing medical condition such as diabetes, asthma, heart disease etc. may continuously be in contact with their medical supervisor.
8. Effect of COVID-19 on global GDP
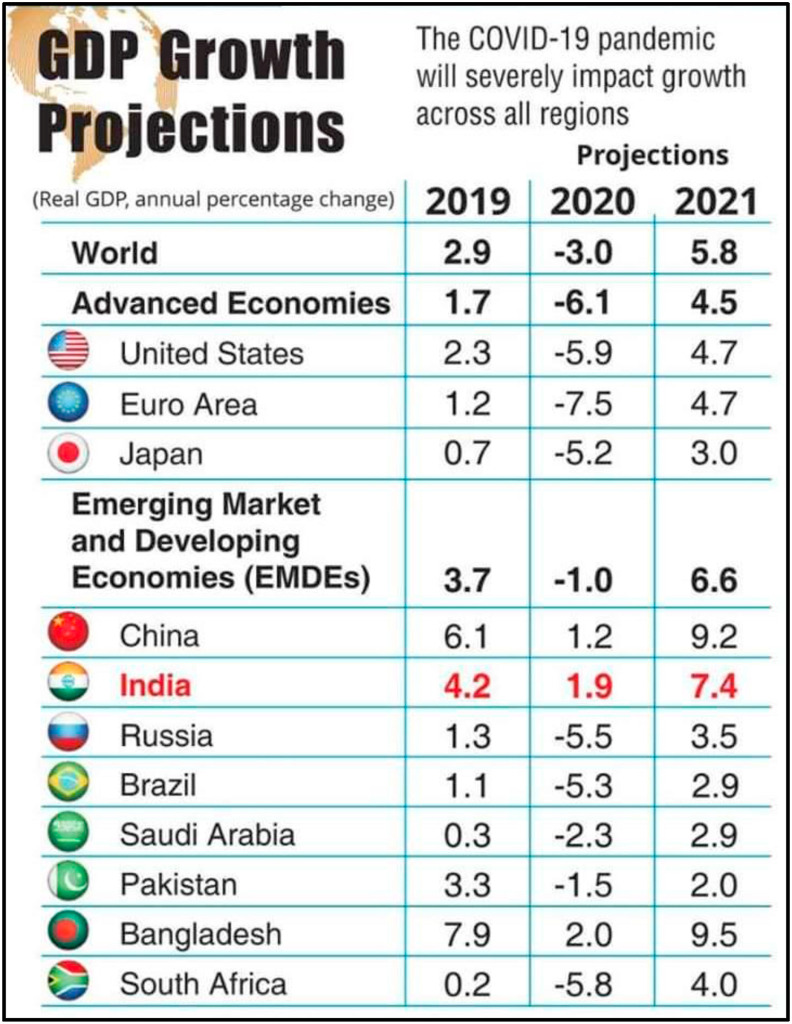
Global economy will suffer the worst financial crisis, said the International Monetary Fund as the planet is struggling with the COVID-19 pandemic. IMF has estimated that in 2020 the growth for global GDP is estimated to fall to −3% (Fig. 3 ) and will rebound to 5.8% in 2021. The cumulative global loss due to COVID-19 in year 2020 and 2021 is expected to be around 9 trillion dollar.50 IMF has projected that GDP of China will be around 1.2% in 2020 and will grow to 9.2% in 2021. India is expected to grow in 2020 and 2021 at GDP of 1.9% and 7.4% respectively. The UN ‘Economic and Social Commission for Asia and the pacific (ESCAP) 2020 said that COVID-19 having a great impact over economic, tourism, aviation sector and financial linkages. According to the SBI Ecowrap report, the extension of the lockdown would result in economic loss of 6% of the nominal Gross Value Added (GVA). If we talk about the positive growth, the emerging Asia is projected to be the only region with positive growth rate expected in 2020.
Fig. 3.
Impact of COVID-19 on GDP growth rate; according to World Economic Growth Projection.50
9. Conclusion and future prospect
Person-to-person contact was minimized to control the spread of COVID-19 infection. Guideline was published by WHO for healthcare workers, medical staffs, public and researchers those who are handling COVID-19 samples. There are no specific drugs or effective vaccine for COVID-19, so we have to rely exclusively in prevention and control measures such as social distancing, wearing mask whenever go outside. Various research conducted in vitro against COVID-19, that shows drug remdesivir and chloroquine are highly effective in controlling the infection. At present, it is important to control infection and cut off the transmission route. S protein is considered as an important viral antigen for developing vaccine. We should promote research to develop vaccine and reduce mortality for the safety of human lives and also to maintain the economic growth rate of the world.
Conflicts of interest
The author has none to declare.
Acknowledgement
Authors are thankful to Department of Biotechnology, Radha Govind University for pursuing research activities.
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Section: frequently asked questions (FAQs) on coronaviruses (COVID-19) https://www.who.int/news-room/q-a-detail/q-a-coronaviruses
- 6.Gupta N., Praharaj I., Bhatanagar T., et al. Severe acute respiratory illness surveillance for coronavirus disease 2019, India, 2020. Indian J Med Res. 2020 doi: 10.4103/ijmr.IJMR_1035_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Director-General's opening remarks at the media briefing on COVID-19. 11 M arch 2020. https://www.who.int/dg/speeches/detail/who-directorgeneral-s-opening-remarks-atthe-media-briefing-on-covid-19-11-march-2020 [Google Scholar]
- 8.Singh A.K., Singh A., Shaikh A., et al. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr.: Clin Res Rev. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai C.C., Liu Y.H., Wang C.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W., Morse J.S., Lalonde T., et al. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.26434/chemrxiv.11728983.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Wang Y.M., Xu J.Y., et al. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jiehe He Huxi Zazhi. 2020;5(43):E002. doi: 10.3760/cma.j.1001-0939.2020.0002. [DOI] [PubMed] [Google Scholar]
- 13.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almedia J.D., Berry D.M., Cunningham C.H., et al. Virology: coronavirus. Nature. 1968;220:650. [Google Scholar]
- 16.Forni D., Cagliani R., Clerici M., et al. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan J.F., Kok K.H., Zhu Z., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel corona virus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Science Direct Medicine and dentistry/coronavirinae. https://www.sciencedirect.com/topics/medicine-and-dentistry/coronavirinae
- 20.The New York Times. https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html
- 21.Ren L.L., Wang Y.M., Wu Z.Q., et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chinese Med J (Engl) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R., Ghosh A., Singh A.K., et al. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab. Syndr.: Clin Res Rev. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worldometers https://www.worldometers.info/coronavirus/
- 24.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlos W.G., Dela Cruz C.S., Cao B., et al. Novel wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201(4):7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 26.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health, Family and Welfare Advisory & Strategy for use of rapid antibody based blood test. https://www.mohfw.gov.in/pdf/Advisory&StrategyforUseofRapidAntibodyBasedBloodTest.pdf
- 29.Lei J., Li J., Li X., et al. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020:200236. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan L.T., Nguyen T.V., Luong Q.C., et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hindson J. COVID-19: faecal-oral transmission? Nat Rev Gastroenterol Hepatol. 2020;17(5):259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okba N.M.A., Müller M.A., Li W., et al. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Food & Drug Administration FDA combating COVID-19 with medical device. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
- 34.WHO emergency use listing for in vitro diagnostics (IVDs) detecting SARS-CoV-2 nucleic acid. https://www.who.int/diagnostics_laboratory/200514_eul_sars_cov2_product_list.pdf
- 35.Kumar V., Jung Y.S., Liang P.H. Anti-SARS coronavirus agents: a patent review (2008–present) Expert Opin Ther Pat. 2013;23(10):1337–1348. doi: 10.1517/13543776.2013.823159. [DOI] [PubMed] [Google Scholar]
- 36.Li G., Clercq E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 37.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du L., He Y., Zhou Y., et al. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z.Y., Kong W.P., Huang Y., et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang S., He Y., Liu S. SARS vaccine development. Emerg Infect Dis. 2005;11(7):1016–1020. doi: 10.3201/1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widjaja I., Wang C., vanHaperen R., et al. Towards a solution to MERS: protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg Microb Infect. 2019;8(1):516–530. doi: 10.1080/22221751.2019.1597644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji W., Wang W., Zhao X., et al. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol. 2020 doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clinical trials gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19.
- 44.Le T.T., Andreadakis Z., Kumar A., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 45.Rajendran K., Krishnasamy N., Rangarajan J., et al. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Rev. 2020 doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO . 2014. Use of Convalescent Whole Blood or Plasma Collected from Patients Recovered from Ebola Virus Disease for Transfusion, as an Empirical Treatment during Outbreaks.http://apps.who.int/iris/rest/bitstreams/604045/retrieve [Google Scholar]
- 47.Arabi Y., Balkhy H., Hajeer A.H. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. SpringerPlus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Food and Drug Administration Recommendation for investigational COVID-19 convalescent plasma, content current as of 05/01/2020. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
- 49.Kai D., Bende L., Cesheng L., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci Unit States Am. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IMFBlog The great lockdown: worst economic downturn since the great depression. https://blogs.imf.org/2020/04/14/the-great-lockdown-worst-economic-downturn-since-the-great-depression/