Abstract
Epigenetics has long been a hot topic in the field of scientific research. The scope of epigenetics usually includes chromatin remodelling, DNA methylation, histone modifications, non‐coding RNAs and RNA modifications. In recent years, RNA modifications have emerged as important regulators in a variety of physiological processes and in disease progression, especially in human cancers. Among the various RNA modifications, m6A is the most common. The function of m6A modifications is mainly regulated by 3 types of proteins: m6A methyltransferases (writers), m6A demethylases (erasers) and m6A‐binding proteins (readers). In this review, we focus on RNA m6A modification and its relationship with urological cancers, particularly focusing on its roles and potential clinical applications.
Keywords: epigenetics, m6A, RNA modification, urological cancers
1. INTRODUCTION
Epigenetics is commonly defined as the study of reversible and heritable phenotype alterations that do not involve DNA sequence changes. 1 The scope of epigenetics usually includes chromatin remodelling, DNA methylation, histone modifications, non‐coding RNAs and RNA modifications. Epigenetics is well studied in cancer. In our previous studies, we elucidated that both DNA methylation and non‐coding RNAs are involved in the carcinogenesis of urological cancers (prostate cancer, bladder cancer and renal cell carcinoma). 2 , 3 , 4 In recent years, RNA modifications have emerged as important regulators in a variety of physiological processes and disease progression, especially in human cancers. 5 Common RNA modifications include 5‐methylcytosine (m5C), N6‐methyladenosine (m6A) and N7‐methylguanosine (m7G). 6
Among the various RNA modifications, m6A is the most common. 7 Recent studies have illustrated that m6A is involved in many cellular processes, including human cancers. 8 , 9 , 10 , 11 In this review, we focus on the relationship between RNA m6A modification and urological cancers, especially their roles and potential clinical applications.
2. RNA m6A MODIFICATION
Transcriptome‐wide analysis revealed that m6A modification may affect 7676 mammalian genes. 12 Most m6A modification sites are located in the 3′‐untranslated regions (3′‐UTRs) and stop codons, presenting as a consensus sequence of RRACH (R = G or A; H = A, C, or U). 9 The function of m6A modifications is mainly regulated by three types of proteins, writers, erasers and readers. Writers are m6A methyltransferases, including METTL3, METTL14, WTAP, METTL16, VIRMA, RBM15 and ZC3H13. 13 Erasers are m6A demethylases, including FTO and ALKBH5. 14 , 15 Readers are m6A‐binding proteins, including YTHDFs, YTHDCs, IGF2BPs, HNRNPA2B1 and EIF3. 6 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 The molecular structure and potential function of m6A regulators have been described in detail in previous reviews. 11 , 26 , 27 , 28 In brief, m6A methyltransferases and demethylases alter the m6A modification of target RNA and further recruited m6A‐binding proteins to determine the fate of target RNA. These m6A modified RNAs may further play important roles in biological processes.
3. ROLES OF RNA m6A MODIFICATION IN UROLOGICAL CANCERS
Emerging evidence shows that RNA m6A methylation is closely associated with the progression of urological cancers, including their carcinogenesis, proliferation and metastasis. Herein, we briefly review recent studies of m6A methylation in urological cancers (Table 1).
TABLE 1.
The roles of RNA m6A in urological cancers
| Cancer type | M6A regulators | Roles in cancer | Biological function | Mechanisms |
|---|---|---|---|---|
| Prostate cancer | YTHDF2 29 | Oncogene | Promoting proliferation and metastasis | Regulated by miR‐493‐3p |
| METTL3 30 | Oncogene | Promoting proliferation | Regulating Hedgehog pathway | |
| Bladder cancer | METTL3 31 | Oncogene | Promoting proliferation and metastasis | Regulating via AFF4/NF‐κB/MYC signalling network in m6A‐dependent way |
| METTL3/YTHDF2 32 | Oncogene | Promoting proliferation and metastasis | Inhibiting the expression of SETD7 and KLF4 in m6A‐YTHDF2‐dependent way | |
| METTL3 33 | Oncogene | A prognostic indicator | — | |
| METTL3 34 | Oncogene | Promoting malignant transformation | Regulating via METTL3‐YTHDF1‐CDCP1 axis | |
| METTL3 35 | Oncogene | Promoting proliferation and progression | Regulating via METTL3‐YTHDF1/3‐ITGA6 axis | |
| METTL3 36 | Oncogene | Promoting carcinogenesis | Regulating pri‐miR221/222 process in m6A‐dependent way | |
| METTL3 37 | Oncogene | Biomarker | — | |
| Renal cell carcinoma | METTL3 38 | Tumour suppressing gene | Inhibiting proliferation and metastasis/biomarker | Regulating via EMT and PI3K‐Akt‐mTOR pathways |
| METTL14 39 | Tumour suppressing gene | Biomarker | Regulating PTEN | |
| METTL14 40 | Tumour suppressing gene | Inhibiting metastasis | P2RX6 activation promoted metastasis via ATP‐induced Ca2+ influx modulating ERK1/2 phosphorylation and MMP9 pathway. | |
| METTL3/METTL14 41 | Tumour suppressing gene | Biomarkers | Regulating of mTOR pathway | |
| METTL3/METTL14 42 | Tumour suppressing gene | Biomarkers | — | |
| FTO/ALKBH5 43 | Tumour suppressing gene | Biomarkers | — | |
| FTO 44 | Tumour suppressing gene | Suppressing carcinogenesis | Regulating via FTO‐PGC‐1α signalling axis |
3.1. Prostate cancer
Prostate cancer is one of the most commonly diagnosed cancers worldwide and is especially prevalent in developed countries. 45 In our previous study, we found that the expression of YTHDF2 in prostate cancer was up‐regulated, and the increased expression of YTHDF2 was related to prostate cancer proliferation and metastasis. 29 The up‐regulation of YTHDF2 in prostate cancer was possibly contributed to the regulation of miR‐493‐3p, which increased the m6A level and inhibited tumour carcinogenesis by down‐regulating its downstream target YTHDF2 in prostate cancer. In our ongoing study, we found that METTL3 in prostate cancer was up‐regulated and contributed to the carcinogenesis of prostate cancer. METTL3 inhibited the expression of LHPP and NKX3‐1 in an m6A‐YTHDF2‐dependent manner to further promote AKT phosphorylation‐induced tumour progression in prostate cancer (Figure 1). Cai et al 30 also demonstrated that the METTL3 promoted proliferation and metastasis of prostate cancer. They further demonstrated that METTL3 regulated m6A modification and GLI1 expression, an important component of the hedgehog pathway.
FIGURE 1.
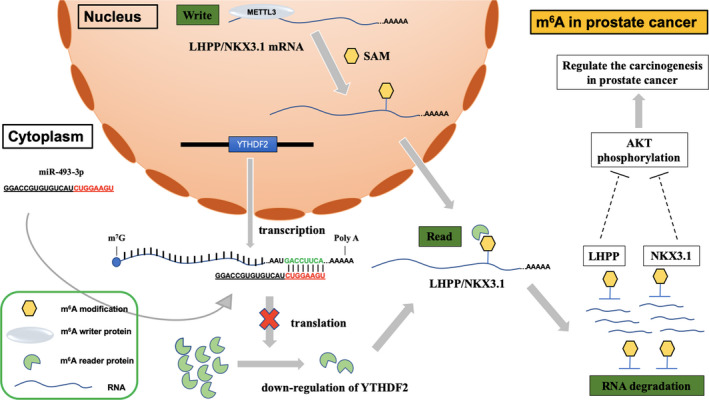
The possible mechanism of m6A methylation in prostate cancer. miR‐493‐3p increased the m6A level and inhibited tumour carcinogenesis by down‐regulating its downstream target YTHDF2 in prostate cancer, and METTL3 inhibited the expression of LHPP and NKX3‐1 in an m6A‐YTHDF2‐dependent manner to further promote AKT phosphorylation‐induced tumour progression in prostate cancer
3.2. Bladder cancer
Bladder cancer is one of most commonly diagnosed cancers worldwide, especially in men. 46 Both the incidence and mortality of bladder cancer have increased rapidly in recent years. 47 The m6A methyltransferase METTL3 seems to play important roles in the carcinogenesis of bladder cancer. The role of METTL3 in bladder cancer has been exclusively studied in many research centres. Cheng et al 31 found that the expression of METTL3 was elevated in bladder cancer and further identified AF4/FMR2 family member 4 (AFF4), key regulators of the NF‐κB pathway (IKBKB and RELA) and MYC as direct downstream targets of METTL3. METTL3 promoted the proliferation and metastasis of bladder cancer via the AFF4/NF‐κB/MYC signalling network in an m6A‐dependent manner. Similarly, in our previous study, we also found that the expression of METTL3 and YTHDF2 was up‐regulated in bladder cancer and showed that METTL3 inhibited the expression of SETD7 and KLF4 in an m6A‐YTHDF2‐dependent manner to further promote the proliferation and metastasis of bladder cancer. 32 In addition, a nine‐gene panel that included METTL3 was identified as a prognostic indicator for the recurrence of muscle invasive bladder cancer. 33 Yang et al 34 showed that METTL3 and ALKBH5 regulated m6A modification of the 3′‐UTR of the oncogene CDCP1 mRNA and that YTHDF1 recognized m6A modification to promote CDCP1 translation in bladder cancer. Similarly, Jin et al 35 found that METTL3 and ALKBH5 regulated the m6A modification of the 3′‐UTR of the oncogene ITGA6 mRNA and that YTHDF1/3 recognized m6A modification to promote ITGA6 translation in bladder cancer. Recent studies have showed that METTL3 also regulates m6A modifications of non‐coding RNAs (miRNAs, lincRNAs and circRNAs). 48 , 49 , 50 In bladder cancer, METTL3 regulates pri‐miR221/222 processing in an m6A‐dependent manner to promote carcinogenesis. 36 In addition, a high expression pattern of METTL3 is demonstrated in bladder cancer via bioinformatic analysis. 37 Therefore, METTL3 serves as an oncogene in the carcinogenesis of bladder cancer (Figure 2).
FIGURE 2.
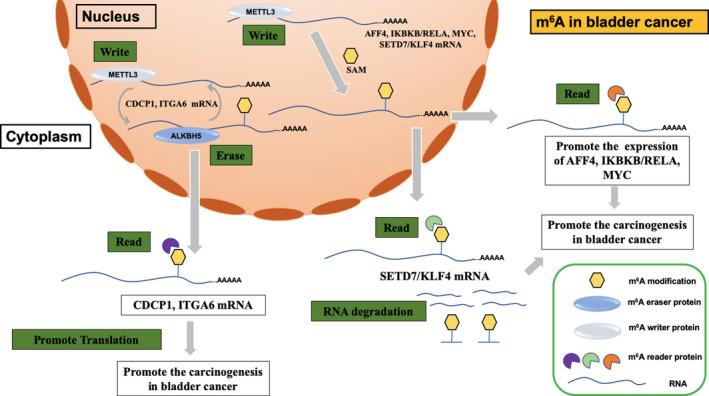
The possible mechanism of m6A methylation in bladder cancer. METTL3 promoted the AFF4/NF‐κB/MYC signalling network, the translation of CDCP1 and ITGA6, and inhibited the expression of SETD7 and KLF4 in an m6A‐dependent manner to further promote the carcinogenesis in bladder cancer. Different readers functioned differently and played crucial roles in bladder cancer (YTHDF1/2/3)
3.3. Renal cell carcinoma
The incidence of renal cell carcinoma is still increasing rapidly worldwide. 51 Although mortality is decreasing in developed countries, it is still a major problem in developing countries. Li et al 38 found that the expression of METTL3 in renal cell carcinoma was down‐regulated and that the decreased expression of METTL3 was related to poor prognosis. They further elucidated that METTL3 inhibited proliferation and metastasis in renal cell carcinoma via epithelial‐to‐mesenchymal transition (EMT) and PI3K‐Akt‐mTOR pathways. In addition, down‐regulation of METTL14 in renal cell carcinoma was related to poor prognosis, and that METTL14 regulated PTEN expression via m6A modification, which indicated that METTL14 could possibly serve as a prognostic biomarker. 39 A similar METTL14 expression pattern was found in renal cell carcinoma, and METTL14 further suppressed P2RX6 activation regulated metastasis of renal cell carcinoma via ATP‐induced Ca2+ influx modulating ERK1/2 phosphorylation and MMP9 signalling. 40 Zhou et al 41 further used the TCGA database to analyse the gene signatures and prognostic values of m6A regulators in renal cell carcinoma. They found that patients with any copy number variations (CNVs) of the m6A regulatory genes had worse overall survival (OS) and disease‐free survival (DFS) than those without CNVs; in addition, deletions of METTL3 and METTL14 were independent risk factors for poor OS. They also elucidated that decreased expression of METTL3 was related to activation of the mTOR pathway. Similar results were also observed by another team. 42 They established a risk signature for predicting the prognosis of renal cell carcinoma based on METTL14 and METTL3. Interestingly, the down‐regulated expression of ALKBH5 and FTO was found to be related to poor overall survival and cancer‐specific survival in renal cell carcinoma, which implied that ALKBH5 and FTO could serve as potential prognostic biomarkers. 43 In another study, the expression of FTO was also found to be down‐regulated in renal cell carcinoma, and FTO suppressed carcinogenesis of renal cell carcinoma via the FTO‐PGC‐1α signalling axis. 44 To determine the potential downstream targets of m6A regulatory genes, m6A sequencing, RNA sequencing and bioinformatics analysis were used to demonstrate the first m6A transcriptome‐wide map of renal cell carcinoma and to identify differentially expressed mRNAs with m6A modifications. 52 This could help to develop identify m6A‐related genes that can be exploited in new therapeutic strategies for renal cell carcinoma. The possible mechanism of m6A methylation in renal cell carcinoma is summarized in Figure 3.
FIGURE 3.
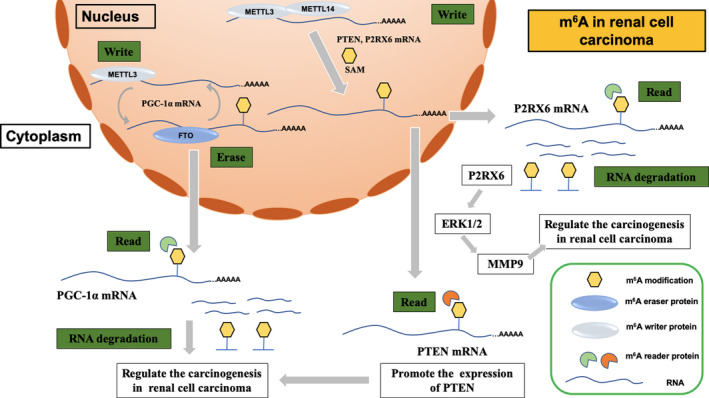
The possible mechanism of m6A methylation in renal cell carcinoma. METTL14 regulated PTEN and P2RX6 expression via m6A modification in renal cell carcinoma, and FTO suppressed carcinogenesis of renal cell carcinoma via the FTO‐PGC‐1α signalling axis
4. DISCUSSION
4.1. Potential application of m6A modification in urological cancers
4.1.1. m6A modifications as biomarkers
An increasing number of studies have indicated that m6A regulators could possibly be novel biomarkers in cancer diagnosis and prognostic evaluation. As an important m6A methyltransferase, METTL3 is well studied in many types of cancers. The expression of METTL3 is increased in gastric cancer and associated with poor prognosis, indicating that the expression of METTL3 is an independent prognostic factor and effective predictor in gastric cancer. 53 , 54 Similar results were also observed in hepatocellular carcinoma, pancreatic cancer, breast cancer, etc. 55 , 56 , 57
As we mentioned before, METTL3 plays critical roles in both prostate cancer and bladder cancer, and the expression of METTL3 is elevated in these cancers. Taken together, considering the expression pattern of METTL3 in prostate cancer, bladder cancer and other cancers, and the role of METTL3 in the carcinogenesis of prostate cancer and bladder cancer, METTL3 could be a promising biomarker and prognostic indicator in prostate cancer and bladder cancer. However, unlike the expression pattern in prostate cancer, bladder cancer and most other types of tumours, METTL3 seems to be down‐regulated in renal cell carcinoma. Although its internal biological mechanisms need further elucidation, we found that METTL3 could possibly be used as a diagnostic biomarker and independent prognostic factor.
As crucial m6A‐binding proteins, the YTH domain family members play important roles in many types of cancers. The expression of YTHDF2 is up‐regulated in hepatocellular carcinoma, pancreatic cancer, etc, 55 , 58 , 59 the expression of YTHDF1 is up‐regulated in colorectal cancer, hepatocellular carcinoma, ovarian cancer, lung cancer, etc, 60 , 61 , 62 , 63 and both YTHDF1 and YTHDF2 are independent risk factors for OS.
As we mentioned before, YTHDF1 and YTHDF2 play critical roles in both bladder cancer and prostate cancer. The expression of YTHDF1/2 is elevated in bladder cancer, and the expression of YTHDF2 is elevated in prostate cancer. Taken together, considering the expression pattern of YTHDF1/2 in prostate cancer, bladder cancer and other cancers, and the role of YTHDF1/2 in the carcinogenesis of prostate cancer and bladder cancer, YTHDF1/2 could be a promising biomarker and prognostic indicator in prostate cancer and bladder cancer.
4.1.2. m6A modifications as therapeutic targets
The crucial roles of m6A modifications in urological cancers indicate that these modifications may become novel antitumour therapeutic targets. As crucial m6A regulators, METTL3 and YTHDF2 act as oncogenes in both prostate cancer and bladder cancer. The carcinogenesis of METTL3 and YTHDF2 in prostate cancer and bladder cancer mainly contributes to the inhibition of antitumour genes and the promotion of oncogenes, resulting in tumour development. Considering oncogenic role in prostate cancer and bladder cancer, METTL3 and YTHDF2 present opportunities for the development of effective targeted therapeutics. Small‐molecule METTL3 and YTHDF2 inhibitors could be designed and synthetised to examine the antitumour effects and safety in both prostate cancer and bladder cancer. Future studies could highlight the broad potential of targeting METTL3 and YTHDF2 for both prostate cancer and bladder cancer. In contrast, both m6A methyltransferases (METTL3 and METTL14) and m6A demethylases (FTO) act as tumour suppressor genes in renal cell carcinoma, indicating that the recruited m6A‐binding protein and potential downstream target play important roles in tumour development. However, in our ongoing research, we found that the m6A methyltransferase WTAP and the m6A demethylase ALKBH5 act as oncogenes in renal cell carcinoma. Therefore, due to the pathological diversity of renal cell carcinoma, the actual role of m6A regulators in it and its subtypes needs further elucidation.
PD‐1/PD‐L1‐related immunotherapy has proven to be effective in many types of tumours. 64 Recent studies illustrated that PD‐1/PD‐L1‐related immunotherapy was effective in urological cancers. 65 , 66 , 67 , 68 FTO is demonstrated to promote carcinogenesis and anti‐PD‐1 resistance in melanoma, suggesting that FTO could be a potential therapeutic target in immunotherapy, 69 and the deletion of YTHDF1 enhances the therapeutic efficacy of PD‐L1 checkpoint blockade, indicating that the m6A‐binding protein YTHDF1 could be another therapeutic target in antitumour immunotherapy. 70 In addition, Wang et al 71 identified the function of the m6A methyltransferase METTL3 in increasing the translation of immune transcripts.
As we mentioned before, m6A modification regulators, including METTL3, FTO and YTHDF1, play important roles in the carcinogenesis of urological cancers, suggesting that m6A modifications may be potential therapeutic targets for immunotherapy.
Chemotherapy and radiotherapy are commonly used in bladder cancer and prostate cancer, respectively. It is important to identify the chemoradiation sensitivity of each patient via certain indicators, and m6A regulators could be such indicators. METTL3 was found to increase the sensitivity of cells to anticancer reagents such as gemcitabine, 5‐fluorouracil, cisplatin and irradiation in pancreatic cancer, indicating the potential role of METTL3 in chemo‐ and radiotherapy resistance. 72 In addition, FTO was found to enhance chemoradiotherapy resistance in cervical squamous cell carcinoma via β‐catenin. 73
As we mentioned before, both METTL3 and FTO play crucial roles in carcinogenesis in both bladder cancer and renal cell carcinoma. Taken together, these findings suggest that m6A regulators could be potential therapeutic targets for patients receiving chemo‐ and radiotherapy.
4.1.3. miRNAs and m6A modifications
In addition to the functions of m6A modifications in mRNA, recent studies have shown that m6A modifications also have roles in regulating non‐coding RNAs, especially microRNAs (miRNAs). 24 , 48 , 49 , 50 miRNAs are mainly processed by the microprocessor complex, which includes RNA‐binding protein DGCR8 and ribonuclease type III DROSHA. 74 Alarcón et al 48 found that METTL3 methylated pri‐miRNAs, facilitating their recognition and processing by DGCR8, indicating that the m6A regulator could possibly be a key factor in the initiation of miRNA biogenesis. They further identified that HNRNPA2B1 bound to m6A‐modified sites in a group of pri‐miRNAs, interacted with DGCR8, and promoted the maturation of pri‐miRNAs. 24 In addition, METTL14 was identified to interact with DGCR8 and regulate the pri‐miR‐126 mature process in an m6A‐dependent manner in hepatocellular carcinoma. 75 Pri‐miR‐25 in pancreatic cancer could be matured by smoking via enhanced m6A modification, which was catalysed by METTL3. 76 A similar mechanism was found in bladder cancer. METTL3 interacted with DGCR8 and regulated the pri‐miR‐221/222 maturation process. 36
Taken together, considering the crucial roles of m6A regulators and miRNAs in urological cancers and the potential regulatory mechanisms between m6A regulators and miRNAs, miRNAs and microprocessor proteins (such as DGCR8) could be potential therapeutic targets in urological cancers. However, future studies are needed to further clarify the underlying mechanisms.
4.1.4. Alternative splicing and m6A modifications
Alternative splicing is the process of generating numerous mRNA variants from a single gene transcript, leading to proteome complexity and diversification. 77 Alternative splicing exists in almost 95% of human genes and exerts functions in many biological aspects, including chromatin modification, signal transduction and carcinogenesis. 78 In addition to cis‐regulatory elements, trans‐acting splicing factors including m6A regulators play critical roles in the alternative splicing process. 9 , 79 Previous studies indicated that dysregulation of m6A regulators drastically affects the process of alternative splicing. METTL3 regulated MyD88 alternative splicing on the lipopolysaccharide‐induced inflammatory response. 80 FTO is involved in the alternative splicing process by triggering the inclusion of alternatively spliced exons and regulating the expression of terminal exons. 81 HNRNPG interacts with RNA polymerase II to coregulate alternative splicing in m6A‐enriched exonic regions. 82 HNRNPA2B1 phenocopies the effect of METTL3 to play an important role in alternative splicing on pri‐miRNA processing. 24 YTHDC1 interacts with alternative splicing factors SRSF3 and SRSF10, and YTHDC1/SRSF3, and SRSF10 regulates splicing in an m6A‐dependent manner. 83 In prostate cancer, YTHDC1 regulates CD44 alternative splicing, which is associated with carcinogenesis. 84
Previous studies illustrated that alternative splicing plays important roles in urological cancers. In prostate cancer, androgen receptor spliced variants (AR‐Vs) have been implicated in the carcinogenesis of metastatic prostate cancer, which contribute to resistance to both anti‐androgen therapy and radiotherapy. 85 In bladder cancer, Xie et al 86 illustrated that PTBP1 promotes bladder cancer lymph node metastasis and cell proliferation via an alternative splicing dependent mechanism and that PTBP1 could be a novel prognostic marker and therapeutic target. In renal cell carcinoma, SF3B3 modulates EZH2 alternative splicing to promote carcinogenesis, and SF3B3 could be a potential prognostic factor and therapeutic target in renal cell carcinoma. 87
Therefore, understanding the role of the m6A‐regulated alternative splicing in the carcinogenesis of urological cancers and exploring the therapeutic application of manipulating alternative splicing to improve urological cancer patient care are of great importance in the future.
4.2. Brief summary
The regulatory functions of m6A modifications include RNA degradation, translation, processing and splicing. Although m6A modifications are also involved in the carcinogenesis of many types of cancers, including urological cancers, the potential mechanisms should be further studied. METTL3 has different expression patterns in different urological cancers. Considering the dual role of METTL3 as an oncogene in prostate cancer and bladder cancer and as a tumour suppressor gene in renal cell carcinoma, its underlying mechanisms need to be further studied. In addition, the mechanisms by which m6A‐binding proteins co‐ordinate their different functions in certain types of cancers need to be further elucidated. Additionally, some m6A modification–based enzyme inhibitors have demonstrated potential effects on cancer; however, there is still a long way to go before m6A‐based cancer therapy can be applied in the clinic. 88
5. CONCLUSIONS
Epigenetics has long been a hot topic in the field of scientific research. 89 RNA m6A modifications are of great importance in urological cancers and serve as diagnostic and prognostic biomarkers that regulate carcinogenesis and metastasis, indicating their potential as therapeutic targets. However, further studies are still necessary to elucidate the underlying mechanisms in urological cancers so that these findings can be translated from bench to bedside in the future.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Xiao Wang: Writing‐original draft (lead). Haiyun Xie: Data curation (equal). Yufan Ying: Data curation (equal). Danni Chen: Writing‐review & editing (lead). Jiangfeng Li: Project administration (lead).
ACKNOWLEDGEMENT
This study was supported by Zhejiang Provincial Natural Science Foundation of China (LY20H160022), Zhejiang Province Medical and Health Scientific Research Project (2019RC033), the National Natural Science Foundation of China (81802564) and China Postdoctoral Science Foundation (2018M632489).
Wang X, Xie H, Ying Y, Chen D, Li J. Roles of N6‐methyladenosine (m6A) RNA modifications in urological cancers. J Cell Mol Med. 2020;24:10302–10310. 10.1111/jcmm.15750
Contributor Information
Xiao Wang, Email: zjuwangxiao@zju.edu.cn.
Danni Chen, Email: dr_chendanni@zju.edu.cn.
Jiangfeng Li, Email: lijf@zju.edu.cn.
REFERENCES
- 1. Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396‐398. [DOI] [PubMed] [Google Scholar]
- 2. Liang Z, Wang X, Xu X, et al. MicroRNA‐608 inhibits proliferation of bladder cancer via AKT/FOXO3a signaling pathway. Mol Cancer. 2017;16(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu X, Wu J, Li S, et al. Downregulation of microRNA‐182‐5p contributes to renal cell carcinoma proliferation via activating the AKT/FOXO3a signaling pathway. Mol Cancer. 2014;13:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Wang X, Li J, et al. c‐Met, CREB1 and EGFR are involved in miR‐493‐5p inhibition of EMT via AKT/GSK‐3beta/snail signaling in prostate cancer. Oncotarget. 2017;8(47):82303‐82313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boccaletto P, Machnicka MA, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018, 46(D1):D303‐D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Lu Z, Gomez A, et al. N6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batista PJ. The RNA Modification N(6)‐methyladenosine and Its Implications in Human Disease. Genomics Proteomics Bioinformatics. 2017;15(3):154‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng X, Chen K, Luo GZ, et al. Widespread occurrence of N6‐methyladenosine in bacterial mRNA. Nucleic Acids Res. 2015;43(13):6557‐6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dominissini D, Moshitch‐Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature. 2012;485(7397):201‐206. [DOI] [PubMed] [Google Scholar]
- 10. He L, Li J, Wang X, et al. The dual role of N6‐methyladenosine modification of RNAs is involved in human cancers. J Cell Mol Med. 2018;22(10):4630‐4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen XY, Zhang J, Zhu JS. The role of m(6)A RNA methylation in human cancer. Mol Cancer. 2019;18(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia G, Fu YE, Zhao XU, et al. N6‐methyladenosine in nuclear RNA is a major substrate of the obesity‐associated FTO. Nat Chem Biol. 2011;7(12):885‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng G, Dahl J, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Zhao BS, Roundtree IA, et al. N(6)‐methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N(6)‐methyladenosine‐modified RNA. Cell Res. 2017;27(3):315‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li A, Chen Y‐S, Ping X‐L, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roundtree IA, Luo G‐Z, Zhang Z, et al. YTHDC1 mediates nuclear export of N(6)‐methyladenosine methylated mRNAs. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu PJ, Zhu Y, Ma H, et al. Ythdc2 is an N(6)‐methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27(9):1115‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller S, Glaß M, Singh AK, et al. IGF2BP1 promotes SRF‐dependent transcription in cancer in a m6A‐ and miRNA‐dependent manner. Nucleic Acids Res. 2019;47(1):375‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Chim B, Su Y, et al. Enhancement of LIN28B‐induced hematopoietic reprogramming by IGF2BP3. Genes Dev. 2019;33(15–16):1048‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li T, Hu P‐S, Zuo Z, et al. METTL3 facilitates tumor progression via an m(6)A‐IGF2BP2‐dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A‐dependent nuclear RNA processing events. Cell. 2015;162(6):1299‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer KD, Patil DP, Zhou J, et al. 5' UTR m(6)A promotes cap‐independent translation. Cell. 2015;163(4):999‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6‐methyladenosine and its role in cancer. Mol Cancer. 2019;18(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma S, Chen C, Ji X, et al. The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. 2019;12(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu BB, Wang XY, Gu XY, et al. N(6)‐methyladenosine (m(6)A) RNA modification in gastrointestinal tract cancers: roles, mechanisms, and applications. Mol Cancer. 2019;18(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Meng S, Xu M, et al. Downregulation of N(6)‐methyladenosine binding YTHDF2 protein mediated by miR‐493‐3p suppresses prostate cancer by elevating N(6)‐methyladenosine levels. Oncotarget. 2018;9(3):3752‐3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai J, Yang F, Zhan H, et al. RNA m(6)A methyltransferase METTL3 promotes the growth of prostate cancer by regulating hedgehog pathway. OncoTargets and Therapy. 2019;12:9143‐9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng M, Sheng L, Gao Q, et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF‐kappaB/MYC signaling network. Oncogene. 2019;38(19):3667‐3680. [DOI] [PubMed] [Google Scholar]
- 32. Xie H, Li J, Ying Y, et al. METTL3/YTHDF2 m(6) A axis promotes tumorigenesis by degrading SETD7 and KLF4 mRNAs in bladder cancer. J Cell Mol Med. 2020;24(7):4092‐4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han Y, Zheng Q, Tian Y, Ji Z, Ye H. Identification of a nine‐gene panel as a prognostic indicator for recurrence with muscle‐invasive bladder cancer. J Surg Oncol. 2019;119(8):1145‐1154. [DOI] [PubMed] [Google Scholar]
- 34. Yang F, Jin H, Que B, et al. Dynamic m(6)A mRNA methylation reveals the role of METTL3‐m(6)A‐CDCP1 signaling axis in chemical carcinogenesis. Oncogene. 2019;38(24):4755‐4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin H, Ying X, Que B, et al. N6‐methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine. 2019;47:195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han J, Wang J‐Z, Yang X, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri‐miR221/222 maturation in m6A‐dependent manner. Mol Cancer. 2019;18(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen M, Nie ZY, Wen XH, Gao YH, Cao H, Zhang SF. m6A RNA methylation regulators can contribute to malignant progression and impact the prognosis of bladder cancer. Biosci Rep. 2019;39(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li X, Tang J, Huang W, et al. The M6A methyltransferase METTL3: acting as a tumor suppressor in renal cell carcinoma. Oncotarget. 2017;8(56):96103‐96116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Q, Zhang H, Chen Q, Wan Z, Gao X, Qian W. Identification of METTL14 in kidney renal clear cell carcinoma using bioinformatics analysis. Dis Markers. 2019;2019:5648783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gong D, Zhang J, Chen Y, et al. The m(6)A‐suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP‐induced Ca(2+) influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp & Clin Cancer Res. 2019;38(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou J, Wang J, Hong B, et al. Gene signatures and prognostic values of m6A regulators in clear cell renal cell carcinoma ‐ a retrospective study using TCGA database. Aging. 2019;11(6):1633‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J, Zhang C, He W, Gou X. Effect of m(6)A RNA methylation regulators on malignant progression and prognosis in renal clear cell carcinoma. Front Oncol. 2020;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strick A, von Hagen F, Gundert L, et al. The m6A erasers ALKBH5 and FTO are prognostic biomarkers in patients with clear cell renal carcinoma. BJU Int. 2020. [DOI] [PubMed] [Google Scholar]
- 44. Zhuang C, Zhuang C, Luo X, et al. N6‐methyladenosine demethylase FTO suppresses clear cell renal cell carcinoma through a novel FTO‐PGC‐1alpha signalling axis. J Cell Mol Med. 2019;23(3):2163‐2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer J Clin. 2020, 70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 46. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 47. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96‐108. [DOI] [PubMed] [Google Scholar]
- 48. Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6‐methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou C, Molinie B, Daneshvar K, et al. Genome‐wide maps of m6A circRNAs identify widespread and cell‐type‐specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20(9):2262‐2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang D, Qiao J, Wang G, et al. N6‐Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46(8):3906‐3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Znaor A, Lortet‐Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519‐530. [DOI] [PubMed] [Google Scholar]
- 52. Chen Y, Zhou C, Sun Y, He X, Xue D. m(6)A RNA modification modulates gene expression and cancer‐related pathways in clear cell renal cell carcinoma. Epigenomics. 2020;12(2):87‐99. [DOI] [PubMed] [Google Scholar]
- 53. Wang Q, Chen C, Ding Q, et al. METTL3‐mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2019. [DOI] [PubMed] [Google Scholar]
- 54. Yue B, Song C, Yang L, et al. METTL3‐mediated N6‐methyladenosine modification is critical for epithelial‐mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen M, Wei L, Law C‐T, et al. RNA N6‐methyladenosine methyltransferase‐like 3 promotes liver cancer progression through YTHDF2‐dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67(6):2254‐2270. [DOI] [PubMed] [Google Scholar]
- 56. Cai X, Wang X, Cao C, et al. HBXIP‐elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let‐7g. Cancer Lett. 2018;415:11‐19. [DOI] [PubMed] [Google Scholar]
- 57. Xia T, Wu X, Cao M, et al. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract. 2019;215(11):152666. [DOI] [PubMed] [Google Scholar]
- 58. Yang Z, Li J, Feng G, et al. MicroRNA‐145 modulates N(6)‐methyladenosine levels by targeting the 3'‐untranslated mRNA region of the N(6)‐methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292(9):3614‐3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen J, Sun Y, Xu X, et al. YTH domain family 2 orchestrates epithelial‐mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16(23):2259‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nishizawa Y, Konno M, Asai A, et al. Oncogene c‐Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9(7):7476‐7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao X, Chen Y, Mao Q, et al. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21(4):859‐868. [DOI] [PubMed] [Google Scholar]
- 62. Liu T, Wei Q, Jin J, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shi Y, Fan S, Wu M, et al. YTHDF1 links hypoxia adaptation and non‐small cell lung cancer progression. Nat Commun. 2019;10(1):4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature. 2017;541(7637):321‐330. [DOI] [PubMed] [Google Scholar]
- 65. Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE‐012): a non‐randomised, open‐label, phase 1b study. Lancet Oncol. 2017;18(2):212‐220. [DOI] [PubMed] [Google Scholar]
- 66. Vaughn DJ, Bellmunt J, Fradet Y, et al. Health‐related quality‐of‐life analysis from KEYNOTE‐045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol. 2018;36(16):1579‐1587. [DOI] [PubMed] [Google Scholar]
- 67. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. New Engl J Med. 2019;380(12):1116‐1127. [DOI] [PubMed] [Google Scholar]
- 68. Antonarakis ES, Piulats JM, Gross‐Goupil M, et al. Pembrolizumab for treatment‐refractory metastatic castration‐resistant prostate cancer: multicohort, open‐label phase II KEYNOTE‐199 study. J Clin Oncol. 2020;38(5):395‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang S, Wei J, Cui Y‐H, et al. m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti‐PD‐1 blockade. Nat Commun. 2019;10(1):2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han D, Liu J, Chen C, et al. Anti‐tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566(7743):270‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang H, Hu X, Huang M, et al. Mettl3‐mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun. 2019;10(1):1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Taketo K, Konno M, Asai A, et al. The epitranscriptome m6A writer METTL3 promotes chemo‐ and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52(2):621‐629. [DOI] [PubMed] [Google Scholar]
- 73. Zhou S, Bai ZL, Xia D, et al. FTO regulates the chemo‐radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta‐catenin through mRNA demethylation. Mol Carcinog. 2018;57(5):590‐597. [DOI] [PubMed] [Google Scholar]
- 74. Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231‐235. [DOI] [PubMed] [Google Scholar]
- 75. Ma JZ, Yang F, Zhou CC, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6)‐methyladenosine‐dependent primary MicroRNA processing. Hepatology. 2017;65(2):529‐543. [DOI] [PubMed] [Google Scholar]
- 76. Zhang J, Bai R, Li M, et al. Excessive miR‐25‐3p maturation via N(6)‐methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10(1):1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463(7280):457‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high‐throughput sequencing. Nat Genet. 2008;40(12):1413‐1415. [DOI] [PubMed] [Google Scholar]
- 79. Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10(11):741‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Feng Z, Li Q, Meng R, Yi B, Xu Q. METTL3 regulates alternative splicing of MyD88 upon the lipopolysaccharide‐induced inflammatory response in human dental pulp cells. J Cell Mol Med. 2018;22(5):2558‐2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6‐methyladenosine demethylase FTO targets pre‐mRNAs and regulates alternative splicing and 3'‐end processing. Nucleic Acids Res. 2017;45(19):11356‐11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou KI, Shi H, Lyu R, et al. Regulation of co‐transcriptional pre‐mRNA splicing by m(6)A through the low‐complexity protein hnRNPG. Mol Cell. 2019;76(1):70‐81 e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507‐519. [DOI] [PubMed] [Google Scholar]
- 84. Luxton HJ, Simpson BS, Mills IG, et al. The oncogene metadherin interacts with the known splicing proteins YTHDC1, Sam68 and T‐STAR and plays a novel role in alternative mRNA splicing. Cancers. 2019;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Paschalis A, Sharp A, Welti JC, et al. Alternative splicing in prostate cancer. Nat Rev Clin Oncol. 2018;15(11):663‐675. [DOI] [PubMed] [Google Scholar]
- 86. Xie R, Chen XU, Chen Z, et al. Polypyrimidine tract binding protein 1 promotes lymphatic metastasis and proliferation of bladder cancer via alternative splicing of MEIS2 and PKM. Cancer Lett. 2019;449:31‐44. [DOI] [PubMed] [Google Scholar]
- 87. Chen KE, Xiao H, Zeng J, et al. Alternative splicing of EZH2 pre‐mRNA by SF3B3 contributes to the tumorigenic potential of renal cancer. Clin Cancer Res. 2017;23(13):3428‐3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang J, Tsoi H, Li X, et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP‐WT1‐TBL1 axis. Gut. 2016;65(9):1482‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361(6409):1346‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]


