Abstract
Background
The release of pro-inflammatory cytokines, resulting in cytokine storm syndrome, contributes to the morbidity and mortality associated with COVID-19 disease. This study aimed to compare the effects of intravenous (IV) and subcutaneous (SC) tocilizumab, an IL-6 receptor antagonist, on respiratory parameters and clinical outcome in patients with COVID 19.
Methods
We performed a retrospective cohort study of hospitalized patients with COVID-19 treated with either IV or SC tocilizumab from March 26, 2020, to May 18, 2020. Respiratory parameters seven days after receiving tocilizumab therapy were compared to baseline measurements. All patients were assessed until discharged from the hospital.
Results
Tocilizumab was administered to 125 patients: 65 received IV, and 60 received SC therapy. At day seven, 52% of the IV group patients demonstrated improvement in respiratory parameters, compared to 28% in the SC group (P = 0.01). Mortality rates at days seven and 28 were 15% and 37%, respectively, in the IV group and 17% and 50%, respectively, in the SC group (PNS). The i n-hospital mortality rate was 38% for the IV group versus 57% for the SC group (P = 0.04). More than 90% of patients in each group received corticosteroids; however, significantly more patients received convalescent plasma in the IV group.
Conclusions
At the doses used in this study, IV tocilizumab is preferred over SC therapy to treat cytokine storm syndrome due to COVID-19.
Keywords: COVID-19, Cytokine storm, Tocilizumab
Introduction
The novel coronavirus, SARS-CoV-2, emerged in Wuhan, China, in December 2019, and spread rapidly worldwide, causing COVID-19 disease. As of July 2020, there have been 10 million cases reported, with 500,000 fatalities (https://www.who.int/emergencies/ diseases/novel-coronavirus-2019/situation-reports). While the majority of COVID-19 cases are mild and self-limiting, severe disease and death can occur. Risk factors for progression to critical illness and death include advanced age, underlying cardiac or renal disease, and obesity (Wu et al. 2020; Petrilli et al., 2020). Progressive illness is characterized by massive alveolar damage, progressive respiratory failure, and multi-organ dysfunction (Xu et al., 2020a, Chen et al., 2020a). Post-mortem analyses have shown an overactivation of TH17 and CD8 T cells with the release of pro-inflammatory cytokines resulting in immune injury and cytokine storm.
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that has been shown to be elevated in patients with severe disease (Chakraborty et al., 2020, Luo et al., 2020; Alzghari and Acuña, 2020), and a potential target to reduce disease progression. Tocilizumab is a recombinant humanized monoclonal antibody that is directed specifically against the interleukin-6 receptor (IL-6R) and works by binding to both soluble and membrane-bound IL-6R, resulting in inhibition of IL-6-mediated signaling through these receptors (Le et al., 2018, Antwi-Amoabeng et al., 2020). Tocilizumab is FDA approved for use in patients with rheumatoid arthritis, systemic juvenile idiopathic arthritis, giant cell arteritis, and life-threatening cytokine release syndrome associated with the use of chimeric antigen receptor T-cells. Several studies have documented favorable outcomes following tocilizumab therapy in patients with severe COVID-19 disease. Xu et al. reported the use of tocilizumab (administered as a one-time 400 mg intravenous dose) in 21 patients with COVID-19 that resulted in no deaths, with 90% of their patients discharged home (Xu et al., 2020b). Subsequent studies have also demonstrated benefit, with reductions in overall mortality, particularly in patients with more advanced disease (requiring mechanical ventilation) (Toniati et al., 2020, Klopfenstein et al., 2020, Rossotti et al., 2020, Somers et al., 2020, Guaraldi et al., 2020). However, not all reports have been so favorable, especially in critically ill patients (Luo et al., 2020). In addition, adverse effects (including superinfections and prolongation of hospital stay) have been noted (Rossotti et al., 2020, Somers et al., 2020, Guaraldi et al., 2020).
Both intravenous (IV) and subcutaneous (SC) formulations of tocilizumab have been used to treat the cytokine storm due to COVID-19, with apparent equal effect (Guaraldi et al., 2020). It is noteworthy that the pharmacokinetic profiles of the two formulations differ significantly. SC injection has an absorption half-life of approximately four days, resulting in Cmax's delayed achievement (Tocilizumab package insert, 2017). In patients with rheumatoid arthritis, administration of 162 mg tocilizumab SC weekly and biweekly resulted in maximum serum levels of 9.3 ± 5.1 μg/mL and 5.8 ± 4.1 μg/mL, respectively (Lee et al., 2014). In contrast, 8 mg/kg of tocilizumab given IV weekly resulted in a maximum serum concentration of 136 ± 34 μg/mL (Lee et al., 2014). Whether a more delayed but sustained effect following SC administration or a more intensive but shorter-lived effect following IV administration is preferable in managing cytokine storm is unknown.
In this report, the respiratory and clinical outcomes of patients treated with either IV or SC tocilizumab therapy for COVID-19 are compared.
Materials and methods
Study patients
Consecutive patients receiving tocilizumab for COVID-19 related illness between March 26, 2020, through May 18, 2020, underwent a standardized chart review. Our medical center, a large tertiary care facility located in Brooklyn, serves a predominantly minority and underserved population. All admitted patients with suspected or proven COVID-19 illness who were in respiratory distress (typically defined as a peripheral oxygen saturation ≤ 93 % on room air) were considered eligible for tocilizumab therapy.
Standard of care treatment developed at our institution included hydroxychloroquine 400 mg twice a day for one day, followed by 200 mg twice a day for an additional four days plus azithromycin 500 mg once, followed by 250 mg oral once daily for an additional four days. Concomitant with tocilizumab therapy, short courses (typically 3–5 days) of corticosteroids were encouraged; corticosteroid dosing was often left to the primary care providers' discretion.
Tocilizumab was administered at 400 mg IV, typically as a single dose, based on initial reports (Xu et al., 2020a). When the intravenous formulation was unavailable, the subcutaneous formulation was used. At our institution, a decision to use an SC dose of 324 mg (given as two simultaneous doses of 162 mg) was based on known pharmacokinetic data (Zhang et al., 2013). It should be noted this was the same dosage used in another comparator study (Guaraldi et al., 2020).
The study was approved by the Institutional Review Board at SUNY-Downstate Medical Center and the System to Track and Approve Research at NYC Health and Hospitals.
Data extraction
Patients treated with tocilizumab were retrospectively identified by a review of pharmacy records. A subsequent review of the electronic medical record was performed for each patient to obtain demographic, clinical, and laboratory information. The respiratory parameters for the 24 h preceding the tocilizumab dose, and on days three and seven post-administration, were recorded. Two respiratory-based criteria were used to assess response to tocilizumab therapy:
-
1)
to detect subtle changes in respiratory parameters, definitions of ventilatory response were taken to mirror National Healthcare Safety Network (NHSN) definitions for ventilatory-associated events. For every 24 h under review, the highest levels of oxygen requirement (i.e., FiO2) or PEEP that were sustained for at least one hour were recorded. Patients were considered to have ventilatory improvement if there was a decrease in FiO2 of ≥ 20% or PEEP of ≥ 3 cm H2O (with a PEEP setting of 0−5 cm H2O as the lowest setting). Patients were also considered to have responded if there was an incremental decrease in oxygen requirement reflected by a change from a higher to a lower category of oxygen support: mechanical ventilation (highest category), BiPAP/CPAP, high flow nasal cannula, low-flow facemask, low flow nasal cannula, and room air (the lowest category).
-
2)
To determine more overt changes in respiratory parameters, on day seven the disease severity scale employed by Li et al., 2020, was used. This ordinal scale consists of six clinical points: 6= death; 5= hospitalization with mechanical ventilation; 4= hospitalization with non-invasive ventilation or high-flow oxygen therapy; 3= hospitalization with other oxygen therapy; 2= hospitalization without oxygen therapy; and 1= discharged or achieved discharge criteria. Improvement was defined as a reduction by at least two points in the disease severity scale.
The following additional clinical information was recorded: 1) prior history of diabetes mellitus, hypertension, and ischemic heart disease; 2) receipt of corticosteroids and convalescent plasma during the 7-day observation period, and 3) duration of symptoms prior to tocilizumab administration. The following inflammatory markers prior to and within seven days post tocilizumab therapy were collected: C-reactive protein, D-dimers, ferritin, IL-6, lactate dehydrogenase, and procalcitonin. Changes in basic laboratory values and positive cultures of blood were also noted during the 7-day observation period. Cytokine release syndrome grades were determined according to the criteria of Lee et al., 2018. As of July 15, 2020, all patients had been discharged from the acute care medical service. Survival data were calculated from the day of tocilizumab administration to either death or discharge from the hospital.
Statistical analysis
The primary endpoints were changes in ventilatory status at days three and seven following tocilizumab therapy. Secondary endpoints were survival rates at days seven, 28, and during the hospital stay. Fisher’s exact test and student’s t-test were used to compare categorical and continuous values, respectively, between groups. Student’s t-test for paired values was used to compare pre- and post-tocilizumab laboratory values. Survival curves were created using the Kaplan-Meier method and compared using the log-rank test. A P value of ≤ 0.05 was considered significant.
Results
There were 125 patients included in the study; 65 received IV, and 60 received SC tocilizumab. Overall, 87 (70%) patients were African American persons. A nasopharyngeal swab was positive by RT-PCR for SARS-CoV-2 in 117 patients, with the remaining eight patients highly suspected of having COVID-19 illness. Overall, 82 patients were receiving supplemental oxygen and/or considered to have severe disease, and 43 were on mechanical ventilation and considered to have a critical illness. The baseline characteristics were generally comparable between the patients in the IV and SC groups (Table 1 ); there tended to be more females who received IV tocilizumab. At the time of IV tocilizumab therapy, 36 (55%) patients met grade 3, and 27 (42%) patients met grade 4 cytokine release syndrome criteria. Similarly, at the time of SC tocilizumab therapy, 32 (53%) patients met grade 3, and 26 (43%) met grade 4 cytokine release syndrome criteria.
Table 1.
Baseline characteristics of the patients in the intravenous (IV) and subcutaneous (SC) –treated tocilizumab groups.
| IV group (n = 65) | SC group (n = 60) | |
|---|---|---|
| Age, years, mean ± SD | 58.9 ± 17.9 | 57.2 ± 15 |
| Female, No. (%) | 27 (42%) | 15 (8%) |
| African American, No. (%) | 43 (66%) | 44 (73%) |
| Body mass index, kg/m2, mean ± SD | 32.5 ± 10.6 | 33.0 ± 10.1 |
| Comorbidities | ||
| Diabetes, No. (%) | 25 (38%) | 29 (48%) |
| Hypertension, No. (%) | 43 (66%) | 35 (58%) |
| Renal disease, No. (%) | 8 (12%) | 7 (12%) |
| Mechanical ventilation, No. (%) | 22 (34%) | 21 (35%) |
| Cytokine release syndrome grade, mean ± SD | 3.4 ± 0.6 | 3.4 ± 0.6 |
| Duration of symptoms, days, mean ± SD (median) | 11.0 ± 9.6 (8) | 13.8 ± 7.5 (13) |
| Laboratory values | ||
| White blood cell count K/μL, mean ± SD | 13.2 ± 5.1 | 12.7 ± 6,3 |
| Absolute neutrophil count K/μL, mean ± SD | 11.1 ± 4.6 | 10.7 ± 5.6 |
| Hemoglobin g/dL, mean ± SD | 11.4 ± 2.2 | 11.7 ± 2.0 |
| Platelets K/μL, mean ± SD | 273 ± 122 | 257 ± 127 |
| Potassium mmol/L, mean ± SD | 4.4 ± 0.7 | 4.7 ± 0.9 |
| Creatinine mg/dL, mean ± SD | 2.1 ± 2.6 | 2.7 ± 3.7 |
| Alanine aminotransferase U/L, mean ± SD | 53.7 ± 87.9 | 71.2 ± 91.8 |
| C-reactive protein mg/L, mean ± SD | 194 ± 97 | 209 ± 155 |
| D-Dimers ng/mL, mean ± SD | 8,359 ± 16,121 | 11,029 ± 18,444 |
| Ferritin ng/mL, mean ± SD | 1205 ± 708 | 1384 ± 734 |
| Interleukin-6 pg/mL, mean ± SD | 340 ± 655 | 124 ± 157a |
| Lactate acid dehydrogenase U/L, mean ± SD | 705 ± 388 | 696 ± 321 |
| Procalcitonin ng/mL, mean ± SD | 6.37 ± 18.8 | 2.92 ± 8.04 |
P = 0.04 comparing the IV and SQ groups.
Several laboratory values have been shown to be predictors of mortality in patients with COVID-19 (Garcia et al., 2020), including blood levels of potassium, creatinine, D-dimers, lactate, and P/F ratios. The baseline laboratory values for potassium, creatinine, and D-dimer were similar for the IV and SC groups (Table 1). Lactate levels (2.4 ± 1.6 vs. 2.1 ± 1.1 mmol/L) and P/F ratios (148 ± 101 vs. 148 ± 89) were also similar between the IV and SC groups, respectively. The percentage of patients with known ischemic heart disease, also an indicator of higher mortality (Garcia et al., 2020), was also similar in the IV and SC groups: five of 65 (8%) vs. three of 60 (5%), respectively. IL-6 levels have also been correlated with poor respiratory outcomes (Herold et al., 2020, Chen et al., 2020b), and baseline levels were significantly higher in the group that received IV therapy vs. SC therapy (340 ± 655 vs. 124 ± 157 pg/mL, P = 0.04). Concomitant use of corticosteroids was high in both groups: 94% in the IV group and 90% in the SC group. However, patients in the IV group tended to receive higher daily doses (≥ 500 mg of methylprednisolone or equivalent) of corticosteroids than those in the SC group (40 of 65 vs. 23 of 60, P = 0.01). Finally, compared to patients in the SC group, more patients in the IV group received convalescent plasma during the seven days following tocilizumab therapy (33 of 65 vs. five of 60, P < 0.001).
Responses to therapy using NHSN-based definitions (criterion one) were determined. On day three following tocilizumab therapy, 34 of the 65 (52%) patients in the IV group showed improvement in respiratory parameters, compared with 19 of 60 (32%) in the SC group (P = 0.03). At day seven, the difference in respiratory improvement persisted, with 34 of 65 (52%) in the IV group versus 17 of 60 (28%) of patients in the SC group showing improvement (P = 0.01). Improvements in respiratory parameters at day seven were more often seen for those on mechanical ventilation: 13 of 22 patients that received IV therapy improved, compared to four of 21 patients that received SC therapy (P = 0.01). For patients who were not on mechanical ventilation, 21 of 43 patients in the IV group had improved respiratory parameters, compared to 13 of 39 in the SC group (P NS). Similarly, 12 of the 43 patients in the IV group initially not on mechanical ventilation subsequently required mechanical ventilation on day seven, compared to 16 of 39 patients in the SC group (P NS).
Using the six-point disease severity scale to assess the response to therapy (criterion two), more favorable outcomes on day seven were noted in the IV group. At day seven, 16 (25%) of patients in the IV group had a two-point reduction, compared to six (10%) of patients in the SC group (P = 0.04).
Improvements in the cytokine release syndrome grades also favored the patients in the IV therapy group. Among the survivors at day seven, the average cytokine release syndrome grade fell from 3.33 ± 0.55 to 2.78 ± 1.23, P = 0.0003) for the IV group. At day seven, the average cytokine release syndrome grade fell from 3.38 ± 0.57 to 3.18 ± 1.02, P NS) for the SC group. Among the survivors at day seven in the IV group, the number of patients with cytokine release syndrome grades 3 or 4 fell from 53 of 55 (96%) to 35 of 55 (64%), P < 0.0001. Among the survivors at day seven in the SC group, the number of patients with cytokine release syndrome grades 3 or 4 fell from 48 of 50 (96%) to 38 of 50 (76%), P = 0.008.
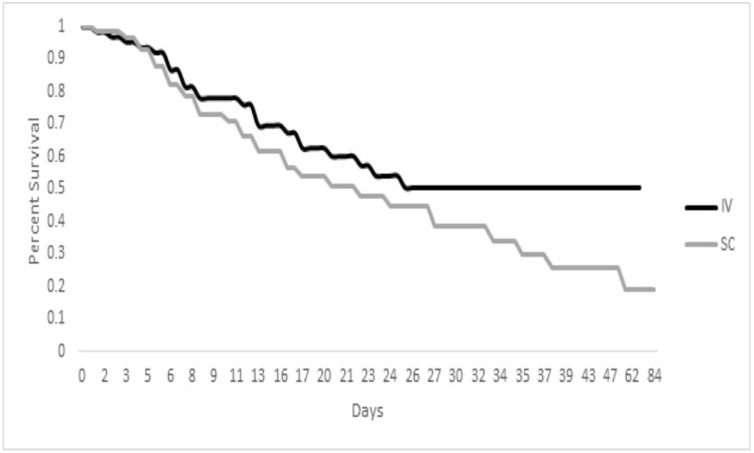
Mortality rates were similar between the two groups seven days following tocilizumab therapy: ten of 65 (15%) in the IV group versus ten of 60 (17%) in the SC group P NS). By day 28, 24 of 65 patients in the IV group had died, compared to 30 of 60 in the SC group (P = 0.15). When followed to hospital discharge, 25 of 65 (38%) in the IV group did not survive hospitalization vs. 34 of 60 (57%) in the SC group (P = 0.05). In the IV group, 27 of 65 patients were discharged home (42%) vs. 15 of 60 (25%) in the SC group (P = 0.06). Kaplan-Meier survival plots for the two treatments are shown in Fig. 1 ; a trend towards improved survival was noted with the IV group compared to the SC group (P = 0.11).
Fig. 1.
Kaplan-Meier estimates of survival among patients treated with intravenous (IV) and subcutaneous (SC) tocilizumab. Long-rank test for intergroup differences in survival rate distribution P = 0.11.
Laboratory values were also assessed in the survivors on day seven to determine if there were any differences in possible toxicities related to IV vs. SC tocilizumab therapy. Laboratory values at baseline were generally similar to values at day seven for patients in the IV group: white blood cell count (12.8 ± 5.1 vs. 14.7 ± 10.3 K/μL), absolute neutrophil count (10.7 ± 4.6 vs. 12.0 ± 9.5 K/μL), hemoglobin (11.5 ± 2.3 vs. 11.0 ± 2.2 g/dL), platelets (268 ± 117 vs. 274 ± 121 K/μL) and creatinine 1.85 ± 2.34 vs. 1.51 ± 1.76 mg/L). However, alanine aminotransferase levels did rise significantly on day seven, from 57.6 ± 95 to 107 ± 112 U/L (P < 0.001). Laboratory values at baseline were all similar to those at day seven for patients in the SC group: white blood cell count (13.2 ± 6.4 vs. 13.8 ± 8.5 K/μL), absolute neutrophil count (11.0 ± 5.7 vs. 11.1 ± 7.2 K/μL), hemoglobin (11.9 ± 1.9 vs. 11.1 ± 2.7 g/dL), platelets (266 ± 135 vs. 271 ± 130 K/μL), creatinine (2.41 ± 3.4 vs. 2.36 ± 2.80 mg/L) and alanine aminotransferase 73.7 ± 98.7 vs. 91.6 ± 112.4 U/L). During the 7-day post-treatment period, there were twelve positive blood cultures among the IV group patients versus five in the SC group. Of the 17 patients with positive blood cultures, six cultures grew coagulase-negative staphylococci, likely representing contamination.
In an attempt to identify factors that might predict improvement in respiratory parameters at day seven, baseline laboratory data were compared (Table 2 ) between the groups of patients showing and lacking (including death) improvement. C-reactive protein levels were significantly higher in patients in the IV group that did not respond (Table 2); for the patients with baseline laboratory values, 20 of 29 non-responders had C-reactive protein levels > 200 mg/L compared to 10 of 29 responders (P = 0.02). The other baseline laboratory markers of inflammation were similar in the responders and non-responders. For those in the IV group, 32 of the 34 patients who had improved respiratory parameters received corticosteroids, compared to 29 of 31 patients who did not have a clinical response (P NS). Similarly, 17 of the 34 responders received convalescent plasma, vs. 16 of the 31 non-responders (P NS).
Table 2.
Comparison of characteristics between patients that did (responders) and did not (non-responders) have improvement in respiratory parameters at seven days following tocilizumab therapy.
| IV Group (n = 65) |
SC Group (n = 60) |
|||
|---|---|---|---|---|
| Responders (n = 34) | Non-responders (n = 31) | Responders (n = 17) | Non-responders (n = 43) | |
| Age, years, mean ± SD | 58.4 ± 17.0 | 59.4 ± 19.0 | 53 ± 17.6 | 58.9 ± 13.7 |
| Sex, No. (%), female | 12 (35%) | 15 (48%) | 3 (18%) | 12 (29%) |
| Race, No. (%), African American | 21 (59%) | 22 (52%) | 13 (76%) | 31 (73%) |
| Body mass index kg/m2, mean ± SD | 33.4 ± 11.1 | 31.4 ± 10.1 | 34.1 ± 9.5 | 32.6 ± 10.4 |
| Comorbidities | ||||
| Diabetes, No. (%) | 12 (35%) | 13 (42%) | 4 (24%) | 25 (58%)a |
| Hypertension, No. (%) | 24 (71%) | 19 (61%) | 7 (41%) | 28 (65%) |
| Renal disease, No. (%) | 1 (3%) | 6 (19%)a | 2 (12%) | 4 (9%) |
| Duration of symptoms, days, mean ± SD | 11.3 ± 12.2 | 10.6 ± 6.0 | 14.6 ± 8.2 | 13.4 ± 7.3 |
| C-reactive protein mg/L, mean ± SD | 166 ± 90 | 224 ± 95a | 175 ± 97.2 | 223 ± 172 |
| D-dimers ng/mL, mean ± SD | 6509 ± 14,383 | 10,332 ± 17,825 | 5712 ± 14,719 | 13,283 ± 19,549 |
| Ferritin ng/mL, mean ± SD | 1051 ± 800 | 1365 ± 570 | 1298 ± 619 | 1420 ± 783 |
| Interleukin-6 pg/mL, mean ± SD | 289 ± 628 | 391 ± 691 | 149 ± 222 | 115 ± 131 |
| Lactic acid dehydrogenase U/L, mean ± SD | 647 ± 255 | 758 ± 383 | 608 ± 385 | 732 ± 288 |
| Procalcitonin ng/mL, mean ± SD | 5.44 ± 19.4 | 7.31 ± 18.6 | 2.36 ± 5.93 | 3.17 ± 8.9 |
P≤0.05 comparing values between the two groups.
Lastly, laboratory markers of inflammation were analyzed before and during the seven days following tocilizumab therapy to identify trends correlated with responses involving the respiratory parameters (Table 3 ). For both responders and non-responders in the IV and SC groups, C-reactive protein levels fell significantly following therapy (Table 3). IL-6 levels rose in all groups; however, this reached statistical significance only in the non-responders in the SC group. None of the other inflammation markers collected within seven days following therapy were significantly different from pre-treatment values.
Table 3.
Laboratory markers of inflammation before and following tocilizumab therapy in patients that did and did not have improvement in respiratory parameters at day seven.
| IV Group | ||||||
|---|---|---|---|---|---|---|
| Responders (n = 34) |
Non-responders (n = 31) |
|||||
| Number | Before | After | Number | Before | After | |
| C-reactive protein mg/L, mean ± SD | 23 | 163 ± 95 | 47 ± 87a | 20 | 228 ± 100 | 77 ± 102a |
| D-dimers ng/mL, mean ± SD | 28 | 7116 ± 15,291 | 2216 ± 2432 | 23 | 11,629 ± 19,429 | 6963 ± 12,935 |
| Ferritin ng/mL, mean ± SD | 26 | 1064 ± 845 | 1010 ± 611 | 20 | 1376 ± 597 | 1450 ± 574 |
| Interleukin-6 pg/mL, mean ± SD | 11 | 109 ± 116 | 179 ± 259 | 9 | 465 ± 526 | 779 ± 748 |
| Lactic acid dehydrogenase U/L, mean ± SD | 20 | 585 ± 195 | 556 ± 223 | 17 | 874 ± 449 | 845 ± 363 |
| Procalcitonin ng/mL, mean ± SD | 17 | 8.32 ± 24.7 | 5.47 ± 14.3 | 14 | 3.94 ± 6.71 | 2.18 ± 3.58 |
| SC Group | ||||||
|---|---|---|---|---|---|---|
| Responders (n = 17) |
Non-responders (n = 43) |
|||||
| Number | Before | After | Number | Before | After | |
| C-reactive protein mg/L, mean ± SD | 13 | 165 ± 97.4 | 32.3 ± 28.8a | 29 | 194 ± 106 | 79.5 ± 94.8a |
| D-dimers ng/mL, mean ± SD | 14 | 6409 ± 16,199 | 3019 ± 4092 | 32 | 13,805 ± 19,371 | 7894 ± 13,555 |
| Ferritin ng/mL, mean ± SD | 13 | 1275 ± 601 | 1061 ± 630 | 31 | 1491 ± 766 | 1364 ± 617 |
| Interleukin-6 pg/mL, mean ± SD | 5 | 141 ± 236 | 563 ± 775 | 16 | 97.2 ± 86.1 | 738 ± 1011a |
| Lactic acid dehydrogenase U/L, mean ± SD | 9 | 488 ± 226 | 480 ± 196 | 18 | 759 ± 328 | 705 ± 236 |
| Procalcitonin ng/mL, mean ± SD | 5 | 5.60 ± 10.4 | 2.12 ± 3.54 | 12 | 1.29 ± 1.52 | 8.88 ± 13.5 |
P≤0.05 comparing values between the two groups.
Discussion
It is becoming increasingly evident that targeting the cytokine storm syndrome in patients with COVID-19 related illness can improve outcomes. Elevated IL-6 levels have been identified as a risk factor for adverse outcomes, including worsening respiratory status and death (Rossotti et al., 2020, Ruan et al., 2020). Considerable data have accumulated regarding the use of tocilizumab therapy for patients with serious or critical illness due to COVID-19. Tocilizumab has been found to be associated with improved outcomes in patients with COVID-19 related respiratory disease, particularly for patients with a critical illness (i.e., requiring mechanical ventilation) (Rossotti et al., 2020, Somers et al., 2020). In one study, both IV (8 mg/kg for two doses) and SC (324 mg as a single dose) were found comparable in reducing mortality compared to standard of care (Guaraldi et al., 2020).
In this report, we found divergent outcomes in patients administered IV vs. SC tocilizumab. We attempted to identify subtle differences in respiratory parameters during the first week of therapy by including the National Safety Healthcare Network criteria of ventilator-associated events. As might be anticipated, given the pharmacokinetic differences of IV and SC tocilizumab, greater improvements in respiratory parameters were observed at three and seven days in the group of patients receiving IV therapy. This improvement in respiratory function subsequently translated into improved clinical outcomes - compared to those patients that received SC therapy, patients that received IV therapy had lower in-hospital mortality.
Over 90% of the patients in our study concomitantly received short courses of corticosteroids. Corticosteroid dosing was higher in patients who received IV tocilizumab, possibly contributing to the differences found with the SC group. However, doses of corticosteroids in both the IV and SC groups typically equaled or exceeded six mg of dexamethasone per day, a dose that is beneficial in reducing mortality in patients with advanced COVID-19 disease (Collaborative Recovery group, 2020). The combination of corticosteroids and tocilizumab may have an additive effect in treating cytokine storm syndrome (Ramiro et al., 2020); this effect may be observed only with IV tocilizumab therapy.
Laboratory markers of inflammation are often used in the assessment of patients with COVID-19. In addition to IL-6, elevated levels of C-reactive protein, D-dimers lactate dehydrogenase, and procalcitonin have been associated with poor prognosis (Wu et al., 2020, Ruan et al., 2020, Chen et al., 2020c, Zhou et al., 2020). In this report, patients who received IV tocilizumab and failed to improve respiratory parameters had higher C-reactive protein levels than patients who did improve. Patients with extremely elevated C-reactive protein levels may likely require a more aggressive strategy (e.g., multiple doses of tocilizumab). Laboratory markers of inflammation are often used to gauge the clinical response to therapy in patients with COVID-19. We did not find trends in inflammation markers that differentiated patients who did or did not have improvement of respiratory parameters seven days after treatment. In our report, C-reactive protein levels fell acutely in both patients that did and did not have an improvement in respiratory parameters seven days following tocilizumab therapy. A decrease in C-reactive protein levels has been observed following tocilizumab therapy with or without corticosteroids (Xu et al., 2020b, Rossotti et al., 2020).
Our study has several limitations. In addition to being a single-center study, corticosteroids were administered to over 90% of our patients, as noted above. More patients in the IV group received convalescent plasma during the seven days following tocilizumab therapy. While it is possible that convalescent plasma therapy favorably influenced outcomes in the IV group, we feel this is unlikely since 1) patients had symptoms on average for more than ten days, a time when the intrinsic antibody response is already occurring, and 2) a recent study suggested an overall lack of efficacy in clinical improvement (Li et al., 2020). Moreover, significantly improved clinical outcomes with convalescent plasma therapy has been restricted to patients not requiring mechanical ventilation (Li et al., 2020). In our study, the greatest benefit of IV tocilizumab therapy was observed in the subgroup requiring mechanical ventilation.
Compared to SC administration, tocilizumab administered by the IV route in patients with complicated COVID-19 resulted in improved respiratory parameters at days three and seven and reduced in-hospital mortality. Tocilizumab IV should be favored over the SC route in managing patients with COVID-19.
Author contributions
MK, SS, and JQ performed the study concept and design. MK, SS, KB, AK, and AG participated in data acquisition. MK and MA performed overall data analysis and interpretation of data. JQ performed the statistical analysis. MK, MA, and JQ wrote the draft of the manuscript. All authors contributed to the critical revision and final approval of the manuscript.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to have influenced the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Institutional Review Board at SUNY Downstate Medical Center.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgments
None.
References
- Alzghari S.K., Acuña V.S. Supportive treatment with tocilizumab for COVID-19: A systematic review. J Clin Virol. 2020;127(Jun(104380)) doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwi-Amoabeng D., Kanji Z., Ford B., Beutler Bd, Riddle Ms, Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review. J Med Virol. 2020;(May) doi: 10.1002/jmv.26038. 10.1002/jmv.26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Agoramoorthy G. COVID-19: Consider IL6 receptor antagonist for the therapy of cytokine storm syndrome in SARS-CoV-2 infected patients. J Med Virol. 2020;(May) doi: 10.1002/jmv.26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(Feb(10223)):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Liang W., Jiang M., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(Jul(1)):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., et al. Detectable serum SARS-Co-V-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P.D.W., Fumeaux T., Guerci P., et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25(2020):100449. doi: 10.1016/j.eclinm.2020.100449. doi: 10/1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheum. 2020;2(Aug(8)) doi: 10.1016/S2665-9913(20)30173-30179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Amreich C., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(Jul(1)):128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Zayet S., Lohse A., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;50(Aug(5)):397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R.Q., Yuan W., Shord S.S., et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T Cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(Aug(8)):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Gardner R., Porter D.L., et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(Jul(2)):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. A randomized clinical trial. J Am Med Assoc. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qui L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92(Apr(7)):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Brit Med J. 2020;369(May):m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro S., Mostard R.L.M., Magro-Checa C., et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;9 doi: 10.1136/annrheumdis-2020-218479. 1143-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recovery collaborative group Dexamethasone in hospitalized patients with Covid-19- Preliminary report. N Eng J Med. 2020 doi: 10.1056//NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossotti R., Travi G., Ughi N., et al. Safety and efficacy of anti-IL-6 receptor tocilizumab use in severe and critical patients affected by coronavirus disease 19: A comparative analysis. J Infect. 2020 doi: 10.1016/jinf/2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(May(5)):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers E.C., Eschenauer G.A., Troost J.P., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(Jul(7)):102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(Mar(7)):834–843. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(May(20)):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Zhang J., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(Apr(4)):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Gerogy A., Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013;51(Jun(6)):443–455. doi: 10.5414/CP201819. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(Mar(10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]