Abstract
Objectives
There is scarce data available on the prognostic application of chest CT. The main purpose of this study was to evaluate the performance of a semi-quantitative CT severity score in identifying the risk of mortality in COVID-19 patients.
Methods
This retrospective cohort study was performed on 262 hospitalized COVID-19 patients. The CT severity score was assessed by two independent radiologists using a method previously used to score the severity of acute respiratory distress syndrome on thin slice lung CT.
Results
Multivariate regression analysis showed increasing odds of in-hospital death associated with older age, and the presence of coronary artery disease at the time of admission. The mean CT severity score was 7.5 in the survivor group and 14.5 in the deceased group. Overall, the lower zones were the most frequently affected sites in COVID-19. There was significant difference between the survivor and deceased groups regarding CT severity scores. Multivariate regression analysis showed increasing odds of in-hospital death associated with higher CT severity score at admission.
Conclusions
Our results show that mortality was significantly higher in patients with higher CT severity score even after adjustment for clinical, demographics and laboratory parameters. However, this study is performed retrospectively and needs to be validated in a prospective study.
Keywords: COVID-19, Survival, Mortality, Computed tomography
1. Introduction
1.1. Background
In December 2019, a series of patients with pneumonia of unknown cause was reported in Wuhan, China. Molecular analysis of lower tract samples from the patients showed the causative organism to be a virus from corona virus family. On February 11, 2020, the virus was designated by the World Health Organization (WHO) as corona virus disease 2019 (COVID-19). WHO announced the pandemic in March 11, 2020. As of May 2, 2020, there were 3.4 million confirmed cases and 239,000 deaths reported globally [1].
Although real time reverse-transcriptase polymerase chain reaction (RT-PCR) is considered the gold standard for diagnosis of COVID-19 infection, chest computed tomography (CT) has been reported to be diagnostic in cases of false negative results of RT-PCR. It is not only a diagnostic tool, but also it has great significance in monitoring disease progression and evaluating therapeutic efficacy [2]. During the first weeks of outbreak, RT-PCR results took days to be prepared which significantly impacted the emergency department dynamics. As the result, in those centers where CT was more available, CT was employed to assist with diagnosis instead of less available RT-PCR test [3]. Several studies have reported the CT findings of COVID-19 pneumonia. However, the outcome of patients has not been definite in many of these series. Therefore, the estimation of risk factors for severe disease and death in these earlier studies are not very strong, and there are limited data available about the prognostic application of chest CT.
There are certain downsides to CT such as extended cleaning times that shut down a CT scanner after a COVID-positive patient is scanned, increased staff exposures, transport of a potentially unstable patient out of the department and limited CT scanners in some emergency departments. Taking these into account, CT scan is not recommended as the first imaging modality in many patient scenarios [2]. Yet, in a considerable number of patients, lung CT scan is already obtained at the time of presentation to the emergency department (i.e. CT is performed at the sending hospital or on an outcome basis), or it is requested by the emregency physician. Information about the prognostic value of CT scan could assist emergency physicians in triaging patients, while allocating limited intensive care resources.
The aim of this study was to describe the relationship between COVID-19 mortality and chest CT scan findings obtained at the time of admission. The main purpose of this study was to evaluate the performance of a semi-quantitative CT severity score in identifying the risk of mortality in COVID-19.
2. Methods
2.1. Patient selection
This retrospective cohort study was performed in a large tertiary referral academic hospital designated to COVID-19 patients, in which only COVID-19 patients were admitted at the time of recent epidemy. Institutional review boards approved this retrospective study, and patient informed consent was waived. From February 20, 2020 to March 10, 2020, during the first weeks of COVID-19 outbreak, 262 consecutive adult patients (≥ 18 years old) hospitalized with laboratory-confirmed COVID-19 infection, who were either discharged or deceased by April 1, 2020, entered the study. The inclusion criteria were positive RT-PCR test before or after hospitalization, routine blood tests and CT performed at admission in the emergency department. The exclusion criteria were receiving any empirical treatment other than the standard protocol published by health authorities, or normal CT scan at admission. The standard treatment protocol at the time of conducting this study included Hydroxychloroquine, azitromycine and Ribanavir/Ritonavir in selected patients. Those receiving empirical treatments (e.g. prone positioning, Remdesivir, hemoperfusion) were excluded from the study.
2.2. Data collection
Clinical and laboratory data for each patient were extracted and recorded. The outcome measured was in-hospital death. The patients were categorized into two groups: those who expired during hospitalization (deceased group) and those who survived and were discharged from the hospital -i.e. patients who were afebrile for at least 72 h, had stable vital signs and did not benefit from further hospitalization. (survivor group). Clinical data included demographic characteristics (age and gender), symptoms at presentation and underlying disease and comorbidities (hypertension, coronary arterial disease, diabetes mellitus, chronic lung disease, chronic renal disease, chronic liver disease, immunodeficiency and cancer). Blood examinations at admission included complete blood count (CBC), renal and liver function tests, lactate dehydrogenase (LDH), serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
2.3. Image acquisition and viewing
Computed tomography scans were obtained using a commercially available helical 16-MDCT scanner (Neusoft, Neuviz 16). Patients were examined in the supine position with both arms extended above the head. The scans were taken in the caudocranial direction, spanning the entire chest from the diaphragmatic dome up to the lung apices. All scans were performed without intravenous contrast administration. All chest CT images were transferred from PACS into a dedicated workstation and analyzed by OSIRIX MD™ (version 10.0.1) software and a medical monitor. From each dataset, multiplanar reconstructions were generated on the standard three orthogonal planes—axial, coronal, and sagittal. All CT images were evaluated by two radiologists (MPR and BA) with 18 and 7 years of experience in thoracic CT interpretation, respectively, who were blind to the clinical data and laboratory findings. They reached the conclusion by consensus.
2.4. Image interpretation
The extent of involvement on thin-slice CT images was evaluated by the two readers. We used the method previously applied by Wang et al. [4]. This method uses lung opacification as a criterion for extent of the disease in the lungs. Each lung was divided into three zones: the upper zone (above the carina), the middle zone (from the carina to the inferior pulmonary vein), and the lower zone (below the inferior pulmonary vein). The degree of involvement in each zone was scored as follows: a score of 0 denoted no involvement; 1, < 25% involvement; 2, 25% to less than 50% involvement; 3, 50% to less than 75% involvement; and 4, ≥ 75% involvement. Total score ranged from 0 to 24.
The CT findings were also classified using Radiology Society of North America consensus statement on Reporting Chest CT findings Related to COVID-19 [5]. CT images were also assessed in line with the descriptors defined by the Fleischner Society [4,6] regarding the presence of alveolar pattern characterized as ground-glass opacity (GGO) (increased parenchymal attenuation without obstruction of underlying vasculature), consolidation (increased parenchymal attenuation with obstruction of underlying vasculature), or a combination of consolidation and GGO. Ground glass opacity was further divided as pure GGO, crazy paving or reverse halo/Atoll sign. The presence of rounded GGO/consolidation, halo sign, architectural distortion or parenchymal lines were also evaluated. Additionally, the presence of pleural effusion, pneumothorax, pneumomediastinum, mediastinal lymphadenopathy (short axis diameter > 10 mm) and pleural thickening were also noted. The laterality and distribution of parenchymal abnormalities both in the transverse (central, peripheral, diffuse and random) and longitudinal planes (upper zone, middle zone, lower zone and random) were evaluated. The outer third of the lung was defined as peripheral, and the inner two-thirds were defined as central.
2.5. Statistical analysis
Data analysis was performed using SPSS software (version 23.0, SPSS Inc., Chicago, Ill).
We summarized continuous variables using mean ± SD or median and interquartile range (IQR) when appropriate. Categorical variables are presented as n (%). The difference in the demographic data including underlying disease of patients and frequencies and patterns of CT findings between two clinical groups were compared with Mann–Whitney U test, chi square, and Fischer exact tests using permutation method for multiple comparisons. In all statistical analyses, P < 0.05 was considered statistically significant.
Variables with a p value <0.5 in the univariate analysis were entered into multivariate logistic regression analysis to identify independent risk factors in the mortality of COVID-19 pneumonia.
3. Results
3.1. Clinical and laboratory findings
Table 1 shows the demographic and clinical data on 262 patients with confirmed COVID-19 pneumonia at the time of admission. Of the 262 cases, 206 (78.6%) were discharged, and 56 (21.4%) died in the hospital. The median age of patients was 58 (43–67) years, ranging from 20 to 97 years. Most patients were male (65.6%). The most common clinical symptoms were fever (182/262, 69.5%) and cough (159/262,60.7%). Hypertension was the most common underlying disease (109 [41.6%] patients), followed by coronary heart disease (78 [29.8%]) and diabetes (48 [18.3%]). There was a significant difference in terms of patient age (P < 0.001), and presence of coronary artery disease (P < 0.001), hypertension (P < 0.001), diabetes (P = 0.003) and chronic renal failure (P = 0.002) between the two groups. Leukopenia (white cell counts less than 4*109/L) occurred in 47 (17.9%) and lymphocytopenia (defined as lymphocyte counts less than 109/L) occurred in 146 (55.7%) patients. Detailed patient characteristics are shown in Table 1.
Table 1.
Patient demographic, comorbidities, symptoms and laboratory findings at admission
| Variables | All patients (n = 262) |
survivors (n = 206) | deceased (n = 56) | P value | |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 58 (43–67) | 56 (41–65) | 66 (58–77) | <0.000* | |
| Gender, n (%) | Male | 172 (65.6) | 117 (62.6) | 55 (73.3) | 0.64** |
| Female | 90 (34.4) | 70 (37.4) | 20 (26.7) | ||
| Comorbidities | |||||
| Diabetes | 48 (18.3) | 30 (14.6) | 18 (32.1) | 0.003** | |
| Hypertension | 109 (41.6) | 69 (33.5) | 40 (71.4) | <0.001** | |
| Coronary artery disease | 78 (29.8) | 44 (21.4) | 34 (60.7) | <0.001*** | |
| Chronic lung disease | 40 (15.3) | 27 (13.1) | 13 (23.2) | 0.06** | |
| Chronic liver disease | 0 (0) | 0 (0) | 0 (0) | – | |
| Chronic renal failure | 16 (6.1) | 7 (3.4) | 9 (16.1) | 0.002*** | |
| Malignancy | 7 (2.7) | 7 (3.4) | 0 (0) | 0.18*** | |
| Immunosuppression | 6 (2.3) | 6 (2.9) | 0 (0) | 0.23*** | |
| Post-partum | 2 (0.8) | 2 (1) | 0 (0) | – | |
| Symptoms | Fever | 182 (69.5) | 142 (68.9) | 40 (71.4) | 0.72** |
| Cough | 159 (60.7) | 126 (61.2) | 33 (58.9) | 76** | |
| Dyspnea | 102 (38.9) | 80 (39%) | 22 (29.3) | 0.5** | |
| Myalgia | 29 (11.1) | 26 (12.6) | 3 (5.4) | 0.12** | |
| Symptom onset (days), median (IQR) | 4 (3–6) | 4 (3–7) | 4 (3–5) | 0.054* | |
| Hypoxia at admission | 160 (61.1) | 114 (55.3) | 46 (82.1) | <0.001** | |
| ICU admission (overall) | 64 (24.4) | 18 (8.7) | 46 (82.1) | <0.001⁎⁎ | |
| ICU admission from beginning of hospitalization | 19 (7.3) | 5 (2.4) | 14 (25) | <0.001⁎⁎ | |
| Intubation (overall) | 58 (22.1) | 7 (3.4) | 51 (91.1) | <0.001⁎⁎ | |
| Intubation (in the ED) | 9 (3.4) | 1 (0.5) | 8 (14.3) | <0.001⁎⁎⁎ | |
| Hospital stay | 8 (5–13) | 7 (5–11) | 13 (8–19) | <0.001⁎ | |
| Laboratory findings | WBC (*10^9/L), median (IQR) | 6.7 (4.8–9.6) | 6.3 (4.5–9) | 7.7 (6–12) | 0.007* |
| LYM% | 16 (9–23) | 17 (11–24) | 11 (6.4–19) | <0.001* | |
| Lymphocyte count (*10^9/L), median (IQR) | 0.96 (0.75–1.4) | 1 (0.77–1.4) | 0.9 (0.7–1.2) | 0.03* | |
| Platelet count (*10^9/L), median (IQR) | 182 (145–247) | 186 (145–253) | 169 (132−222) | 0.11* | |
| LDH (U/L), median (IQR) | 676.5 (473.8–848.5) | 579 (431–761) | 836 (675–1115) | <0.001* | |
| CRP (mg/L), median (IQR) | 97 (43–156.5) | 87 (33–149) | 143 (100–180) | <0.001* | |
| ESR (mm/h), median (IQR) | 54.5 (35–83.2) | 53 (34–83) | 60 (46–84) | 0.32* | |
| AST (U/L), median (IQR) | 33.5 (24–53.75) | 30 (24–50) | 49 (33–78) | 0.001* | |
| ALT (U/L), median (IQR) | 28 (16.5–48) | 28 (16–43.7) | 35 (17–67) | 0.25* | |
| BUN (mg/dL), median (IQR) | 16 (12–23.4) | 15 (11−20) | 23 (14–29) | <0.001* | |
| Cr (mg/dL), median (IQR) | 1 (0.8–1.2) | 1 (0.8–1.1) | 1.1 (0.9–1.4) | <0.001* | |
| Hb (g/dL), median (IQR) | 13.85 (12.3–15) | 13.7 (12.2–14.9) | 14.3 (13–15.5) | 0.08* | |
*: Mann-Whitney U test, **: Chi Square test, ***: Fischer's exact test.
WBC: White blood count, LDH: Lactate dehydrogenase, CRP: C reactive protein, ESR: erythrocyte sedimentation rate, AST: Aspartate aminotransferase, ALT: alanine aminotransferase, BUN: blood urea nitrogen, Cr: Serum creatinine, Hb: hemoglobin, LYM%: lymphocyte percentage.
Out of 262 patients, 64 were admitted to the ICU, from which 19 were admitted directly from the ED to the ICU ward, and in 45 patients ICU admission occurred later in the course of hospital stay. A total of 58 patients were intubated, from which 9 were intubated in the ED (Table 1).
The median hospital stay was 7 days (IQR, 5-11 days) in the survivor group. The median time from admission to death was 13 days (IQR, 8–19 days) for the deceased group.
In univariable analysis, odds of in-hospital death were higher in patients with diabetes, hypertension, renal failure or coronary heart disease (Table 3). Age, hypoxemia at admission, lymphocyte percentage less than 20%, and elevated LDH were also associated with death (Table 3).
Table 3.
Computed tomography findings
| CT findings | Overall (n = 262) | survivors (n = 206) | deceased (n = 56) | P value | |
|---|---|---|---|---|---|
| CT severity score | 8 (6–12) | 7.5 (6–11) | 14.5 (10–21) | <0.001* | |
| Parenchymal infiltrate | GGO | 67 (25.6) | 56 (27.2) | 11 (19.6) | 0.26** |
| Consolidation | 79 (30.1) | 65 (31.6) | 14 (25) | ||
| GGO and consolidation | 116 (44.3) | 85 (41.3) | 31 (55.4) | ||
| RSNA pattern | Typical | 143 (54.6) | 113 (54.9) | 30 (53.6) | 0.81** |
| Indeterminate | 81 (30.9) | 62 (30.1) | 19 (33.9) | ||
| Atypical | 38 (14.5) | 31 (15) | 7 (12.5) | ||
| Bilateral | 253 (96.6) | 198 (96.1) | 55 (98.2) | 0.39*** | |
| Longitudinal distribution | Upper zone | 21 (8) | 16 97.8) |
5 (8.9) | 0.23** |
| Middle zone | 38 (14.5) | 33 (16) | 5 (8.9) | ||
| Lower zone | 100 (38.2) | 82 (39.8) | 18 (32.1) | ||
| Random | 103 (39.3) | 75 (36.4) | 28 (50) | ||
| Axial distribution | Peripheral | 101 (38.5) | 90 (43.7) | 11 (19.6) | 0.094*** |
| Central | 4 (1.5%) | 4 (1.9) | 0 (0) | ||
| Diffuse | 131 (50) | 91 (44.2) | 40 (71.4) | ||
| Random | 26 (9.9) | 21 (10.2) | 5 (8.9) | ||
| Number of involved lobes | 5 (5–5) | 5 (5–5) | 5 (5–5) | 0.36* | |
| Round infiltrate | |||||
| Halo sign | 19 (7.3) | 17 (8.3) | 2 (3.6) | 0.18*** | |
| Reverse halo sign | 26 (9.9) | 21 (10.2) | 5 (8.9) | 0.78** | |
| Crazy paving | 77 (29.4) | 54 (26.2) | 23 (41.1) | 0.03** | |
| Architectural distortion | 150 (57.3) | 131 (63.6) | 19 (33.9) | <0.001** | |
| Parenchymal lines | 93 (35.5) | 85 (41.3) | 8 (14.3) | <0.001** | |
| Pleural effusion | 26 (9.9) | 19 (9.2) | 7 (12.5) | 0.47** | |
| Lymphadenopathy | 6 (2.3) | 3 (1.5) | 3 (5.4) | 0.11*** | |
| Pleural thickening | 30 (11.5) | 26 (12.6) | 4 (7.1) | 0.49*** | |
| Pericardial effusion | 1 (0.4) | 0 (0) | 1 (1.3) | – | |
*: Mann-Whitney U test, **: Chi Square test, ***: Fischer's exact test.
Multivariate regression analysis showed increasing odds of in-hospital death associated with older age (odds ratio 1.05, 95% CI 1.03–1.07, per year increase; P = 0.01), and the presence of coronary artery disease (odds ratio 9, 95% CI 1.4–60.8); P = 0.02) at admission.
3.2. Imaging findings
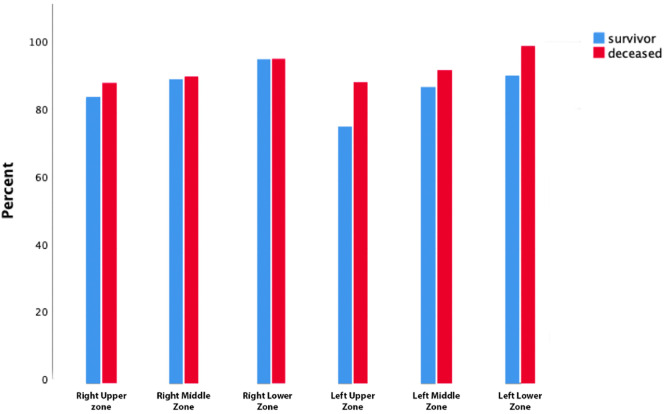
The mean CT severity score was 7.5 (6–11) in the survivor group and 14.5 (10−21) in the deceased group (P < 0.001). Fig. 1 displays the total number of patients with lung opacities in each lung zone. Overall, the lower zones (right, 252/262, [96.2%]; left 244/262, [93.1%]) were the most frequently involved sites in COVID-19. There were significant differences between the survivors and deceased groups regarding CT severity scoring in each lung zone, (P < 0.001) (Fig. 1, Table 2 ). The median left lung score was 3 [[2], [3], [4], [5]], right lung score was 4 [[3], [4], [5], [6]] and total CT severity score was 7.5 (6–11) in the survivor group, while the median left lung score was 7 (4–11), right lung score was 8 (5–11) and total CT severity score was 14.5 (10–21) in the deceased group. The lower lobe scores were higher than the middle and upper lobe score in each group (Fig. 2 ).
Fig. 1.
The frequency of involvement in each lung zone.
Table 2.
Comparison of scores of each lung zone between the two groups
| Variable | Overall (n = 262) | Survivors (n = 206) | Deceased (n = 56) | P |
|---|---|---|---|---|
| Right Upper Zone | <0.001 | |||
| 0 | 37 (14.1) | 31 (15) | 6 (10.7) | |
| 1 | 123 (46.9) | 111 (53.9) | 12 (21.4) | |
| 2 | 48 (18.3) | 37 (18) | 11 (19.6) | |
| 3 | 30 (11.5) | 17 (8.3) | 13 (23.2) | |
| 4 | 24 (9.2) | 10 (4.9) | 14 (25) | |
| Right Middle Zone | <0.001 | |||
| 0 | 25 (9.5) | 20 (9.7) | 5 (8.9) | |
| 1 | 114 (43.5) | 106 (51.5) | 8 (14.3) | |
| 2 | 68 (26) | 54 (26) | 14 (25) | |
| 3 | 33 (12.6) | 19 (9.2) | 14 (25) | |
| 4 | 22 (8.4) | 7 (3.4) | 15 (26.8) | |
| Right Lower Zone | <0.001 | |||
| 0 | 10 (3.8) | 8 (3.9) | 2 (3.6) | |
| 1 | 124 (47.3) | 111 (53.9) | 13 (23.2) | |
| 2 | 61 (23.3) | 54 (26.2) | 7 (12.5) | |
| 3 | 30 (11.5) | 21 (10.2) | 9 (16.1) | |
| 4 | 37 (14.1) | 12 (5.8) | 37 (14.1) | |
| Left Upper Zone | <0.001 | |||
| 0 | 55 (21) | 49 (23.8) | 6 (10.7) | |
| 1 | 142 (54.2) | 122 (59.2) | 20 (35.7) | |
| 2 | 30 (11.5) | 18 (8.7) | 12 (21.4) | |
| 3 | 14 (5.3) | 9 (4.4) | 5 (8.9) | |
| 4 | 21 (8) | 8 (3.9) | 13 (23.2) | |
| Left Middle Zone | <0.001 | |||
| 0 | 29 (11.1) | 25 (12.1) | 4 (7.1) | |
| 1 | 113 (43.1) | 101 (49) | 12 (21.4) | |
| 2 | 73 (27.9) | 59 (28.6) | 14 (25) | |
| 3 | 11 (4.2) | 7 (3.4) | 4 (7.1) | |
| 4 | 36 (13.7) | 14 (6.8) | 22 (39.3) | |
| Left Lower Zone | <0.001 | |||
| 0 | 18 (6.9) | 18 (8.7) | 0 (0) | |
| 1 | 136 (51.9) | 119 (57.8) | 17 (30.4) | |
| 2 | 50 (19.1) | 40 (19.4) | 10 (17.9) | |
| 3 | 22 (8.4) | 16 (7.8) | 6 (10.7) | |
| 4 | 36 (13.7) | 23 (41.1) | 13 (6.3) |
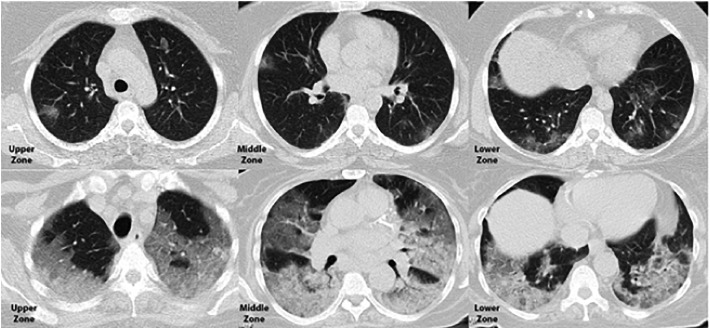
Fig. 2.
Axial Chest CT scan in a 44-year-old woman who was discharged from the hospital (above) and a 77-year-old woman who passed away (below) provided a side-by-side comparison of CT severity score.
Computed tomography findings of COVID-19 pneumonia are summarized in Table 4. The most frequent CT appearance was a combination of GGO and consolidation which was seen in 116 (44.3%) patients. Other common parenchymal abnormalities observed were GGO (25.6%, 67 patients) and consolidation (30.1%, 79 patients). Parenchymal abnormalities were distributed bilaterally in 253 patients (96.6%), whereas unilateral involvement was seen in 9 patients (3.4%). The abnormalities showed lower zone predominance (39.3%, 103 patients) or random distribution (38.2%, 100 patients) in the longitudinal plane. In the transverse plane, the lung abnormalities mostly showed peripheral (50%, 131 patients) or diffuse (38.5%, 90 patients) involvement.
Table 4.
Univariable and Multivariable analysis
| Variable |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | p value | |
| Age | 1.05 | 1.03–1.07 | 0.000 | 1.07 | 1.02–1.16 | 0.009 |
| Coronary artery disease | 5.7 | 3–10.7 | 0.000 | 6.7 | 1.08–42.2 | 0.04 |
| Hypertension | 5 | 2.6–9.5 | 0.000 | 2.1 | 0.42–11.2 | 0.35 |
| Diabetes | 2.8 | 1.4–5.5 | 0.003 | 3.2 | 0.5–21.2 | 0.22 |
| Chronic Renal failure | 5.4 | 1.9–15.4 | 0.000 | 29 | 0.3–3162 | 0.16 |
| Hypoxia at admission | 3.7 | 1.8–7.7 | 0.000 | 0.73 | 0.13–3.9 | 0.71 |
| WBC | 1.03 | 0.98–1.08 | 0.22 | – | – | – |
| LYM count | 0.4 | 0.2–0.8 | 0.01 | – | – | – |
| LYM% less than 20% | 2.5 | 1.3–4.6 | 0.004 | 4.6 | 0.9–23.9 | 0.07 |
| Hb | 1 | 0.96–1.03 | 0.9 | – | – | – |
| Plt | 1 | 0.99–1 | 0.12 | – | – | – |
| AST | 1 | 0.99–1 | 0.39 | – | – | – |
| ALT | 1 | 0.99–1 | 0.28 | – | – | – |
| LDH | 1.003 | 1.001–1.005 | 0.000 | 1.001 | 0.99–1.004 | 0.58 |
| CRP | 1 | 0.99–1.01 | 0.968 | – | – | – |
| ESR | 1 | 1–1.01 | 0.69 | – | – | – |
| Cr | 1 | 0.92–1.1 | 0.88 | – | – | – |
| CT severity score | 1.3 | 1.1–1.6 | 0.007 | 1.4 | 1.14–1.7 | 0.001 |
In univariable analysis, odds of in-hospital death were higher in patients with higher CT severity score and those with crazy paving pattern on the CT at admission (Table 3 ).
As patients requiring intubation or ICU admission may have a prognosis that is worse than those not requiring this at the beginning of their hospital course, we excluded these critical patients, and repeated the tests for those not requiring intubation or ICU admission at the beginning of their hospitalization. After excluding the critically ill patients, the median CT severity score was 7 (IQR, 6–11) in the survivor, and 14 (10−20) in the deceased group (P < 0.001).
There was a significant correlation between the time from admission to death and CT severity score (P: 0.03, correlation coefficient − 0.29). CT severity score was significantly correlated with time to ICU admission (P: 0.001, correlation coefficient: −0.38), and time to intubation (P: 0.035, correlation coefficient: −0.28). There was no significant correlation between CT severity score and the time of onset of symptoms (P: 0.08).
Multivariate regression analysis showed increasing odds of in-hospital death associated with higher CT severity score (odds ratio 1.3, 95% CI 1.1–1.7; P = 0.01) at admission.
3.3. ROC curve analysis
The ROC curve analysis for CT severity score is shown in Fig. 3 . The area under the curve for discriminating survivors from deceased was 0.839 (standard error 0.03; CI, 0.78–0.9), and optimal CT severity score threshold for identifying deceased patients was 10, with 84% (CI, 71.7%–92.4%) sensitivity and 66% (59.1%–72.5%) specificity. Increasing the threshold for CT severity score to 12 would return a sensitivity of 69.6% (55.9%–81.2%) and a specificity of 80.1% (74%–85.3%).
Fig. 3.
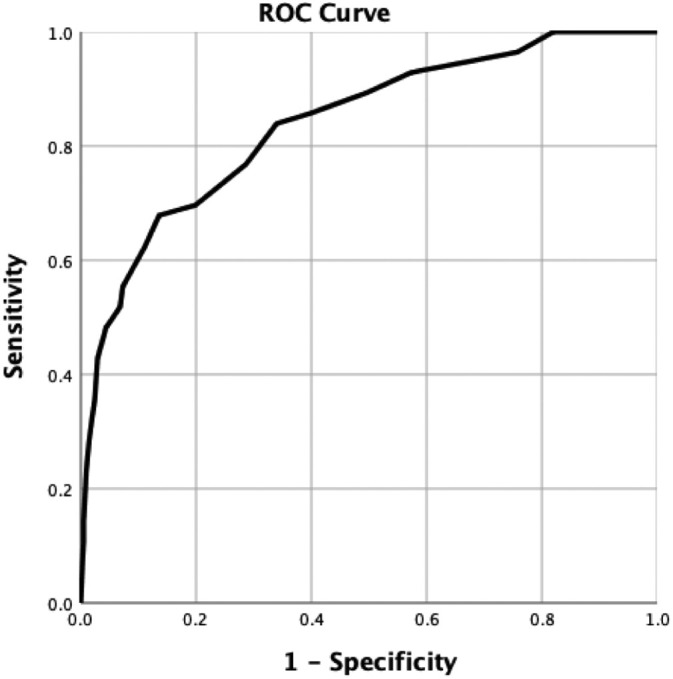
ROC curve for CT severity score sensitivity and specificity for in-hospital mortality.
4. Discussion
In this study we evaluated 262 patients with laboratory confirmed COVID-19 pneumonia. All patients were admitted to the designated university hospital in Mashhad, Iran from February 20, 2020 to March 10, 2020. COVID-19 pneumonia has been reported to be associated with variable mortality ranging from 11% to 15% in different studies [1,7]. The higher mortality rate reported in this study is probably due to inclusion of only hospitalized patients, and exclusion of outpatients with mild disease. The results of the present study identified several risk factors for mortality in patients hospitalized with COVID-19. In particular, older age, coronary artery disease, and CT severity score on admission were associated with higher odds of in-hospital death. Additionally, hypoxemia, hypertension, diabetes, elevated levels of blood C-reactive protein and lactate dehydrogenase, lymphopenia and crazy paving pattern on the chest CT scan at admission were more commonly seen in deceased group.
The predominant parenchymal abnormalities in our patients were areas of GGO, consolidation or both, with lower lung zone predominance in longitudinal plane and peripheral distribution in transverse plane. The distribution of the lesions was not shown to be associated with mortality.
In this study, we applied a semi-quantitative scoring method previously used by Zhou et al. [8] and Wang et al. [4] to score the degree of involvement using a system previously described for severity of acute respiratory distress syndrome on thin section lung CT scan [9]. CT severity score was previously reported as a risk factor for mortality in ARDS [9]. However, there is little data available on the prognostic value of CT in COVID-19. The results of our study show that mortality was significantly higher in patients with higher CT severity score even after adjustment for both clinical, demographics and laboratory parameters. CT severity score could discriminate admitted patients with higher in-hospital mortality with acceptable accuracy (area under the curve of 0.839). This is especially significant for the judgment of clinical condition and has important value in the disposition of patients. Patient with higher CT severity may benefit from early ICU admission. This can help in patient disposal in the emergency departments, especially in the settings with limited ICU resources. It is important to keep in mind that nucleic acid testing using polymerase chain reaction (PCR) is the reference standard test for diagnosis of COVID-19 infection. Although this study highlight the importance of chest CT as a prognostication tools, we should emphasize that chest CT is neither a necessary nor sufficient as diagnostic and prognostic tool, and can be only of use in patients for whom chest CT is available at the time of admission.
A CT severity score equal or greater than 10 had a sensitivity of 84% and specificity of 66% for in-hospital mortality. Increasing the cut-off threshold to led to a sensitivity of 69.6% and specificity of 80.1%. The optimal cut-off needs to be validated prospectively.
We also found a significant correlation between the CT severity score and rapidity of decline in clinical condition (time to death, time to ICU admission, and time to intubation). Though, these correlations are not so strong, and need to be validate by studies with larger sample sizes.
To sum up, we suggest that CT scan can be of prognostic use in patients who already have it at the time of admission, and can help as an adjunct tool for patient prognostification. Patients with greater CT severity score may beneift from more intensive hospital care. However, our study is performed retrospectively and needs to be validated in a prospective study.
The study has some limitations. First, it is a retrospective analysis performed in a single center. Second, the inter-rater agreement for CT severity score was not calculated as the two readers evaluated the images together. Third, as body mass index was not routinely calculated at patients' admission, this variable was not included in this analysis.
5. Conclusion
In conclusion, CT severity score was an independent predictor of death in patients with COVID-19 pneumonia. Patients with higher CT severity score at presentation may benefit from more intensive hospital care, regardless of their clinical condition.
Funding source
None.
Declaration of Competing Interest
The authors declare no conflicts of interests.
Acknowledgment
This research was supported by the Chancellor for Research of Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection 2020 Available from. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 3.Sverzellati N., Milanese G., Milone F., Balbi M., Ledda R.E., Silva M. Integrated radiologic algorithm for COVID-19 pandemic. J. Thorac. Imaging. 2020;35(4):228–233. doi: 10.1097/RTI.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;200843 doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson S., Kay F.U., Abbara S., Bhalla S., Chung J.H., Chung M., et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology. 2020;2 doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou S., Wang Y., Zhu T., Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am. J. Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 9.Ooi G.C., Khong P.L., Müller N.L., Yiu W.C., Zhou L.J., Ho J.C., et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]