Abstract
Background
Dengue is a viral disease, transmitted by infected Aedes aegypti and Aedes albopictus female mosquitoes. Worldwide, 96 million infections were estimated in 2010. The dengue virus comprises four distinct serotypes (DENV‐1, DENV‐2, DENV‐3, and DENV‐4) which belong to the genus Flavivirus. Determining the serotypes during dengue outbreaks is crucial for its effective management in terms of diagnostics improvement and polyvalent vaccine development. The aim of the present study is to determine the prevalence rate of dengue virus serotypes in the samples collected from patients during the 2017 outbreak in Khyber Pakhtunkhwa, Pakistan.
Methods
A total of 800 ELISA‐positive samples were collected, of which 513 (290 males, 223 females) samples were confirmed positive by PCR.
Results
Out of 513, 25 were found serotype 1 (5%), 196 were serotype 2 (38%), 192 were serotype 3 (37%), 56 were serotype 4 (11%), and 44 (8%) were found to have mix serotypes.
Conclusion
We can conclude that serotypes 2 and 3 of dengue virus were the predominated serotypes of dengue virus in the 2017 outbreak in Peshawar, capital city of Khyber Pakhtunkhwa, Pakistan.
Keywords: dengue, dengue virus, KP‐Pakistan, prevalence, serotyping
1. INTRODUCTION
Dengue is mosquito‐borne viral infection, which affects about 390 million people every year. 1 In the last decade, 120 countries are facing great challenge to prevent dengue virus (DENV) transmission and 2.5 billion world populations are at risk of dengue infection according to WHO report. 3 During the dengue outbreaks in Pakistan, more than 55 000 individuals were hospitalized and many of them are children. Since 2006, dengue epidemics have occurred every year and have become a major health problem in Pakistan. 2
Dengue is endemic in most tropical and subtropical countries, 4 and most of the dengue spread has caused by large number of human migration. Aedes aegypti mosquito is native to Africa but worldwide migration caused the spread of dengue, and Ae. Aegypti became one of major vector of dengue spread. 4 The epidemiology and spread of DENV became a serious burden in South East Asia after Second World War. 5 The first recorded epidemic of dengue hemorrhagic fever (DHF) was recorded in Philippines (Manila) in 1953‐1954, followed by second emergence in 1956, 5 in Bangkok in 1958, and in Thailand in 1960s. 5 , 6 Until 1970s, only nine countries reported the presence of DHF which increased in 1995. 7
The first case of dengue in Pakistan was reported from Hub, in Baluchistan in 1960, at that time Pakistan population was 45.9 million. From 1960 to 1980, there were only 12 dengue cases reported in Pakistan. 8 In Pakistan, the first outbreak of DENV was detected in 1994, in which two distinct serotypes DENV‐2 and DENV‐3 had been identified in Karachi. 9
Dengue virus is a positive single‐stranded RNA virus composed of about 11 kilobase (kb) that codes for 3411 amino acids polypeptide molecule. The polypeptide gives rise to structural proteins (membrane, envelope, and capsid) which are responsible for the basic structure of virus and are not take part in the viral genome replication and to non‐structural proteins (NS5, NS4B, NS4A, NS3, NS2B, NS2A, NS1) which are expressed inside the infected cells. 10 Globally, the variants of dengue virus have been classified into four serotypes named DENV‐1, DENV‐2, DENV‐3, and DENV‐4, belonging to family named “Flaviviridae” of “Flavivirus” genus. The four serotypes of dengue virus have been originated from sylvatic strains, found in South East Asia forests. 11 Each serotype of DENV consists of different genetic composition and undergoes genetic mutation that makes difficult to produce vaccines against all four serotypes. If patients gain immunity against one serotype, then immunity will be maintained against that serotype, but immunity against another serotype would be temporary. 12
Dengue virus infection can cause a wide spectrum of clinical manifestations, which differ from dengue fever (DF) and severe dengue disease (SDD), which includes dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). 2
Dengue fever (DF) is characterized with high fever onset. In young children, neurological disturbances caused by dehydration may occur during febrile phase. 13 DF can cause extreme weakness along with myalgia, leucopenia, petechiae rash, arthralgia, and eye pain. 14 DF is also called breakbone fever due to severe pain in muscles and joints. There is no difference between the symptoms of DF and DHF during early stages. Although DHF leads to manifestations of viral hemorrhagic, vascular permeability that causes plasma leakage and thrombocytopenia. 15 On the other hand, dengue shock syndrome (DSS) is different from DHF because it causes plasma leakage, a process in which the protein‐rich fluid component of the blood leaks from blood vessels into interstitial spaces and leads to shock. 16 Plasma leakage is the major cause of mortality and morbidity in patients with DSS.
The conditions of about 5%‐10% of patients with DF can become severe; hospitalization is required for the patients suffering from DHF. About 30%‐40% of children suffering from DHF can contract DSS. Treatment is symptomatic and supportive. Fatality rate due to DSS/DHF can reach 5%. The patients recovered from DF acquired immunity for the life time, against the serotype of dengue that had caused infection in the patient. 17
Determining the dengue serotypes during a particular outbreak of the disease is very important not only for its effective management but above all for improving diagnostics and for the development of polyvalent vaccine. Considering the current scenario of dengue infection in Pakistan, it is of prime importance to investigate the prevalence of serotypes in this region. This study aims to figure out the prevalence of DENV serotypes in the samples collected from the dengue patients during the 2017 outbreaks in Peshawar, KP.
2. MATERIALS AND METHODS
2.1. Sample collection
The study was approved by the Ethics Committee of the IBGE, UAP. A total of 800 patients from various healthcare centers, showing DF symptoms during 2017 outbreak, were enrolled in the study. About 4 mL blood was collected from DF‐positive samples in EDTA tubes after the approval of informed consent from the selected patients. Collected blood samples were screened for the presence of dengue virus active infection according to standard biosafety protocol. The demographic and clinical history was recorded on a proforma.
2.2. Viral RNA isolation
The RNA was extracted from 150 µL serum sample using Favorgen viral RNA extraction kit (Favorgen Biotech Corp), following the manufacturer's instructions. Extracted RNA was either stored at −80°C or processed immediately. Method introduced by Lanciotti and colleagues in 1992 was used for single‐tube multiplex PCR, rapid detection, and typing of dengue virus in clinical samples. 18
2.3. cDNA synthesis, amplification, and DENV serotyping
Four microlitre of extracted RNA was reverse transcribed with the help of Moloney‐murine leukemia virus (M‐MLV). PCR mix comprised of 4.0 μL 5× Buffer, 2.0 μL 5 pm outer antisense primer, 2.0 μL dNTPs (10 mmol/L), 1.0 μL MMLV (200 U/μL), and 1.0 μL dH2O to adjust the volume to 10 μL. The condition for RT‐PCR was as follows: initial denaturing for 42 minutes at 42°C, annealing at 60°C for 1 minutes, final temperature 92°C for 2 minutes, and holding temperature 22°C for 2 minutes. Two‐step PCR (1st and 2nd round) was carried out to amplify the synthesized cDNA and dengue virus serotyping. cDNA was amplified using forward and reverse primers: D1 represents the forward (sense) primers while D2 represents the reverse (antisense) primers. In the 2nd round of PCR, the reverse primers were replaced with four serotype‐specific primers. D1 and type specific 1 (TS1) were used for the identification of serotype 1, which amplify a region of 482 bp; D1 along with type specific 2 (TS2) was used to identify serotype 2, which amplify a region of 119 bp; and D1 and type specific 3 (TS3) amplified a region of 290 bp. Serotype 4 was amplified by D1 and type specific 4 (TS4), and a region of 392 bp was amplified. The primers used are shown in Table 1. PCR‐amplified products were detected by using agarose gel. 2% agarose gel was prepared in TBE buffer (1×), which was pre‐stained with 3 µL ethidium bromide that was used as a florescent dye. After electrophoresis, photographs were taken under ultraviolet light transilluminator. A 100 bp of DNA ladder was used as a DNA marker.
Table 1.
Primers used for dengue virus serotyping Lanciotti et al 18
| Primer Name | Sequence (5′–3′) | bp amplified |
|---|---|---|
| D1 (Dengue Forward) | TCAATATGCTGAAACGCGCGAGAAACCG | 511 |
| D1 (Dengue Reverse) | TTGCACCAACAGTCAATGTCTTCAGGTTC | |
| TS1 (Type specific 1) | CGTCTCAGTGATCCGGGGG | 482 |
| TS2 (Type specific 2) | CGCCACAAGGGCCATGAACAG | 119 |
| TS3 (Type specific 3) | TAACATCATCATGAGACAGAGC | 290 |
| TS4 (Type specific 4) | CTCTGTTGTCTTAAACAAGAGA | 392 |
3. RESULTS
Patients with clinical manifestations of dengue and confirmed IgM (first antibodies to appear after primary DENV infection) or IgG‐positive samples were analyzed for the presence of active DENV. Initial screening was done for anti‐DENV using ELISA. Confirmation of active DENV infection was performed by RT‐PCR. Out of 800 collected samples, 513 (290 males, 223 females) samples (64.12%) were found to have active dengue virus (DENV) infection (Figure 1A).
Figure 1.
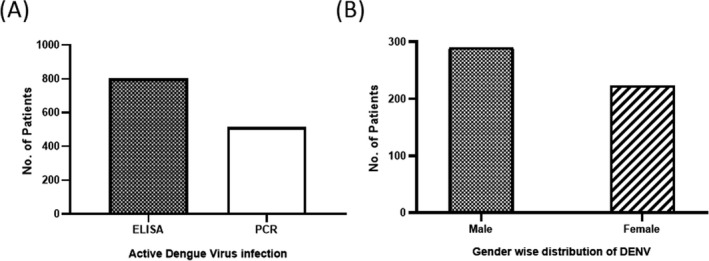
Active dengue virus infection. A, 800 samples were IgM/IgG positive, which was confirmed by ELISA (filled bar). All ELISA‐positive samples were further confirmed by RT‐PCR for active DENV infection (empty bar). B, Shows active infection in male and female
PCR‐positive patients were further analyzed for serotype‐specific detection with already reported primers. 18 Among 513 PCR‐positive patients, 290 were males and 223 were females (Figure 1B). The patients were grouped into 3 classes according to age. First group was <15 years old; second group includes patients between 15 and 45 years old while the third group consisted of patients older than 45 years. Highest prevalence (60%) was found in the second group of patients with age from 15 to 45 years, while in case of younger age group (<15 years old) lowest DENV prevalence (15%) was recorded (Figure 2). Male:female ratio was quite similar in all age groups.
Figure 2.
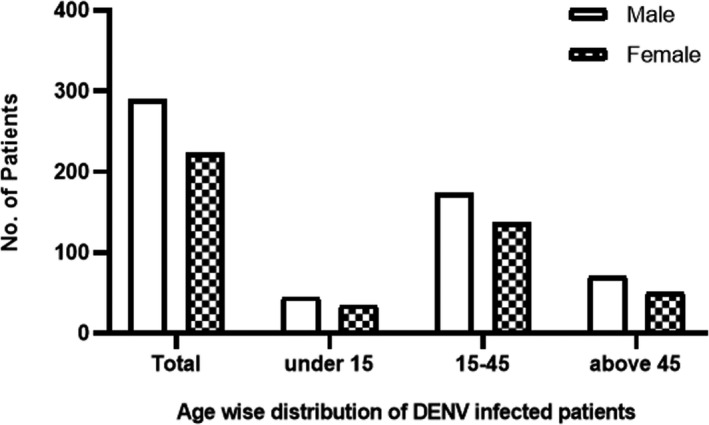
Age‐wise distribution of DENV‐infected patients. Empty bars represent the number of male patients belonging to different age groups, while bars with patterns show the female patients in three age groups
Using serotype‐specific primers, specific serotypes were determined, as illustrated in Figure 3. Out of 513 patients, serotype 1 was the least prevalent serotype with only 4% cases, serotypes 2 and 3 were predominant serotypes accounting for 38% and 37%, respectively, whereas 10% positive DENV patients have serotype 4. Mixed circulating serotype was detected in approximately 9% patients.
Figure 3.
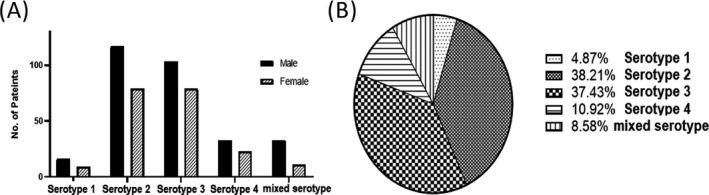
Serotype distribution of DENV in Peshawar, KPK. A, All DENV‐positive samples were further analyzed to identify the specific serotype. Bar graph represents the serotype distribution in male and female patients. B, Pie chart shows the percentage of all serotypes in DENV‐positive samples
4. DISCUSSION
Dengue is a fast emerging pandemic‐prone viral disease in many parts of the world. Dengue is caused by virus of Flaviviridae family, and there are 4 distinct, but closely related, serotypes that cause dengue (DENV‐1, DENV‐2, DENV‐3, and DENV‐4). 19 The aim of our study is to investigate which DENV serotype was prevalent in the 2017 outbreak. DENV is first identified in 1982 in Pakistan 20 ; however since 2006, dengue has become the most crucial vector‐borne disease in Pakistan. Once infected, humans become the main vectors and multipliers of the virus, acting as viral source for uninfected mosquitoes. At present, the main method to prevent the transmission of DENV is the control of mosquito populations. 2 Over the past decade, the country has faced several outbreaks in which thousands of people have been infected and they even lost their lives. 4 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 In Punjab, a populous province that shares a border with KP, in 2011 a serious epidemic affected over 50 000 people. In 2013, another huge outbreak of infection from KP province was reported with an estimated number of cases of 8546, including 33 deaths in the Swat district according to the WHO report. The prevalent serotypes were serotypes 1, 2, and 3. 29 , 30 , 31 , 32
The dengue virus may have been endemic to Pakistan, which remains unnoticed due to inadequate diagnostic and surveillance facilities. The majority of cases are asymptomatic or mild and self‐managed, so the actual number of dengue cases is underestimated. Many cases are also misdiagnosed as other febrile illnesses.
Dengue infection is very common among travelers from tropical and subtropical regions. The risk of infection in travelers usually depends on exposure to risk factors and the local prevalence of the infection. No prophylaxis is available to prevent dengue. On the other hand, nosocomial transmission may occur in areas where dengue is endemic. This may be especially relevant to healthcare workers who care for patients with dengue with hemorrhage. 33
Viral transmission without a mosquito vector has been rarely reported. The routes of transmission include needlestick injuries, bone marrow transplantation, and intrapartum and vertical transmission.
The KP population is exposed to all kinds of deadly diseases because the etiological factors for most diseases are found mainly in this province. The country has been battered by the war, the last one has been fought against terrorism, and therefore, there have been outbreaks of many deadly diseases. However, many diseases have been controlled and their spread has been overcome, but adequate planning is needed to combat a deadly disease. Efficient strategy should not only neutralize the viral spread but should also include eliminating or reducing risk factors. Although the province's health system is constantly improving, there are many problems to be solved.
The Ministry of Health in Pakistan has taken several response measures, such as vector surveillance and control activities have been initiated, and all provinces have arranged free‐of‐charge diagnostic and clinical management services for the cases. 34
Efficient medical assistance can limit the progression of the disease and can save lives, reducing mortality rates from more than 20% to <1%. 2 Contaminated environment contributes to the onset of viral outbreaks. During the epidemic of July 19, 2017, dengue affected 95 000 people. According to the World Health Organization, in Peshawar, the capital KP province of KP, the largest number of cases was reported with a total of 88 000 suspected cases, including 19 000 confirmed cases and 56 deaths (CFR 0,3%) on October 24, 2017.
The first step to solving a problem is to quantify and identify its nature. Similarly to combat the virus, we first need to find out what its actual prevalence is in a particular area. In particular, in our study eight hundred ELISA‐positive samples were collected, of which 513 (290 males, 223 females) samples were confirmed PCR positive. The highest prevalence was observed in the age group 15‐45 years with 308 patients (60%). Out of 513, 25 had serotype 1 (16 males, 9 females), 196 had serotype 2 (117 males, 79 females), 192 had serotype 3 (103 males, 79 females), 56 had serotype 4 (33 males, 23 females), and mixed serotypes were found in 44 (33 males, 11 females). Hence, we conclude that serotypes 2 and 3 were the prevalent serotypes (38% and 37%, respectively) of dengue in the 2017s outbreak. Our results are in accordance with previous reports suggesting that the age group 15‐45 years is typical of infection in the area, and the serotypes 2 and 3 remain the most common in previous outbreaks in Pakistan as summarized in Table 2. Various studies published in the last decade have reported DENV‐2 and DENV‐3 as predominant serotype except Humayoun and colleagues, which found DENV 4 as prevalent serotype. 4 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28
Table 2.
Most prevalent DENV serotypes during last decade (2008‐2018) in Pakistan
| Year of outbreak | Predominant Serotype | Location | References |
|---|---|---|---|
| 2008 | DENV‐4 | Lahore, Punjab | Humayoun et al 4 |
| 2007‐2009 | DENV‐2 | Lahore | Fatima et al 21 |
| 2010 | DENV‐2 and DENV‐3 | Lahore, Sheikhpura, and Gujranwala | Mahmood 22 |
| 2011‐2013 | DENV‐2 and DENV‐3 |
Punjab Swat, KP |
Ali et al 23 |
| 2011 | DENV‐2 | Lahore | Idrees et al 24 |
| 2011 | DENV‐2 | Lahore | Khan et al 25 |
| 2013 | DENV‐2 and DENV‐3 | Swat, KP | Ali et al 26 |
| 2013 | DENV2 |
Rawalpindi, Lahore, Faisalabad, Multan, Swat |
Shahid et al 27 |
| 2013 | DENV‐3 | Swat, KP | Ali et al 26 |
| 2013 | DENV‐2 and DENV‐3 | Swat | Khan, Ghaffar, & Khan 28 |
| 2017 | DENV2 | Peshawar, KP | This study |
Dengue flourishes in urban poor areas, in the suburbs and in the countryside, but it also affects the more affluent neighborhoods in countries located in the tropics and subtropics.
In Pakistan, people are exposed to comorbidities, infected blood products, and many risk factors. The risk factors are influenced by local spatial variations of rainfall, temperature, relative humidity, degree of urbanization, and quality of vector control services in urban areas.
The recommendation to combat the disease is to inform the local community about risk factors and potentially fatal complications of dengue virus. Awareness seminars and workshops should be held to educate the population. In particular, healthcare workers should be employed for the cause so that they can carry out a massive campaign and inform people to take preventive measures to combat dengue.
In conclusion, the incidence of dengue has grown dramatically in the last decade in Pakistan. In our study, serotypes 2 and 3 were the predominated serotypes of dengue virus in the 2017 outbreak in Peshawar. To prevent and combat the spread of dengue is necessary an efficient monitoring and evaluation strategy. This includes the disease surveillance through the identification and molecular characterization of infected human cases, and the vector surveillance by tracking mosquito populations in areas of potential. In addition, monitoring of behavioral impact is essential for observing whether the strategies aimed to reduce dengue transmission are adopted and supported by the community.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
ETHICAL APPROVAL
The Ethics Committee of the IBGE, UAP, granted approval for the study that was conducted according to the principles of ICH‐GCP and Declaration of Helsinki.
ACKNOWLEDGMENTS
This study was supported by HEC project No: 21‐1818/SRGP/R&D/HEC/2018.
Khan NU, Danish L, Khan HU, et al. Prevalence of dengue virus serotypes in the 2017 outbreak in Peshawar, KP, Pakistan. J Clin Lab Anal. 2020;34:e23371 10.1002/jcla.23371
Contributor Information
Najeeb Ullah Khan, Email: najeebkhan@aup.edu.pk.
Arnolfo Petruzziello, Email: arnolfo.petruzziello@aorncaserta.it.
Annunziata Guzzo, Email: a.guzzo@istitutotumori.na.it.
REFERENCES
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO/ TDR . Dengue: guidelines for diagnosis, treatment, prevention, and control. Special Programme for Research and Training in Tropical Diseases. 2009. 147.
- 3. Rasheed SB, Butlin RK, Boots M. A review of dengue as an emerging disease in Pakistan. Public Health. 2013;127:11‐17. [DOI] [PubMed] [Google Scholar]
- 4. Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis. 2010;14:e54‐e59. [DOI] [PubMed] [Google Scholar]
- 5. Ooi EE, Gubler DJ. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cad Saude Publica. 2009;25(Suppl 1):115‐124. [DOI] [PubMed] [Google Scholar]
- 6. Hammon WM. Dengue hemorrhagic fever–do we know its cause? Am J Trop Med Hyg. 1973;22:82‐91. [DOI] [PubMed] [Google Scholar]
- 7. Chaturvedi UC, Shrivastava R, Nagar R. Dengue vaccines: problems & prospects. Indian J Med Res. 2005;121:639‐652. [PubMed] [Google Scholar]
- 8. Khan J, Khan I, Ghaffar A, Khalid B. Epidemiological trends and risk factors associated with dengue disease in Pakistan (1980–2014): a systematic literature search and analysis. BMC Public Health. 2018;18:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan E, Kisat M, Khan N, Nasir A, Ayub S, Hasan R. Demographic and clinical features of dengue fever in Pakistan from 2003–2007: a retrospective cross‐ sectional study. PLoS One. 2010;5:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pang X, Zhang M, Dayton AI. Development of dengue virus replicons expressing HIV‐1 gp120 and other hete. EBSCOhost. BMC Microbiol. 2001;1:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eryu W, Ni H, Xu R, et al. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol. 2000;74:3227‐3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parkash O, Shueb RH. Diagnosis of dengue infection using conventional and biosensor based techniques. Viruses. 2015;7:5410‐5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Preventive and control of dengue hemmorhagic fever. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. 2009. 160. [PubMed]
- 14. Henchal EA, Putnak JR. The dengue viruses. Clin Microbiol Rev. 1990;3:376‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuiskunen Bäck A, Lundkvist Å. Dengue viruses – an overview. Infect Ecol Epidemiol. 2013;3:19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100‐103. [DOI] [PubMed] [Google Scholar]
- 17. Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid‐impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase‐polymerase chain reaction. J Clin Microbiol. 1992;30:545‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen R, Vasilakis N. Dengue–quo tu et quo vadis? Viruses. 2011;3(9):1562‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayes CG, Baqar S, Ahmed T, Chowdhry MA, Reisen WK. West Nile virus in Pakistan. 1. Sero‐epidemiological studies in Punjab Province. Trans R Soc Trop Med Hyg. 1982;76(4):431‐436. [DOI] [PubMed] [Google Scholar]
- 21. Fatima Z, Idrees M, Bajwa MA, et al. Serotype and genotype analysis of dengue virus by sequencing followed by phylogenetic analysis using samples from three mini outbreaks‐ 2007–2009 in Pakistan. BMC Microbiol. 2011;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahmood N, Rana MY, Qureshi Z, Mujtaba G, Shaukat U. Prevalence and molecular characterization of dengue viruses serotypes in 2010 epidemic. Am J Med Sci. 2012;343:61‐64. [DOI] [PubMed] [Google Scholar]
- 23. Ali A, Ahmad H, Idrees M, Zahir F, Ali I. Circulating serotypes of dengue virus and their incursion into non‐endemic areas of Pakistan; A serious threat. Virol J. 2016;13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Idrees M, Hussain W, Rehman HUR, et al. Dengue virus serotype 2 ( DEN‐2): the causative agent of 2011‐dengue epidemic in Pakistan. Am J Biomed Sci. 2012;4:307‐315. [Google Scholar]
- 25. Khan MA, Ellis EM, Tissera HA, et al. Emergence and diversification of dengue 2 cosmopolitan genotype in Pakistan, 2011. PLoS One. 2013;8:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ali A, Ahmad H, Idrees M, Zahir F, Ali I. Dengue virus serotype 2 and 3 causing high morbidity and mortality in Swat, Pakistan. Biohelikon: Immun Dis. 2013;1:11‐13. [Google Scholar]
- 27. Shahid M, Amin I, Afzal S, et al. Prevalence and molecular detection of dengue virus in 2013 outbreak in KPK and Punjab, Pakistan. Pakistan J. Zool. 2017;49:1119‐1122. [Google Scholar]
- 28. Khan J, Ghaffar A, Khan SA. The changing epidemiological pattern of Dengue in Swat, Khyber Pakhtunkhwa. PLoS One. 2018;13:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haroon M, Jan H, Faisal S, Ali N, Kamran M, Ullah F. Dengue outbreak in Peshawar: clinical features and laboratory markers of dengue virus infection. J Infect Public Health. 2019;12:258‐262. [DOI] [PubMed] [Google Scholar]
- 30. Rai MA. Control of dengue fever in Pakistan. Nature. 2011;479:41. [DOI] [PubMed] [Google Scholar]
- 31. Savioli L, Daumerie D. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. 2013. 2.
- 32. Khan J, Khan I, Ali I, Iqbal A, Salman M. The Role of vertical transmission of dengue virus among field‐captured aedes aegypti and aedes albopictus mosquitoes in Peshawar, Khyber Pakhtunkhwa Pakistan. Pak J Zoolog. 2017;49:3. [Google Scholar]
- 33. Chen LH, Wilson ME. Transmission of dengue virus without a mosquito vector: nosocomial mucocutaneous transmission and other routes of transmission. Clin Infect Dis. 2004;39:56‐60. [DOI] [PubMed] [Google Scholar]
- 34. WHO . Dengue fever – Pakistan. https://www.who.int/csr/don/19‐november‐2019‐dengue‐pakistan/en/


