Abstract
Mucins are a family of multifunctional glycoproteins that mostly line the surface of epithelial cells in the gastrointestinal tract and exert pivotal roles in gut lubrication and protection. Pancreatic cancer is a lethal disease with poor early diagnosis, limited therapeutic effects, and high numbers of cancer‐related deaths. In this review, we introduce the expression profiles of mucins in the normal pancreas, pancreatic precursor neoplasia and pancreatic cancer. Mucins in the pancreas contribute to biological processes such as the protection, lubrication and moisturization of epithelial tissues. They also participate in the carcinogenesis of pancreatic cancer and are used as diagnostic biomarkers and therapeutic targets. Herein, we discuss the important roles of mucins that lead to the lethality of pancreatic adenocarcinoma, particularly MUC1, MUC4, MUC5AC and MUC16 in disease progression, and present a comprehensive analysis of the clinical application of mucins and their promising roles in cancer treatment to gain a better understanding of the role of mucins in pancreatic cancer.
Keywords: diagnosis, mucins, pancreatic cancer, prognosis, therapy
1. INTRODUCTION
Pancreatic cancer (PC) is the fourth leading cause of cancer‐related deaths in the United States and has a 5‐year survival rate of <6%. 1 Pancreatic ductal adenocarcinoma (PDAC) remains the most common type, and nearly 250 000 new cases are diagnosed per year worldwide. 2 In China, it ranks sixth among all cancer types. 3 It is reported that the mortality rate of PC will become the second highest among cancer‐related deaths before 2030. 4 PDAC is not yet fully understood by clinicians and scientists, and its major symptoms, including abdominal pain, obstructive jaundice and Cullen syndrome, are not easily detected at the early stages. Surgery is the only effective treatment for PDAC patients, but most patients miss the optimal time of surgery because of the late diagnosis. 5 Chemotherapy and radiotherapy may improve the poor prognosis slightly but with severe side effects. 3 Despite the increasing research efforts by cancer scientists, there is an urgent need for breakthroughs on the mechanism of oncogenesis and targeted therapy.
Early diagnosis is clinically dependent on one biomarker, carbohydrate antigen 19‐9 (CA19‐9). CA19‐9, an epitope of sialylated Lewis group antigen, is elevated in approximately 80% of patients. Patients diagnosed with locally advanced or metastatic disease usually have much higher levels of CA19‐9. 2 Antigens such as CA19‐9 are composed of oligosaccharide structures present on heavily glycosylated mucins born by the antigen. 6 Mucins are produced by various epithelial cells that are located on serine or threonine residues of the mucin core protein backbone. There are repetitive short stretches in the protein backbone termed tandem repeat regions (TRRs), while many O‐glycosidic or N‐glycosidic linkages are connected to the backbone. 7 Furthermore, there are multiple functional domains in mucin family members including von Willebrand factor D domain (vWD), nidogen‐like domain (NIDO), von Willebrand factor like domain (vWF‐like), epidermal growth factor (EGF) and sea urchin sperm protein‐enterokinase‐agrin (SEA). These extended structures are used for communication with cellular receptors, extracellular matrix and signalling mediators 8 , 9 (Figure 1).
FIGURE 1.
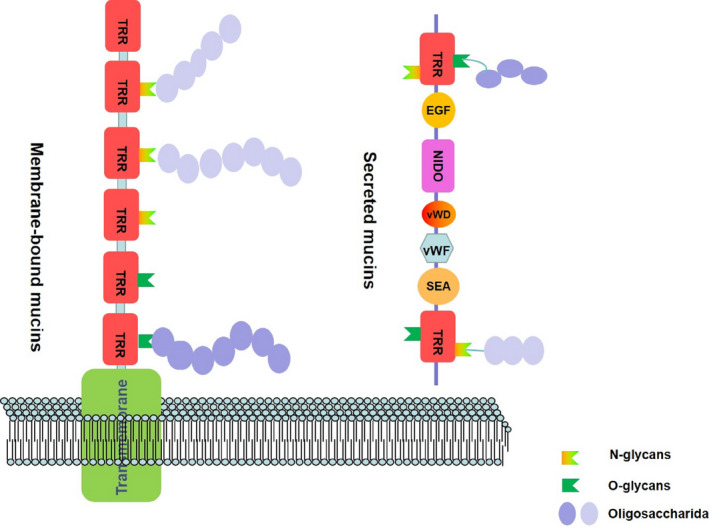
The structure of membrane‐bound mucin and secreted mucin. EGF, epidermal growth factor; NIDO, nidogen‐like domain; SEA, sea urchin sperm protein‐enterokinase‐agrin; TRR, tandem repeat region; vWD, von Willebrand factor D domain; vWF, von Willebrand factor like domain
Genes encoding mucins are denoted by the first three letters, “MUC,” followed by the number in which they were discovered chronologically. 10 Mucins are divided into two categories: membrane‐bound mucins and secreted mucins. The first class includes MUC1, MUC3, MUC4 and MUC16, which have been frequently investigated. MUC12, MUC13, MUC17 and MUC21 are also included in this group but limited studies have been performed on these. The protein structure of these mucins is composed of an N‐terminal portion and a transmembrane domain, which has many phosphorylation sites that are involved in signal transduction. 10 , 11 The latter group of secreted mucins include MUC2, MUC5AC, MUC5B, MUC6 and MUC7, which are important for the formation of mucin heterodimers or homodimers. 9 Furthermore, another mucin, MUC18 (melanoma cell adhesion molecule), belongs to the immunoglobulin superfamily. 10 As well as protecting the barriers of mucous membranes, mucins also have important roles in cellular regeneration, differentiation, migration, adhesion and signalling. 8 , 12
Mucins may represent a diagnostic parameter for the early detection of PC and act as specific discriminated biomarkers among PC, pancreatic intraepithelial neoplasia (PanIN) and pancreatitis. Our review aims to summarize and update the role of mucins in the pathogenesis of PC and focuses on the diagnostic, prognostic and therapeutic functions of these glycoproteins in PDAC.
2. MUCINS IN THE NORMAL PANCREAS
Exploring the distribution of certain mucins in normal pancreatic tissue may help to determine their biological significance. Much research has focused on mucin expression in normal and pathological pancreatic tissue to reveal a significant role in cancer pathogenesis. 11 , 12 , 13 , 14 MUC1 has been widely studied for many years because of its oncogenic features in various cancers including PC. 15 In normal pancreatic tissue, MUC1 is expressed in the intralobular ducts and centrally in the interlobular ducts, while the glycoforms of MUC1 are undetectable in the main pancreatic duct. MUC2 and MUC4 expression is also undetectable in the normal pancreas. 16 MUC3 expressed in the main pancreatic duct is reduced to low levels at 13 weeks of gestation, and MUC6 can be detected from 13 weeks in the pancreatic duct system, and is mainly detected in small interlobular ducts and developing acini, 7 and also in normal adult pancreas tissue. 16 , 17 , 18 Furthermore, while MUC5AC is not detectable, MUC5B is found in normal pancreatic tissue and the normal pancreatic duct, 5 , 13 , 14 unlike MUC7, which is also undetectable in the normal pancreas. 7 Other mucin genes, such as MUC11, MUC12 and MUC17 can be detected in normal pancreatic tissues. However, their specific expression pattern in the development of PC is unclear. 19 , 20 It was reported that there is relatively low expression of MUC20 and MUC21 in normal pancreatic tissue. 21 , 22
3. MUCINS IN PRECURSOR LESIONS OF THE PANCREAS
Pancreatic ductal adenocarcinoma is the major type of pancreatic malignancy and the transformation model for this disease recently focused on the concept of intraepithelial neoplasia, termed PanIN. According to the morphological pattern, PanINs are divided into PanIN‐1, PanIN‐2 and PanIN‐3 based on the different cellular morphologies and architectural atypia. 23 The profile of mucin expression in the pancreas is affected by the pathological conditions, and mucin exerts individualized functions in the development of premalignant and malignant neoplasms. MUC1 is reported to be expressed in the apical membrane of the intralobular duct in normal pancreatic tissue. However, aberrant MUC1 expression can be detected during the early stages of PC and gradually increases with the formation of invasive carcinoma. 11 , 24 , 25 Other studies revealed that MUC1 RNA levels are low in the tissue of both chronic pancreatitis and normal pancreas, while high MUC1 expression indicates the development of PC or PanIN progression. 5 As for mucin expression in intraductal papillary‐mucinous neoplasm (IPMN), MUC1 tends to promote the development of PDAC and the potential for metastasis. 26 Similarly, MUC4 is minimally expressed in PanIN and chronic pancreatitis but is highly expressed in PC cell lines, 14 , 27 and aberrant overexpression of MUC4 was also associated with the progression of PanIN to PC, 28 , 29 while the intensity of expression increased in parallel with the severity of cellular dysplasia. 29 MUC5B is weakly expressed in pancreatic precursor lesions, while MUC5AC is gradually expressed during PanIN transformation. Since MUC5AC is seldomly expressed in the normal pancreas, 30 it has value as a biomarker of early neoplastic lesions, which correlate with poor prognosis. 31 Although MUC6 can be detected in the foetal pancreas in the early period of gestation, 32 , 33 it was reported have no involvement in the prognosis of PC patients. 34
4. MUCINS IN PANCREATIC CANCER
Mucins form a protective coat around cancer cells and have critical roles in the carcinogenesis of PC. 35 Several studies of the abnormal expression of mucins in PDAC indicated their potential roles in its pathogenesis, such as restricting intracellular drug uptake, facilitating immune escape, up‐regulating signal pathways or even serving as prognostic biomarkers. 36 , 37 , 38 PDAC is characterized by the differential expression or glycosylation of the mucin family as it transforms from healthy tissue to cancer, as outlined in Table 1.
TABLE 1.
Mucins expression in different pancreatic neoplasms
| Normal | PanIN | Pancreatic cancer | Pancreatitis | IPMN | MCN | |||
|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||
| MUC1 | + | + | ++ | +++ | +++ | ++ | ± | ± |
| MUC2 | − | − | − | − | − | − | ++ | NA |
| MUC3 | ± | + | + | + | ++ | ± | ± | NA |
| MUC4 | − | + | ++ | +++ | +++ | − | ± | ++ |
| MUC5AC | − | + | ++ | +++ | +++ | ± | + | + |
| MUC5B | ± | NA | NA | NA | +++ | ++ | ± | NA |
| MUC6 | + | ++ | ++ | ++ | +++ | ++ | ++ | NA |
| MUC7 | NA | NA | NA | NA | + | + | NA | NA |
| MUC11/12 | ± | NA | NA | NA | NA | NA | NA | NA |
| MUC13 | ± | NA | NA | NA | +++ | NA | NA | NA |
| MUC16 | − | + | ++ | ++ | +++ | NA | NA | NA |
| MUC17 | + | ++ | ++ | ++ | + | NA | NA | NA |
| MUC20 | + | NA | NA | NA | NA | NA | NA | NA |
| MUC21 | + | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: −, negative; +, low; ++, moderate; +++, intense; ±, negative or positive depending the reports; IPMN, intraductal papillary‐mucinous neoplasms; MCN, mucinous cystic neoplasm; NA, no available data; PanIN, pancreatic intra‐epithelial neoplasia.
4.1. MUC1
Many studies have focused on MUC1 as an oncogene, not only in PDAC but also in different cancer types. 39 , 40 , 41 MUC1 is expressed mainly along on the luminal cell surface and partially along the baso‐lateral surface, and is detected in PDAC exhibiting aggressive behaviour. 42 High MUC1 expression is detected in more than 60% of PC cases and is significantly associated with tumour size. Furthermore, the higher the level of MUC1 expression, the poorer the prognosis in patients with PC. 43 In addition, PC manifests high expression rates of MUC1 glycoforms (MUC1/MY.1E12, 98%; MUC1/DF3, 96%; MUC1/HMFG1, 76%; and MUC1/CORE, 66%). 44
The cytoplasmic tail of MUC1 (MUC1‐C) is widely involved in carcinogenesis and is correlated with PC progression by promoting the aggressive and metastatic phenotype. 45 MUC1‐C contains multiple phosphorylated residues and interacts with signal transducers, cytokines, growth factors and other receptor tyrosine kinases to activate downstream signalling cascades. 46 For example, integrin‐linked kinase, platelet‐derived growth factor receptor‐β and Met receptor tyrosine kinase modulate MUC1‐C and enhance its oncogenic properties in PC. 47 , 48 , 49 Moreover, MUC1 in PC binds to β‐catenin and EGFR to activate cell proliferation via the Wnt/ß‐catenin or MAPK pathways. 50 , 51 MUC1 in PDAC significantly prompts tube formation, vessel generation and metastasis through the NRP1–VEGF axis 52 and can also induce epithelial‐mesenchymal transition to facilitate metastasis. 51
Additionally, because of the loss of apical‐basal polarity in cancers, aberrantly glycosylated MUC1 was demonstrated to regulate metabolism via several aspects, including directly influencing metabolic gene expression, modulating the activity of metabolic enzymes and even indirectly regulating reactive oxygen species (ROS) and autophagy. 53 , 54 The observations of MUC1‐induced metabolic alteration of PC have also strengthened its role as an oncoprotein. MUC1 interacting with HIF‐1α induced metabolic reprogramming to impart gemcitabine chemoresistance in PC. 54 , 55 Furthermore, MUC1 was reported to promote chemoresistance in PC cells by inhibiting BRCA1 to enhance glycolysis. 25 MUC1 also has a role in response to radiotherapy by enhancing biosynthesis and glycolysis in PC cells to destroy radiation‐induced cytotoxicity. 56 Moreover, glucose limitation in MUC1‐up‐regulated PC cells disrupts the synthesis of pyrimidine nucleotides and increases the consumption of glutamine, resulting in a metabolic switch. 57 MUC1 oncoprotein could also facilitate the pro‐adaptive stress response axis through cytidine deaminase‐mediated pyrimidine metabolic reprogramming and ROS alterations, therefore regulating cancer cell survival and chemotherapy response in PC. 58
4.2. MUC4
MUC4 is overexpressed in most PCs, or even as early as the PanIN‐I stages. 29 , 59 Several studies reported that MUC4 imparts several oncogenic properties and is detected in 32%–89% of PDAC patients. 28 , 29 , 60 These differences can be attributed to the use of different MUC4‐positive thresholds. MUC4, regarded as the tumour‐associated mucin of the pancreas, might be a promising biomarker to discriminate PC from pancreatitis. The survival rate of PC patients with high MUC4 expression was significantly lower than that of patients with low MUC4 expression. 61 Moreover, MUC4 acts as an important factor in modulating the chemoresistance of gemcitabine in PC cells. Down‐regulation of MUC4 can sensitize highly metastatic PC cells to gemcitabine in vitro. 62 In addition, MUC4 was also found to be expressed in PC stem cells, indicating its important role not only in maintaining cell proliferation but also in increasing the chemoresistance of PC cells. 63 Interestingly, MUC4 inhibits apoptosis by interfering with caspase protein and cytochrome C present in the mitochondria, 64 , 65 and MUC4/β‐catenin can suppress the progression and metastasis of PC by interfering GCNT3, a glycosyltransferase. 66 Recently, it was identified as a new tumour‐related antigen for immunotherapy in PC. The recombinant MUC4 domain and the elicited cellular and humoral anti‐MUC4 response indicate its application as a candidate vaccine for PC therapy. 67
4.3. MUC5AC
MUC5AC is highly expressed in different grades of PanIN and in IPMN and PDAC. 68 , 69 Among 132 cases of PDAC, 92% were positive for MUC5AC, 60 MUC5AC mRNA expression was also higher in tumoral tissues compared with para‐tumoral tissue, 70 which might be correlated with PDAC invasion, suggesting its role in the acceleration of cancer progression. In vivo xenograft research indicates that low levels of MUC5AC expression suppress tumorigenicity and inhibit neutrophil‐induced apoptosis. 71 Down‐regulation of MUC5AC could also result in decreased growth and metastasis, while up‐regulation of MUC5AC accelerated high‐grade PanIN to invasive cancer. 72 Furthermore, MUC5AC seems to be a sensitive biomarker of early pancreatic neoplasms, providing another link with unfavourable prognosis. 30 , 31 In addition, the combination of MUC5AC and CA19‐9 presented optimal performance and improved specificity compared with CA19‐9 to differentiate PC from healthy controls. 73 , 74 However, contrary results revealed that the expression level of MUC5AC is correlated with promising clinical events in PC. 75 , 76
4.4. MUC16
MUC16, also known as CA‐125 because it carries a CA‐125 epitope, 77 is a widely expressed tumour antigen observed in ovarian cancer that is usually elevated in PC, although it has relatively low specificity. MUC16 was proven to exert a critical role in the formation, progression, metastases and relapse of PC. 78 , 79 MUC16 expression was found to be remarkably lower in low‐grade dysplasia compared with high‐grade dysplasia, 78 while it was much higher in metastatic foci than that at primary sites; thus, we can conclude that it may have a pivotal function in the metastasis of PC. 79 Clinically, it is also combined with CA19‐9 to evaluate the severity and prognosis of PC. 80 , 81 MUC16 is involved in many oncogenic signalling pathways, such as activating LMO2 and JAK2 to promote proliferation, and up‐regulation of MUC16 stimulates mTOR and c‐MYC to reprogram PC metabolism, enhancing glycolysis and cell proliferation. 82 , 83 MUC16 can also activate the AKT and MAPK pathways to promote metastasis, 83 and the MUC16‐C terminal can promote the enrichment of Tregs through IL‐6 activation of the JAK–STAT pathway in PC. 84 It was also reported that MUC16 might correspond with the up‐regulation of other mucins, such as MUC1 and MUC4 in PC. 85 Overall, the involvement of MUC16 in the carcinogenesis of PC has been widely identified.
4.5. Others
Mucins in pancreatic disease, particularly MUC1, ‐4, ‐5 and ‐16 have gained extensive attention because of their important roles in carcinogenesis, differential diagnosis and prognosis prediction. Other mucin family members have also been studied in the field of PC. MUC3 has cysteine‐rich domains that can inhibit apoptosis and enhance the progression of PC cells, 86 whereas MUC6 shows no effects on patient survival in PC. 34 MUC12 and MUC11 share sequence homologies and are found in pancreatic tissues, although there is limited evidence. 5 Few studies of MUC15, ‐17 and ‐20 have been published, although some reports have revealed that their expression in PC might exceed that in the normal pancreas. 87 , 88
5. ROLE OF MUCIN IN THE CARCINOGENESIS OF PANCREATIC CANCER
Although the oncogenic mechanism underlying pancreatic malignancy remains unclear, several studies have identified the critical role of mucins in PC formation, such as prompting tumorigenicity, enhancing metastasis and producing chemoresistance by O (or N)‐linked oligosaccharides. 37 In summary, mucin‐mediated interactions, oncogenic signalling pathways and genetic alterations contribute to PC carcinogenesis.
5.1. Molecular mechanisms of mucin in pancreatic cancer
Mucins have important roles at different stages of PC by altering their expression and glycosylation during the transition from healthy tissue to neoplasia in the pancreas. Apical mucins are brought into proximity with receptor tyrosine protein kinases (RTKs) through the loss of polarity of epithelial cells. RTKs are important in signalling pathways that promote cell proliferation and cancer metastases. 46 , 89 The nuclear localization of mucins is associated with poorly differentiated cancers, high metastasis and unfavourable prognosis. 90 , 91 , 92 Mucins also modify the exfoliation of primary cancer cells, 93 , 94 accelerate cellular migration, 15 enhance proliferation 95 and act as mediators of cellular adhesion during metastasis. 96 The pancreatic tumour microenvironment (TME) contains fibroblasts, pancreatic stellate cells, collagen, fibronectin and some cytokines, and importantly, mucin has many interactions with the TME to mediate immune evasion, oncogenic signalling and angiogenesis. 93 , 97
5.2. Mucin‐related signalling pathways in pancreatic cancer
Transmembrane mucins participate in various signalling pathways to promote carcinogenesis and pathogenesis, either via the extracellular domain or by directly interacting with receptors. 32 , 46 MUC1‐C, a powerful and representative mucin because of the presence of multiple phosphorylation sites, represents a hub protein of different signalling cascades, including the c‐SRC‐mediated signalling pathway, RAS‐ERK pathway, LEF/TCF‐dependent Wnt pathway, p53 pathway, MAP kinase pathway and HIF‐1α pathway. 39 , 46 , 50 , 54 In addition, MUC4 is gaining attention as an important molecule and is reported to bind with ERBB2 to activate the PI3K‐AKT and RAS‐ERK pathways. 46 , 63 In addition, MUC16 was verified to enhance tumour progression and immune regulation through the mTOR, c‐MYC, MAPK and JAK‐STAT pathways. 82 , 83 , 84 These roles of mucins have been comprehensively reviewed elsewhere.
5.3. Genetic alteration of mucins
A pan‐mucin genomic study across multiple cancers was conducted to identify novel genomic alterations in mucins and their functions in carcinogenesis based on The Cancer Genome Atlas (TGCA) dataset. DNA mutational analysis revealed the T112P mutation in 50% of stage II pancreatic MUC1 mutations. MUC16 also has a high mutational rate in cancers, and amino acid‐altering mutations were observed in 43% of PC specimens, with 14.9% leading to deletions or frameshifts. 98
6. MUCIN AS A DIAGNOSTIC TOOL IN THE DETECTION OF PANCREATIC CANCER
Most patients with PC are diagnosed at an advanced stage, especially in borderline resectable stages or metastases. Therefore, sensitive diagnostic markers for early‐stage disease are urgently required. The presence of sialylated Lewis antigen on the surface of mucins is related to the advanced stages of malignant tumours. 99 CA19‐9 recognizes the sialylated Lewis a/b antigen on MUC1. CA19‐9 remains the only marker recommended by the Food and Drug Administration (FDA) in the USA for monitoring PC. However, nearly 10%‐20% patients are Lewis a/b‐negative, resulting in a sensitivity of less 95%. 100 To further enhance the diagnostic value of CA19‐9, it is combined with other mucins such as MUC5AC. 64 , 72
Additionally, endoscopic ultrasonography‐guided, fine‐needle aspiration (EUS‐FNA) is also applied to the diagnosis before surgery as a minimally invasive method. MUC16 and MUC4 have high specificity in diagnosing PDAC from healthy controls with 63% and 67% sensitivity by EUS‐FNA, respectively. 101 The latest research showed that MUC4 levels in the cystic fluid of IPMN patients could accurately discriminate high‐ from low‐risk cystic neoplasms with high sensitivity. 102 In another study of EUS–FNA, up‐regulation of MUC1 (77.5%), MUC2 (10.0%) and MUC5AC (80.0%) was reported in patients with PC, and positive ratios of MUC1 (25.0%), MUC2 (31.3%) and MUC5AC (43.8%) were detected in benign pancreatic diseases, respectively. 103 Moreover, MUC7 was detected in aspirated material from 73% PC patients, indicating its potential as a biomarker to identify PC. 104
In addition to EUS biopsy, circulating tumour cells have gained increasing attention as potential biomarkers for diagnosis. With respect to biomarkers in circulating blood, MUC16 has been shown to be effective in predicting recurrent disease, while overexpressed MUC4 indicates early metastasis. 105 , 106 Even in pancreatic juice, MUC1, MUC4, MUC5AC, MUC6 and MUC16 have been identified as candidate biomarkers for PC through detection of vesicle‐associated proteins. 107 Analysis of mucin expression as well as other biomarkers contributes to providing superior diagnostic information and enhanced monitoring of risk of disease recurrence.
7. MUCINS AS PROGNOSTIC INDICATORS IN PANCREATIC CANCER
The overall survival curves associated with members of the mucin family were depicted by online Kaplan‐Meier plotter (www.kmplot.com), a novel interactive website that aims to compare gene expression levels and prognostic conditions according to TCGA. The median was selected as the group cut‐off value for survival plots. Correlations between the mRNA levels of the mucin family and the overall survival of patients with PC is shown in Figure 2. Among the available data, 12 mucins were analysed, eight of which were significantly associated with prognosis: MUC1, MUC4, MUC5AC, MUC15, MUC 16, MUC17, MUC20 and MUC 21. However, further studies are required to consider the impact of pathological stage, sex and degree of differentiation on the prognostic value of mucin. Additionally, although the relationship between mRNA expression and prognosis has been clarified, further analyses are needed at the protein level. Considering the important roles played by mucins in PC and their prognostic value as determined using the online database, it is necessary to evaluate the function of a panel of multiple mucins for PC.
FIGURE 2.
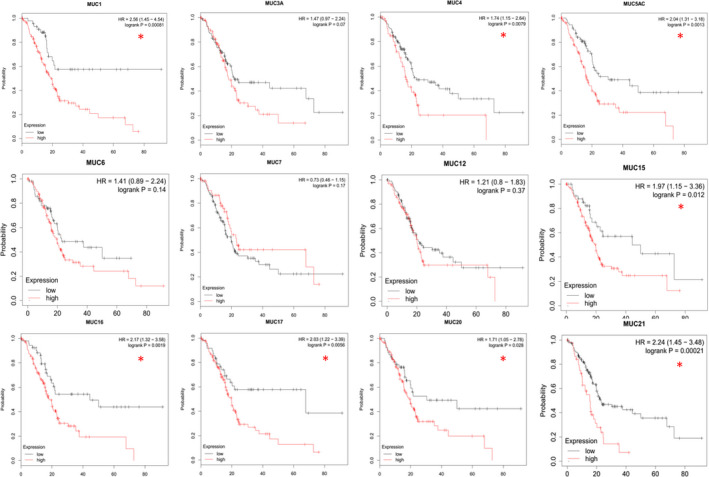
The overall survival curves of mucins in pancreatic cancer depicted by Kaplan‐Meier plotter (*P < .05)
8. MUCIN‐BASED THERAPEUTIC STRATEGIES
Mucins are involved in many malignant processes including evasion, invasion and metastatic by affecting oncogenic signalling, cell survival, proliferation and resistance to chemotherapeutics. 38 , 62 Furthermore, several mucins have been linked with tumour progression, chemoresistance and prognosis in PC. Because of these attributes, mucin‐based therapy has also been applied for PC strategies including vaccines, antibodies, gene therapy and mucolytic agents (Figure 3).
FIGURE 3.
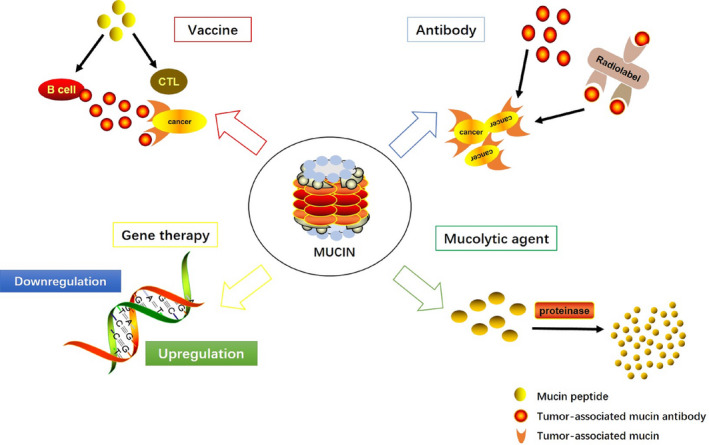
Mucins play important roles in the therapy of pancreatic cancer. The red arrow indicates the application of mucin‐based vaccines. The blue arrow shows the antibody targeting mucins for therapy. The yellow arrow implicates the gene therapy by altering the expression of mucins. The green arrow manifests the mechanism of mucolytic agents
8.1. Vaccines
MUC1 has garnered great interest during the course of mucin research, and the clinical application of MUC1 as a vaccine for activating the immune response against tumour‐associated antigens has been investigated. Interestingly, MUC1‐specific cytotoxic T‐lymphocytes prompted clinical studies of MUC1‐targeting vaccines in PC, and a subsequent animal experiment revealed that MUC1 could provide immune protection against cancer cells in wild‐type mice. 108 The relationship between MUC1 and T‐cell numbers are important in the immune response. 109 Many vaccine formulations of MUC1 have been administered to advanced‐stage patients. For example, a vaccine using MUC1‐TRR peptide, 110 a synthetic MUC1 peptide carried by Bacillus Calmette‐Guérin 111 or even Freund's adjuvant 112 is used to strengthen the antigen presentation of dendritic cells and activate cytotoxic T cells. Further mucin‐specific cytotoxic T‐lymphocytes with no isotype‐transforming antibody response continue to be investigated. Many experiments of MUC1 peptide‐loaded dendritic cells were conducted in subsequent clinical trial phases, revealing that this novel vaccine preparation could be well tolerated without recurrence. 113 Recently, MUC1‐based glycosylated tricomponent antitumour vaccines demonstrated a clear reduction in tumour burden by eliciting both cellular and humoral immune reactions. 114 Furthermore, MUC4‐targeted cancer therapies have been reported and many prospective immunotherapies have been considered. 67 Vaccines linked with HLA‐A1 and HLA‐A2 MUC4 epitopes activate cellular immunity and humoral immunity. 115 In addition, the existence of MUC4 splice variants, autoantibodies against abnormally glycosylated MUC4 and T‐cell clones against MUC4 mutations strengthen its importance as a tumour‐associated antigen in vaccine research. 67
8.2. Antibodies
Some anticancer therapeutics based on mucin antibody binding to tumour antigens or targeting PC have also been explored. A new MUC1 antibody named PankoMab (Berlin, Germany) is considered to have beneficial features. 116 PankoMab can specifically recognize the carbohydrate‐induced conformational epitope of PC, differentiating between malignant and non‐malignant mucin‐related antigens while producing huge antibody‐dependent killing responses. It also stimulates MUC1‐expressing PC cells to produce strong antibody‐dependent cytotoxicity. Even for cancers originating from glandular cells or squamous epithelia, the humanized form of PankoMab showed a strong response in an IHC analysis of 137 surgical specimens. 117 Another way to eliminate cancer cells is the combination of MUC1 antibodies and radioisotopes. Radiolabeled antibody PAM4, which specifically targets MUC1, has been studied in PC. 118 Tumour targeting by 131iodine (131I)‐PAM4, 99mTechnetium (99mTc)‐PAM4, and the humanized form of 90Y‐PAM4 (h90Y‐PAM4) was shown to be partially effective with acceptable adverse effects. 119 , 120 And an α‐immunoconjugate of a monoclonal antibody, also known as C595, which can discern the TRR on MUC1, has been adopted as a valuable approach. 121 However, although some evidence for their effectiveness is promising, the side effects of radiotherapy such as neutropenia and thrombocytopenia as well as the high cost should be considered. 122
8.3. Gene therapy
Several ongoing clinical studies proved that down‐regulation of the expression of selected mucins could be a new therapeutic approach for PC. Knockdown of MUC1 by short interfering RNA inhibited the proliferation of PC cell lines, and injection of these cells into the pancreatic tissue of an animal model reduced the incidence of metastasis. 123 Similarly, MUC4 interference by siRNA in CD18/HPAF PC cells also inhibited proliferation in an orthotopic mouse model. 90 Except for targeting down‐regulated mucins, the mucin gene promoter also will induce tumour cell death in MUC1‐expressing PC. 124 Although some studies showed the potential effect of gene therapies, they neglected to address the challenge of delivering the gene into the human body and are also not clinically approved.
8.4. Mucolytic agents
It has been reported that changes in the dense mucin mesh could alter the absorption of cytotoxic drugs. Mucins constitute a barrier on the cell surface that can infect drug uptake. 125 Therefore, drug‐dissolving mucins such as bromelain and N‐acetylcysteine (NAC) have been applied. 126 , 127 Bromelain is a cysteine proteinase composed of thiol endopeptidases that destroy the glycosidic bonds of mucins, while NAC denatures the disulphide bonds of mucus to decrease mucus viscosity, facilitating its clearance. 128 The combination of such two drugs can be used as an adjunct to cytotoxic drugs because of its effect on the lysis of mucin on the cell surface. 129
9. CONCLUSIONS
Pancreatic adenocarcinoma is a worldwide challenge with high rates of incidence and mortality. Although many efforts have been made to identify the main mechanism of PC occurrence and development, the exact molecular processes underlying the aggressive nature of this devastating disease remain to be elucidated. Mucins, well‐known and promising glycoproteins, have been investigated in the pathogenesis and progression of this malignancy, and their expression is useful in the differential diagnosis of PC, premalignant lesions and benign pancreatic tumours. Mucins have been identified as being more valuable in ameliorating the unfavourable survival status of PC than previously thought. Moreover, they also participate in pancreatic carcinogenesis, indicating their significance as future therapeutic targets.
Because the association between PC and mucins has been studied for several years both in the laboratory and in clinical trials, it is clear that the mucin family has significant functions in the carcinogenesis of PC and in therapy targeting PC. In addition, the potential functions of other mucins contributing to the carcinogenesis of PC, such as MUC4, MUC5 and MUC16, have also recently been investigated in detail. Although the roles of MUC1, MUC4, MUC5AC and MUC16 in PC have already been described, evidence for the significance of other mucins is still not available, and new members of the mucin family are likely to be identified. However, no mucin alone can enhance the entire biological process of PC, and optimal therapy for PC could consist of combined agents that target a series of mucins. In conclusion, the data presented in this review provide insights into the utilization of mucin family members as biomarkers and therapeutic targets in PC, and indicate further potential research areas examining new functional roles, biomarker functions and therapeutic drugs against mucins in PC.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Shunda Wang: Data curation (lead); Formal analysis (lead); Methodology (lead); Writing‐original draft (lead). Lei You: Visualization (lead). Menghua Dai: Conceptualization (equal); Supervision (equal). Yupei Zhao: Conceptualization (equal); Supervision (equal).
ACKNOWLEDGEMENTS
We thank H. Nikki March, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Wang S, You L, Dai M, Zhao Y. Mucins in pancreatic cancer: A well‐established but promising family for diagnosis, prognosis and therapy. J Cell Mol Med. 2020;24:10279–10289. 10.1111/jcmm.15684
Funding information
The project was funded by Chinese Academy of Medical Science (CAMS) Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2016‐I2M‐3‐005). Another supportive source of this work was from the National Major Research and Development Programs of the Ministry of Science and Technology of the People's Republic of China during the 13th Five‐Year Plan Period (Grant No. 2017YFC1308602).
Contributor Information
Menghua Dai, Email: daimh@pumch.cn.
Yupei Zhao, Email: zhao8028@263.net.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 3. He Y, Zheng R, Li D, Zeng H, Zhang S, Chen W. Pancreatic cancer incidence and mortality patterns in China, 2011. Chin J Cancer Res. 2015;27:29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol. 2012;9:435‐444. [DOI] [PubMed] [Google Scholar]
- 5. Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033‐4040. [PubMed] [Google Scholar]
- 6. Kim YS, Gum JR Jr, Crawley SC, Deng G, Ho JJ. Mucin gene and antigen expression in biliopancreatic carcinogenesis. Ann Oncol. 1999;10(Suppl 4):51‐55. [PubMed] [Google Scholar]
- 7. Reid CJ, Harris A. Developmental expression of mucin genes in the human gastrointestinal system. Gut. 1998;42:220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192‐D1206. [DOI] [PubMed] [Google Scholar]
- 9. Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77‐99. [DOI] [PubMed] [Google Scholar]
- 10. Chakraborty S, Bonthu N, Swanson BJ, Batra SK. Role of mucins in the skin during benign and malignant conditions. Cancer Lett. 2011;301:127‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189‐222. [DOI] [PubMed] [Google Scholar]
- 13. Balague C, Audie JP, Porchet N, Real FX. In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology. 1995;109:953‐964. [DOI] [PubMed] [Google Scholar]
- 14. Balague C, Gambus G, Carrato C, et al. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology. 1994;106:1054‐1061. [DOI] [PubMed] [Google Scholar]
- 15. Kohlgraf KG, Gawron AJ, Higashi M, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011‐5020. [PubMed] [Google Scholar]
- 16. Kloppel G, Kosmahl M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol. 2006;44:249‐250. [DOI] [PubMed] [Google Scholar]
- 17. Kim GE, Bae HI, Park HU, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052‐1060. [DOI] [PubMed] [Google Scholar]
- 18. Lan MS, Hollingsworth MA, Metzgar RS. Polypeptide core of a human pancreatic tumor mucin antigen. Cancer Res. 1990;50:2997‐3001. [PubMed] [Google Scholar]
- 19. Gum JR Jr, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane‐tethered mucin. Biochem Biophys Res Commun. 2002;291:466‐475. [DOI] [PubMed] [Google Scholar]
- 20. Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down‐regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083‐4089. [PubMed] [Google Scholar]
- 21. Higuchi T, Orita T, Nakanishi S, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up‐regulated in injured kidney. J Biol Chem. 2004;279:1968‐1979. [DOI] [PubMed] [Google Scholar]
- 22. Itoh Y, Kamata‐Sakurai M, Denda‐Nagai K, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74‐83. [DOI] [PubMed] [Google Scholar]
- 23. Hruban RH, Adsay NV, Albores‐Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579‐586. [DOI] [PubMed] [Google Scholar]
- 24. Klausen P, Kovacevic B, Toxvaerd A, et al. Subtyping of intraductal papillary mucinous neoplasms ‐ pitfalls of MUC1 immunohistochemistry. APMIS. 2019;127:27‐32. [DOI] [PubMed] [Google Scholar]
- 25. Fu X, Tang N, Xie WQ, Mao L, Qiu YD. MUC1 promotes glycolysis through inhibiting BRCA1 expression in pancreatic cancer. Chin J Nat Med. 2020;18:178‐185. [DOI] [PubMed] [Google Scholar]
- 26. Terris B, Dubois S, Buisine MP, et al. Mucin gene expression in intraductal papillary‐mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632‐637. [DOI] [PubMed] [Google Scholar]
- 27. Choudhury A, Moniaux N, Winpenny JP, Hollingsworth MA, Aubert JP, Batra SK. Human MUC4 mucin cDNA and its variants in pancreatic carcinoma. J Biochem. 2000;128:233‐243. [DOI] [PubMed] [Google Scholar]
- 28. Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791‐796. [DOI] [PubMed] [Google Scholar]
- 29. Park HU, Kim JW, Kim GE, et al. Aberrant expression of MUC3 and MUC4 membrane‐associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–e54. [DOI] [PubMed] [Google Scholar]
- 30. Zen Y, Sasaki M, Fujii T, et al. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct–an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350‐358. [DOI] [PubMed] [Google Scholar]
- 31. Boonla C, Wongkham S, Sheehan JK, et al. Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer. 2003;98:1438‐1443. [DOI] [PubMed] [Google Scholar]
- 32. Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087‐1095. [DOI] [PubMed] [Google Scholar]
- 33. Buisine MP, Devisme L, Degand P, et al. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48:1667‐1676. [DOI] [PubMed] [Google Scholar]
- 34. Jinfeng M, Kimura W, Hirai I, Sakurai F, Moriya T, Mizutani M. Expression of MUC5AC and MUC6 in invasive ductal carcinoma of the pancreas and relationship with prognosis. Int J Gastrointest Cancer. 2003;34:9‐18. [DOI] [PubMed] [Google Scholar]
- 35. Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142‐151. [DOI] [PubMed] [Google Scholar]
- 36. Jonckheere N, Skrypek N, Van Seuningen I. Mucins and pancreatic cancer. Cancers (Basel). 2010;2:1794‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin‐based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong W, Ekmu B, Wang X, Lu Y, Wan L. AGR2‐induced glucose metabolism facilitated the progression of endometrial carcinoma via enhancing the MUC1/HIF‐1alpha pathway. Hum Cell. 2020;33:790‐800. [DOI] [PubMed] [Google Scholar]
- 40. Liu X, Mao D, Deng G, et al. Nondestructive analysis of tumor‐associated membrane protein MUC1 in living cells based on dual‐terminal amplification of a DNA ternary complex. Theranostics. 2020;10:4410‐4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mirili C, Paydas S, Kilic EB, et al. Prognostic significance of EGFR, MUC1 and PD‐L1 expressions in cases with triple negative breast cancer. J BUON. 2020;25:159‐167. [PubMed] [Google Scholar]
- 42. Osako M, Yonezawa S, Siddiki B, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191‐2199. [DOI] [PubMed] [Google Scholar]
- 43. Pillai K, Akhter J, Chua TC, Morris DL. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 2013;31:241‐250. [DOI] [PubMed] [Google Scholar]
- 44. Luttges J, Zamboni G, Longnecker D, Kloppel G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942‐948. [DOI] [PubMed] [Google Scholar]
- 45. Banerjee S, Mujumdar N, Dudeja V, et al. MUC1c regulates cell survival in pancreatic cancer by preventing lysosomal permeabilization. PLoS One. 2012;7:e43020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh PK, Hollingsworth MA. Cell surface‐associated mucins in signal transduction. Trends Cell Biol. 2006;16:467‐476. [DOI] [PubMed] [Google Scholar]
- 47. Huang HL, Wu HY, Chu PC, et al. Role of integrin‐linked kinase in regulating the protein stability of the MUC1‐C oncoprotein in pancreatic cancer cells. Oncogenesis. 2017;6:e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh PK, Wen Y, Swanson BJ, et al. Platelet‐derived growth factor receptor beta‐mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67:5201‐5210. [DOI] [PubMed] [Google Scholar]
- 49. Singh PK, Behrens ME, Eggers JP, et al. Phosphorylation of MUC1 by Met modulates interaction with p53 and MMP1 expression. J Biol Chem. 2008;283:26985‐26995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nath S, Roy LD, Grover P, Rao S, Mukherjee P. Mucin 1 regulates cox‐2 gene in pancreatic cancer. Pancreas. 2015;44:909‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trehoux S, Duchene B, Jonckheere N, Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42–44 MAPK, Akt, Bcl‐2 and MMP13 pathways. Biochem Biophys Res Commun. 2015;456:757‐762. [DOI] [PubMed] [Google Scholar]
- 52. Zhou R, Curry JM, Roy LD, et al. A novel association of neuropilin‐1 and MUC1 in pancreatic ductal adenocarcinoma: role in induction of VEGF signaling and angiogenesis. Oncogene. 2016;35:5608‐5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mehla K, Singh PK. MUC1: a novel metabolic master regulator. Biochim Biophys Acta. 2014;1845:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chaika NV, Gebregiworgis T, Lewallen ME, et al. MUC1 mucin stabilizes and activates hypoxia‐inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci U S A. 2012;109:13787‐13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shukla SK, Purohit V, Mehla K, et al. MUC1 and HIF‐1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32 71‐87.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gunda V, Souchek J, Abrego J, et al. MUC1‐mediated metabolic alterations regulate response to radiotherapy in pancreatic cancer. Clin Cancer Res. 2017;23:5881‐5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gebregiworgis T, Purohit V, Shukla SK, et al. Glucose limitation alters glutamine metabolism in MUC1‐overexpressing pancreatic cancer cells. J Proteome Res. 2017;16:3536‐3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olou AA, King RJ, Yu F, Singh PK. MUC1 oncoprotein mitigates ER stress via CDA‐mediated reprogramming of pyrimidine metabolism. Oncogene. 2020;39:3381‐3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moschovis D, Bamias G, Delladetsima I. Mucins in neoplasms of pancreas, ampulla of Vater and biliary system. World J Gastrointest Oncol. 2016;8:725‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108‐124. [DOI] [PubMed] [Google Scholar]
- 61. Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down‐regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xie K, Zhi X, Tang J, et al. Upregulation of the splice variant MUC4/Y in the pancreatic cancer cell line MIA PaCa‐2 potentiates proliferation and suppresses apoptosis: new insight into the presence of the transcript variant of MUC4. Oncol Rep. 2014;31:2187‐2194. [DOI] [PubMed] [Google Scholar]
- 65. Zhu Y, Zhang JJ, Liang WB, et al. Pancreatic cancer counterattack: MUC4 mediates Fas‐independent apoptosis of antigen‐specific cytotoxic T lymphocyte. Oncol Rep. 2014;31:1768‐1776. [DOI] [PubMed] [Google Scholar]
- 66. Gupta R, Leon F, Thompson CM, et al. Global analysis of human glycosyltransferases reveals novel targets for pancreatic cancer pathogenesis. Br J Cancer. 2020;122(11):1661‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gautam SK, Kumar S, Dam V, Ghersi D, Jain M, Batra SK. MUCIN‐4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Semin Immunol. 2020;47:101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243‐254. [DOI] [PubMed] [Google Scholar]
- 69. Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary‐mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201‐210. [DOI] [PubMed] [Google Scholar]
- 70. Ohuchida K, Mizumoto K, Yamada D, et al. Quantitative analysis of MUC1 and MUC5AC mRNA in pancreatic juice for preoperative diagnosis of pancreatic cancer. Int J Cancer. 2006;118:405‐411. [DOI] [PubMed] [Google Scholar]
- 71. Hoshi H, Sawada T, Uchida M, et al. MUC5AC protects pancreatic cancer cells from TRAIL‐induced death pathways. Int J Oncol. 2013;42:887‐893. [DOI] [PubMed] [Google Scholar]
- 72. Hoshi H, Sawada T, Uchida M, et al. Tumor‐associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol. 2011;38:619‐627. [DOI] [PubMed] [Google Scholar]
- 73. Zhang J, Wang Y, Zhao T, et al. Evaluation of serum MUC5AC in combination with CA19‐9 for the diagnosis of pancreatic cancer. World J Surg Oncol. 2020;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaur S, Smith LM, Patel A, et al. A combination of MUC5AC and CA19‐9 Improves the diagnosis of pancreatic cancer: a multicenter study. Am J Gastroenterol. 2017;112:172‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Higashi M, Yokoyama S, Yamamoto T, et al. Mucin expression in endoscopic ultrasound‐guided fine‐needle aspiration specimens is a useful prognostic factor in pancreatic ductal adenocarcinoma. Pancreas. 2015;44:728‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328‐341. [DOI] [PubMed] [Google Scholar]
- 77. Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737‐740. [DOI] [PubMed] [Google Scholar]
- 78. Takaori K, Hruban RH, Maitra A, Tanigawa N. Pancreatic intraepithelial neoplasia. Pancreas. 2004;28:257‐262. [DOI] [PubMed] [Google Scholar]
- 79. Haridas D, Chakraborty S, Ponnusamy MP, et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One. 2011;6:e26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu HX, Li S, Wu CT, et al. Postoperative serum CA19‐9, CEA and CA125 predicts the response to adjuvant chemoradiotherapy following radical resection in pancreatic adenocarcinoma. Pancreatology. 2018;18:671‐677. [DOI] [PubMed] [Google Scholar]
- 81. Abe T, Koi C, Kohi S, et al. Gene variants that affect levels of circulating tumor markers increase identification of patients with pancreatic cancer. Clin Gastroenterol Hepatol. 2020;18:1161‐1169.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Muniyan S, Haridas D, Chugh S, et al. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer. 2016;7:110‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shukla SK, Gunda V, Abrego J, et al. MUC16‐mediated activation of mTOR and c‐Myc reprograms pancreatic cancer metabolism. Oncotarget. 2015;6:19118‐19131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fan K, Yang C, Fan Z, et al. MUC16 C terminal‐induced secretion of tumor‐derived IL‐6 contributes to tumor‐associated Treg enrichment in pancreatic cancer. Cancer Lett. 2018;418:167‐175. [DOI] [PubMed] [Google Scholar]
- 85. Chakraborty S, Baine MJ, Sasson AR, Batra SK. Current status of molecular markers for early detection of sporadic pancreatic cancer. Biochim Biophys Acta. 2011;1815:44‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ho SB, Dvorak LA, Moor RE, et al. Cysteine‐rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology. 2006;131:1501‐1517. [DOI] [PubMed] [Google Scholar]
- 87. Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17. Complete coding sequence and organization. J Biol Chem. 2006;281:23676‐23685. [DOI] [PubMed] [Google Scholar]
- 88. Komatsu H, Tanji E, Sakata N, et al. A GNAS mutation found in pancreatic intraductal papillary mucinous neoplasms induces drastic alterations of gene expression profiles with upregulation of mucin genes. PLoS One. 2014;9:e87875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622‐630. [DOI] [PubMed] [Google Scholar]
- 90. Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309‐320. [DOI] [PubMed] [Google Scholar]
- 91. Gupta BK, Maher DM, Ebeling MC, et al. Increased expression and aberrant localization of mucin 13 in metastatic colon cancer. J Histochem Cytochem. 2012;60:822‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xu H, Inagaki Y, Seyama Y, et al. Expression of KL‐6/MUC1 in pancreatic ductal carcinoma and its potential relationship with beta‐catenin in tumor progression. Life Sci. 2011;88:1063‐1069. [DOI] [PubMed] [Google Scholar]
- 93. Besmer DM, Curry JM, Roy LD, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71:4432‐4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti‐adhesive glycoprotein. Int J Cancer. 2000;87:480‐486. [DOI] [PubMed] [Google Scholar]
- 95. Tinder TL, Subramani DB, Basu GD, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181:3116‐3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhao Q, Barclay M, Hilkens J, et al. Interaction between circulating galectin‐3 and cancer‐associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Senapati S, Chaturvedi P, Chaney WG, et al. Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17:267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. King RJ, Yu F, Singh PK. Genomic alterations in mucins across cancers. Oncotarget. 2017;8:67152‐67168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Geng Y, Marshall JR, King MR. Glycomechanics of the metastatic cascade: tumor cell‐endothelial cell interactions in the circulation. Ann Biomed Eng. 2012;40:790‐805. [DOI] [PubMed] [Google Scholar]
- 100. Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem. 1997;272:24198‐24202. [DOI] [PubMed] [Google Scholar]
- 101. Horn A, Chakraborty S, Dey P, et al. Immunocytochemistry for MUC4 and MUC16 is a useful adjunct in the diagnosis of pancreatic adenocarcinoma on fine‐needle aspiration cytology. Arch Pathol Lab Med. 2013;137:546‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Maker AV, Hu V, Kadkol SS, et al. Cyst fluid biosignature to predict intraductal papillary mucinous neoplasms of the pancreas with high malignant potential. J Am Coll Surg. 2019;228:721‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang Y, Gao J, Li Z, Jin Z, Gong Y, Man X. Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound‐guided fine‐needle aspiration specimens of the pancreas. Int J Cancer. 2007;121:2716‐2722. [DOI] [PubMed] [Google Scholar]
- 104. Carrara S, Cangi MG, Arcidiacono PG, et al. Mucin expression pattern in pancreatic diseases: findings from EUS‐guided fine‐needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359‐1363. [DOI] [PubMed] [Google Scholar]
- 105. Urey C, Andersson B, Ansari D, et al. Low MUC4 expression is associated with survival benefit in patients with resectable pancreatic cancer receiving adjuvant gemcitabine. Scand J Gastroenterol. 2017;52:595‐600. [DOI] [PubMed] [Google Scholar]
- 106. Liu L, Xu HX, Wang WQ, et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis‐associated burden. Oncotarget. 2016;7:5943‐5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Osteikoetxea X, Benke M, Rodriguez M, et al. Detection and proteomic characterization of extracellular vesicles in human pancreatic juice. Biochem Biophys Res Commun. 2018;499:37‐43. [DOI] [PubMed] [Google Scholar]
- 108. Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315‐321. [PubMed] [Google Scholar]
- 109. Turner MS, Cohen PA, Finn OJ. Lack of effective MUC1 tumor antigen‐specific immunity in MUC1‐transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild‐type Th cells. J Immunol. 2007;178:2787‐2793. [DOI] [PubMed] [Google Scholar]
- 110. Barratt‐Boyes SM, Vlad A, Finn OJ. Immunization of chimpanzees with tumor antigen MUC1 mucin tandem repeat peptide elicits both helper and cytotoxic T‐cell responses. Clin Cancer Res. 1999;5:1918‐1924. [PubMed] [Google Scholar]
- 111. Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB‐AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yamamoto K, Ueno T, Kawaoka T, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25:3575‐3579. [PubMed] [Google Scholar]
- 113. Lepisto AJ, Moser AJ, Zeh H, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955‐964. [PMC free article] [PubMed] [Google Scholar]
- 114. Li M, Wang Z, Yan B, et al. Design of a MUC1‐based tricomponent vaccine adjuvanted with FSL‐1 for cancer immunotherapy. Medchemcomm. 2019;10:2073‐2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wu J, Wei J, Meng K, et al. Identification of an HLA‐A*0201‐restrictive CTL epitope from MUC4 for applicable vaccine therapy. Immunopharmacol Immunotoxicol. 2009;31:468‐476. [DOI] [PubMed] [Google Scholar]
- 116. Danielczyk A, Stahn R, Faulstich D, et al. PankoMab: a potent new generation anti‐tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55:1337‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fan XN, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor‐related MUC1 epitope (TA‐MUC1) with various human carcinomas. Pathol Res Pract. 2010;206:585‐589. [DOI] [PubMed] [Google Scholar]
- 118. Cardillo TM, Blumenthal R, Ying Z, Gold DV. Combined gemcitabine and radioimmunotherapy for the treatment of pancreatic cancer. Int J Cancer. 2002;97:386‐392. [DOI] [PubMed] [Google Scholar]
- 119. Cardillo TM, Ying Z, Gold DV. Therapeutic advantage of (90)yttrium‐ versus (131)iodine‐labeled PAM4 antibody in experimental pancreatic cancer. Clin Cancer Res. 2001;7:3186‐3192. [PubMed] [Google Scholar]
- 120. Gold DV, Cardillo T, Goldenberg DM, Sharkey RM. Localization of pancreatic cancer with radiolabeled monoclonal antibody PAM4. Crit Rev Oncol Hematol. 2001;39:147‐154. [DOI] [PubMed] [Google Scholar]
- 121. Qu CF, Songl YJ, Rizvi SM, et al. In vivo and in vitro inhibition of pancreatic cancer growth by targeted alpha therapy using 213Bi‐CHX.A"‐C595. Cancer Biol Ther. 2005;4:848‐853. [DOI] [PubMed] [Google Scholar]
- 122. Han S, Jin G, Wang L, et al. The role of PAM4 in the management of pancreatic cancer: diagnosis, radioimmunodetection, and radioimmunotherapy. J Immunol Res. 2014;2014:268479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tsutsumida H, Swanson BJ, Singh PK, et al. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976‐2987. [DOI] [PubMed] [Google Scholar]
- 124. Ring CJ, Blouin P, Martin LA, Hurst HC, Lemoine NR. Use of transcriptional regulatory elements of the MUC1 and ERBB2 genes to drive tumour‐selective expression of a prodrug activating enzyme. Gene Ther. 1997;4:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 125. Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5‐FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. Eur J Cancer. 2009;45:164‐173. [DOI] [PubMed] [Google Scholar]
- 126. Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5‐FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150‐160. [DOI] [PubMed] [Google Scholar]
- 127. Amini A, Masoumi‐Moghaddam S, Ehteda A, Liauw W, Morris DL. Potentiation of chemotherapeutics by bromelain and N‐acetylcysteine: sequential and combination therapy of gastrointestinal cancer cells. Am J Cancer Res. 2016;6:350‐369. [PMC free article] [PubMed] [Google Scholar]
- 128. Pillai K, Akhter J, Chua TC, Morris DL. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int J Cancer. 2014;134:478‐486. [DOI] [PubMed] [Google Scholar]
- 129. Amini A, Masoumi‐Moghaddam S, Ehteda A, Liauw W, Morris DL. Depletion of mucin in mucin‐producing human gastrointestinal carcinoma: results from in vitro and in vivo studies with bromelain and N‐acetylcysteine. Oncotarget. 2015;6:33329‐33344. [DOI] [PMC free article] [PubMed] [Google Scholar]


