Abstract
The obesity paradox, which suggests a survival advantage for the obese in heart failure (HF) has sparked debate in the medical community. Studies demonstrate a survival advantage in obese patients with HF, including those with advanced HF requiring continuous inotropic support for palliation or disease modifying therapy with a left ventricular assist device (LVAD) or heart transplantation (HT). Importantly, the obesity paradox is affected by the level of cardiorespiratory fitness (CRF). It is now recommended that HF patients with body mass index ≥35 kg/m2 achieve at least 5–10% weight loss, in order to improve symptoms and cardiac function, though more robust data are urgently needed. CRF may be the single best predictor of overall health and small improvements in fitness levels may lead to improved outcomes in HF. In addition to implications of obesity in chronic HF, we also discuss management of obese patients with advanced HF and their implications for therapies such as LVAD implantation and HT.
Keywords: Obesity, Heart failure, Cardiovascular disease, Heart transplant, Left ventricular assist device, Cardiorespiratory fitness
Abbreviations: AA, African American; ACC, American College of Cardiology; AF, Atrial Fibrillation; AHA, American Heart Association; BMI, Body Mass Index; BNP, Brain Natriuretic Peptide; BTT, Bridge to Transplant; CAD, Coronary Artery Disease; Coronavirus Disease 2019, COVID-19; CPX, Cardiopulmonary Exercise Testing; CRF, Cardiorespiratory Fitness; CV, Cardiovascular; CVD, Cardiovascular Disease; DM, Diabetes Mellitus; HF, Heart Failure; HFpEF, Heart Failure with Preserved Ejection Fraction; HFrEF, Heart Failure with Reduced Ejection Fraction; HFSA, Heart Failure Society of America; HR, Hazard Ratio; HT, Heart Transplant; HTN, Hypertension; ISHLT, International Society for Heart Lung Transplantation; LV, Left Ventricular; LVAD, Left Ventricular Assist Device; MET, Metabolic Equivalent; RCT, Randomized Controlled Trial; Vo2, Oxygen Uptake
Introduction
Severe obesity with a body mass index (BMI) ≥ 40 kg/m2 has increased 4-fold, and morbid obesity with BMI ≥ 50 kg/m2 has increased >10-fold in the last quarter century in the US population.1 Obesity is a known independent risk factor for heart failure (HF).2 HF continues to grow due to our aging population, and obesity contributes to an even steeper rise in prevalence of HF which is projected to increase nearly 50% between 2012 and 2030.3 Approximately 29–40% of HF patients are overweight and 30–49% are obese.4 Obesity is significantly more prevalent in the HF with preserved ejection fraction (HFpEF) population compared to those with reduced ejection fraction (HFrEF), with >80% HFpEF patients classified as overweight or obese.5 Although obesity is not considered to result in a cardiomyopathy (either primary or secondary) in isolation, others have suggested that an obesity cardiomyopathy be defined as one occurring entirely or predominantly due to obesity.6 , 7 Although precise mechanisms are not known, excessive adipose accumulation associated with greater absolute lean mass may result in an increase in circulating blood volume, with subsequent increase in cardiac output, cardiac work, systolic blood pressure, lipotoxicity-induced cardiac myocyte injury and myocardial lipid accumulation, all of which have been implicated as potential mechanisms resulting in the cardiomyopathy manifestation.7
Cardiovascular (CV) diseases (CVD) associated with obesity
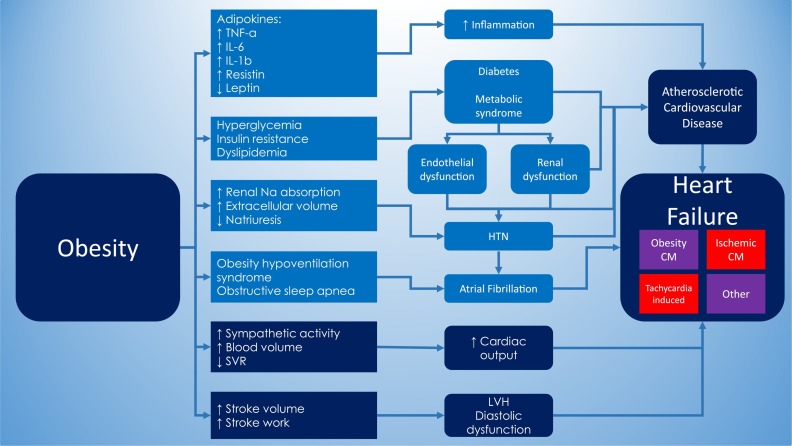
Obesity is strongly associated with multiple forms of CVD and CVD risk factors, such as hypertension (HTN), atherosclerosis, metabolic syndrome, diabetes mellitus (DM), dyslipidemia, systemic inflammation and obstructive sleep apnea,8 , 9 which too are associated with the development of HF. In addition, increased adiposity can independently induce alterations in cardiac structure and function,8 which worsens the impact of obesity on the CV system. Therefore, overweight and obese patients are more likely to develop CVDs, such as coronary artery disease (CAD), HTN, atrial fibrillation (AF) and HF.9 Fig. 1 summarizes some of the pathophysiological changes and CV risk factors associated with obesity and HF.
Fig. 1.
– Pathophysiological changes and CV risk factors associated with obesity and HF.
CM = Cardiomyopathy, HTN = Hypertension, IL = Interleukin, LVH = Left ventricular hypertrophy, Na = Sodium, SVR = Systemic vascular resistance, TNF = Tumor necrosis factor.
AF is more prevalent in obese populations. The Women's Health Study included over 34,000 participants and found a 4.7% increase in AF risk with each 1 kg/m2 unit increase in BMI, and participants in this study who became obese also had a 41% adjusted increase in AF risk compared to those not developing obesity.10 Obese patients also have a higher prevalence of HTN compared with lean subjects.11 Obesity causes increases in renal sodium absorption, impairment in natriuresis and ultimately leads to extracellular volume expansion and elevated blood pressure.12 CAD risk is therefore increased in obesity because of the associations between obesity and HTN, DM, metabolic syndrome and dyslipidemia, all of which too, are risk factors for CAD, which can lead to HF.
Regarding HF, the Framingham Heart Study of 5881 patients reported an increase of HF prevalence of 5% in men and 7% in women for every 1-unit increase in BMI.13 Khan et al.14 studied the association of lifetime risk of CVD with BMI, analyzing 3.2 million person-years follow-up from 1964 to 2015. This study found that obesity was associated with significantly increased risk of CV morbidity and mortality, and that the strongest association was between BMI and HF, with a 5-fold increase in incident HF with morbid obesity. It appears that the increased risk of HF due to obesity can be mediated by improved fitness levels, as demonstrated by Kokkinos et al.15 This study assessed cardiorespiratory fitness and BMI in over 20,000 US male veterans and found that HF risk was significantly higher in the obese category [hazard ratio (HR) 1.22], which was no longer significant following adjustment for metabolic equivalents (METs). When compared to the least fit group, HF risk declined progressively with increased CRF in all BMI categories, suggesting that fitness is more important than BMI regarding HF risk.
Obesity paradox
Obesity is undoubtedly associated with increased risk of CVD, morbidity and mortality. However, several studies have found that obese patients have better survival compared to normal weight and particularly underweight individuals, a phenomenon known as the “obesity paradox”. The obesity paradox has been demonstrated for various forms of CVD and CVD risk factors, such as HTN,16 CAD,17 AF,18 pulmonary arterial hypertension,19 ST-elevation myocardial infarction,20 and HF.21 The obesity paradox is typically seen in mild obesity, and far less in severely obese subjects, who seem to lose any potential survival advantage associated with obesity.8 , 9 , 22 , 23 Recently, however, even those with severe obesity have shown to present favorable outcomes compared to leaner patients.24
Explaining potential mechanisms of the obesity paradox are beyond the scope of this review and have been reported previously.12 , 22 , 25 Some have argued that the observance of an obesity paradox is simply due to research biases, such as lead-time bias,26 as obese patients may be more symptomatic and seek medical care earlier compared to normal weight individuals. Obese HF patients have been shown to have significantly lower brain natriuretic peptide (BNP) levels than non-obese HF patients (205 +/− 22 and 335 +/− 39 pg/ml, respectively; p = 0.0007), despite having similar severity of HF.27 Obese HF patients may therefore lose the physiologic benefits of BNP (vasodilation, natriuresis), which may lead to earlier symptom development and diagnosis of HF. These patients may therefore receive life-saving therapies earlier in their disease course which increases their survival advantage. The obesity paradox may simply be a representation of the poor prognosis in patients with CVD who develop frailty or cachexia (“lean paradox”),11 as low BMI has been associated with increased all-cause and CV mortality in the HF population.28 Patients with higher muscle mass and low adiposity may also be misclassified as “obese”, as many studies rely on BMI to classify obesity, which does not take into account body composition.
Obesity paradox in HF
The obesity paradox was first described by Horwich et al.21 in 2001 in their study of 1203 patients with systolic HF, finding that a BMI >27.8 kg/m2 had significantly improved risk-adjusted heart transplant (HT)-free survival. The worst outcomes in this study were found in the underweight followed by normal weight HF patients. Since this initial finding, several subsequent studies have found evidence of an obesity paradox.
One study involving 108,927 patients with decompensated HF showed a 10% reduction in mortality for every 5-unit increase in BMI.29 Shah et al.30 evaluated 6142 patients with acute HF across four continents and found that for every 5-unit increase in BMI, there was an 11% decrease in 30-day mortality and a 9% decrease at 1 year. Mandviwala et al.31 studied 2501 ambulatory HFpEF patients in 153 Veterans Affairs medical centers over 2 years and found that overweight and obese patients had improved survival compared to normal BMI, however time to HF hospitalization was shorter with increasing BMI. This study showed that increasing BMI was independently associated with an increased risk of HF hospitalization and similar risk for all-cause hospitalization, despite demonstrating an obesity paradox for survival. Despite evidence of a mortality benefit, other studies have also shown increased hospitalization risk in obese patients with both HFpEF and HFrEF.28 , 32 , 33
A recent study has also found evidence of an obesity paradox in HF patients using home inotrope therapy for palliation. Benjamin et al.34 tested the obesity paradox in patients with American College of Cardiology (ACC)/American Heart Association (AHA) stage D HF using continuous IV milrinone. For this study, class 1 obesity indicated a BMI of 30–34.9 kg/m2 and class 2/3 obesity were grouped together (BMI ≥ 35 kg/m2). Compared to non-obese patients, class 1 obesity had a mortality HR of 0.68 while class 2/3 obesity HR was 1.21. In the adjusted model, HR was 0.85 in class 1 obesity and 1.77 in class 2/3. These results are similar to other studies demonstrating evidence of an obesity paradox; a survival advantage tends to exist in those with mild obesity, which is often lost with more severe obesity.
Explanations for the obesity paradox in HF include the increased blood pressure in obese patients which allows them to tolerate more life-saving guideline directed medical therapy.22 Also, HF is a catabolic state, and obese patients with HF may demonstrate a survival advantage due to increased metabolic reserve.35 , 36 However, the use of BMI in the majority of studies demonstrating an obesity paradox has been criticized in the literature.
The use of BMI to classify obesity has been criticized due to the inability of BMI to differentiate between fat, muscle and skeletal weight.9 The current gold standards for assessing body composition are CT and MRI, which are thought to provide the most accurate qualitative and quantitative information on adiposity and lean mass, but their use is limited by expense, availability and radiation.37., 38., 39. Despite its criticisms, BMI is still the most used anthropometric index in the literature and remains a strong predictor of CVD mortality.35
Studies have also found evidence of an obesity paradox in HF patients using measures of adiposity other than BMI, such as waist circumference or measurements of skin folds to estimate total body fat. One study in 209 HF patients used the average of 3 skin folds to measure body fat, finding every 1% absolute increase in percent body fat was associated with >13% reduction in major clinical events.40 Increased waist circumference has also been associated with improved outcomes in advanced HFrEF.37 Conversely, a more recent analysis of HFpEF patients showed that increased waist circumference was associated with approximately 50% increase in both all-cause and CVD mortality, suggesting abdominal obesity and/or visceral adiposity play a more detrimental role in HFpEF.41
Cardiorespiratory fitness
CRF is perhaps the single most important indicator of overall health status, including those who are obese and/or with various forms of CVD, including HF. Hill and Lupton first described CRF as the maximum amount of oxygen uptake that is transported and utilized by working tissue during strenuous exercise.42 Cardiopulmonary exercise testing (CPX) is the gold standard for evaluating CRF,3 and allows for breath-by-breath measurements of oxygen uptake (Vo2), carbon dioxide output and ventilation.43
CPX does have limitations, as peak Vo2 may underestimate CRF in obese patients.44 , 45 Obese patients with preserved CRF may therefore be misclassified as “unfit”, leading to bias in study outcomes. It has been suggested that peak Vo2 adjusted by fat-free mass may overcome this problem. Using this method, a “lean peak Vo2” of >19 was a stronger prognosticator than the commonly used threshold of 14.46 CRF is also often evaluated with simpler exercise stress modalities and the measurement of achieved METs. Kodama et al.47 found that for every 1-MET increase in fitness, all-cause mortality and CAD as well as CVD events were reduced by 13% and 15%, respectively.
The HF-ACTION trial48 supports the notion that even small improvements in CRF may lead to substantial improvements in clinical outcomes. This trial showed that improved CRF achieved with exercise training can improve clinical outcomes such as all-cause mortality and all-cause hospitalizations. Adherence to the exercise program was a significant challenge with a minority of patients (30%) achieving their weekly exercise targets, leading to a much smaller improvement in peak Vo2 than predicted (4%). However, these positive results showing improved clinical outcomes despite lower than expected improvement in fitness levels emphasize how even small improvements in CRF can significantly improve outcomes. A post-hoc analysis found that nearly 50% of HF-ACTION patients were obese, and that exercise training was associated with non-significant reductions in all-cause mortality or hospitalizations in all weight categories (normal weight, overweight and obesity classes I, II and III).49 Importantly, this study found a similar effect of exercise training across BMI categories; exercise was neither more helpful nor more dangerous in overweight or obese patients with HF. HF-ACTION is the largest randomized controlled trial (RCT) to confirm not only the safety but also clinical benefit of exercise training in HFrEF, and supports the current Class I guideline recommendation from the ACC/AHA that exercise training or regular physical activity is safe and effective for patients with HF who are able to participate to improve functional status.50
Multiple authors have found that in fitness studies, an obesity paradox exists for un-fit individuals, and tends to disappear in fit populations. For example, Lavie et al.51 evaluated 2066 patients with systolic HF and found that BMI was a significant predictor of improved survival in the low fitness group defined as peak Vo2 < 14, but not in the high CRF group. More recently, a study from the Henry Ford Exercise Training Project found no relationship between BMI and mortality in the subgroup with exercise tolerance at or above 4 METs, while the obesity paradox was observed in the low exercise capacity group.52 These studies suggest that in the unfit population, obese HF patients have better outcomes, whereas this benefit is lost when compared to groups with preserved fitness. These findings suggested that the obesity paradox exists in unfit HF patients and that preserved CRF may negate the obesity paradox, perhaps by improving prognosis in the non-obese population,37 however, results from HF-ACTION challenge this notion, as exercise training improved outcomes in HFrEF patients across all BMI categories.
Weight loss
Weight loss has been shown to reduce the incidence of HF. For example, Pandey et al.53 found that among patients with DM, higher baseline or sustained improvements in CRF and weight loss were associated with lower risk of HF. However, official recommendations regarding weight loss for patients with HF have been lacking largely due to a paucity of data regarding the safety or efficacy of intentional weight loss in HF. In addition, unintentional weight loss has been shown to be detrimental in patients with HF, even in patients with mild HF in whom weight loss of 5% or more was a significant predictor of CV death or hospitalization for HF.54 At the time of the 2013 ACC/AHA/HFSA (Heart Failure Society of America) HF guidelines, there were no large-scale studies demonstrating safety or efficacy of weight loss with diet, exercise or bariatric surgery in obese HF patients.50
The 2016 European Society of Cardiology guidelines for HF state that obesity should be managed according to guidelines for CVD prevention.2 These guidelines state explicitly that in patients with HF and moderate obesity (BMI < 35 kg/m2), weight loss cannot be recommended, and in those with BMI of 35–45 kg/m2, weight loss may be considered to manage symptoms and exercise capacity.2 The ACC/AHA/HFSA guidelines also provide a class 1 recommendation suggesting that conditions that may lead to or contribute to HF such as obesity, DM, tobacco use and known cardiotoxic agents should be controlled or avoided.7 Overall, the current HF guidelines seem to agree that obesity should be treated or managed in HF patients similarly to the general population, however, firm guidance regarding how to manage obesity in HF patients is lacking, due to the paucity of data.
The 2013 AHA/ACC guidelines for the management of overweight and obesity in adults do not specifically discuss the management of patients with HF.55 According to these guidelines, the need to lose weight is based on BMI ≥ 30 kg/m2, or BMI 25–29.9 kg/m2 with additional risk factors.55 All patients with HF who are overweight or obese therefore meet these guideline definitions for the need to lose weight. The dose-response relationship between weight loss and lipid profiles and blood pressure are shown in Table 1 . There are clear weight loss thresholds that provide meaningful results for certain CVD risk factors, however, thresholds which are safe and effective need to be established for the HF population.
Table 1.
Weight loss for CVD risk factors according to 2013 overweight/obesity guidelines55.
| Those at risk for diabetes | |
| Average weight loss 2.5–5.5 kg | Reduce risk of diabetes by 30–60% |
| Patients with diabetes | |
| 5–10% weight loss | Hemoglobin A1c reduction of 0.6–1% and reduced need for diabetes medications |
| Impact on lipid profile | |
| 3 kg weight loss | Reduces Triglycerides at least 15 mg/dl |
| 5–8 kg weight loss | Low-density Lipoprotein reduced 5 mg/dl and High-density Lipoprotein increases 2–3 mg/dl |
| <3 kg weight loss | Modest and variable improvements |
| Impact on blood pressure | |
| 5% weight loss | Mean reduction of 3 and 2 mmHg in Systolic and Diastolic blood pressure, respectively |
| <5% weight loss | Modest and variable reduction |
Intentional weight loss for the obese can lead to improvements in multiple physiologic abnormalities associated with CVD, such as reductions in circulating blood volume, left ventricular (LV) stroke volume, cardiac output and LV work.12 Small pilot studies in HFrEF patients have suggested that intentional weight loss achieved by dietary modification may improve cardiac systolic and diastolic function, and reduce cardiac chamber sizes,56 , 57 however, RCTs are lacking.
Updated guidance for nutrition and weight management in HF
At the time of publication of the American and European HF guidelines in 2013 and 2016, respectively,2 , 50 evidence supporting an obesity paradox in HF combined with a lack of compelling data suggesting safety and efficacy of weight loss in obese HF patients led to very little guidance regarding weight management. Fortunately, in 2019 the HFSA published a consensus statement regarding nutrition, obesity and cachexia in patients with HF.58 This document states that at least 5–10% weight loss is recommended for HF patients with BMI ≥ 35 kg/m2. However, this recommendation for 5–10% weight loss is based on small RCT studies and the positive effects of weight loss on AF, insulin resistance, LV hypertrophy and reduced incidence of HF. Although robust RCT data are still lacking, this document gives clinicians more confidence recommending weight loss to improve symptoms and functional status in obese HF patients.
This consensus statement also suggests that HF patients should be offered at least 1 session from a registered dietitian nutritionist or other health professional with specialist nutritional knowledge for nutritional evaluation and education. Unfortunately, reimbursement limits access to a registered dietitian nutritionist in the US because the centers for Medicare and Medicaid services only cover these services for patients with DM, chronic kidney disease or renal transplant.58 Table 2 summarizes recommendations for exercise, diet and weight loss according to professional societies. Finally, independent of weight loss, improving quality of diet toward a prudent plant-based diet rich in fruits and vegetables and unsaturated fatty acids has been suggested to be beneficial in patients with HF,59., 60., 61., 62., 63., 64. although large RCTs are required to confirm these preliminary findings. There is an ongoing RCT which will assess potential improvements in CRF following increased consumption of unsaturated fatty acids in HFpEF patients (NCT: 03966755).
Table 2.
Recommendations for exercise and weight loss according to professional societies.
| 2013 and 2017 ACC/AHA/HFSA Guidelines for HF | 2016 ESC Guidelines for HF | 2016 ISHLT Guidelines | 2019 HFSA Consensus Statement | 2013 ACC/AHA Obesity Guidelines | |
|---|---|---|---|---|---|
| Exercise | Exercise training (or regular physical activity) is recommended as safe and effective for patients with HF who are able to participate to improve functional status (Class I) Cardiac rehabilitation can be used in clinically stable patients with HF to improve functional capacity, quality of life and mortality (Class IIa) | Regular aerobic exercise is encouraged to improve functional capacity and symptoms in HF patients (Class I) Regular aerobic exercise is encouraged in stable HFrEF patients to reduce risk of HF hospitalization (Class I) | Not discussed | Physical activity if HF symptoms permit for target weight loss of 5–10% | HF patients not discussed specifically General guidance: Weight loss for BMI ≥ 30 kg/m2 Weight loss for BMI 25–29.9 kg/m2 with additional risk factors |
| Weight loss | Conditions that may lead to or contribute to HF such as obesity, DM, tobacco use and known cardiotoxic agents should be controlled or avoided (Class I) | Obesity should be managed according to guidelines for CVD prevention (no classification) HF patients with BMI < 35 kg/m2: Weight loss cannot be recommended HF patients with BMI 35–45 kg/m2: Weight loss may be considered (no classification) | Pre-transplant BMI > 30 kg/m2 or percent ideal body weight > 140% are associated with poor outcomes after HT Pre-transplant BMI > 30 kg/m2 or ideal body weight > 140%: It is reasonable to recommend weight loss to achieve BMI < 30 or percent ideal body weight < 140% before listing for HT (Class IIa) Pre-transplant BMI > 35 kg/m2: It is reasonable to recommend weight loss to achieve BMI ≤ 35 before listing for HT (Class IIa) Severe (Class III obesity) is a contraindication to HT | At least 5–10% weight loss is recommended for HF patients with BMI ≥ 35 kg/m2a | |
| Bariatric Surgery | Not discussed | Not discussed | Not discussed | Bariatric surgery is reasonable to reduce incident HF and CV mortality for patients with BMI ≥ 40 kg/m2, BMI ≥ 35 kg/m2 and 1 or more obesity related comorbidities, or BMI ≥ 30 and type 2 DM with inadequate glycemic control Selected patients with BMI ≥ 35 kg/m2 and NYHA class II-III with or without an LVAD, whose HT depends on weight loss: Bariatric surgery can be considered within an experienced multidisciplinary team; laparoscopic sleeve preferred to avoid multiple surgical anastomoses of Roux-en-Y | |
| Diet | Sodium restriction is reasonable for patients with symptomatic HF to reduce congestive symptoms (Class IIa) | Not discussed | Not discussed | HF patients should be offered at least 1 session from a registered dietary nutritionist or other health professional with specialist nutritional knowledge for nutritional evaluation and education No specific weight loss diet is recommended Generally, negative energy balance of 500–750 kcal/d or absolute intake of 1200–1500 kcal/d for women and 1500–1800 kcal/d for men, aiming for a loss of 1–2 lb./wk |
ACC = American College of Cardiology, AHA = American Heart Association, BMI = Body Mass Index, CV = Cardiovascular, CVD = Cardiovascular Disease, DM = Diabetes Mellitus, ESC = European Society of Cardiology, HF = Heart Failure, HFrEF = Heart Failure with reduced Ejection Fraction, HFSA = Heart Failure Society of America, HT = Heart Transplant, ISHLT = International Society for Heart and Lung Transplantation, LVAD = Left Ventricular Assist Device, NYHA = New York Heart Association.
No robust RCT data, further study is urgently needed.
Bariatric surgery
Bariatric surgery as a means for weight loss can assist obese patients in reaching their weight loss goal to undergo HT, as obese HF patients are often denied HT until they obtain a certain BMI threshold, often <35 kg/m2.65 However, a large transplant center reported unexpected poor outcomes in 3 patients who underwent Roux-en-Y gastric bypass prior to HT; 2 died due to severe allograft rejection, the third experienced cardiac arrest at home.66 At this same institution, 2 patients who underwent sleeve gastrectomy prior to HT had no evidence of significant rejection during their 10-year and 15-year annual evaluations. Impaired medication absorption may explain these findings post-Roux-en-Y surgery, though the data is sparse.
Previous studies have concluded that bariatric surgery is safe and highly effective in obese patients with severe HF with substantial improvements in cardiac function and symptoms. A retrospective study of 21 HF patients undergoing bariatric surgery and followed-up for 12 months revealed significant weight loss of 26 kg (95% CI; 5–78.5, p < 0.001), significant improvement in LV ejection fraction (10 +/− 11.9%, p < 0.001) and significant reduction of New York Heart Association classification.67
In addition to reduced HF incidence, bariatric surgery has also been shown to reduce other adverse CVD outcomes. A nationwide nested cohort study of 7402 patients (3701 undergoing bariatric surgery and 3701 not undergoing surgery) followed-up for a median 11.2 years found that bariatric surgery was associated with significantly lower incidence of fatal and non-fatal myocardial infarction and stroke, lower incident HF and mortality.68 Bariatric surgery is not discussed in the current American or European HF guidelines, or in the current HT listing guidelines, though these more recent data may suggest bariatric surgery can be used as meaningful therapy, or a bridge to transplant, which has been shown to be safe and effective.65 , 69., 70., 71., 72. Should patients with HF improve significantly after bariatric surgery, would Guideline Directed Medical Therapy need adjusting is not known.
Left ventricular assist device
Because obesity remains a relative contraindication to HT, obese patients with advanced HF may receive an LV assist device (LVAD) as destination therapy or bridge to weight loss in order to become listed for HT. Jaiswal et al.73 analyzed data from 2620 patients from the interagency registry for mechanically assisted circulatory support (INTERMACS) who had BMI ≥ 35 kg/m2, finding that these patients tended to be young, non-white females with dilated cardiomyopathy and received an LVAD as destination therapy. Survival was similar among BMI groups, however, obese patients had significantly higher risk for infection, device malfunction or thrombosis, cardiac arrhythmia and hospital readmissions, but lower bleeding risk. Significant weight loss (10% or more) was only achieved by 18.6% of patients with BMI ≥ 35 kg/m2 during LVAD support. Weight loss worsened bleeding risk without altering risk for infection, arrhythmia and device complications.
Obese patients may be at higher risk of post-surgical and device related complications following LVAD implantation. The HeartWare ADVANCE trial (n = 382) with 48 patients (13%) classified as severely obese found no difference in survival at 2 year follow-up, but had higher risk of driveline infection and acute renal dysfunction.74 In a meta-analysis of 15 observational studies (n = 26,842), Khan et al.75 found that obese patients who received an LVAD had significantly decreased all-cause mortality at both 6 months (RR = 0.79, p < 0.001) and one year (RR = 0.87, p = 0.008) compared with non-obese patients. However, there was no significant difference in all-cause mortality at 2 or 3 years, and obese patients had significantly higher risk of device-related infections, pump thrombosis and right sided HF compared to the non-obese.
Another study including 30 severely obese patients with BMI ≥ 40 kg/m2 who underwent LVAD implantation found higher rates of pump thrombosis and acute kidney injury in the severely obese group.76 Though the severely obese patients in this study had higher rates of complications, no differences were found in 30-day or 1-year survival, even after adjusting for age and clinical risk factors. Considering the findings of the meta-analysis by Khan et al.75 in which there was evidence of an obesity paradox in the short-term following LVAD implantation but not at 2 or 3 years, it would be interesting to see if the severely obese patients in this study would also lose their survival advantage at 2 or 3 years or even demonstrate increased mortality at longer-term follow-up.
A less invasive surgical approach for LVAD implantation using a left thoracotomy and upper hemisternotomy has been investigated in a trial of 42 patients, 27 of whom had BMI < 30 kg/m2 and 15 patients had BMI ≥ 30 kg/m2.77 The obese and non-obese groups were similar except the obese group was significantly younger (58.5 vs 46.1 years). Perioperative and short-term outcomes were similar except longer ventilator time in the obese cohort, and one obese patient experienced wound dehiscence. 6-month survival was comparable between cohorts, though the age difference between groups may have led to bias. Further evidence is required to determine if this less invasive surgical approach for LVAD implantation can decrease post-operative and device complications in the obese population.
LVAD has been used as a bridge to HT (BTT) due to organ scarcity and is capable of improving wait list survival, however, LVAD used as BTT confers a significantly higher risk of early post-transplantation mortality.78 When comparing LVAD BTT patients to those who were medically managed, BMI > 30 kg/m2 was significantly associated with 2-fold increased risk of 1-year mortality.78 While LVAD use as BTT in obese HF patients may be with the intention of affording time to achieve sufficient weight loss to be listed for HT, LVAD implantation has also been associated with weight gain.79 , 80 Some studies have found that LVAD implantation combined with bariatric surgery can be a safe method to bridge obese patients to HT using sleeve gastrectomy or gastric banding.81 , 82 However, these are very small studies (n = 5, combined) and require further research before providing firm conclusions.
Heart transplant
A pre-HT BMI > 35 kg/m2 is associated with worse outcomes after HT.83 While some studies have shown improved survival in overweight HT patients, these patients also suffered from more graft rejections, coronary artery vasculopathy and DM.84 Because HF incidence continues to increase while organs remain in limited supply, optimal strategies for improving outcomes following HT are of utmost importance. Alyaydin et al.85 analyzed 172 HT patients for mean 13.2 years and analyzed their patients as survivors and non-survivors. It was found that non-survivors obtained hearts from more obese donors, which would suggest that non-survivors were more likely to be obese, as recipients and donors are often matched by body size to obtain an appropriately sized organ. Hence, these findings may also suggest that obesity is associated with increased mortality following HT.
The 2016 International Society for Heart Lung Transplantation (ISHLT) updated guidelines state that overall, pre-transplant BMI > 30 kg/m2 or percent ideal body weight > 140% are associated with poor outcomes after HT, and that it is reasonable to recommend weight loss to achieve BMI < 30 kg/m2 or percent ideal body weight < 140% target before listing for transplant (Class IIa).83 Obese patients who undergo HT have earlier high-grade acute rejection and higher 5-year mortality compared with normal weight or overweight HT patients.86 The ISHLT HT candidacy guidelines support weight reduction to achieve optimal post-HT outcomes.83 Severe (class III obesity) is a contraindication for HT and efforts must be made to achieve BMI ≤ 30 kg/m2 before listing for HT.83 This weight loss goal can be achieved with the aid of a dietary nutritionist, through dietary modification, exercise or bariatric surgery, though Roux-en-Y gastric bypass has been associated with worse outcomes compared to sleeve gastrectomy in some large HT centers, and clear thresholds for safe and effective weight loss in the obese HF population are needed.
Racial, socioeconomic Factors, COVID-19 and the obesity-HF connection
The syndrome of Coronavirus disease 2019 (COVID-19) is intricately linked with CVD complications and particularly HF (both in terms of worsening pre-existing disease as well as in the development of de-novo disease driven by hyperinflammation).87 Similarly, obesity is associated with unfavorable outcomes in COVID-19 and confers worse prognosis and greater propensity for severe illness.88 , 89 Thus, it is likely that the obese may be more predisposed to severe COVID-19 illness due to the underlying influence on the CV continuum and HF. Socio-economic factors and specifically African American (AA) race adds another complex layer of risk with the observation that 2/3rd of COVID-19 related deaths have occurred in the AA populations of Louisiana, Alabama, Milwaukee county, Chicago, Philadelphia and Detroit, among other regions.87 , 90 , 91 In a recent analysis from Louisiana, AA race was not associated with a higher in-hospital mortality than white race, after accounting for differences in socioeconomic and demographic factors.92 Several reasons for these disproportionate outcomes have been suggested, including the higher rates of obesity, HTN and DM among the AA population, which increase HF, as well as worsen outcomes in COVID-19.90 It is very likely that the confluence of these intertwined factors are not just restricted to the COVID-19 crisis but have simply been magnified by the attention upon the pandemic. Among other suggested causes for these observations is the issue of medical mistrust among AA and other minority populations as well as lower healthcare access, which may lead to delayed diagnosis and worse prognosis.93 Correcting racial disparities in healthcare will benefit society manifold; primarily from a humanitarian perspective, as well as economically, considering the financial burden of a pandemic.
Addressing some of these racial issues, as well as many other lifestyle factors, including physical inactivity, sedentary behavior and obesity, is a critical factor in the prevention of HF. We recently demonstrated that a structured dietary and physical activity program in an underserved, mostly AA population, produced a 5% weight loss and 7.1% in those with 80% adherence,94 which should go a long way to lessen obesity, HTN, DM, and HF related morbidity and mortality.
Conclusion
Obesity is associated with HF, and weight loss has been shown to reduce incident HF. Current guidelines provide little guidance regarding weight loss in the obese HF population, however, the HFSA recently published a consensus statement in which 5–10% weight loss is recommended for HF patients with BMI ≥ 35 kg/m2. This recommendation is based on small RCTs and more robust data is urgently needed. There are clear weight loss thresholds that provide meaningful results for certain CVD risk factors (e.g. blood pressure, lipids, insulin resistance), however, thresholds which are safe and effective need to be clearly established for the HF population.
Exercise is generally considered to be safe and effective in the HF population. The HF-ACTION trial found non-significant reductions in all-cause mortality or hospitalizations across all BMI categories, suggesting that exercise training is safe, and may modestly benefit overweight and obese HF patients in terms of weight loss and quality of life. As such, HF-ACTION was the first RCT to confirm not only the safety but also the clinical benefit of exercise training in HFrEF, supporting the current guideline recommendations for regular physical activity in the HF population. Regular physical activity receives a class I recommendation from both the American and European HF guidelines.
Obesity is a relative contraindication to HT and obese patients tend to be at higher risk for post-surgical and device related complications following LVAD implantation. Though some studies have shown a short-term survival advantage in obese patients following LVAD implantation, long-term data are not available, and this advantage may be lost at 2–3 years. Other studies have shown improved survival in overweight HT patients, however these patients also suffered from increased complications such as graft rejection and coronary vasculopathy. Bariatric surgery has been shown to improve outcomes in some patients with advanced HF and in those undergoing LVAD implantation, though more data is needed in order to establish firm conclusions.
Disclosures/Conflicts of Interest
Dr. Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association and by the Clinical and Translational Science Awards Program UL1TR002649 from National Institutes of Health to Virginia Commonwealth University. Dr. Mehra is a consultant for Abbott (fees paid to Brigham and Women's Hospital), Portola, Bayer, Baim Institute for Clinical Research and Triple Gene; a trial steering committee member for Abbott, Medtronic, Roivant and Janssen; a scientific advisory board member for Leviticus, NupulseCV and FineHeart; and a DSMB member for Mesoblast. In addition he is Editor in Chief of the Journal of Heart and Lung Transplantation.
References
- 1.Piché M.E., Tchernof A., Després J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Carbone S., Lavie C.J., Elagizi A., Arena R., Ventura H.O. The impact of obesity in heart failure. Heart Fail Clin. 2020;16(1):71–80. doi: 10.1016/j.hfc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt B., Aguilar D., Deswal A., et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation. 2016;134:e535–e578. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 5.Lewis G.A., Schelbert E.B., Williams S.G., et al. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70(17):2186–2200. doi: 10.1016/j.jacc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Maron B.J., Towbin J.A., Thiene G., et al. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113:1807–1816. doi: 10.1161/circulationaha.106.174287. [DOI] [PubMed] [Google Scholar]
- 7.Yancy C.W., Jessup M., Bozkurt B., et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 8.Oktay A.A., Lavie C.J., Kokkinos P.F., Parto P., Pandey A., Ventura H.O. The interaction of cardiorespiratory fitness with obesity and the obesity paradox in cardiovascular disease. Prog Cardiovasc Dis. 2017;60(1):30–44. doi: 10.1016/j.pcad.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Lavie C., Arena R., Alpert M., Milani R., Ventura H. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol. 2018;15(1):45–56. doi: 10.1038/nrcardio.2017.108. [DOI] [PubMed] [Google Scholar]
- 10.Tedrow U.B., Conen D., Ridker P.M., et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation. The WHS (Women's Health Study) J Am Coll Cardiol. 2010;55(21):2319–2327. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavie C.J., McAuley P.A., Church T.S., Milani R.V., Blair S.N. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63(14):1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Parto P., Lavie C.J., Arena R., Bond S., Popovic D., Ventura H.O. Body habitus in heart failure: Understanding the mechanisms and clinical significance of the obesity paradox. Future Cardiol. 2016;12(6):639–653. doi: 10.2217/fca-2016-0029. [DOI] [PubMed] [Google Scholar]
- 13.Kenchaiah S., Evans J.C., Levy D., et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 14.Khan S.S., Ning H., Wilkins J.T., et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–287. doi: 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokkinos P., Faselis C., Franklin B., et al. Cardiorespiratory fitness, body mass index and heart failure incidence. Eur J Heart Fail. 2019;21(4):436–444. doi: 10.1002/ejhf.1433. [DOI] [PubMed] [Google Scholar]
- 16.Uretsky S., Messerli F.H., Bangalore S., et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120(10):863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Sierra-Johnson J., Romero-Corral A., Somers V.K., et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15(3):336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 18.Lavie C.J., Milani R.V., Ventura H.O. Obesity and cardiovascular disease. Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal M.A., Shah M., Garg L., Lavie C.J. Relationship between obesity and survival in patients hospitalized for hypertensive emergency. Mayo Clin Proc. 2018;93(2):263–265. doi: 10.1016/j.mayocp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Neeland I.J., Das S.R., Simon D.N., et al. The obesity paradox, extreme obesity, and long-term outcomes in older adults with ST-segment elevation myocardial infarction: Results from the NCDR. Eur Hear J - Qual Care Clin Outcomes. 2017;3:183–191. doi: 10.1093/ehjqcco/qcx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwich T.B., Fonarow G.C., Hamilton M.A., MacLellan W.R., Woo M.A., Tillisch J.H. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795. doi: 10.1016/S0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 22.Lavie C.J., Sharma A., Alpert M.A., et al. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58(4):393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Lavie C.J., Oktay A.A., Milani R.V. The obesity paradox and obesity severity in elderly STEMI patients. Eur Hear J - Qual Care Clin Outcomes. 2017;3(3):166–167. doi: 10.1093/ehjqcco/qcx018. [DOI] [PubMed] [Google Scholar]
- 24.Vaishnav J., Chasler J.E., Lee Y.J., et al. Highest obesity category associated with largest decrease in N-terminal pro-B-type natriuretic peptide in patients hospitalized with heart failure with preserved ejection fraction. J Am Heart Assoc. 2020;9(15) doi: 10.1161/JAHA.119.015738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbone S., Canada J.M., Billingsley H.E., Siddiqui M.S., Elagizi A., Lavie C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc Health Risk Manag. 2019;15:89–100. doi: 10.2147/VHRM.S168946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Schutter A., Lavie C.J., Milani R.V. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease-the obesity paradox. Prog Cardiovasc Dis. 2014;56(4):401–408. doi: 10.1016/j.pcad.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Mehra M.R., Uber P.A., Park M.H., et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43(9):1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A., Lavie C.J., Borer J.S., et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115(10):1428–1434. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Fonarow G.C., Srikanthan P., Costanzo M.R., Cintron G.B., Lopatin M. ADHERE scientific advisory committee and investigators. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the acute decompensated heart failure national registry. Am Heart J. 2007;153(1):74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Shah R., Gayat E., Januzzi J.L., Jr., et al. Body mass index and mortality in acutely decompensated heart failure across the world: A global obesity paradox. J Am Coll Cardiol. 2014;63(8):778–785. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 31.Mandviwala T.M., Basra S.S., Khalid U., et al. Obesity and the paradox of mortality and heart failure hospitalization in heart failure with preserved ejection fraction. Int J Obes. 2020 doi: 10.1038/s41366-020-0563-1. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Cox Z.L., Lai P., Lewis C.M., Lenihan D.J. Limits of the obesity paradox: obese patients with heart failure are at higher risk of hospitalization. J Card Fail. 2017;23(8S):S110–S111. doi: 10.1016/j.cardfail.2017.07.324. [DOI] [Google Scholar]
- 33.Carbone S., Lavie C.J. Disparate effects of obesity on survival and hospitalizations in heart failure with preserved ejection fraction. Int J Obes (Lond) 2020;44(7):1543–1545. doi: 10.1038/s41366-020-0579-6. [DOI] [PubMed] [Google Scholar]
- 34.Benjamin M.M., Sundararajan S., Sulaiman S., Kindel T., Joyce D., Mohammed A.A. Testing the obesity paradox in patients on long-term milrinone infusion for end-stage heart failure. Am J Cardiovasc Dis. 2019;9(4):59–64. [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega F.B., Lavie C.J., Blair S.N. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 36.Kachur S., Lavie C.J., De Schutter A., Milani R.V., Ventura H.O. Obesity and cardiovascular diseases. Minerva Med. 2017;108(3):212–228. doi: 10.23736/S0026-4806.17.05022-4. [DOI] [PubMed] [Google Scholar]
- 37.Horwich T.B., Fonarow G.C., Clark A.L. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61(2):151–156. doi: 10.1016/j.pcad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Cornier M.A., Després J.P., Davis N., et al. Assessing adiposity: A scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 39.Carbone S., Billingsley H.E., Rodriguez-Miguelez P., et al. Lean mass abnormalities in heart failure: The role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol. 2019 doi: 10.1016/j.cpcardiol.2019.03.006. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie C.J., Osman A.F., Milani R.V., Mehra M.R. Body composition and prognosis in chronic systolic heart failure: The obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/S0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 41.Tsujimoto T., Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70:2739–2749. doi: 10.1016/j.jacc.2017.09.1111. [DOI] [PubMed] [Google Scholar]
- 42.Hill A.V., Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. QJM. 1923;16(62):135–171. doi: 10.1093/qjmed/os-16.62.135. [DOI] [Google Scholar]
- 43.Balady G.J., Arena R., Sietsema K., et al. Clinician's guide to cardiopulmonary exercise testing in adults: A scientific statement from the American heart association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 44.Krachler B., Savonen K., Komulainen P., Hassinen M., Lakka T.A., Rauramaa R. VO2max/kg is expected to be lower in obese individuals! Int J Cardiol. 2015 doi: 10.1016/j.ijcard.2015.04.100. Epub2015 Apr 15. [DOI] [PubMed] [Google Scholar]
- 45.Carbone S., Popovic D., Lavie C.J., Arena R. Obesity, body composition and cardiorespiratory fitness in heart failure with preserved ejection fraction. Future Cardiol. 2017;13(5):451–463. doi: 10.2217/fca-2017-0023. [DOI] [PubMed] [Google Scholar]
- 46.Mancini D.M., Eisen H., Kussmaul W., Mull R., Edmonds L.H., Wilson J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. doi: 10.1161/01.CIR.83.3.778. [DOI] [PubMed] [Google Scholar]
- 47.Kodama S., Saito K., Tanaka S., et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor C.M., Whellan D.J., Lee K.L., et al. Efficacy and safety of exercise training in patients with chronic heart failure HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwich T.B., Broderick S., Chen L., et al. Relation among body mass index, exercise training, and outcomes in chronic systolic heart failure. Am J Cardiol. 2011;108(12):1754–1759. doi: 10.1016/j.amjcard.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 51.Lavie C.J., Cahalin L.P., Chase P., et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88(3):251–258. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAuley P.A., Keteyian S.J., Brawner C.A., et al. Exercise capacity and the obesity paradox in heart failure: the FIT (Henry Ford exercise testing) project. Mayo Clin Proc. 2018:S0025–S6196. doi: 10.1016/j.mayocp.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 53.Pandey A., Patel K.V., Bahnson J.L., et al. Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: An analysis from the look AHEAD trial. Circulation. 2020;141(16):1295–1306. doi: 10.1161/CIRCULATIONAHA.119.044865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okuhara Y., Asakura M., Orihara Y., et al. Effects of weight loss in outpatients with mild chronic heart failure: Findings from the J-MELODIC study. J Card Fail. 2019;25(1):44–50. doi: 10.1016/j.cardfail.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Jensen M.D., Ryan D.H., Apovian C.M., et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults. J Am Coll Cardiol. 2013;2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Alpert M.A., Terry B.E., Mulekar M., et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80(6):736–740. doi: 10.1016/S0002-9149(97)00505-5. [DOI] [PubMed] [Google Scholar]
- 57.Alpert M.A., Lambert C.R., Panayiotou H., et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76(16):1194–1197. doi: 10.1016/S0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 58.Vest A.R., Chan M., Deswal A., et al. Nutrition, obesity, and cachexia in patients with heart failure: A consensus statement from the Heart Failure Society of America scientific statements committee. J Card Fail. 2019;25(5):380–400. doi: 10.1016/j.cardfail.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Hummel S.L., Karmally W., Gillespie B.W., et al. Home-delivered meals postdischarge from heart failure hospitalization. Circ Heart Fail. 2018;11(8) doi: 10.1161/CIRCHEARTFAILURE.117.004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen K.E., Billingsley H.E., Carbone S. Nutrition, heart failure, and quality of life: Beyond dietary sodium. JACC Heart Fail. 2020;8(9):765–769. doi: 10.1016/j.jchf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billingsley H., Rodriguez-Miguelez P., Del Buono M.G., Abbate A., Lavie C.J., Carbone S. Lifestyle interventions with a focus on nutritional strategies to increase cardiorespiratory fitness in chronic obstructive pulmonary disease, heart failure, obesity, sarcopenia, and frailty. Nutrients. 2019;11(12):2849. doi: 10.3390/nu11122849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carbone S., Billingsley H.E., Canada J.M., et al. Unsaturated fatty acids to improve cardiorespiratory fitness in patients with obesity and HFpEF: The UFA-preserved pilot study. JACC Basic to Transl Sci. 2019;4(4):563–565. doi: 10.1016/j.jacbts.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carbone S., Canada J.M., Buckley L.F., et al. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. JACC Basic to Transl Sci. 2017;2(5):513–525. doi: 10.1016/j.jacbts.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Billingsley H.E., Hummel S.L., Carbone S. The role of diet and nutrition in heart failure: A state-of-the-art narrative review. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.08.004. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choudhury R.A., Foster M., Hoeltzel G., et al. Bariatric surgery for congestive heart failure patients improves access to transplantation and long-term survival. J Gastrointest Surg. 2020 doi: 10.1007/s11605-020-04587-6. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Ahluwalia M., Givertz M.M., Mehra M.R. Bariatric surgery and heart transplantation outcomes: A note of caution. J Hear Lung Transplant. 2020;39(9):986–987. doi: 10.1016/j.healun.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Yang T.W.W., Johari Y., Burton P.R., et al. Bariatric surgery in patients with severe heart failure. Obes Surg. 2020 doi: 10.1007/s11695-020-04612-2. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 68.Moussa O., Ardissino M., Heaton T., et al. Effect of bariatric surgery on long-term cardiovascular outcomes: A nationwide nested cohort study. Eur Heart J. 2020;41(28):2660–2667. doi: 10.1093/eurheartj/ehaa069. [DOI] [PubMed] [Google Scholar]
- 69.Lim C.P., Fisher O.M., Falkenback D., et al. Bariatric surgery provides a “bridge to transplant” for morbidly obese patients with advanced heart failure and may obviate the need for transplantation. Obes Surg. 2016;26(3):486–493. doi: 10.1007/s11695-015-1789-1. [DOI] [PubMed] [Google Scholar]
- 70.daSilva-deAbreu A., Alhafez B.A., Curbelo-Pena Y., et al. Bariatric surgery in obese patients with ventricular assist devices. BMC Res Notes. 2020;13:382. doi: 10.1186/s13104-020-05221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.daSilva-deAbreu A., Garikapati K., Alhafez B.A., et al. Laparoscopic sleeve gastrectomy in patients with obesity and ventricular assist devices: A comprehensive outcome analysis. Obes Surg. 2020 doi: 10.1007/s11695-020-04948-9. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 72.daSilva-deAbreu A., Alhafez B.A., Patel H., et al. Laparoscopic sleeve gastrectomy in patients with ventricular assist devices, beyond just bridging to heart transplantation. Obes Surgs. 2020 doi: 10.1007/s11695-020-04966-7. In press. [DOI] [PubMed] [Google Scholar]
- 73.Jaiswal A., Truby L.K., Chichra A., et al. Impact of obesity on ventricular assist device outcomes: Obesity and VAD outcomes. J Card Fail. 2020;26(4):287–297. doi: 10.1016/j.cardfail.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kiernan M.S., Najjar S.S., Vest A.R., et al. Outcomes of severely obese patients supported by a centrifugal-flow left ventricular assist device: Outcomes of HVAD patients with severe obesity. J Card Fail. 2020;26(2):120–127. doi: 10.1016/j.cardfail.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Khan M.S., Yuzefpolskaya M., Memon M.M., et al. Outcomes associated with obesity in patients undergoing left ventricular assist device implantation: A systematic review and meta-analysis. ASAIO J. 2020;66(4):401–408. doi: 10.1097/MAT.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 76.Lee A.Y., Tecson K.M., Lima B., et al. Durable left ventricular assist device implantation in extremely obese heart failure patients. Artif Organs. 2019;43(3):234–241. doi: 10.1111/aor.13380. [DOI] [PubMed] [Google Scholar]
- 77.Voorhees H.J., Sorensen E.N., Pasrija C., Kaczorowski D., Griffith B.P., Kon Z.N. Outcomes of obese patients undergoing less invasive LVAD implantation. J Card Surg. 2019;34(12):1465–1469. doi: 10.1111/jocs.14307. [DOI] [PubMed] [Google Scholar]
- 78.Truby L.K., Farr M.A., Garan A.R., et al. Impact of bridge to transplantation with continuous-flow left ventricular assist devices on posttransplantation mortality. Circulation. 2019;140(6):459–469. doi: 10.1161/circulationaha.118.036932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emani S., Brewer R.J., John R., et al. Patients with low compared with high body mass index gain more weight after implantation of a continuous-flow left ventricular assist device. J Hear Lung Transplant. 2013;32(1):31–35. doi: 10.1016/j.healun.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 80.Mano A., Kilic A., Lampert B.C., Smith S.A., Whitson B., Hasan A.K. Impact of change in body mass index on outcomes after left ventricular assist device implantation in obese patients. ASAIO J. 2019;65(7):668–673. doi: 10.1097/MAT.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 81.Greene J., Tran T., Shope T. Sleeve gastrectomy and left ventricular assist device for heart transplant. JSLS J Soc Laparoendosc Surg. 2017;21(3) doi: 10.4293/JSLS.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gill Combined ventricular assist device placement with adjustable gastric band (VAD-BAND): A promising new technique for morbidly obese patients awaiting potential cardiac transplantation. J Clin Med Res. 2012;4(2):127–129. doi: 10.4021/jocmr814w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mehra M.R., Canter C.E., Hannan M.M., et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35(1):1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 84.Jalowiec A., Grady K.L., White-Williams C. Clinical outcomes in overweight heart transplant recipients. Hear Lung J Acute Crit Care. 2016;45(4):298–304. doi: 10.1016/j.hrtlng.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alyaydin E., Welp H., Reinecke H., Tuleta I. Predisposing factors for late mortality in heart transplant patients. Cardiol J. 2020 doi: 10.5603/cj.a2020.0011. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavie C.J., Mehra M.R., Ventura H.O. Body composition and advanced heart failure therapy: Weighing the options and outcomes. JACC Hear Fail. 2016;4(10):769–771. doi: 10.1016/j.jchf.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Mehra M.R., Ruschitzka F. COVID-19 illness and heart failure: A missing link? JACC Hear Fail. 2020;8(6):512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and outcomes in COVID-19: When an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma A., Garg A., Rout A., Lavie C.J. Association of obesity with more critical illness in COVID-19. Mayo Clin Proc. 2020;95(9):2036–2048. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanchis-Gomar F., Lippi G., Lavie C.J. Why is COVID-19 especially impacting the African American population? Ann Med. 2020;52(7):331–333. doi: 10.1080/07853890.2020.1808695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferdinand K.C., Nasser S.A. African-American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75(21):2746–2748. doi: 10.1016/j.jacc.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Price-Haywood E.G., Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Evans M.K. COVID's color line — infectious disease, inequity, and racial justice. N Engl J Med. 2020;383:408–410. doi: 10.1056/NEJMp2019445. [DOI] [PubMed] [Google Scholar]
- 94.Katzmarzyk P.T., Martin C.K., Newton R.L., et al. Weight loss in underserved patients - a cluster-randomized trial. N Engl J Med. 2020;383(10):909–918. doi: 10.1056/NEJMoa2007448. [DOI] [PMC free article] [PubMed] [Google Scholar]