Abstract
With the outbreak of a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the public healthcare systems are facing great challenges. Coronavirus disease 2019 (COVID‐19) could develop into severe pneumonia, acute respiratory distress syndrome and multi‐organ failure. Remarkably, in addition to the respiratory symptoms, some COVID‐19 patients also suffer from cardiovascular injuries. Dipeptidyl peptidase‐4 (DPP‐4) is a ubiquitous glycoprotein which could act both as a cell membrane‐bound protein and a soluble enzymatic protein after cleavage and release into the circulation. Despite angiotensin‐converting enzyme 2 (ACE2), the recently recognized receptor of SARS‐CoV and SARS‐CoV‐2, which facilitated their entries into the host, DPP‐4 has been identified as the receptor of middle east respiratory syndrome coronavirus (MERS‐CoV). In the current review, we discussed the potential roles of DPP‐4 in COVID‐19 and the possible effects of DPP‐4 inhibitors on cardiovascular system in patients with COVID‐19.
Keywords: cardiovascular system, COVID‐19, DPP‐4 inhibitors
1. INTRODUCTION
Since early December 2019, the outbreak of the coronavirus disease 2019 (COVID‐19) occurred. With the rapid spread in the world, the number of infected cases has grown exponentially. Recently, the development in the detection technologies of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has given rise to a better understanding of the clinical characteristics and molecular epidemiology of COVID‐19. It is noteworthy that according to the present clinical data, cardiac injury is one of the most common complications in patients with COVID‐19, which is also associated with poor prognosis. 1
Dipeptidyl peptidase‐4 (DPP‐4) is a widely expressed glycoprotein that could not only act as a cell membrane‐bound receptor but also as a soluble enzymatic protein. The enzymatic functions of DPP‐4 were well‐recognized. DPP‐4 has a variety of substrates, including incretin hormones, cytokines, chemokines, neuropeptides and growth factors. DPP‐4 is widely expressed in the blood vessels, myocardium and myeloid cells. It has been reported that the polymorphisms of DPP‐4 were associated with the risk of myocardial infarction in patients with atherosclerosis. 2
Previous studies showed that membrane‐associated human DPP‐4 was a functional receptor of middle east respiratory syndrome coronavirus (MERS‐CoV), which interacted with MERS‐CoV through the spike glycoprotein S1b domain to promote viral entry. 3 Moreover, by blocking spike protein S1 binding to DPP‐4, recombinant human adenosine deaminase (ADA) could inhibit MERS‐CoV infection of cells transfected with human DPP‐4. 4 Considering the similar outer membrane spike glycoproteins among the coronavirus, it is possible that DPP‐4 might also be a functional receptor of SARS‐CoV‐2. Like angiotensin‐converting enzyme 2 (ACE2), DPP‐4 played important roles in cardiovascular physiological processes and metabolism homeostasis. Here, we review the potential roles of DPP‐4 in COVID‐19, especially in the cardiovascular injury of COVID‐19 patients.
2. HUMAN DPP‐4 MIGHT BE A SARS‐CoV‐2 RECEPTOR
DPP‐4 could present as a membrane binding protein or a cleaved soluble enzyme protein. It has been reported that membrane‐associated human DPP‐4, as a functional MERS‐CoV receptor, interacted with MERS‐CoV through the spike glycoprotein S1b domain to facilitate the entry of MERS‐CoV. 3 Moreover, blocking spike protein S1 or the receptor‐binding domain (RBD) of the MERS‐CoV Spike protein could directly against MERS‐CoV binding to human DPP‐4, thereby prevent MERS‐CoV infection. 5 Considering the similarity among MERS‐CoV and the other coronavirus, it has been speculated that membrane‐associated human DPP‐4 might also be a functional SARS‐CoV‐2 receptor. 6 Moreover, Naveen et al detected that the S1 domain of SARS‐CoV‐2 spike glycoprotein, the key immunoregulatory factor for hijacking and virulence, potentially interacted with the human DPP‐4 by overall homo‐trimer model structure. 7 However, whether DPP‐4 is indeed a direct receptor of SARS‐CoV‐2 remains to be further verified.
3. THE ROLE OF DPP‐4 IN CARDIOVASCULAR INJURY
One of the major functions of DPP‐4 is degrading incretin hormones, including incretin hormones (glucagon‐like peptide‐1 [GLP‐1] and gastric inhibitory polypeptide [GIP]), cytokines, chemokines, neuropeptides and growth factors. 8 It is well‐known that GLP‐1 and GIP promote insulin secretion from the pancreatic β cells and suppress glucagon secretion from other cells. Therefore, the inhibitors of DPP‐4 are widely used to treat diabetes. Moreover, further studies find that DPP‐4 is also involved in the cardiovascular complications of diabetes.
In atherosclerosis, the key pathogenesis is chronic inflammation characterized by accumulation of plaques within the arteries. 9 It was discovered that DPP‐4 enhanced monocytes migration to atherosclerotic plaque and down‐regulated the expression of adiponectin, which promoted inflammation and the formation of atherosclerotic plaques. 10 Moreover, DPP‐4 could down‐regulate stromal‐derived factor 1 (SDF‐1), a chemoattractant for multiple cell types, 11 while inhibition of SDF‐1‐mediated chemical protection and proliferation of hematopoietic stem cells and progenitor cells would inhibit neovascularization and the recovery of tissue damage. 12 In vein endothelial cells, by inhibiting the GLP‐1R signalling pathway, DPP‐4 could also promote the development of atherosclerosis. 13
In addition to atherosclerosis, DPP‐4 also plays a negative role in the process of heart failure. DPP‐4 could degrade brain natriuretic peptide (BNP), which is secreted from the cardiac ventricles in response to stretch, 14 into a less potent metabolite BNP (3‐32), resulting in a loss of the BNP‐mediated protective effects on the heart. Moreover, DPP‐4 could also inhibit the activation of GLP‐1R, which localized in the cardiac atria, reduce the secretion of atrial natriuretic peptide and increase the blood pressure. 15 Thus, DPP‐4 inhibitor therapy may have additional favourable influences on cardiovascular system.
4. THE POTENTIAL EFFECTS OF DPP‐4 ON CARDIOVASCULAR SYSTEM IN COVID‐19
As mentioned above, DPP‐4 might be the functional SARS‐CoV‐2 receptor which facilitates the entry of SARS‐CoV‐2 into the host cells, including cardiomyocytes. Although the presence of SARS‐CoV‐2 in the heart was confirmed by biopsy, as well as myocarditis was observed in certain COVID‐19 patients, the underlying mechanism of SARS‐CoV‐2‐induced cardiac injury still needs further investigation. SARS‐CoV‐2 might damage the cardiomyocytes directly, and the cardiomyocytes might be injured indirectly via the systemic cytokine storm or the interactions between organs.
Evidence from severely ill patients with COVID‐19 suggested that the release of cytokines and chemokines was delayed in respiratory epithelial cells, dendritic cells (DCs) and macrophages at the early stage of SARS‐CoV‐2 infection. 16 Later, it was found that high levels of pro‐inflammatory cytokines (IL‐6, IL‐10 and TNF‐α), lymphopenia (reduced CD4+ and CD8+ T cells) and low levels of antiviral factors (interferon, IFNs) were positively associated with the severity of COVID‐19. 17 Similar findings were also observed in SARS‐CoV and MERS‐CoV infected human airway epithelial cells, THP‐1 cells, human peripheral blood monocyte‐derived macrophages and DCs. 18 Although inflammation initially only damages limited organs, such as the lungs, an over‐activated inflammatory response will spread all over the body rapidly, including the heart. This maybe one of the reasons why the plasma levels of cardiac injury biomarkers usually positively correlated with the plasma levels of inflammatory markers.
During the infection of SARS‐CoV‐2, DPP‐4 might play important roles. First, in MERS‐CoV related studies, Ahmed et al found that spike glycoprotein MERS‐CoV suppressed macrophage responses via DPP‐4‐mediated induction of interleukin‐1 receptor‐associated kinase (IRAK)‐M and peroxisome proliferators‐activated receptor γ (PPARγ) at the early stage of infection. 19 They also demonstrated that indeed the immunosuppressive effects of the S glycoprotein could be ameliorated by sitagliptin, a DPP‐4 inhibitor, via down‐regulating IRAK‐M and PPARγ. Meanwhile, Tetsurou et al found that DPP‐4 could enhance the transcription of IL‐6 and TNF‐α in THP‐1 cells and monocytes. 20 They also found that stimulation with a combination of DPP‐4 and LPS would up‐regulate ERK in the cytosol and c‐Fos, NF‐kB p65, NF‐kB p50 and CUX1 in the nucleus, which enhanced the transcription of TNF‐α and IL‐6 in a DPP‐4 enzyme activity‐dependent manner (Figure 1). Moreover, Hiromura et al demonstrated that DPP‐4 exerted a pro‐inflammatory effect in mouse and human macrophages, and caveolin‐1, a binding protein of DPP‐4, was essential for the anti‐inflammatory effects of DPP‐4 inhibitors. 21
Figure 1.
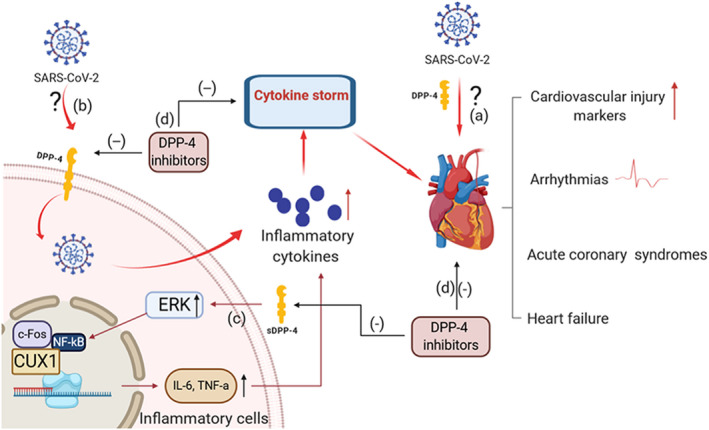
The potential roles of DPP‐4 in COVID‐19 related cardiovascular injury. A, DPP‐4 might promote the production of inflammatory storm mediated by SARS‐CoV‐2 and promote damage to the cardiovascular system. B, DPP‐4 might be the functional SARS‐CoV‐2 receptor which facilitates entry into host cells, assisting the direct myocardial injury of SARS‐CoV‐2. C, DPP‐4 up‐regulated ERK in the cytosol and c‐Fos, NF‐kB p65, NF‐kB p50 and CUX1 in the nucleus enhance the expressions of TNF‐α and IL‐6 in a DPP‐4 enzyme activity‐dependent manner. D, DPP‐4 inhibitors might alleviate the COVID‐19 related cardiovascular injury. CUX1, CUT‐like homeobox 1; ERK, extracellular signal‐regulated kinase; NF‐kb, nuclear factor‐kappa B
Proper inflammation is an essential part of an effective immune response which eliminates the pathogens and ultimately leads to tissue repair and restoration of homeostasis. However, SARS‐CoV‐2 might induce excessive and prolonged cytokine responses in severely ill cases with ARDS or MODS, even death. Although the direct involvement of DPP‐4 in SARS‐CoV‐2 infection needs to be clarified, there is evidence suggesting that DPP‐4 inhibitors might be of potential benefits in patients with COVID‐19.
5. THE FUNCTION OF DPP‐4 INHIBITORS IN COVID‐19
Early control of cytokine storms through immunomodulators, cytokine antagonists or immunoadsorption to reduce the infiltration of inflammatory cells is the key to improve the prognosis and reduce the mortality of COVID‐19 patients.
DPP‐4 inhibitors, such as sitagliptin, alogliptin, vildagliptin, saxagliptin and linagliptin, which selectively inhibit the catalytic activity of cell‐associated and circulating soluble DPP‐4, are widely used drugs against diabetes. DPP‐4 inhibitors may be of potential use for severe COVID‐19 by suppressing T cell proliferation and the production of pro‐inflammatory cytokines. 22 For example, Takeshi et al 23 showed that sitagliptin alleviated lung injury by inhibiting pro‐inflammatory cytokines IL‐1β, TNF‐α and IL‐6 in an experimental model of ARDS, which was also the main cause of SARS‐CoV‐2‐induced death. Ta et al 24 detected that alogliptin reduced Toll‐like receptor 4 (TLR4)‐mediated up‐regulation of IL‐6 and IL‐1β in diabetic ApoE−/− mice. Moreover, sitagliptin interacted with one of the predicted binding sites (V341) of SARS‐CoV‐2, which might modify the SARS‐CoV‐2‐DPP‐4 interaction. 7 In addition, previous studies revealed that DPP‐4 inhibitors could improve cardiovascular function directly.
It was worth mentioning that some reports published recently showed that the role of DPP‐4 inhibitors in type 2 diabetes (T2D) patients with COVID‐19 was controversial. Eleftheriou et al 25 suggested that DPP‐4 inhibitors might be beneficial to COVID‐19 infections with diabetes. In addition, Scheen et al suggested that no negative sign was identified regarding the use of DPP‐4 inhibitors and the outcome of COVID‐19 patients. 26 Moreover, by retrieving information on exposure to DPP‐4 inhibitors among patients with diabetes hospitalized for COVID‐19 at an outbreak hospital in Italy, Fadini et al found no evidence that DPP‐4 inhibitors may be more useful for infected patients with diabetes. 27 Bouhanick et al 28 suggested that DPP4 inhibitors should be used with caution or discontinued upon admission to hospital of unstable patients and critically ill patients. Although the reported effects of DPP‐4 on COVID‐19 patients with diabetes are controversial, these studies mainly focus on the mortality and did not assess the potential effects of DPP‐4 inhibitors on cardiovascular injury in COVID‐19 patients.
Taken together, DPP‐4 might be a functional SARS‐CoV‐2 receptor which facilitates viral entry into host cells, assisting the direct myocardial injury or inflammatory storm production mediated by SARS‐CoV‐2. Moreover, DPP‐4 inhibitors might alleviate the COVID‐19 related cardiovascular injury including arrhythmia, acute coronary syndrome and heart failure (Figure 1).
6. CONCLUSION
In this review, we put forward a hypothesis that DPP‐4 might participate in the process of SARS‐CoV‐2 infection. This may promote COVID‐19 progression towards an hyperinflammatory state, which could damage the cardiovascular system. Meanwhile, DPP‐4 inhibitors could inhibit the over‐activated inflammatory caused by SARS‐CoV‐2 and thus improve cardiovascular function. Moreover, more clinical and laboratory evidence about the effects of DPP‐4 inhibitors on COVID is urgently needed.
CONFLICTS OF INTEREST
None.
AUTHOR CONTRIBUTION
Hengzhi Du: Conceptualization (equal); Investigation (lead); Writing‐original draft (lead). Dao Wen Wang: Funding acquisition (lead); Supervision (equal). Chen Chen: Conceptualization (lead); Supervision (lead); Writing‐review & editing (lead).
ACKNOWLEDGEMENTS
We thank all our colleagues from the Division of Cardiology, Tongji Hospital, as well as all the medical staff fighting against COVID‐19, for their tremendous efforts.
Du H, Wang DW, Chen C. The potential effects of DPP‐4 inhibitors on cardiovascular system in COVID‐19 patients. J Cell Mol Med. 2020;24:10274–10278. 10.1111/jcmm.15674
Funding information
This work was supported by grants from the National Natural Science Foundation of China (nos. 81822002 and 31771264) and the Natural Science Foundation of Hubei Province (2018CFB715). The funders had no role in study design, data collection and analysis, manuscript preparation or decision to publish.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aghili N, Devaney JM, Alderman LO, Zukowska Z, Epstein SE, Burnett MS. Polymorphisms in dipeptidyl peptidase IV gene are associated with the risk of myocardial infarction in patients with atherosclerosis. Neuropeptides. 2012;46:367‐371. [DOI] [PubMed] [Google Scholar]
- 3. Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature. 2013;495:251‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM‐17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167‐176. [DOI] [PubMed] [Google Scholar]
- 5. Tang XC, Agnihothram SS, Jiao Y, et al. Identification of human neutralizing antibodies against MERS‐CoV and their role in virus adaptive evolution. Proc Natl Acad Sci USA. 2014;111:E2018‐E2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):457‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vankadari N, Wilce JA. Emerging WuHan (COVID‐19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54:249‐264. [DOI] [PubMed] [Google Scholar]
- 9. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317‐325. [DOI] [PubMed] [Google Scholar]
- 10. Shah Z, Kampfrath T, Deiuliis JA, et al. Long‐term dipeptidyl‐peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broxmeyer HE, Capitano M, Campbell TB, Hangoc G, Cooper S. Modulation of hematopoietic chemokine effects in vitro and in vivo by DPP‐4/CD26. Stem Cells Dev. 2016;25:575‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhong J, Rajagopalan S. Dipeptidyl peptidase‐4 regulation of SDF‐1/CXCR4 axis: implications for cardiovascular disease. Front Immunol. 2015;6:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sivertsen J, Rosenmeier J, Holst JJ, Vilsboll T. The effect of glucagon‐like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9:209‐222. [DOI] [PubMed] [Google Scholar]
- 14. Krupicka J, Janota T, Kasalova Z, Hradec J. Natriuretic peptides – physiology, pathophysiology and clinical use in heart failure. Physiol Res. 2009;58:171‐177. [DOI] [PubMed] [Google Scholar]
- 15. Kim M, Platt MJ, Shibasaki T, et al. GLP‐1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567‐575. [DOI] [PubMed] [Google Scholar]
- 16. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID‐19. J Infect. 2020;80(6):607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130:2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tynell J, Westenius V, Ronkko E, et al. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte‐derived macrophages and dendritic cells. J Gen Virol. 2016;97:344‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Qahtani AA, Lyroni K, Aznaourova M, et al. Middle east respiratory syndrome corona virus spike glycoprotein suppresses macrophage responses via DPP4‐mediated induction of IRAK‐M and PPARgamma. Oncotarget. 2017;8:9053‐9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikeda T, Kumagai E, Iwata S, Yamakawa A. Soluble CD26/dipeptidyl peptidase IV enhances the transcription of IL‐6 and TNF‐alpha in THP‐1 cells and monocytes. PLoS One. 2013;8:e66520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiromura M, Nohtomi K, Mori Y, et al. Caveolin‐1, a binding protein of CD26, is essential for the anti‐inflammatory effects of dipeptidyl peptidase‐4 inhibitors on human and mouse macrophages. Biochem Biophys Res Commun. 2018;495:223‐229. [DOI] [PubMed] [Google Scholar]
- 22. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;e33213321 10.1002/dmrr.3321. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawasaki T, Chen W, Htwe YM, Tatsumi K, Dudek SM. DPP4 inhibition by sitagliptin attenuates LPS‐induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315:L834‐L845. [DOI] [PubMed] [Google Scholar]
- 24. Ta NN, Schuyler CA, Li Y, Lopes‐Virella MF, Huang Y. DPP‐4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E‐deficient mice. J Cardiovasc Pharmacol. 2011;58:157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eleftheriou P, Amanatidou D, Petrou A, Geronikaki A. In silico evaluation of the effectivity of approved protease inhibitors against the main protease of the novel SARS‐CoV‐2 virus. Molecules. 2020;25(11):2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID‐19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020. 10.1016/j.diabet.2020.05.008. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fadini GP, Morieri ML, Longato E, et al. Exposure to dipeptidyl‐peptidase‐4 inhibitors and COVID ‐19 among people with type 2 diabetes: A case‐control study. Diabetes Obes Metab. 2020. 10.1111/dom.14097. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouhanick B, Cracowski J‐L, Faillie J‐L. Diabetes and COVID‐19. Therapies. 2020. 10.1016/j.therap.2020.05.006. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


