Abstract
Calcium deposition in vascular smooth muscle cells (VSMCs) is a form of ectopic ossification in blood vessels. It can result in rigidity of the vasculature and an increase in cardiac events. Here, we report that the microRNA miR‐134‐5p potentiates inorganic phosphate (Pi)‐induced calcium deposition in VSMCs by inhibiting histone deacetylase 5 (HDAC5). Using miRNA microarray analysis of Pi‐treated rat VSMCs, we first selected miR‐134‐5p for further evaluation. Quantitative RT‐PCR confirmed that miR‐134‐5p was increased in Pi‐treated A10 cells, a rat VSMC line. Transfection of miR‐134‐5p mimic potentiated the Pi‐induced increase in calcium contents. miR‐134‐5p increased the amounts of bone runt‐related transcription factor 2 (RUNX2) protein and bone morphogenic protein 2 (BMP2) mRNA in the presence of Pi but decreased the expression of osteoprotegerin (OPG). Bioinformatic analysis showed that the HDAC5 3′untranslated region (3′UTR) was one of the targets of miR‐134‐5p. The luciferase construct containing the 3′UTR of HDAC5 was down‐regulated by miR‐134‐5p mimic in a dose‐dependent manner in VSMCs. Overexpression of HDAC5 mitigated the calcium deposition induced by miR‐134‐5p. Our results suggest that a Pi‐induced increase of miR‐134‐5p may cause vascular calcification through repression of HDAC5.
Keywords: histone deacetylase 5, microRNA, miR‐134‐5p, vascular calcification, vascular smooth muscle cells
1. INTRODUCTION
Certain chronic stresses and other metabolic or cardiovascular diseases may cause an abnormal deposition of calcium phosphate crystals in blood vessels. Those stresses can be initiated by atherosclerosis, chronic kidney disease and diabetes. This ectopic ossification induces remodelling and rigidity of the blood vessels, which causes ischaemia and impairment of circulation. 1 By examining the anatomy of the blood vessel, we can distinguish whether the vascular calcification is intimal calcification or medial calcification. Intimal calcification is often associated with atherosclerosis, whereas medial calcification is related to metabolic diseases such as diabetes mellitus or chronic kidney disease. 2 Although the main causative diseases may differ, no clear pathophysiologic differences within the vascular smooth muscle cells (VSMCs) have been described, which suggests that the extracellular stimuli share a common final intracellular signal pathway or pathways during calcification.
Noncoding RNA represents DNA sequences that are transcribed but not translated into proteins. Although their roles are still being investigated, recent advances show that noncoding RNAs have a unique function to regulate pathophysiologic events as well as cellular homeostasis. 3 Noncoding RNA can be classified into groups by differences in length, structure and function. Usually, sequences spanning less than 200 nucleotides are named small noncoding RNAs, and microRNAs (miRNAs) are one of the small noncoding RNAs. miRNAs commonly act as negative regulators of other coding genes by inducing degradation of target gene mRNA. They bind to the 3′UTR of the target mRNA, resulting in either degradation of mRNA or inhibition of translation. 4 It is noteworthy that a perfect match between the miRNA and the target 3′UTR is not needed and that a single coding gene can provide multiple sites for many miRNAs. 5 This broad specificity results in diverse and complex regulation of cellular functions by miRNAs. Indeed, it has been reported that over 90% of human genes are under the control of miRNAs. 6
Likewise, many functions of VSMCs have been reported to be regulated by miRNAs. Indeed, atherosclerosis and VSMC proliferation provide good examples of miRNA‐mediated regulation. 7 We found that miR‐132, miR‐34c and miR‐124 modulate VSMC proliferation and thereby affect atherogenesis. 8 , 9 , 10 Vascular calcification is another disease that is tightly regulated by miRNA. Indeed, miRNAs may function as negative regulators in vascular calcification 11 , 12 or as positive initiators. 13 , 14 Considering the complexity of miRNA‐mediated regulation of diseases, however, further investigation is needed to understand the development of vascular calcification.
HDAC5, a member of the Class II histone deacetylases (HDACs), plays a role in cellular and epigenetic processes that control the progression of diverse diseases, including cancer, 15 cardiac diseases 16 and vascular calcification. 14 Several studies have reported that miRNAs such as miR‐124/miR‐9, miR‐2861 and miR‐589 target HDAC5 in neurons, 17 bone 18 and lung. 19 For example, Xia et al reported that miR‐2861 directly targets HDAC5 and promotes osteogenic transdifferentiation of VSMCs by inhibiting the expression of RUNX2. 14 In the present study, we investigated the role of miR‐134‐5p in calcium deposition in VSMCs. We found that HDAC5 expression is down‐regulated by miR‐134‐5p, thereby increasing calcium deposition in VSMCs. These findings give novel insight into a role of miR‐134‐5p as a regulator of HDAC5 in calcium deposition in VSMCs.
2. MATERIALS AND METHODS
All experimental procedures were approved by the Chonnam National University Medical School Research Institutional Animal Care and Use Committee and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
2.1. miRNA mimic, small interfering RNA (siRNA) and antibodies
miR‐134‐5p mimic, control miRNA, HDAC5 siRNA and scramble siRNA were purchased from Bioneer Corp. Antibodies against RUNX2 (23981, Abcam) and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (G9545, Sigma) were used at 1:1000 dilution.
2.2. Cell cultures
Rat VSMCs were isolated from thoracic aorta of 6‐7‐week‐old male Sprague‐Dawley rats after anaesthesia with 2,2,2‐Tribromoethanol (240 mg/kg; intraperitoneal injection) (T48402, Sigma). The aorta was washed using ice‐cold phosphate‐buffered saline (PBS) before incubation in Ham's F12 medium (12‐615F, Lonza) with 0.2% collagenase I (LS004196, Worthington) at 37°C for 30 minutes. The aorta was opened longitudinally, and the intima was scraped from the luminal surface. Tissue samples were minced in Ham's F12 media containing 300 U/mL penicillin and 300 U/mL streptomycin and then incubated in 0.2% collagenase I solution at 37°C for 30 minutes. The rat VSMCs were cultured in DMEM (LM001‐05, Welgene) with 10% foetal bovine serum (FBS) (S001‐07, Welgene) and antibiotics (15240062, ThermoFisher Scientific). Rat VSMCs were used at passages 2‐6.
A10 cells, derived from embryonic rat aorta, were purchased from American Type Culture Collection (CRL‐1476) and have been used as a model system of rat VSMCs. The A10 cells were cultured in DMEM with 10% FBS. All cells were maintained in an incubator under a humidified atmosphere with 5% CO2 at 37°C.
2.3. Induction of vascular calcification in vitro
The cell culture medium supplemented with 2 mmol/L Pi was changed every 2 days for up to 6 days to induce calcification. The cells were washed twice with PBS before quantification of calcium deposition.
2.4. miRNA and mRNA microarray and bioinformatics
To investigate changes in levels of miRNA in Pi‐treated rat VSMCs, miRNA microarray was performed after pooling of three samples. RNA was isolated as described below. To reduce the experimental error, two pooled samples were independently used for the microarray and the averaged values were evaluated further.
The mRNA samples were analysed by utilizing the mRNA microarray (Agilent Microarray, Agilent‐028282), and the results were previously deposited in Gene Expression Omnibus (GEO) database under accession code GSE74755. 20 Putative target mRNAs of miR‐134‐5p were screened using Targetscan (http://www.targetscan.org/), 21 microRNA.org (http://www.microrna.org) 22 and mirDB (http://mirdb.org/). 23
For the clustering analysis of miRNA microarray data, Cluster 3.0 was used for the unsupervised hierarchical clustering. 24 The analysis result was visualized by Java Treeview. 25 In the clustering analysis, microarray signals were median‐centred and normalized for genes and arrays. Average linkage analysis was performed using the centred‐correlation method.
2.5. Quantification of calcium deposition
Cells were decalcified in 0.6 N HCl at 4°C for 24 hours. The calcium content of the HCl supernatants was determined using QuantiChromTM Calcium Assay Kit (DICA‐500, BioAssay Systems) according to the manufacturer's protocol. Briefly, the samples had been mixed with working reagent, and then the absorbance of the mixture at 570 nm was measured using ELx808 Absorbance Reader (BTELX808, BioTek Instruments). Decalcified cells were lysed with 0.1 N NaOH/0.1% SDS to extract proteins. The protein content was quantified with BCA Protein Assay kit (23225, ThermoFisher Scientific). The calcium content was normalized against the protein content.
2.6. Reverse transcription and quantitative real‐time polymerase chain reaction (qRT‐PCR)
Total RNA was extracted using either TRIzol Reagent (15596026, Invitrogen) or NucleoSpin® RNA/Protein (740933.250, Macherey‐Nagel) following the manufacturer's protocols. mRNAs were reverse‐transcribed using the SuperScript™ First‐Strand Synthesis System and random hexamer primer (11904018, SO142, ThermoFisher Scientific). The cDNAs were then analysed by qPCR using a QuantiTect SYBR Green PCR Kit (204141 and Qiagen), gene‐specific primers and a Rotor gene Q real‐time PCR cycler (9001550, Qiagen). 18S rRNA was used as an expression control. Pre‐designed qPCR primer for 18S rRNA was purchased from ThermoFisher Scientific (Rn03928990_g1). Custom‐designed primers for HDAC5 (sense 5′‐TCC CGT CCG TCT GTC TGT TA‐3′, antisense 5′‐GAC ATG CCA TCC GAC TCG TT‐3′), BMP2 (sense:5′‐TCA CCC CGG CTG TGA TGC GA‐3′, antisense: 5′‐ACC CGC AAC CCT CCA CAA CC‐3′) and OPG (sense: 5′‐GGC AGG GCA TAC TTC CTG TTG CC‐3′, antisense: 5′‐TCG GTT GTG GGT GCG GTT GC‐3′) were purchased from Bioneer (Daejon, Korea).
The cDNA of miR‐134‐5p was synthesized by adding a poly (A) tail to the 3′ end and ligating an ‘adapter’ to the 5′ end of an miRNA followed by reverse transcription with universal primer using the TaqMan Advanced miRNA cDNA Synthesis Kit (A28007, Applied Biosystems). The cDNAs were then analysed by qPCR using a QuantiTect SYBR Green PCR Kit (204141, Qiagen, Hilden, Germany) with gene‐specific primers for miR‐134‐5p and 18S rRNA (4427975 and 4333760F, Applied Biosystems).
2.7. Western blot analysis
Cellular proteins were prepared with lysis buffer [50 mmol/L Tris (pH 8.0), 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP‐40 (28324, ThermoFisher Scientific), 1 mmol/L dithiothreitol (DTT), 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3PO4 and protease inhibitor (11 697 498 001, Hoffmann‐La Roche [Basel, Switzerland])]. The proteins were separated by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and then transferred to a polyvinylidene difluoride membrane (Millipore), followed by blocking with 5% skim milk (232100, BD Difco) in TRIS‐buffered saline‐Tween 20 (20605, ThermoScientific Fisher) (TBST). The membranes were then incubated with primary antibodies overnight at 4°C on a rocker. After three washes in TBST, the membranes were incubated with horseradish peroxidase‐conjugated secondary antibodies (7076, 7074, Cell Signaling) for 1 hour at room temperature. The peroxidase activity was visualized by enhanced chemiluminescence using Western Blotting Luminol Reagent (sc‐2048 Santa Cruz Biotechnology) and FUJIFILM Luminescent Image Analyzer LAS‐3000 (Fujifilm Life Science). Quantification of Western blot analysis was performed after retrieving the density of the bands using Scion Image software (Scion Corporation) after more than three independent sets of experiments.
2.8. Cloning
The coding sequence of rat HDAC5 was cloned onto pcDNA6/myc‐His vector (V22120, ThermoFisher Scientific) for overexpression of HDAC5 in mammalian cells. A DNA fragment corresponding to the 3′UTR of rat HDAC5 containing the putative binding site for miR‐134‐5p was cloned into psiCHECK™‐2 vector (C8021A, Promega) for the luciferase assay.
2.9. Luciferase assay
The Renilla luciferase vector, with firefly luciferase as an internal control, was co‐transfected with miR134‐5p mimic into A10 cells, and luciferase activity was measured by using the Luciferase Assay System (E1500, Promega) following the manufacturer's protocols. Renilla luciferase activity was normalized against firefly luciferase activity.
2.10. Statistical analysis
Data are presented as mean ± SEM Statistical significance was determined by Student's t tests or one‐way ANOVA, followed by Tukey's honestly significant difference multiple‐comparison post hoc test using PASW Statistics 19 software (SPSS, an IBM Company).
3. RESULTS
3.1. Screening of vascular calcification‐associated miRNAs and their possible targets
We used microRNA array analysis to look for miRNAs associated with vascular calcification. We first treated rat VSMCs with Pi to induce sufficient calcium deposition (Figure 1A). We next performed miRNA microarray (GSE 130 486). Among eight significantly altered miRNAs, we noticed that miR‐134‐5p was increased over 600‐fold (Figure 1B). The up‐regulation of miR‐134‐5p was further confirmed by qRT‐PCR (Figure 1C). We were interested in miR‐134‐5p because it has been reported that it is one of the miRNAs up‐regulated in the serum of patients with coronary artery calcification. 26 Also, in endothelial cells, miR‐134‐5p is related to tumour angiogenesis 27 and endothelium‐associated cardiac tube formation during development. 28 More recently, miR‐134‐5p was shown to be involved in VSMC phenotypic switching and migration and in the progression of thoracic aortic dissection. 29 However, its role in calcium deposition in VSMCs has not been reported.
FIGURE 1.
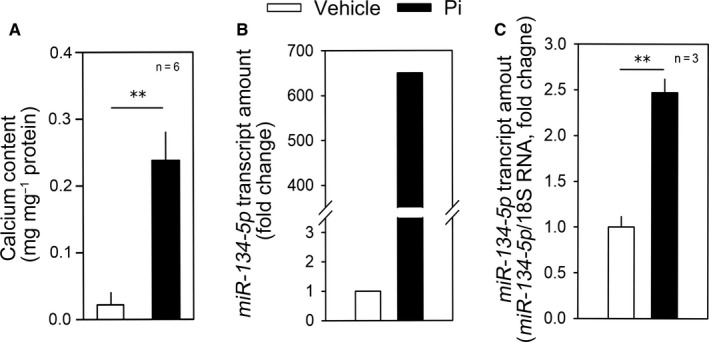
miR‐134‐p5 is up‐regulated by treatment with inorganic phosphate. A, Treatment with inorganic phosphate (Pi) significantly induced calcium deposition in rat vascular smooth muscle cells (RVSMCs). B, microRNA microarray analysis showing the increase in miR‐134‐5p in response to Pi in rat VSMCs. Two values were averaged. C, Treatment with Pi for 6 d significantly increased the miR‐134‐5p transcript amount in A10 cells. Pi, inorganic phosphate. **P < .01
3.2. miR‐134‐5p induces calcium deposition
We next investigated the role of miR‐134‐5p in calcium deposition in VSMCs. Transfection of miR‐134‐5p mimic did not increase calcium deposition (left two bars in Figure 2A). However, it potentiated Pi‐induced vascular calcification. Treatment with Pi for 6 days induced calcium deposition, which was further potentiated by transfection of miR‐134‐5p mimic (right two bars in Figure 2A). Alizarin red S staining showed that Pi‐induced calcium deposition was enhanced by transfection of miR‐134‐5p mimic (Figure 2B).
FIGURE 2.
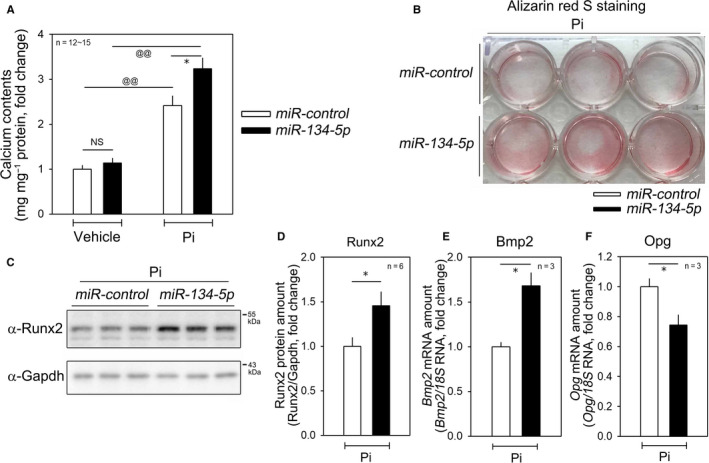
miR‐134‐5p potentiates vascular calcification. A, In the absence of Pi, transfection of miR‐134‐5p mimic did not significantly affect calcium contents in A10 cells. However, in the presence of Pi, miR‐134‐5p enhanced calcium deposition. B, Alizarin red S staining in Pi‐treated A10 cells. C‐D, Transfection of miR‐134‐5p mimic increased the protein expression of RUNX2. C, Representative Western blot image. D, Quantitative results. E, Transfection of miR‐134‐5p mimic increased the mRNA amount of bone morphogenic protein 2 (BMP2) in the presence of Pi in A10 cells. F, miR‐134‐5p decreased the mRNA amount of osteoprotegerin (OPG). * P < .05, @@ P < .01. NS, not significant
Vascular calcification is a form of ectopic bone formation and shares a common pathway with osteogenic signals in bone. 30 Among the bone‐forming signals, RUNX2 is a key player in osteoblast differentiation. 31 As in bone formation, RUNX2 is highly associated with vascular calcification. 32 Interestingly, miR‐134‐5p increased the RUNX2 protein amount in the presence of Pi (Figure 2C,D). Likewise, bone morphogenic protein 2 (BMP2) was significantly increased by treatment with miR‐134‐5p mimic (Figure 2E). Osteoprotegerin (OPG) is a key regulator of bone formation 33 and is also associated with vascular calcification. 34 We observed that miR‐134‐5p mimic down‐regulated the expression of OPG (Figure 2F), suggesting that down‐regulation of a counter‐calcification signal may also participate in the miR‐134‐5p‐induced vascular calcification.
3.3. miR‐134‐5p targets HDAC5
miRNAs induce degradation of their target mRNAs through direct binding to their 3′UTR, and the miRNAs and their target genes are reciprocally regulated. 4 Thus, by in silico analysis as well as mRNA microarray, we searched for candidate targets of miR‐134‐5p that were down‐regulated in response to vascular calcification. Among the candidates, the 3′UTR of HDAC5 had a complementary sequence to miR‐134‐5p as shown in Figure 3A. mRNA array also showed that HDAC5 was down‐regulated by Pi treatment (Figure 3B). The Pi‐induced reduction in the HDAC5 transcript amount was further confirmed by qRT‐PCR (Figure 3C).
FIGURE 3.
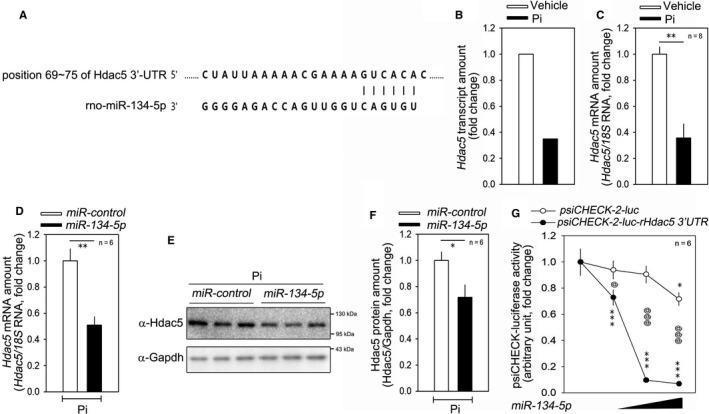
HDAC5 is a post‐transcriptional target of miR‐134‐5p. A, Sequence alignments of the HDAC5 3™UTR and miR‐134‐5p. B, mRNA microarray result showing that the HDAC5 mRNA amount was down‐regulated in Pi‐treated rat VSMCs. Two values were averaged. C, qRT‐PCR results. D, Transfection of miR‐134‐5p mimic reduced the mRNA level of HDAC5. qRT‐PCR results. E, miR‐134‐5p mimic reduced the protein amount of HDAC5 as determined by Western blot analysis. F, Quantification results are shown. G, miR‐134‐5p mimic significantly attenuated the luciferase activity driven by the HDAC5 3’UTR. Either psiCHECK™‐2‐luc rat HDAC5 3’UTR or psiCHECK™‐2‐luc empty vector was used to measure luciferase activity. * and @ p < 0.05. ** p < 0.01. *** and @@@ p < 0.001.
We observed that miR‐134‐5p could directly reduce the HDAC5 mRNA level (Figure 3D) and its protein level (Figure 3E). The quantitative results for the changes in protein level are shown in Figure 3F. Next, we generated a luciferase construct by inserting the 3′UTR of rat HDAC5 into psiCHECK™‐2 vector. miR‐134‐5p mimic was transfected together with either psiCHECK™‐2‐rHDAC5 3′UTR plasmid or empty psiCHECK™‐2 vector. The miR‐134‐5p mimic successfully attenuated luciferase activity of psiCHECK™‐2‐luc‐rHDAC5 3′UTR, in a dose‐dependent manner, whereas it failed to do so with the empty vector (Figure 3G).
3.4. HDAC5 inhibits Pi‐induced vascular calcification
HDAC5 was previously reported to inhibit RUNX2 induced by EGFR 35 and TGF‐β. 36 It has been also reported that miR‐2861 inhibits HDAC5, thereby inhibiting the calcification of VSMCs. 14 We therefore looked into the effect of HDAC5 on calcium deposition in VSMCs. In our experimental model, HDAC5 reduced Pi‐induced calcium deposition in VSMCs, as determined by Alizarin red S staining (Figure 4A). Transfection of HDAC5 did not significantly alter basal calcium contents in the absence of Pi (3 d, left two bars in Figure 4B). However, HDAC5 abolished Pi‐induced vascular calcification (right two bars in Figure 4B). By contrast, HDAC5‐knockdown by HDAC5 siRNA potentiated the increase in calcium deposition induced by Pi treatment for 2 days (Figure 4C and right two bars in Figure 4D).
FIGURE 4.
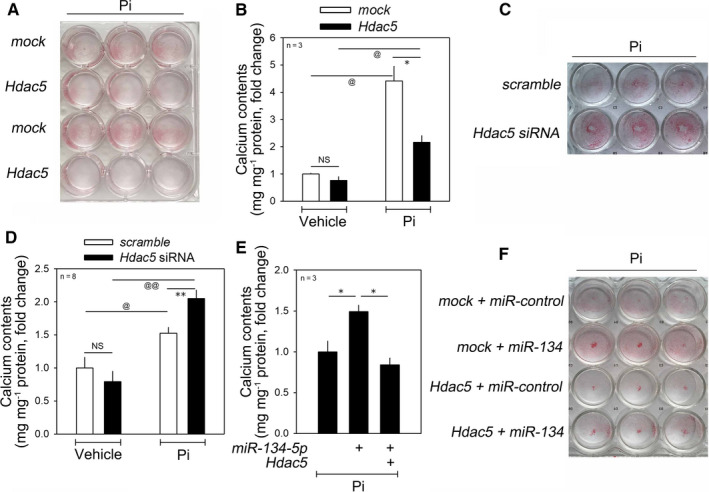
HDAC5, a novel anti‐calcification factor, mitigates miR‐134‐5p‐induced calcium deposition in the presence of Pi. A, Alizarin red S staining assay. B, Transfection of HDAC5 attenuated the increase in calcium deposition induced by treatment with Pi for 4 d. C, Alizarin red S staining. D, Knocking down of HDAC5 by HDAC5 siRNA significantly enhanced the increase in vascular calcification induced by Pi treatment for 4 d. E, miR‐134‐5p‐induced increase in calcium contents was abrogated by simultaneous overexpression of HDAC5. F, Alizarin red S staining. * and @ P < .05, @@ P < .01. NS, not significant
3.5. HDAC5 overexpression mitigates miR‐134‐5p‐mediated calcium deposition
To check whether miR‐134‐5p‐mediated vascular calcification is dependent on HDAC5, we tested whether transfection of HDAC5 could interfere with the effect of miR‐134‐5p. In the presence of Pi, transfection of miR‐134‐5p further enhanced the calcium deposition; the potentiation, however, was completely blunted by HDAC5 (Figure 4E). Alizarin red S staining further showed that miR‐134‐5p‐induced calcium deposition was attenuated by transfection of HDAC5 (Figure 4F).
4. DISCUSSION
The present work illustrates a new mechanism of vascular calcification involving miR‐134‐5p and its target HDAC5. The main finding of this work is that stimulation of vascular calcification induces the expression of miR‐134‐5p, which in turn potentiates Pi‐induced calcium deposition by increasing RUNX2 and decreasing OPG (Figure 5). We also showed that miR‐134‐5p leads to the down‐regulation of HDAC5 and that HDAC5 works as an anti‐calcification mediator in VSMCs.
FIGURE 5.
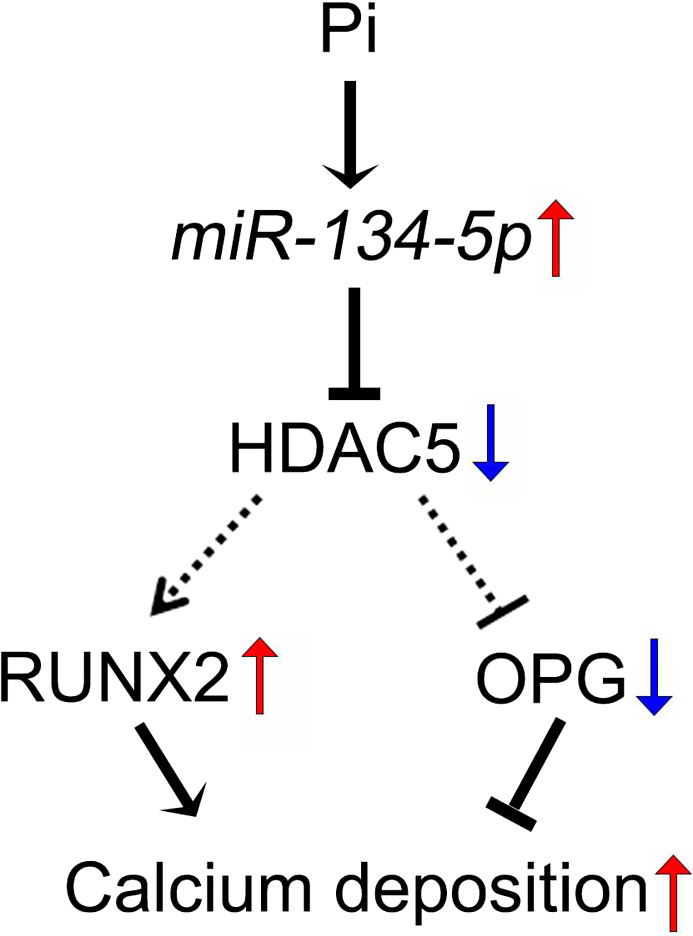
Diagram of miR‐134‐5p/HDAC5‐mediated calcium deposition in VSMCs. Treating VSMCs with Pi increased miR‐134‐5p transcription, thereby suppressing HDAC5 expression, followed by an increase in RUNX2 and decrease in OPG to induce vascular calcification
Given that they participate in the pathomechanisms of diverse diseases, noncoding RNAs are extensively involved in vascular calcification. For example, our research group previously reported that the long noncoding RNA Lrrc75a‐as1 works as a negative regulator of vascular calcification. 37 More recently, we also showed that circular RNAs such as circSamd4a reduce calcium deposition in VSMCs by absorbing miRNAs. 38 In both of those previous studies, we noted that miRNAs are involved in the mechanisms of action of both the long noncoding and circular RNAs. Indeed, the most extensively investigated noncoding RNAs are miRNAs. 39 Concerning the phenotypic effects of those RNAs, both positive 13 , 14 and negative 11 , 12 effects on vascular calcification have been reported.
Numerous studies have proposed a function for miRNAs in human diseases such as cardiovascular diseases. In addition, many miRNAs have been recognized as clinical, diagnostic and prognostic biomarkers, including in cancer. For example, miR‐143/145 has been proposed as a marker of progression and metastasis of breast malignancy. 40 Recently, several studies suggested miR‐134 as a biomarker of cancer, because expression of miR‐134 is markedly altered in many cancerous tissues. 41 , 42 It has been reported that miR‐134 plays a key role in human carcinoma and participates in many aspects of cancer progression and has been associated with cancer prognosis in a variety of cancers. 41 In addition, miR‐134 directly regulates multiple target genes such as DPD, 43 FOXM1, 44 KRAS 45 and STAT5B, 46 depending on type of carcinoma, through complicated signalling pathway including the MAPK/ERK signalling pathway, the EGFR pathways and the Notch pathway. 41 However, the effects of miR‐134 in cell proliferation and cancer progression are in debate, depending on the tumour type and target genes, whether tumour suppressor 47 , 48 or inducer. 49 , 50 It is noteworthy that miR‐134 was first described to have a role in hippocampal neurons by regulating synaptic plasticity and memory. 51 Recently, target genes of miR‐134 such as CREB, 52 LIMK1 53 and PUM2 54 have been identified in several studies.
The function of miR‐134 has also been investigated in cardiovascular diseases, although fewer studies have been reported than for the nervous system or cancer. In the embryo, miR‐134 regulates endothelium‐linked cardiac tube formation through down‐regulation of polycomb complex protein BMI‐1. 28 Wu et al reported that miR‐134 directly targets myeloid ecotropic insertion site 2 and promotes human cardiomyocyte precursor cell proliferation. 55 Indeed, miR‐134 might be induced in circumstances where active cardiomyocyte proliferation is required, since it is up‐regulated in acute myocardial infarction and could be an early marker of the disease. 56 The function of miR‐134 in myocardial infarction is to accelerate myocardial hypoxia/reoxygenation injury by targeting nitric oxide synthase 3, encoding endothelial nitric oxide synthase, which generates nitric oxide and plays a protective role in the cardiovascular system. 57 Xiao et al revealed that inhibition of miR‐134 could protect myocardial ischaemia/reperfusion injury through up‐regulation of NOS3 and activation of the PI3K/AKT pathway. 57
miR‐134 seems to actively participate in the progression of atherosclerosis not only in cardiomyocytes but also by targeting angiopoietin‐like 4 in macrophages. 58 It is noteworthy that miR‐134‐5p is up‐regulated in the serum of patients with coronary artery calcification and could be used as a biomarker. 26 However, to our knowledge, no research to investigate the mechanism of miR‐134 in vascular calcification has been done. In the only report showing the function of miR‐134 in VSMCs in the development of transverse aortic dissection, 29 Wang et al found that miR‐134‐5p is down‐regulated in thoracic aortic dissection. They also observed that miR‐134‐5p induces VSMC differentiation by inducing the switch to the contractile phenotype and that STAT5B/ITGB1 is post‐transcriptionally down‐regulated. 29 In the present study, we clearly demonstrated that miR‐134‐5p can potentiate Pi‐induced vascular calcification, although it alone did not induce calcium deposition. Indeed, miR‐134‐5p is a novel positive regulator of vascular calcification and remodelling.
HDAC5 is known to reduce RUNX2 activity in osteoblasts and miRNAs such as miR‐2861 modulate osteoblast differentiation by targeting HDAC5. 18 Since RUNX2 is a key player in both vascular calcification and bone formation, it is plausible that HDAC5 negatively regulates RUNX2 and thereby inhibits vascular calcification. Direct evidence between HDAC5 and vascular calcification, however, has not been fully demonstrated, whereas the involvement of HDAC4, an alternative class II HDAC, in vascular calcification has been reported. 59 Rather, HDAC5 in association with HDAC4 is involved in VSMC hypertrophy and atherogenesis. 60 One report showed that miR‐2861 induces vascular calcification by targeting HDAC5. 14 In the current study, we focused on down‐regulation of HDAC5 by miR‐134‐5p in bone‐like VSMCs, which resulted in vascular calcification. Although the binding of miR‐134‐5p to the HDAC5 3′UTR was sequence‐specific, this does not rule out the possibility that miR‐134‐5p reduces luciferase activity by binding to other, non‐predicted sites in the HDAC5 3′UTR. Therefore, a luciferase construct with modified or deleted target sequences of miR‐134‐5p may further confirm the specificity of the inhibition if the modification recovers luciferase activity. However, another remaining question is how HDAC5 is associated with vascular calcification, that is, whether suppression of HDAC5 by miR‐134‐5p directly or indirectly regulates the expression of osteogenic genes including RUNX2 and OPG in VSMCs, as well whether inhibition of miR‐134‐5p targeting HDAC5 effectively prevents vascular calcification in vivo. Thus, further studies are required to understand the precise mechanism by which the miR‐134‐5p/HDAC5 axis participates in vascular calcification. Those mechanisms could provide novel therapeutic approaches to treat diseases related to vascular calcification.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Nakwon Choe: Data curation (equal); Investigation (lead); Writing‐original draft (equal); Writing‐review & editing (supporting). Sera Shin: Investigation (supporting). Hosouk Joung: Investigation (supporting). Juhee Ryu: Investigation (supporting). Young‐Kook Kim: Conceptualization (supporting); Data curation (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Youngkeun Ahn: Conceptualization (supporting); Data curation (supporting). Hyun Kook: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Supervision (equal); Visualization (lead); Writing‐original draft (equal); Writing‐review & editing (equal). Duk‐Hwa Kwon: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Supervision (equal); Visualization (equal); Writing‐review & editing (lead).
ACKNOWLEDGEMENTS
The authors are grateful for Jennifer Holmes at Medical Editing Services for her excellent language editing and careful reading. This work was supported by National Research Foundation of Korea grants funded by the Korean government (2018R1A2B3001503, 2019R1A4A1028534 and 2017R1C1B2003158).
Choe N, Shin S, Joung H, et al. The microRNA miR‐134‐5p induces calcium deposition by inhibiting histone deacetylase 5 in vascular smooth muscle cells. J Cell Mol Med. 2020;24:10542–10550. 10.1111/jcmm.15670
Contributor Information
Hyun Kook, Email: kookhyun@chonnam.ac.kr.
Duk‐Hwa Kwon, Email: elio9359@hanmail.net.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Gene Expression Omnibus (GEO) database under accession code GSE74755.
REFERENCES
- 1. Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 2. Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93:365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amin N, McGrath A, Chen Y‐PP. Evaluation of deep learning in non‐coding RNA classification. Nature Machine Intelligence. 2019;1:246‐256. [Google Scholar]
- 4. Kim YK, Kook H. Diverse roles of noncoding RNAs in vascular calcification. Arch Pharm Res. 2019;42:244‐251. [DOI] [PubMed] [Google Scholar]
- 5. Hashimoto Y, Akiyama Y, Yuasa Y. Multiple‐to‐multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013;8:e62589.23667495 [Google Scholar]
- 6. Ambros V. The functions of animal microRNAs. Nature. 2004;431:350‐355. [DOI] [PubMed] [Google Scholar]
- 7. Wang YS, Wang HY, Liao YC, et al. MicroRNA‐195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc Res. 2012;95:517‐526. [DOI] [PubMed] [Google Scholar]
- 8. Choe N, Kwon JS, Kim JR, et al. The microRNA miR‐132 targets Lrrfip1 to block vascular smooth muscle cell proliferation and neointimal hyperplasia. Atherosclerosis. 2013;229:348‐355. [DOI] [PubMed] [Google Scholar]
- 9. Choe N, Kwon JS, Kim YS, et al. The microRNA miR‐34c inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by targeting stem cell factor. Cell Signal. 2015;27:1056‐1065. [DOI] [PubMed] [Google Scholar]
- 10. Choe N, Kwon DH, Shin S, et al. The microRNA miR‐124 inhibits vascular smooth muscle cell proliferation by targeting S100 calcium‐binding protein A4 (S100A4). FEBS Lett. 2017;591:1041‐1052. [DOI] [PubMed] [Google Scholar]
- 11. Liao XB, Zhang ZY, Yuan K, et al. MiR‐133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology. 2013;154:3344‐3352. [DOI] [PubMed] [Google Scholar]
- 12. Xu TH, Qiu XB, Sheng ZT, et al. Restoration of microRNA‐30b expression alleviates vascular calcification through the mTOR signaling pathway and autophagy. J Cell Physiol. 2019;234:14306‐14318. [DOI] [PubMed] [Google Scholar]
- 13. Badi I, Mancinelli L, Polizzotto A, et al. miR‐34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (Sirtuin 1) and Axl (AXL receptor tyrosine kinase). Arterioscler Thromb Vasc Biol. 2018;38:2079‐2090. [DOI] [PubMed] [Google Scholar]
- 14. Xia ZY, Hu Y, Xie PL, et al. Runx2/miR‐3960/miR‐2861 positive feedback loop is responsible for osteogenic transdifferentiation of vascular smooth muscle cells. Biomed Res Int. 2015;2015:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsieh TH, Hsu CY, Tsai CF, et al. HDAC inhibitors target HDAC5, upregulate microRNA‐125a‐5p, and induce apoptosis in breast cancer cells. Mol Ther. 2015;23:656‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toth AD, Schell R, Levay M, et al. Inflammation leads through PGE/EP3 signaling to HDAC5/MEF2‐dependent transcription in cardiac myocytes. EMBO Mol Med. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gu X, Fu C, Lin L, et al. miR‐124 and miR‐9 mediated downregulation of HDAC5 promotes neurite development through activating MEF2C‐GPM6A pathway. J Cell Physiol. 2018;233:673‐687. [DOI] [PubMed] [Google Scholar]
- 18. Li H, Xie H, Liu W, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119:3666‐3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regnier A, Alvinerie M, Toutain PL. Clinical evaluation of combined dexamethasone suppression/ACTH stimulation testing in dogs with hyperadrenocorticism, using HPLC for plasma cortisol determination. Zentralbl Veterinarmed A. 1988;35:409‐416. [DOI] [PubMed] [Google Scholar]
- 20. Kwon DH, Eom GH, Ko JH, et al. MDM2 E3 ligase‐mediated ubiquitination and degradation of HDAC1 in vascular calcification. Nat Commun. 2016;7:10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15‐20. [DOI] [PubMed] [Google Scholar]
- 22. Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149‐D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127‐D131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453‐1454. [DOI] [PubMed] [Google Scholar]
- 25. Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics. 2004;20:3246‐3248. [DOI] [PubMed] [Google Scholar]
- 26. Liu W, Ling S, Sun W, et al. Circulating microRNAs correlated with the level of coronary artery calcification in symptomatic patients. Sci Rep. 2015;5:16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Lv Z, Xu J, et al. MicroRNA‐134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. FEBS J. 2018;285:1359‐1371. [DOI] [PubMed] [Google Scholar]
- 28. Miao C, Cao H, Zhang Y, Guo X, Wang Z, Wang J. LncRNA DIGIT accelerates tube formation of vascular endothelial cells by sponging miR‐134. Int Heart J. 2018;59:1086‐1095. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Dong CQ, Peng GY, et al. MicroRNA‐134‐5p regulates media degeneration through inhibiting VSMC phenotypic switch and migration in thoracic aortic dissection. Mol Ther Nucleic Acids. 2019;16:284‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golub EE. Biomineralization and matrix vesicles in biology and pathology. Semin Immunopathol. 2011;33:409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen‐Solal KA, Boregowda RK, Lasfar A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol Cancer. 2015;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Y, Byon CH, Yuan K, et al. Smooth muscle cell‐specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makarovic S, Makarovic Z, Steiner R, Mihaljevic I, Milas‐Ahic J. Osteoprotegerin and vascular calcification: clinical and prognostic relevance. Coll Antropol. 2015;39:461‐468. [PubMed] [Google Scholar]
- 35. Zhu J, Shimizu E, Zhang X, Partridge NC, Qin L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. J Cell Biochem. 2011;112:1749‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF‐beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543‐2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeong G, Kwon DH, Shin S, et al. Long noncoding RNAs in vascular smooth muscle cells regulate vascular calcification. Sci Rep. 2019;9:5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryu J, Kwon DH, Choe N, et al. Characterization of circular RNAs in vascular smooth muscle cells with vascular calcification. Mol Ther Nucleic Acids. 2019;19:31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Esteller M. Non‐coding RNAs in human disease. Nat Rev Genet. 2011;12:861‐874. [DOI] [PubMed] [Google Scholar]
- 40. Piasecka D, Braun M, Kordek R, Sadej R, Romanska H. MicroRNAs in regulation of triple‐negative breast cancer progression. J Cancer Res Clin Oncol. 2018;144:1401‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan JY, Zhang F, Sun CC, et al. miR‐134: a human cancer suppressor? Mol Ther Nucleic Acids. 2017;6:140‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salazar C, Nagadia R, Pandit P, et al. A novel saliva‐based microRNA biomarker panel to detect head and neck cancers. Cell Oncol. 2014;37:331‐338. [DOI] [PubMed] [Google Scholar]
- 43. Hirota T, Date Y, Nishibatake Y, et al. Dihydropyrimidine dehydrogenase (DPD) expression is negatively regulated by certain microRNAs in human lung tissues. Lung Cancer. 2012;77:16‐23. [DOI] [PubMed] [Google Scholar]
- 44. Li J, Wang Y, Luo J, et al. miR‐134 inhibits epithelial to mesenchymal transition by targeting FOXM1 in non‐small cell lung cancer cells. FEBS Lett. 2012;586:3761‐3765. [DOI] [PubMed] [Google Scholar]
- 45. Zhao Y, Pang D, Wang C, Zhong S, Wang S. MicroRNA‐134 modulates glioma cell U251 proliferation and invasion by targeting KRAS and suppressing the ERK pathway. Tumour Biol. 2016;37:11485‐11493. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Kim J, Mueller AC, et al. Multiple receptor tyrosine kinases converge on microRNA‐134 to control KRAS, STAT5B, and glioblastoma. Cell Death Differ. 2014;21:720‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qiu ZA, He GP. MicroRNA‐134 functions as a tumor suppressor gene in gastric cancer. Am J Transl Res. 2016;8:4320‐4328. [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y, Fu Y, Gao Y, et al. microRNA‐134 inhibits melanoma growth and metastasis by negatively regulating collagen triple helix repeat containing‐1 (CTHRC1). Int J Clin Exp Pathol. 2018;11:4319‐4330. [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang L, Huang P, Li Q, Wang D, Xu CX. miR‐134‐5p promotes stage i lung adenocarcinoma metastasis and chemoresistance by targeting DAB2. Mol Ther Nucleic Acids. 2019;18:627‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peng SY, Tu HF, Yang CC, et al. miR‐134 targets PDCD7 to reduce E‐cadherin expression and enhance oral cancer progression. Int J Cancer. 2018;143:2892‐2904. [DOI] [PubMed] [Google Scholar]
- 51. Jimenez‐Mateos EM, Engel T, Merino‐Serrais P, et al. Antagomirs targeting microRNA‐134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine‐induced status epilepticus. Brain Struct Funct. 2015;220:2387‐2399. [DOI] [PubMed] [Google Scholar]
- 52. Feng Z, Zhang L, Wang S, Hong Q. Circular RNA circDLGAP4 exerts neuroprotective effects via modulating miR‐134‐5p/CREB pathway in Parkinson's disease. Biochem Biophys Res Commun. 2020;522:388‐394. [DOI] [PubMed] [Google Scholar]
- 53. Liu W, Wu J, Huang J, et al. Electroacupuncture regulates hippocampal synaptic plasticity via miR‐134‐mediated LIMK1 function in rats with ischemic stroke. Neural Plast. 2017;2017:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fiore R, Rajman M, Schwale C, et al. MiR‐134‐dependent regulation of Pumilio‐2 is necessary for homeostatic synaptic depression. EMBO J. 2014;33:2231‐2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu YH, Zhao H, Zhou LP, et al. miR‐134 modulates the proliferation of human cardiomyocyte progenitor cells by targeting Meis2. Int J Mol Sci. 2015;16:25199‐25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang KJ, Zhao X, Liu YZ, et al. Circulating MiR‐19b‐3p, MiR‐134‐5p and MiR‐186‐5p are promising novel biomarkers for early diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016;38:1015‐1029. [DOI] [PubMed] [Google Scholar]
- 57. Xiao JM, Wang JJ, Sun LL. Effect of miR‐134 against myocardial hypoxia/reoxygenation injury by directly targeting NOS3 and regulating PI3K/Akt pathway. Acta Cir Bras. 2019;34:e201900802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ye Q, Tian GP, Cheng HP, et al. MicroRNA‐134 promotes the development of atherosclerosis via the ANGPTL4/LPL pathway in apolipoprotein E knockout mice. J Atheroscler Thromb. 2018;25:244‐253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59. Abend A, Shkedi O, Fertouk M, Caspi LH, Kehat I. Salt‐inducible kinase induces cytoplasmic histone deacetylase 4 to promote vascular calcification. EMBO Rep. 2017;18:1166‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu X, Ha CH, Wong C, et al. Angiotensin II stimulates protein kinase D‐dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2355‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Gene Expression Omnibus (GEO) database under accession code GSE74755.


