Abstract
Background
The P‐glycoprotein (P‐gp) is one of the mechanisms of Imatinib (IM) resistance in chronic myeloid leukemia (CML). P‐gp has been identified as an efflux pump involved in releasing of IM outside CML cells. To date, the P‐gp involvement in the IM resistance development was not completely understood. Therefore, the present study aimed at measuring the P‐gp expression level on lymphocytes from Tunisian patients with CML and correlating this level with a molecular response to IM.
Method
The expression of P‐gp on peripheral blood lymphocytes from 59 Tunisian patients with CML (27 IM responder patients vs 32 IM non‐responder patients) was evaluated by flow cytometry.
Result
Our finding showed significantly positive expression of P‐gp in the lymphocytes from the IM non‐responder group when compared to the IM‐responder group (P = .001). In IM non‐responder CML patients, the comparison between CCyR achievers and non‐achievers showed a high mean fluorescence intensity (MFI) of P‐gp expression in patients who did not achieve their CCyR (P = .001). The comparison between patients with primary and secondary resistance to IM showed an increasing MFI value in patients with primary resistance to IM (P = .001). Besides, the comparison between nilotinib‐treated and dasatinib‐treated patients proved a high value of MFI in nilotinib‐treated patients (P = .001).
Conclusion
The overexpression of P‐gp on lymphocytes has significantly correlated with the failed molecular response to IM in patients with CML.
Keywords: chronic myeloid leukemia, Imatinib, multidrug resistance, P‐glycoprotein, resistance
1. INTRODUCTION
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm of pluripotent hematopoietic stem cells. 1 CML does appear because of the reciprocal translocation between the long arms of chromosomes 9 and 22 t(9;22) (q34;q11). 2 This translocation results from the formation of a chimeric gene, BCR‐ABL1, which encodes the BCR‐ABL protein with intrinsic tyrosine kinase activity that is critical of leukemogenesis. 3 The discovery of BCR‐ABL protein, which is required for the pathogenesis of this disease, provided the rationale for the therapeutic intervention of an inhibitory agent that targets tyrosine kinase domain of the protein and blocks its phosphorylation. 4
Imatinib mesylate (IM) is the first molecule tyrosine kinase inhibitor (TKI) that is currently successfully used for the treatment of CML. 5 IM is also known as a blocked tyrosine kinase activity of the BCR‐ABL oncoprotein by the competitive inhibitor of ATP‐binding site. 6
Despite IM's striking efficacy, many researchers showed the resistance of this drug has developed over time in a minority of patients with advanced‐stage CML. 7 , 8 , 9
The resistance against IM can result from several mechanisms that can be widely divided into BCR‐ABL‐dependent or BCR‐ABL‐independent. 10 Overall, in these findings, the mutations in the ABL kinase domain and/or amplification of the BCR‐ABL oncogene are greatly observed in the BCR‐ABL‐dependent mechanisms. 11 For the mechanisms of resistance of BCR‐ABL independent to IM, some studies revealed that these mechanisms included decreasing intracellular concentration drug, which can be due to either the bringing of IM by human organic cation transporter 1 (hOCT1) or its export by the P‐glycoprotein (P‐gp). 12 , 13 The influence of efflux transporters on IM pharmacokinetics has widely been investigated, and their increased expression has been usually connected with IM response variability. 12 , 13 The most well‐studied transporter protein is the P‐gp, which has the ability to eject IM from the leukemic cells. 14 This protein is encoded by ATP‐binding cassette, subfamily B, member 1 ABCB1 gene (also known MDR1 [multidrug resistance 1]), and it is located in various tissues as well as in normal peripheral blood lymphocytes and bone marrow cells that are involved in the traffic of IM outside cancer cells. 15 In a considerable number of cancers, overexpression of P‐gp provides the most commonly found mechanism of multidrug resistance (MDR), designing a significant obstacle of failure of cancer chemotherapy. 16 , 17 MDR is a phenomenon that is related to reduced intracellular drug accumulation in leukemic cells resulting from enhanced drug efflux. 18
Within the past few years, the actual role of P‐gp efflux pump in resistance against IM has been widely studied. A number of investigations have proposed that increased P‐gp expression is unlikely to be a primary mechanism of IM resistance in patients with CML. 19 , 20 , 21 However, these results have not been obtained by other investigators. 22 , 23
To clarify these inconsistent and/or controversial findings of these results, the roles of P‐gp in IM resistance need to be more explored. In our study, we tried to find a correlation between the overexpression of P‐gp and the interindividual variability of IM response in CML patients.
2. MATERIALS AND METHODS
2.1. Ethics statement
The protocol of this study was approved relying on the criteria declared by Helsinki. Each patient agreed on his participation in the study.
2.2. Study design
A retrospective case‐control study on Tunisian CML patients has been designed. This study was carried out from June 2015 to January 2016 in the Hematology Department of Hedi Chaker University Hospital, Sfax, Tunisia.
2.3. Patients
Inclusion criteria were as follows: CML patients in chronic phase, availability of clinical data, and patients who have already been treated with IM 400 mg once daily as first‐line therapy. Exclusion criteria were as follows: pregnant women, patients suffering from any other hematological illnesses, patients in accelerated phase or blastic crisis, and particularly patients with BCR‐ABL1 gene mutations.
Demographic, clinical, and biological characteristics (white blood cell [WBC] count, neutrophils count, platelets count, hemoglobin [Hb] concentration, and the spleen size) at diagnosis, Sokal score, TKI‐treated, and therapy failure were extracted retrospectively from the patients' files.
Clinical evolution was performed on the basis overall survival (OS), progression‐free survival (PFS), and MMR achievement, from the date of CML diagnosis and the start of IM treatment with an appropriate length of follow‐up (3‐year).
Sokal score was established to prognosticate the patients of CML at diagnosis. 24 Three risky groups were designated as follows: low risk (score < 0.8), intermediate risk (score 0.8‐1.2), and high risk (score > 1.2).
2.4. Definitions and approaches to measuring responses to Imatinib
According to the European Leukemia Network criteria 2016 (ELN), 25 the IM responses were evaluated by the BCR‐ABL1 gene ratio. One year later of IM treatment, patients are considered as optimal responders to IM if the BCR‐ABL1 gene ratio ≤0.1% (achieve a major molecular response [MMR]) or as IM‐resistant phenotype if the ratio >0.1%. IM non‐responders CML patients can be defined as primary resistance (fail to achieve an optimal response) or as secondary resistance (loss an initiated response).
According to Cortes et al, 26 the complete cytogenetic response (CCyR) took place when the BCR‐ABL1 gene ratio was less than 1% within 6 months from the start of IM treatment.
A complete hematologic response (CHR) is obtained when laboratory values return to normal levels in 3 months from the start of IM therapy.
The BCR‐ABL gene mutation testing was performed exclusively on patients who showed IM resistance.
2.5. Peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells (PBMCs) from CML were isolated by Ficoll‐Hypaque gradient (Eurobio, France) immediately after sampling. Cells were washed once in phosphate‐buffered saline (PBS). After being washed, they are frozen in dimethyl sulfoxide (DMSO) and conserved at −80°C for long‐term storage.
2.6. Determination of P‐gp expression by flow cytometry
The expression of P‐gp was examined on lymphocyte subpopulations in PBMCs by indirect immunofluorescence method. 27 Briefly, 5×105 cells were rinsed twice in PBS with 0.5% bovine serum albumin (BSA) to eliminate the DMSO. Then, these cells were incubated with or without a P‐gp antibody (1:50) (Clone UIC2; GeneTex) for 45 minutes at 4°C. After three washes, cells were sequentially incubated with a saturating concentration (1 µg/mL) of PE anti‐mouse IgG (clone 679.1Mc7; Beckman Coulter) as well as FITC anti‐CD45 (clone J33; Beckman Coulter) on ice for 30 minutes in the darkness. Besides, cells were washed twice in PBS/BSA 0.5% and resuspended in PBS.
Cells were analyzed on the FACS Canto™ II flow cytometer using BD FACS Diva™ software (Becton Dickinson). PBMCs were gated by forward scatter (FSC‐A) vs side scatter (SSC‐A) and FITC anti‐CD45 vs SSC‐A as shown in Figure 1.
Figure 1.
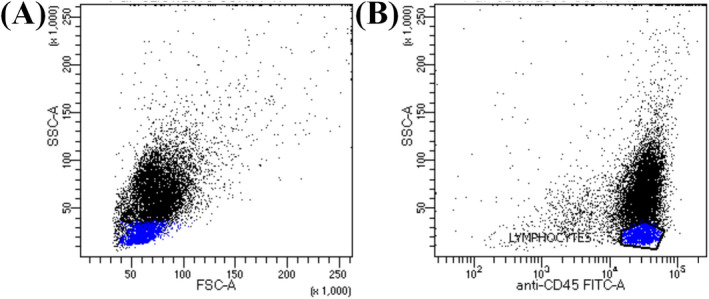
Analysis of P‐gp expression in lymphocytes populations in peripheral blood mononuclear cells from CML patients by flow cytometry. A, FSC versus SSC. B, CD45 versus SSC: lymphocytes were selected by their low SSC and bright CD45. CML, Chronic myeloid leukemia; IM, imatinib; P‐gp, P‐glycoprotein; FSC, forward side scatter; SSC, Side Scatter
The data were revealed as the ratio (relative fluorescence intensity [RFI]) of the mean fluorescence intensity (MFI) of anti‐P‐gp, primary antibody, and PE anti‐mouse IgG, secondary antibody, divided by the MFI of cells treated just with the secondary antibody. 28
2.7. Statistical analysis
The statistical analysis was carried out using Student's t test to assess the statistical significance of the differences between IM responder and those non‐responder patients and the Mann‐Whitney U test when sample sizes are small or/and when not normally distributed. Data were expressed in mean ± standard deviation (SD). P ≤ .05 was considered statistically significant (SPSS 20). The probabilities of OS, PFS, and MMR achievement were calculated using the Kaplan‐Meier method. The log‐rank test was used to compare the Kaplan‐Meier curves. OS was defined as the time interval from the start of IM therapy to either the date of death or the date of the last contact with the patient. PFS was defined as the time interval from the start of IM therapy to the date of disease progression to the advanced phase. MMR achievement was defined as the time interval from the start of IM therapy to the date of obtaining BCR‐ABLIS ≤ 0.10. Follow‐up lasted 3 years.
3. RESULTS
3.1. Clinical characteristics of CML patients
A total of 127 Tunisian subjects with CML have been interrogated from the Hematology Department of Hedi Chaker Hospital. Sixty‐eight patients have been excluded from our study: 58 with BCR‐ABL mutations, 6 in accelerated phase, and 4 in the blastic crisis phase. The final sample size was 59 subjects who met the inclusion criteria and took part in our study.
In our study, patients have been classified into two groups: The first group comprised 27 patients (45.7%) who are considered as optimal responders to IM, while the second group comprised 32 patients (54.3%) who are presented as IM‐resistant phenotype.
Table 1 summarizes the clinical characteristics of both groups of patients with CML. There were no significant differences based on gender, age, and Sokal score between both groups (P < .05). The age of IM responder CML patients ranged from 23 to 80 years, with an average of 50.22 ± 15.23 years and that of IM non‐responders from 24 to 73 years, with an average of 48.53 ± 11.54 years.
Table 1.
Clinical characteristics of CML patients
| Clinical characteristics | CML patients | P value | |
|---|---|---|---|
| IM Responders (n = 27) | IM non‐responders (n = 32) | ||
| Gender | |||
| Male, n | 13 | 18 | .543 |
| Female, n | 14 | 14 | |
| Age at diagnosis (y), mean ± S.D | 50.22 ± 15.23 | 48.53 ± 11.54 | .630 |
| Transcript type | |||
| b2a2, N | 8 | 12 | .390 |
| b3a2, N | 23 | 13 | .006 |
| b2a2 + b3a3, N | 0 | 3 | .540 |
| Sokal score | |||
| Low, N | 8 | 13 | .540 |
| Intermediate, N | 13 | 19 | .470 |
| High, N | 6 | 0 | .190 |
| Achievement of CCyR within 6 mo, N | — | 14 | — |
| Treated with dasatinib, N | — | 11 | — |
| Treated with nilotinib, N | — | 21 | — |
| Primary failure of IM, N | — | 26 | — |
| Secondary failure of IM, N | — | 6 | — |
Abbreviations: CCyR, complete cytogenetic response; CML, chronic myeloid leukemia; IM, imatinib.
We observed a significant increase in the b3a2 transcript type of BCR‐ABL in IM responders as compared with IM non‐responders (P = .006).
Imatinib non‐responder CML patients are classified as patients with primary resistance (n = 26) and patients with secondary resistance (n = 6). The patients who failed in IM has switched to second‐generation TKI as dasatinib (n = 11) or nilotinib (n = 21). Only 14 IM non‐responder patients achieved their CCyR within 6 months (Table 1). All patients achieved a CHR in 3 months following IM therapy.
The test of BCR‐ABL1 gene mutations showed no mutation of the BCR‐ABL kinase domain in IM‐resistant subjects.
3.2. Biological characteristics of CML patients
Table 2 lists the biological characteristics of CML patients. The comparison between the IM responder and IM non‐responder CML patients showed higher levels of WBC and neutrophils in IM non‐responders (P = .005 and P = .01, respectively). We observed more progress of the spleen size for IM non‐responder than for IM responder patients (P = .015).
Table 2.
Biological characteristics of CML patients
| Biological characteristics | CML Patients | P value | |
|---|---|---|---|
| IM Responders (n = 27) | IM non‐responders (n = 32) | ||
| WBC (103/mm3) | 6.20 ± 1.19 | 13 ± 1.03 | .005 |
| Neutrophils (103g/l) | 7.75 ± 1.74 | 14.37 ± 1.33 | .01 |
| Platelets (103/mm3) | 404.16 ± 1.19 | 387.68 ± 1.03 | .839 |
| Hb (g/dL) | 11.65 ± 1.81 | 10.63 ± 1.6 | .18 |
| Spleen size (cm) | 3.69 ± 1.27 | 8.7 ± 1.09 | .015 |
Data presented as mean ± SD and t test applied for the comparisons.
Abbreviations: CML, chronic myeloid leukemia; Hb, hemoglobin; IM, imatinib; WBC, white blood cell.
3.3. P‐gp expression status in CML patients
P‐gp expression ranged from 0.76 to 1.43 with an RFI = 1.1 when all patients were studied. This value of RFI was used as the cutoff point to divide negative (RFI < 1.1) and positive (RFI > 1.1) P‐gp expression. Two instances of a flow cytometric analysis for negative and positive P‐gp expression are shown in Figure 2A,B respectively
Figure 2.
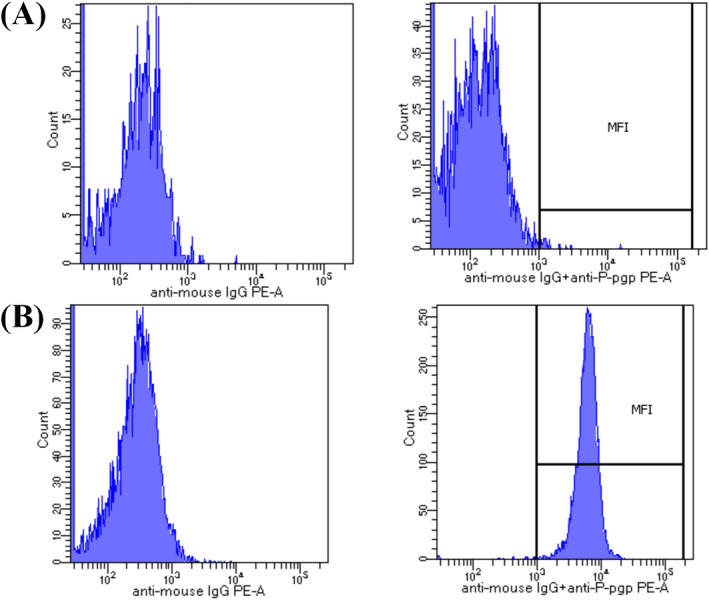
Flow cytometry analysis of CML cells. The results were expressed as the ratio (RFI) of the MFI of UIC2 and PE‐anti‐mouse IgG, divided by the MFI of cells treated just with the PE‐ anti‐mouse IgG. A, An example of negative P‐gp expression in cells incubated with monoclonal antibody anti‐P‐gp UIC2 and PE‐anti‐mouse IgG, secondary antibody. B, An example of positive P‐gp expression in cells incubated with monoclonal antibody anti‐P‐gp UIC2 and PE‐anti‐mouse IgG, secondary antibody. CML, Chronic myeloid leukemia; IM, imatinib; P‐gp, P‐glycoprotein; RFI, relative fluorescence intensity; MFI, mean fluorescence intensity
By analyzing levels of P‐gp expression according to response to IM (responders vs non‐responders), we found that all IM non‐responder CML patients exhibited P‐gp overexpression (RFI > 1.1), while all IM responder patients demonstrated negative expression of P‐gp (RFI < 1.1; P = .001) (Figure 3A; Table 3).
Figure 3.
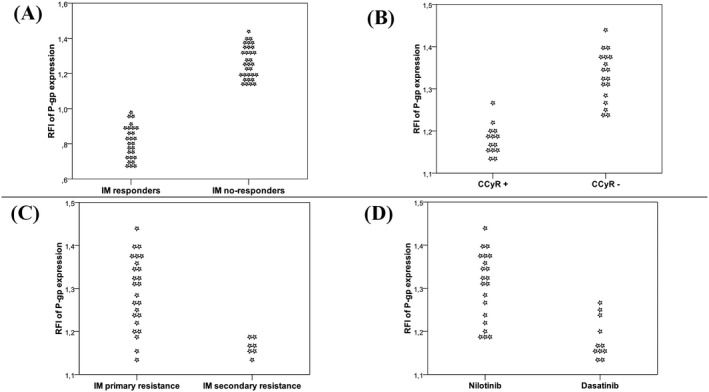
Comparison of P‐gp expression level in CML patients based on clinical patient’s status. Distribution plots demonstrating results of P‐gp level measured by flow cytometry using monoclonal antibodies as described in Materials and methods. A, Comparison of P‐gp expression between CML patients responders and non‐responders to IM. B, Comparison of P‐gp expression between CML patients achieved (CCyR +) and not achieved (CCyR ‐) their CCyR. C, Comparison of P‐gp expression between patients with primary and secondary resistance to IM. D, Comparison of P‐gp expression between patients nilotinib‐treated and patients dasatinib‐treated. CML, Chronic myeloid leukemia; IM, imatinib; P‐gp, P‐glycoprotein; CCyR, Complete Cytogenetic Response
Table 3.
Comparison of relative fluorescence intensity expressed by the median of P‐glycoprotein with CML status
| Relative fluorescence intensity expressed by the median of P‐glycoprotein | |||
|---|---|---|---|
| CML patients responders VS non‐responders to IM | CML patients achieved VS not achieved their CCyR | CML patients with primary VS secondary resistance to IM | Nilotinib‐treated VS dasatinib‐treated CML patients |
| 0.80 ± 0.09 vs 1.26 ± 0.09; P = .001 | 1.17 ± 0.03 vs 1.33 ± 0.05; P = .001 | 1.29 ± 0.08 vs 1.17 ± 0.02; P = .001 | 1.3 ± 0.07 vs 1.18 ± 0.04; P = .001 |
Abbreviations: CCyR, complete cytogenetic response IM, imatinib; CML, chronic myeloid leukemia.
3.4. Relationship between MDR phenotype and expression of P‐gp
In IM non‐responder CML patients, the comparison by Mann‐Whitney U test showed a high RFI of P‐gp expression in patients not achieving their CCyR (P = .001) (Figure 3B), patients with primary IM resistance (P = .001) (Figure 3C), and patients treated with nilotinib (P = .001) (Figure 3D; Table 3).
3.5. Association of expression of P‐glycoprotein with the outcome of patients
Kaplan‐Meier method indicated that the 3‐year probabilities of OS, PFS, and MMR achievement in patients with negative expression were significantly higher compared to those found in patients with positive expression (P < .001, P = .047, and P < .001, respectively) (Figure 4).
Figure 4.
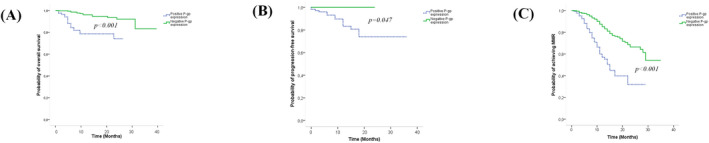
Kaplan‐Meier survival analysis. A, 3‐y overall survival of CML patients, according to P‐gp expression. B, 3‐y progression‐free survival of CML patients, according to P‐gp expression. C, 3‐y achievement of major molecular response of CML patients, according to P‐gp. Kaplan‐Meier analysis demonstrating the results of the P‐gp level measured by flow cytometry using monoclonal antibodies as described in Materials and methods. CML, chronic myeloid leukemia; P‐gp, P‐glycoprotein
4. DISCUSSION
Introduction of IM, as a first anticancer drug treatment for patients with CML, has profoundly enhanced the prognosis of these patients. 5 , 6 Despite the high efficiency of this drug, a fraction of patients with CML developed resistance to IM. 7 , 8 , 9 Various investigations have demonstrated that the responsiveness to IM is due to some factors such as mutations in the ABL kinase domain, amplification in the BCR‐ABL gene, and/or alterations in the expression of drug transporters as P‐gp. 8 , 9 , 10 , 11 , 12 , 13 Our study was conducted to understand the mechanisms of resistance to IM in order to find therapeutic solutions that may help prevent a relapse from this drug in CML. In this review, we have mainly focused on the mechanism of resistance to IM induced by P‐gp overexpression in CML. Luckily, this is the first Tunisian study to measure the level of P‐gp expression on lymphocytes from CML patients.
In our study, the prevalence of resistance to IM was 54.3%. This rate was relatively similar to a Tunisian study (49.2%) 29 but higher than the Korea study (16%). 30 Consequently, we believe that Tunisian patients differ from other populations regarding resistance mechanisms to IM, secondarily to interethnic pharmacokinetic variation in IM response.
Our finding revealed a significant increase in the b3a2 transcript type of BCR‐ABL in IM responders (63.8%) as compared with IM non‐responders (36.2%). Moreover, the findings of Hanfstein et al 31 showed that the probability of achieving MMR was significantly higher in patients with b3a2 transcript. Our result emphasizes that CML patients with b3a2 transcript had less prospect to have failure responses to IM. Consequently, the b3a2 transcript could serve as a clinical and helpful biomarker to prophesy an optimal response to IM in patients with CML.
The level of P‐gp expression was revealed by the RFI that had been defined as the ratio of the MFI of anti‐P‐gp, primary antibody, and PE anti‐mouse IgG, secondary antibody, divided by the MFI of cells treated just with the secondary antibody. 28 The cutoff value (RFI < 1.1 = P‐gp‐negative; RFI > 1.1 = P‐gp‐positive) was used for expression analysis of P‐gp that had been found by the previous studies. 32 , 33
To date, the contribution of P‐gp transporter in influencing the IM therapy response in CML patients remains unclear. However, some investigations have studied the role of P‐gp in IM pharmacokinetics and have shown controversial findings. 19 , 20 , 21 , 22 , 23 Previous works had identified that no consensus exists on the level of P‐gp expression on the surface of normal lymphocytes; hence, these can explain the inconsistency and/or contradictory datum in the literature. 33 , 34 In our research, it was clear that the mode of resistance to IM is proportional to the overexpression of P‐gp that has been reported for the first time in the Tunisian population. We found an increased expression of P‐gp on lymphocytes from IM non‐responder CML patients when compared to IM responder subjects. Our result goes in good analogous with a study reported by Xing‐Xi et al, 22 which examined the level of P‐gp expression in K562 cells and found an overexpressed of P‐gp in K562‐IM cells (exhibiting MDR phenotype) when compared to K562 cells. In contradiction to this investigation, several reports 19 , 20 , 21 have suggested that overexpression of P‐gp in K562 cells only conferred minimal IM resistance. Furthermore, Gambacorti‐Passerini et al 21 found that at 0.5‐1 mmol/L level, which is the physiological plasma concentration of IM in patients given once daily at 400 mg Glivec, K562 cells engineered to overexpress P‐gp did not show IM resistance.
To gain more insight into the interrelationship between the positive expression of P‐gp evidenced in CML patients and the MDR phenom, the level of P‐gp expression was investigated, in a comparative way, in the group of IM‐resistant CML patients. Our results have clearly shown a significantly positive expression of P‐gp: in patients not achieving their CCyR, in subjects with primary resistance to IM, and in patients switched to nilotinib. Our results are in line with a previous work by Laura et al 35 on K562‐Dox cells (ABCB1 overexpressing), which reported that P‐gp overexpression was associated with nilotinib resistance in vitro. Also, a study developed by Raquel et al 36 showed that P‐gp negative expression was associated with the achievement of CCyR.
In definition, P‐gp acts as a drug efflux membrane pump, so it captures drugs like a vacuum cleaner when they pass through the cell membrane and then releases them outside the cell. 37 Consequently, our findings suggest that increased expression of P‐gp on lymphocytes confers acquired IM resistance by functioning as an efflux transporter and whereby reducing the accumulation of IM.
The impact of the P‐gp expression level on clinical evolution has evaluated. Our results suggest that overexpression of P‐gp was a poor prognostic factor in CML patients. The probabilities of OS, PFS, and MMR achievement were lower in CML patients with positive expression of P‐gp. These findings are in accord with the results of Andreas‐Claudius et al 38 on patients with bladder cancer, which found that after 5 years, only 23% of patients with high MDR1 expression were still alive.
Further large‐scale investigations are needed to verify our results and to elucidate the fact that the monitoring of the level of P‐gp expression could be a novel approach to overcome IM resistance.
5. CONCLUSION
So far, the research we carried out has been an attempt to explore the relationship of P‐gp overexpression in the resistance to IM among Tunisian patients with CML. We demonstrate that patients with positive expression of P‐gp have a low prospect of achieving their MMR and a higher risk of developing a resistance to IM. The results presented here suggested that the monitoring of the level of P‐gp expression may potentially identify patients likely to develop IM unresponsiveness or failure to IM therapy.
ACKNOWLEDGMENTS
We would like to thank The Regional Blood Transfusion Center, Sfax, Tunisia, for sample analysis by flow cytometry.
Ammar M, Louati N, Frikha I, et al. Overexpression of P‐glycoprotein and resistance to Imatinib in chronic myeloid leukemia patients. J Clin Lab Anal. 2020;34:e23374 10.1002/jcla.23374
REFERENCES
- 1. Giles FJ, Le Coutre PD, Pinilla‐Ibarz J, et al. Nilotinib in imatinib‐resistant or imatinib‐intolerant patients with chronic myeloid leukemia in chronic phase: 48‐month follow‐up results of a phase II study. Leuk. 2013;27:107‐112. [DOI] [PubMed] [Google Scholar]
- 2. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol. 2016;91:252‐265. [DOI] [PubMed] [Google Scholar]
- 3. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207‐219. [DOI] [PubMed] [Google Scholar]
- 4. Sailaja K, Surekha D, Rao DN, Raghunadharao D, Vishnupriya S. Association of MDR1 gene polymorphism (G2677T) with chronic myeloid leukemia. Biol Med. 2010;2(4):17‐21. [Google Scholar]
- 5. Hochhaus A. Chronic myelogenous leukemia (CML) resistance to tyrosine kinase inhibitors. Ann Oncol. 2006;17:274‐279. [DOI] [PubMed] [Google Scholar]
- 6. Talpaz M. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928‐1937. [DOI] [PubMed] [Google Scholar]
- 7. Walz C, Sattler M. Novel targeted therapies to overcome imatinib mesylate resistance in chronic myeloid leukemia (CML). Crit Rev Oncol Hematol. 2006;57:145‐164. [DOI] [PubMed] [Google Scholar]
- 8. A J, Qian S, Wang G, et al. Chronic myeloid leukemia patients sensitive and resistant to imatinib treatment show different metabolic responses. PLoS ONE. 2010;5(10):e13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esposito N, Colavita I, Quintarelli C, et al. SHP‐1 expression accounts for resistance to imatinib treatment in Philadelphia chromosome‐positive cells derived from patients with chronic myeloid leukemia. Blood. 2011;118(13):3634‐3644. [DOI] [PubMed] [Google Scholar]
- 10. Quintas‐Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16:122‐131. [DOI] [PubMed] [Google Scholar]
- 11. Nestal de Moraes G, Souza PS, Costas FC, Vasconcelos FC, Reis FR, Maia RC. The interface between BCR‐ABL‐dependent and ‐independent resistance signaling pathways in chronic myeloid leukemia. Leuk Res Treat. 2012;2012:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematol Am Soc Hematol Educ Program. 2009;2009(1):461‐476. [DOI] [PubMed] [Google Scholar]
- 13. Thomas J. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104:3739‐3745. [DOI] [PubMed] [Google Scholar]
- 14. Skoglund K, Moreno SB, Baytar M, Jönsson JI, Gréen H. ABCB1 haplotypes do not influence transport or efficacy of tyrosine kinase inhibitors in vitro. Pharmgenomics Pers Med. 2013;6:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silva R, Vilas‐Boas V, Carmo H, et al. Modulation of P‐glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1‐123. [DOI] [PubMed] [Google Scholar]
- 16. Shin SY, Choi BH, Kim J‐R, Kim J‐H, Lee YH. Suppression of P‐glycoprotein expression by antipsychotics trifluoperazine in adriamycinresistant L1210 mouse leukemia cells. Eur J Pharm Sci. 2006;28:300‐306. [DOI] [PubMed] [Google Scholar]
- 17. Siarheyeva A, Liu R, Sharom FJ. Characterization of an Asymmetric occluded state of p‐glycoprotein with two bound nucleotides: implications for catalysis. J Biol Chem. 2010;285:7575‐7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Illmer T, Schaich M, Platzbecker U, et al. P‐glycoprotein‐mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leuk. 2004;18:401‐408. [DOI] [PubMed] [Google Scholar]
- 19. Ferrao PT, Frost MJ, Siah SP, Ashman LK. Overexpression of P‐glycoprotein in K562 cells does not confer resistance to the growth inhibitory effects of imatinib (STI571) in vitro. Blood. 2003;102:4499‐4503. [DOI] [PubMed] [Google Scholar]
- 20. Zong Y, Zhou S, Sorrentino BP. Loss of P‐glycoprotein expression in hematopoietic stem cells does not improve responses to imatinib in a murine model of chronic myelogenous. Leuk. 2005;19:1590‐1596. [DOI] [PubMed] [Google Scholar]
- 21. Gambacorti‐Passerini C, Zucchetti M, Russo D, et al. Alpha 1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin Cancer Res. 2003;9:625‐632. [PubMed] [Google Scholar]
- 22. Xing‐X IP, Amit KT, Hsiang‐C HW, Zhe‐S HC. Overexpression of P‐glycoprotein induces acquired resistance to imatinib in chronic myelogenous leukemia cells. Chin J Cancer. 2012;31(2):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahon FX, Belloc F, Lagarde V, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368‐2373. [DOI] [PubMed] [Google Scholar]
- 24. Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in ‘good‐risk’ chronic granulocytic leukemia. Blood. 1984;63(4):789‐799. [PubMed] [Google Scholar]
- 25. Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leuk. 2016;30(8):1648‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortes J, Quintás‐Cardama A, Kantarjian HM. Monitoring molecular response in chronic myeloid leukemia. Cancer. 2011;117:1113‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu D, Ye D, Fisher M, Juliano Rl. Selective inhibition of P‐glycoprotein expression in multidrug‐ resistant tumor cells by a designed transcriptional regulator. Pharmacol And Experim Ther. 2002;302:963‐971. [DOI] [PubMed] [Google Scholar]
- 28. Silva KL, Vasconcelos FC, Marques‐Santos LF, Kwee JK, Maia RC. CPT‐11 induced cell death in leukemic cells is not affected by the MDR phenotype. Leuk Res. 2003;27:243‐251. [DOI] [PubMed] [Google Scholar]
- 29. Islem BH, Hanene G, Ismail S, et al. hOCT1 gene expression predict for optimal response to Imatinib in Tunisian patients with chronic myeloid leukemia. Cancer Chemother Pharmacol. 2017;79(4):737‐745. [DOI] [PubMed] [Google Scholar]
- 30. Park SH, Park CJ, Kim DY, et al. MRP1 and P‐glycoprotein expression assays would be useful in the additional detection of treatment non‐responders in CML patients without ABL1 mutation. Leuk Res. 2015;39:1109‐1116. [DOI] [PubMed] [Google Scholar]
- 31. Hanfstein B, Lauseker M, Hehlmann R, et al. Distinct characteristics of e13a2 versus e14a2 BCR‐ABL1 driven chronic myeloid leukemia under first‐line therapy with imatinib. Haematologica. 2014;99(9):1441‐1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flavia CV, Geraldo BCJ, Karina LS, et al. Contrasting features of MDR phenotype in leukemias by using two fluorochromes: Implications for clinical practice. Leuk Res. 2006;31(4):445‐454. [DOI] [PubMed] [Google Scholar]
- 33. Cavalcanti GB Jr, Vasconcelos FC, Pinto GF, et al. Coexpression of p53 protein and MDR functional phenotype in leukemias: the predominant association in chronic myeloid leukemia. Cytometry. 2004;61B:1‐8. [DOI] [PubMed] [Google Scholar]
- 34. Neyfakh AA, Serpinskaya AS, Chervonsky AV, Apasov SG, Kazarov AR. Multidrug‐resistance phenotype of a subpopulation of T‐lymphocytes without drug selection. Exp Cell Res. 1989;185:496‐505. [DOI] [PubMed] [Google Scholar]
- 35. Eadie LN, Hughes TP, White DL. ABCB1 overexpression is a key initiator of resistance to tyrosine kinase inhibitors in CML cell lines. PLoS ONE. 2016;11:e0161470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raquel CM, Flavia CV, Paloma SS, Vivian MR. Towards comprehension of the ABCB1/P‐glycoprotein role in chronic myeloid leukemia. Molecules. 2018;23:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chloë D, Charlotte B, Christopher B, Salvador H, Stephan JR, Cyril R. The role of proton dynamics in the development and maintenance of multidrug resistance in cancer. Biochem Biophys Acta. 2013;1832(5):606‐617. [DOI] [PubMed] [Google Scholar]
- 38. Andreas‐Claudius H, Peter W, Christina L, et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia. 2010;12(8):628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]


