Abstract
Background
Neuropilin1 (NRP1) participates in cancer cell proliferation, migration, and metastasis as a multifunctional co‐receptor by interacting with multiple signal pathways, but few studies have addressed the precise function of NRP1 in pancreatic cancer (PACA) cells. We aimed to study whether NRP1 gene silencing involved in the proliferation and migration of PACA cells in vitro.
Methods
A lentiviral vector expressing NRP1 shRNA was constructed and transfected into human PACA cells (CFPAC‐1 and PANC‐1). The expression of NRP1 protein and mRNA was detected by Western blot and quantitative real‐time polymerase chain reaction (qRT‐PCR) assay, respectively. CCK‐8 assay, wound healing assay, and transwell assay were conducted to examine the effect of NRP1 silencing on cells proliferation and migration capability.
Results
Results of qRT‐PCR and Western blot showed successfully established, stably transfected shRNA‐NRP1 cells in PACA cells. The proliferation capacity of PACA cells in NRP1 shRNA group was lower significantly than that in the negative control (NC) group (P < .05). The invasion and migration capability of PACA cells in NRP1 shRNA group was lower significantly than that in the NC group (P < .01).
Conclusions
NRP1‐shRNA lentiviral interference vectors can effectively decrease NRP1 gene expression in PACA cells, thereby inhibiting cells proliferation and migration, which provides a basis for finding a valuable therapeutic target for PACA therapy.
Keywords: cell migration, cell proliferation, lentiviral interference, Neuropilin1, pancreatic cancer
Abbreviations
- ECL
Enhanced chemiluminescence
- EDTA
Ethylenediaminetetraacetic acid
- EMT
Epithelial mesenchymal transition
- FBS
Fetal bovine serum
- NRP1
Neuropilins1
- NSCLC
Non‐small cell lung cancer
- RNAi
RNA interference
- shRNA
Short hairpin RNA
- TGF‐β
Transforming growth factor β
- VEGF
Vascular endothelial growth factor
1. INTRODUCTION
Pancreatic cancer (PACA) is one of the most aggressive human cancer types, accounting for about 2% of all malignant tumors. Owing to the special biological characteristics of the pancreas and its anatomical location, the cancer is associated with high malignancy rate, low early diagnosis rate, and poor prognosis. 1 Globally, the overall 5‐year survival rate of PACA patients is <5%. Although radical surgery is potentially the only optimal curative treatment for PACA, a vast majority of patients (80%) have already lost this chance at the time of diagnosis. 2 , 3 , 4 In combination with the improvement of standard of living and population aging, we are faced with an increasingly serious challenge of PACA.
NRP1 was first found as an axon adhesion protein in the nervous system of frogs, encoding a single type I transmembrane glycoprotein of molecular weight 120‐130 kDa. 5 Later research found that NRP1 gene expression is widespread over a variety of cells and tissues, such as endothelial cells, heart, liver, lung, kidney, pancreas, and skeletal muscle. 6 It is a non‐tyrosine kinase transmembrane glycoprotein located on the cell membrane, acting as co‐receptors for members of the vascular endothelial growth factor (VEGF) family and for secreted Semaphorin3A. In addition, NRP1 also plays a vital role in cardiovascular system, nervous system, immune system, and other aspects of tumor invasion and metastasis through interaction with various ligands, which can promote angiogenesis, neural development, cytoskeleton remodeling, initial immune response, and occurrence and development of tumors. 7 , 8 , 9 There is increasing evidence had indicated that higher expression of NRP1 associated with diverse malignant tumors of human, including hepatocellular carcinoma, 10 breast cancer, 11 gastric cancer, 12 and prostatic cancer. 13 NRP1 can promote tumor angiogenesis, cell proliferation, and cell migration, through a variety of mechanisms that play a vital role in the progression of cancer. 14 , 15 , 16 The role of NRP1 in malignant tumors of human has become a research hotspot in recent years.
However, to our knowledge, the expression level, biological function, and underlying mechanisms of NRP1 in PACA remain poorly understood. 17 The important role of NRP1 in many malignant tumors has prompted us to study whether NRP1 is a potential therapeutic target for PACA. In this research, we observed CFPAC‐1 and PANC‐1 cells in vitro for cell proliferation, invasion, and migration in human PACA after NRP1 knockdown by lentivirus‐mediated RNA interference. Our study provides an experimental basis for gene‐targeted treatment of PACA.
2. MATERIALS AND METHODS
2.1. Human PACA cell lines
CFPAC‐1 and PANC‐1 cells were purchased from the Shanghai Cell Bank and cultured under standard condition containing PMRI 1640 medium (Gibco) supplemented with 1% Penicillin‐Streptomycin (100 IU/mL; Gibco) and 10% fetal bovine serum (Gibco) with 5% CO2 at 37°C. When the cell density was consistent with the density indicated in the previous article, lentivirus transfection and cell function assays were carried out. 18 All operations were carried out in the biosafety cabinet after ultraviolet sterilization.
2.2. Lentivirus vector and transfection
Based on the results of bioinformatics, we selected three short hairpin RNA (shRNA) targeting human NRP1 sequence (sh1 NRP1: 5′‐AAAGCCCCGGGTACCTTACAT‐3′; sh2 NRP1: 5′‐AACACCTAGTGGAGTGATAAA‐3′; sh3 NRP1: 5′‐AACAGCCTTGAATGCACTTAT‐3′). The sh1 NRP1 was finally selected for subsequent experiments. The lentivirus vector used in this experiment was produced and provided by a company (Hanyin Biotechnology Limited Company). More concretely, two single‐stranded DNA were designed and synthesized: 5′‐gatccAAGCCCCGGGTACCTTACATTTCAAGAGAATGTAAGGTACCCGGGGCTTTTTTTTTg‐3′ and 5′‐aattcAAAAAAAAAGCCCCGGGTACCTTATTCTCTTGAAATGTAAGGTACCCGGGGCTTTg‐3′. Then, double‐stranded DNA, formed by annealing the single‐strands, was connected to the linearized vector and was transformed into competent cells. The plasmid was extracted, enzyme digested and sequenced, and the lentivirus was packed into 293T cells. According to the instructions provided by the company, CFPAC‐1 and PANC‐1 cells were transfected transiently with the above lentivirus. We have a scrambled/non‐targeting control (sense, 5′‐GGCTCTAGAAAAGCCTATGC‐3′), which is named NC group, and we set cell lines transfected with the empty vector as a control and it was named Blank group.
2.3. Quantitative real‐time polymerase chain reaction (qRT‐PCR)
In this work, qRT‐PCR was performed according to the previously reported method. 18 , 19 NRP1 and GAPDH primers were synthesized and purchased from Sangon Biotech: NRP1 sense, 5′‐ATCACGTGCAGCTCAAGTGG‐3′, and antisense, 5′‐TCATGCAGTGGGCAGAGTTC‐3′; GAPDH sense, 5′‐AAGGTGAAGGTCGGAGTCAAC‐3′, and antisense, 5′‐GGGGTCATTGATGGCAACAATA‐3′. The experiments were repeated in triplicate. All experimental details were given in Supporting information.
2.4. Protein extraction and Western blot (WB) analysis
In this study, protein extraction and WB assays were carried out according to the previous method. 18 Briefly, the protein extraction reagents and enhanced chemiluminescence kit were purchased from Thermo, Israel. The NRP1 antibody was purchased from Abcam company. Finally, the relative proteins were quantified by Image‐Pro Plus 6.0 (Media Cybernetics). All experimental details were given in Supporting information.
2.5. Cell proliferation, invasion, and migration assays
To determine cell proliferation activity, we performed CCK‐8 assay, experiments in 6 replicates. To determine cell invasion and cell migration capability, we performed transwell assay and wound healing assay, experiments in 3 replicates. The above assays were carried out according to the previous method. 18 , 20 All experimental details were given in Supporting information.
2.6. Statistical analysis
All data were analyzed using SPSS 20.0 software (IBM Corp.), and were summarized and presented as the mean ± SD. Student's t test and ANOVA (Tukey's multiple comparison test) were used to compare statistical significance in the various groups. P < .05 was considered statistically significant difference.
3. RESULTS
3.1. Detection of NRP1 mRNA expression in the target cells after transfection with qRT‐PCR
In order to detect the transfection efficiency of sh1 NRP1, sh2 NRP1, and sh3 NRP1, on the third day after transfection, mRNA was extracted from all types of cells. Then, the expression level of NRP1 mRNA was detected by qRT‐PCR. Results showed that NRP1 mRNA level in three shRNA targeting groups reduced significantly compared to that in the negative control (NC) group (P < .01; Figure 1). Meanwhile, as shown in the Figure 1, the sh1 NRP1 interference worked best.
Figure 1.
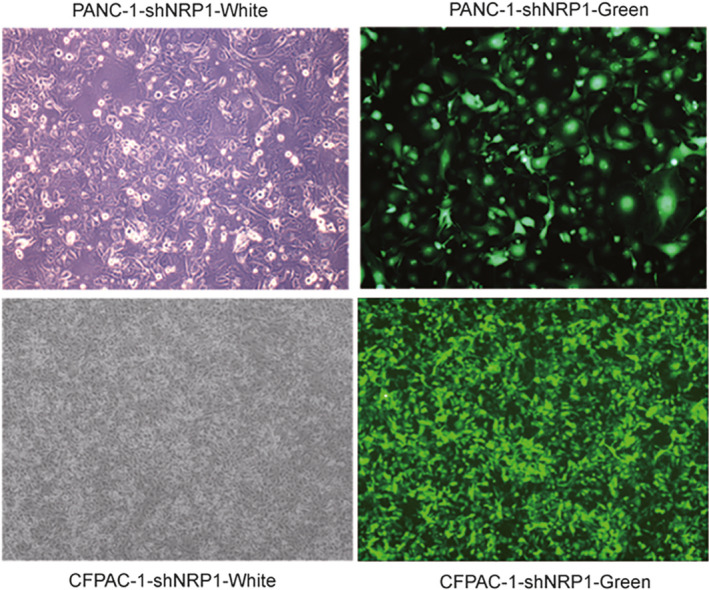
Expression of green fluorescent protein in negative shRNA group and shNRP1 virus transfection group
3.2. Determination of transfection efficiency
In order to further confirm the transfection efficiency of sh1 NRP1, on the third day after transfection, the lentivirus‐transfected PANC‐1 and CFPAC‐1 cells were observed to express NRP1 green fluorescent protein (GFP), under a fluorescence microscope (Olympus Corp.). The fields of view were randomly selected to determine transfection efficiency of PACA cells under the fluorescent microscope, and take pictures of the same under fields of bright vision and fluorescence (Figure 1). Transfection efficiency > 80% at 20 multiplicity of infection (MOI) and the state of good cell growth suggested successful and stable lentivirus transfection, indicating that NRP1 sh1 RNA group may be used for subsequent experiments (Figure 2). Meanwhile, total protein from the sh1 NRP1‐transfected PANC‐1 and CFPAC‐1 cells was extracted and used to detect the expression level of NRP1 by Western blot assays. The expression level of NRP1 protein in the experimental group was remarkably reduced compared to that in the NC group (Figure 3). Based on the above findings, the sh1 NRP1 interference worked best, so the PANC‐1 and CFPAC‐1 cells transfected by sh1 NRP1 were selected for testing in the functional experiments.
Figure 2.
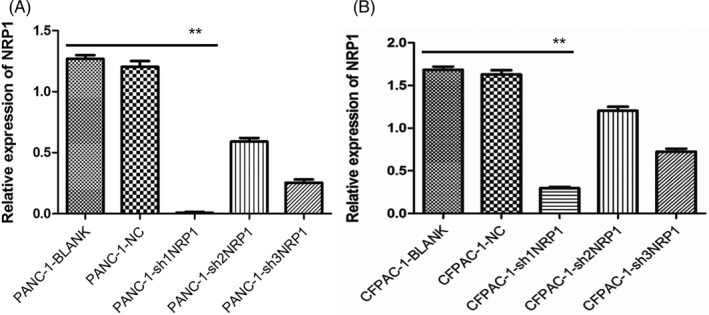
Expression of NRP1 mRNA in (A) PANC‐1 and (B) CFPAC‐1 cells after transfection, as detected by qRT‐PCR
Figure 3.
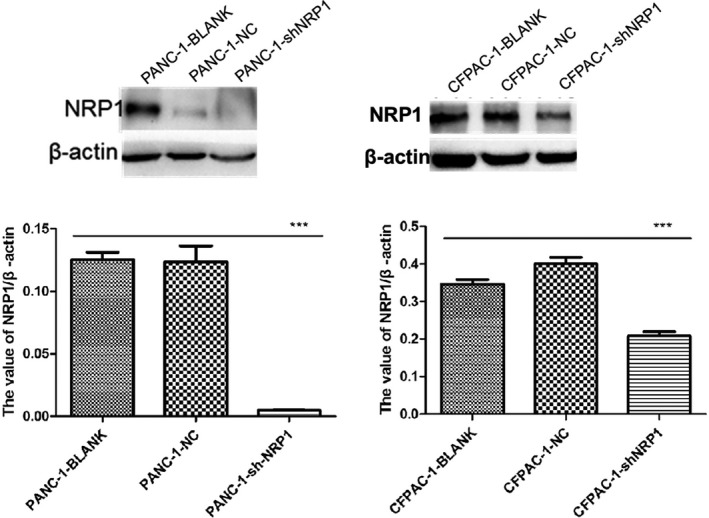
Expression of NRP1 protein in PANC‐1 and CFPAC‐1 cells after transfection, as detected with Western blot
3.3. NRP1 silencing impairs proliferation in PANC‐1 and CFPAC‐1 cell lines in vitro
In the CCK‐8 assay, the proliferation ability of NRP1 shRNA group was apparently worse than that of NC and blank groups in the PANC‐1 cells, especially in the 6 and 7 days (P < .01). CFPAC‐1 cells showed the same trend, and the proliferation ability of NRP1 shRNA group was apparently worse than that of NC and blank groups in the 7 day (P < .05), hence suggesting that the proliferation capacity of NRP1 shRNA group was apparently lower. The results revealed that eliminating NRP1 can continuously and effectively impair proliferation in PANC‐1 and CFPAC‐1 cells (Figure 4).
Figure 4.
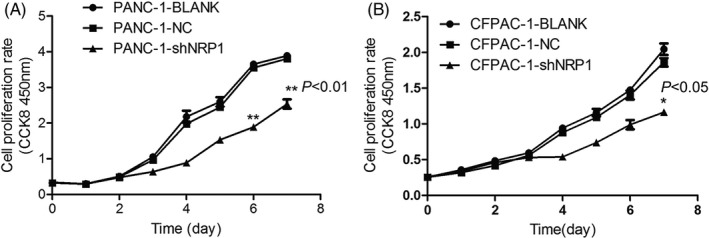
Proliferation of (A) PANC‐1 and (B) CFPAC‐1 cells after NRP1 gene silencing, as detected by CCK8 assay
3.4. NRP1 silencing impairs invasion and migration in PANC‐1 and CFPAC‐1 cell lines in vitro
For the PANC‐1 transwell migration assay, the average number of transmembrane cells was 46.5 ± 3.8 in the NRP1 shRNA group, 228.4 ± 6.7 in the NC group, and 256.1 ± 5.6 in the blank group. Compared to the NC and blank groups, the number of transmembrane cells in the NRP1 shRNA group was reduced significantly (Figure 5, P < .001). CFPAC‐1 cells show the same trend, the average number of transmembrane cells being 42.3 ± 5.2 in the NRP1 shRNA group, 219.6 ± 4.9 in the NC group, and 223.6 ± 5.8 in the blank group. In the PANC‐1 cell wound healing assay, we measured the relative blank area of the three groups at 0, 36, and 72 hours after scratching. The difference between NRP1 shRNA group and other two groups (NC group and blank group) was confirmed to be statistically significant at 72 hours (Figure 6A, P < .01). In the CFPNC‐1 cell, the time is 0, 36, 60, and 90 hours, and the difference between NRP1 shRNA group and other two groups (NC group and blank group) was confirmed to be statistically significant at 90 hours (Figure 6B, P < .001). Results of the above two assays supported each other, and revealed that silencing NRP1 can effectively impair invasion and migration in PANC‐1 and CFPAC‐1 cells.
Figure 5.
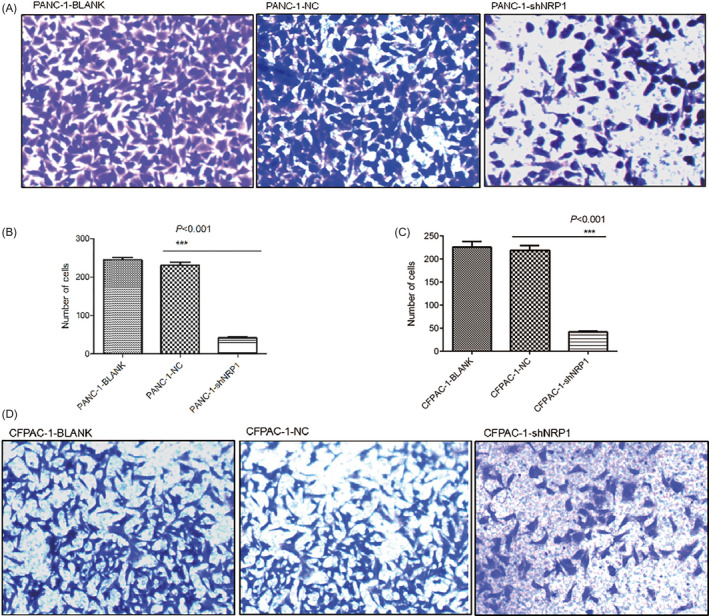
Migration of PANC‐1 and CFPAC‐1 cells after NRP1 gene silencing, as detected by transwell assay
Figure 6.
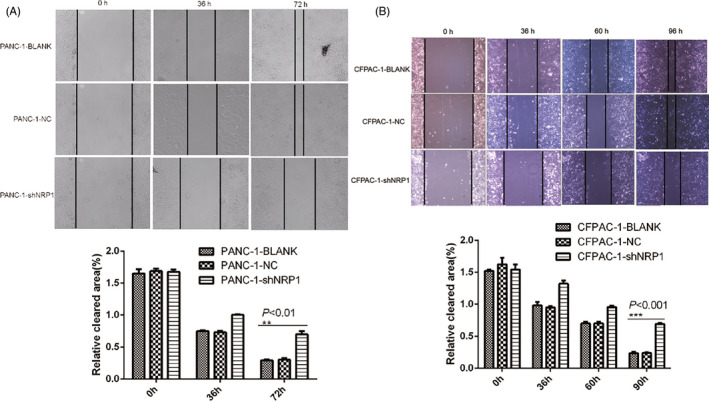
Migration of (A) PANC‐1 and (B) CFPAC‐1 cells after NRP1 gene silencing, as detected by scratch test
4. DISCUSSION
Currently, the expression and role of NRP1 in tumors have attracted the attention of researchers. Much evidence has confirmed NRP1 is overexpression in a range of human malignant tumors and is also closely related to the degree of malignancy. 21 , 22 , 23 Previous published studies have indicated that NRP1 protein is highly expressed in human PACA tissues, and it is closely related to angiogenesis, tumor stage, and poor postoperative overall survival. 24 Although there are some reports about the relationship between NRP1 and PACA, research related to proliferation and migration of NRP1 in PACA has been rarely reported. 24 , 25 , 26 , 27 Therefore, we were interested in the effect of NRP1 on PACA cells function.
To confirm the effect of NRP1 in human PACA cells, RNA interference (RNAi) technology was used to explore the relationship between silencing NRP1 and the ability of proliferation, invasion, and migration in PANC‐1 and CFPAC‐1 cells. RNAi can not only inhibit the expression of certain oncogenes in tumor cells, but also further inhibit tumor proliferation and metastasis thereby aiding the prevention and treatment of cancer with high efficiency, high specificity, and low toxicity features. 28 , 29 We silenced NRP1 gene expression in the present study using lentivirus transfection technology. The expression levels of NRP1 mRNA and protein in PANC‐1 and CFPAC‐1 cells were significantly decreased after shRNA lentiviral vector transfection, hence verifying good interference effect of lentiviral vector. Results showed that PANC‐1 and CFPAC‐1 cells proliferation, invasion, and migration ability of the virus transfected group was significantly reduced compared to that in the NC and blank groups. Based on these findings, we think that NRP1, as a potential promoting factor, plays a significant role in the proliferation, invasion, and migration of PACA cells, and may promote the occurrence, development, and metastasis of PACA.
Previous studies have shown that the silencing of NRP1 in pancreatic ductal adenocarcinoma has multiple cellular and molecular antitumor effects. Although different methods are used, the experimental results are consistent with our results that NRP1 silencing can inhibit the proliferation and migration of pancreatic cancer cells. In addition, we discussed the CFPAC‐1 cell line and to determine cell invasion and cell migration capability, we performed transwell assay and wound healing assay. 17 This study provided a reference for exploring NRP1 gene further for molecular mechanisms of tumor invasion and metastasis, and suggests an important candidate target and new direction for the treatment of PACA. Further research should be undertaken to confirm the effect of overexpression of NRP1 gene on the function of human PACA cells. In future, to develop a full picture of NRP1, more research will be needed, such as in vivo animal experiments. In addition, research needs to be focused on NRP1‐specific function in the occurrence and progression of PACA, mechanism of influence of NRP1 on the proliferation, migration and metastasis of PACA, and contribution of NRP1 to the development of PACA signaling pathway. 30 , 31
5. CONCLUSIONS
This study has shown that NRP1‐shRNA lentiviral interference vectors can effectively decrease NRP1 gene expression in PANC‐1 and CFPAC‐1 cells, thereby inhibiting cell proliferation and migration. These findings contribute in several ways to our understanding of NRP1 and provide a basis for finding a valuable potential therapeutic target for PACA therapy.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest with respect to the research, authorship, and publication of this article.
AUTHOR CONTRIBUTIONS
Li‐hong He, Yong‐lin He, Yue Kang, and Ling‐yun Wang conceived and designed the study, performed the experiments, and wrote the article; Wen‐hang Zuo and Huan Xue analyzed the data, and designed the tables and figures; Yun‐liang Zhang and Yong Meng discussed results and advised during the completion of the study. All authors read and approved the final article.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Sup info
He L‐H, He Y‐L, Zuo W‐H, et al. Neuropilin1 silencing impairs the proliferation and migration of cells in pancreatic cancer. J Clin Lab Anal. 2020;34:e23394 10.1002/jcla.23394
Li‐Hong He, Yong‐Lin He, Wen‐Hang Zuo and Yue Kang contributed equally to the work.
Funding information
This work was supported by The Fundamental Research Funds for the Central Universities (lzujbky‑2018‑k21) and The National Innovation and Entrepreneurship Training Program for Undergraduate (No. 201910730205).
DATA AVAILABILITY STATEMENT
The analyzed data and materials during the study can be obtained from the corresponding author on reasonable request. E‐mail address: doctor1909@163.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846‐4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodmass J, Lipschitz J, McKay A. Physician attitudes and treatment patterns for pancreatic cancer. World J Surg Oncol. 2011;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prud'homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3(9):921‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wild JR, Staton CA, Chapple K, Corfe BM. Neuropilins: expression and roles in the epithelium. Int J Exp Pathol. 2012;93(2):81‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roy S, Bag AK, Singh RK, Talmadge JE, Batra SK, Datta K. Multifaceted role of neuropilins in the immune system: potential targets for immunotherapy. Front Immunol. 2017;8:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu C, Jiang X. Role of NRP‐1 in VEGF‐VEGFR2‐Independent Tumorigenesis. Target Oncol. 2016;11(4):501‐505. [DOI] [PubMed] [Google Scholar]
- 9. Napolitano V, Tamagnone L. Neuropilins controlling cancer therapy responsiveness. Int J Mol Sci. 2019;20(8):2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berge M, Allanic D, Bonnin P, et al. Neuropilin‐1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J Hepatol. 2011;55(4):866‐875. [DOI] [PubMed] [Google Scholar]
- 11. Liu W, Wu T, Dong X, Zeng YA. Neuropilin‐1 is upregulated by Wnt/beta‐catenin signaling and is important for mammary stem cells. Sci Rep. 2017;7(1):10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng Y, Liu YM, Li LC, Wang LL, Wu XL. MicroRNA‐338 inhibits growth, invasion and metastasis of gastric cancer by targeting NRP1 expression. PLoS One. 2014;9(4):e94422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tse BWC, Volpert M, Ratther E, et al. Neuropilin‐1 is upregulated in the adaptive response of prostate tumors to androgen‐targeted therapies and is prognostic of metastatic progression and patient mortality. Oncogene. 2017;36(24):3417‐3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bai J, Zhang Z, Li X, Liu H. MicroRNA‐365 inhibits growth, invasion and metastasis of malignant melanoma by targeting NRP1 expression. Int J Clin Exp Pathol. 2015;8(5):4913‐4922. [PMC free article] [PubMed] [Google Scholar]
- 15. Seifi‐Alan M, Shams R, Bandehpour M, Mirfakhraie R, Ghafouri‐Fard S. Neuropilin‐1 expression is associated with lymph node metastasis in breast cancer tissues. Cancer Manag Res. 2018;10:1969‐1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lampropoulou A, Ruhrberg C. Neuropilin regulation of angiogenesis. Biochem Soc Trans. 2014;42(6):1623‐1628. [DOI] [PubMed] [Google Scholar]
- 17. Borchardt H, Schulz A, Datta K, Muders MH, Aigner A. Silencing of Neuropilins and GIPC1 in pancreatic ductal adenocarcinoma exerts multiple cellular and molecular antitumor effects. Sci Rep. 2019;9(1):15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao ZF, Wu YN, Bai ZT, Zhang L, Zhou Q, Li X. Tumor‐suppressive role of HACE1 in hepatocellular carcinoma and its clinical significance. Oncol Rep. 2016;36(6):3427‐3435. [DOI] [PubMed] [Google Scholar]
- 19. Xue ZX, Zheng JH, Zheng ZQ, et al. Latexin inhibits the proliferation of CD133+ miapaca‐2 pancreatic cancer stem‐like cells. World J Surg Oncol. 2014;12:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li ZF, Xu WW, Li JD, Tao FL, Chen JX, Xu JH. Nucleotide exchange factor SIL1 promotes the progress of breast cancer cells via regulating the cell cycle and apoptosis. Sci Prog. 2019;103(1):36850419891046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YJ, Baek DS, Lee S, et al. Dual‐targeting of EGFR and Neuropilin‐1 attenuates resistance to EGFR‐targeted antibody therapy in KRAS‐mutant non‐small cell lung cancer. Cancer Lett. 2019;466:23‐34. [DOI] [PubMed] [Google Scholar]
- 22. Hang C, Yan HS, Gong C, Gao H, Mao QH, Zhu JX. MicroRNA‐9 inhibits gastric cancer cell proliferation and migration by targeting neuropilin‐1. Exp Ther Med. 2019;18(4):2524‐2530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Chaudhary B, Khaled YS, Ammori BJ, Elkord E. Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol Immunother. 2014;63(2):81‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ben Q, Zheng J, Fei J, et al. High neuropilin 1 expression was associated with angiogenesis and poor overall survival in resected pancreatic ductal adenocarcinoma. Pancreas. 2014;43(5):744‐749. [DOI] [PubMed] [Google Scholar]
- 25. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin‐1 is expressed by endothelial and tumor cells as an isoform‐specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735‐745. [DOI] [PubMed] [Google Scholar]
- 26. Morin E, Sjoberg E, Tjomsland V, et al. VEGF receptor‐2/neuropilin 1 trans‐complex formation between endothelial and tumor cells is an independent predictor of pancreatic cancer survival. J Pathol. 2018;246(3):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsushita A, Gotze T, Korc M. Hepatocyte growth factor‐mediated cell invasion in pancreatic cancer cells is dependent on neuropilin‐1. Cancer Res. 2007;67(21):10309‐10316. [DOI] [PubMed] [Google Scholar]
- 28. Mohr SE, Smith JA, Shamu CE, Neumuller RA, Perrimon N. RNAi screening comes of age: improved techniques and complementary approaches. Nat Rev Mol Cell Biol. 2014;15(9):591‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weng Y, Shi Y, Xia X, Zhou W, Wang H, Wang C. A multi‐shRNA vector enhances the silencing efficiency of exogenous and endogenous genes in human cells. Oncol Lett. 2017;13(3):1553‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417‐427. [DOI] [PubMed] [Google Scholar]
- 31. Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D. Neuropilin‐1‐mediated vascular permeability factor/vascular endothelial growth factor‐dependent endothelial cell migration. J Biol Chem. 2003;278(49):48848‐48860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sup info
Data Availability Statement
The analyzed data and materials during the study can be obtained from the corresponding author on reasonable request. E‐mail address: doctor1909@163.com.


