Abstract
Background and aims
Corona virus diseases 2019 (COVID-19) pandemic spread rapidly. Growing evidences that overweight and obesity which extent nearly a third of the world population were associated with severe COVID-19. This study aimed to explore the association and risk of increased BMI and obesity with composite poor outcome in COVID-19 adult patients.
Methods
We conducted a systematic literature search from PubMed and Embase database. We included all original research articles in COVID-19 adult patients and obesity based on classification of Body Mass Index (BMI) and composite poor outcome which consist of ICU admission, ARDS, severe COVID-19, use of mechanical ventilation, hospital admission, and mortality.
Results
Sixteen studies were included in meta-analysis with 9 studies presented BMI as continuous outcome and 10 studies presented BMI as dichotomous outcome (cut-off ≥30 kg/m2). COVID-19 patients with composite poor outcome had higher BMI with mean difference 1.12 (95% CI, 0.67–1.57, P < 0.001). Meanwhile, obesity was associated with composite poor outcome with odds ratio (OR) = 1.78 (95% CI, 1.25–2.54, P < 0.001) Multivariate meta-regression showed the association between BMI and obesity on composite poor outcome were affected by age, gender, DM type 2, and hypertension.
Conclusion
Obesity is a risk factor of composite poor outcome of COVID-19. On the other hand, COVID-19 patients with composite poor outcome have higher BMI. BMI is an important routine procedure that should always be assessed in the management of COVID-19 patients and special attention should be given to patients with obesity.
Keywords: Body mass index, Covid-19, Obesity, Poor outcome
1. Introduction
The World Health Organization (WHO) has announced COVID-19 outbreak caused by SARS-CoV-2 as a global pandemic on March 11, 2020. It has rapidly spread across China and many other countries since its first emergence in Wuhan, China on December 2019. Currently, there are 21.294.845 confirmed cases globally with the most cases in the European and American regions and total 761.779 deaths [1].
COVID-19 exhibits high morbidity and mortality with fatal complications such as ARDS, acute renal injury, shock, and acute cardiac injury [2,3]. The elderly, people with underlying diseases, and specific health conditions are more susceptible to infection and prone to serious outcomes [4].
Obesity results in a dysregulated immune response to respiratory infections [5]. Obesity also has a great impact on normal lung function. Fat deposits in obesity alter the mechanics of the lungs and chest wall, thus reduce the compliance of the lungs. Many studies also reported excess adiposity is associated with increased production of inflammatory cells and induce airway hyperresponsiveness (AHR). In a patient with ARDS, the work of breathing is increased to meet the body’s oxygen need. This physiological response is complicated by obese state. Thus, obesity may contribute to the increased morbidity associated with obesity in COVID-19 infections [6].
2. Material and methods
2.1. Study selection and eligibility criteria
We included all original research articles with adult COVID-19 patients >18 years old and had information related to body mass index both as categorical (cut-off ≥30 kg/m2) and continuous with related poor outcome. Poor outcome was defined as the presence of one of the following criteria: ICU admission, ARDS, severe COVID-19, use of mechanical ventilation, hospital admission, and mortality. No ethical approval will be needed because data from previous published studies in which informed consent was obtained by primary investigators will be retrieved and analysed. Original research not published in English language, pediatrics subjects (age <18 years old), not available in full text, review articles, and case reports were excluded from this study.
2.2. Literature search
We conducted a systematic literature search from PubMed and Embase database. We use keywords: (1) “COVID-19” OR “SARS-CoV-2 “AND “Obesity”, (2) “COVID-19” OR “SARS-CoV-2” AND “Body Mass Index” OR “BMI”, and (3) “COVID-19” OR “SARS-CoV-2” AND “Malnutrition”. We also performed hand searching and explored the queries through the references cited in some articles in order to include all relevant published articles. We conducted literature research from April 11th and finalized on July 28th, 2020. Duplicate results were removed. The remaining studies were screened for relevance by title and abstract. Further reading and investigation according to inclusion and exclusion was done to search potential relevance studies. The reporting of this systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.3. Data extraction
Data extraction was performed using standardized forms that include generic information (first author, year, place), sample size, study design, age, gender, and measured outcome. Obesity was defined as BMI ≥30 kg/m2. Some studies with limited number of poor outcome was generalized as other composite poor outcome. In addition, we also included age and comorbid factors which consist of hypertension, DM type 2, chronic kidney disease (CKD), malignancy, cardiovascular disease (chronic heart failure and coronary artery disease), lung disease (asthma, pulmonary hypertension, chronic obstructive pulmonary disease) for meta-regression analysis. Data extraction was performed independently by two authors (AYS and P).
2.4. Statistical analysis
Stata version 16 was used for data collection and meta-analysis. Effect size for BMI as continuous outcome was calculated using mean difference. Effect size for BMI as dichotomous outcome (cut-off ≥ 30 kg/m2) were reported as odds ratio using restricted maximum likelihood (REML) with random effect model despite of heterogeneity. Both BMI as continuous and dichotomous outcome reported with its 95% confidence interval. Statistical significance set at ≤0.05 with a two-tailed hypothesis. Funnel plots drawn to evaluate the publication bias was conducted when there are at least 10 included studies. Further test for funnel plot asymmetry using Egger’s test for continous effect size and Harbord’s test for dichotomous effect size were conducted when publication bias indicated. Meta-regression analysis was performed to examine the impact of moderators which consist of age and other comorbid factors.
3. Results
3.1. Study selection
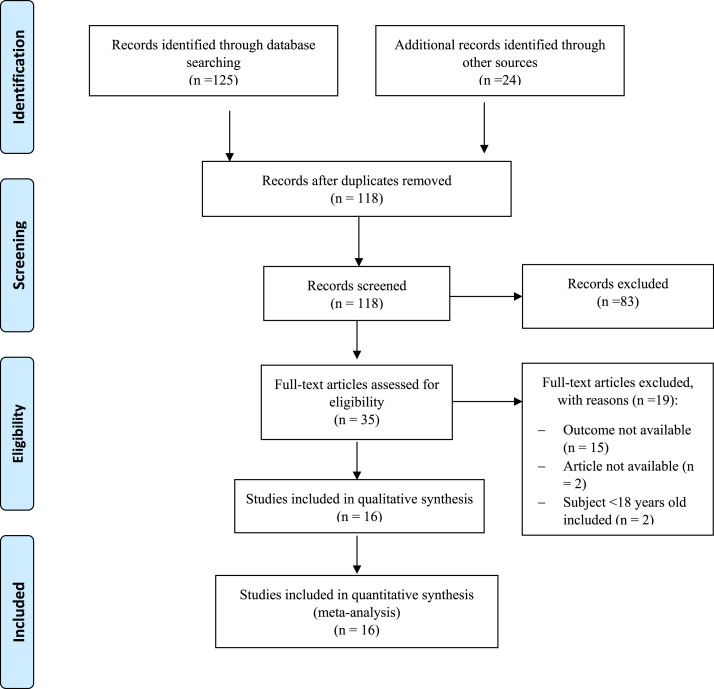
Initial search yield 125 records from an electronic database and 24 records from hand-searching, respectively. Thirty-one records identified as duplicate. As much as 82 records were excluded after screening the title or abstract. After evaluating and assessing 36 potential studies, 20 studies were removed because: outcome of interest not available (n = 15), article not available (n = 2), and subjects didn’t meet inclusion criteria (n = 3). Sixteen Studies were included in this meta-analysis with 9 studies presented BMI as continuous outcome and 10 studies presented BMI as dichotomous outcome. The selection process is shown in Fig. 1 .
Fig. 1.
Prisma flowchart.
3.2. Study characteristics
There were a total of 6690 patients from 16 studies with median age 55.8 years old. Male was more frequent (58%) compared to female (42%). Obesity prevalence ranged from 27.0% to 67.8%. Most studies (68%) were conducted outside China (US, Germany, Switzerland, Mexico, and French) and the remaining were conducted in China (42%). ICU admission was the most frequent outcome of interest (42%). Hypertension was the most frequent comorbid (46.7%, n = 15) followed by DM type 2 (41.3%, n = 9). The basic characteristics of the study are shown in Table 1 .
Table 1.
Characteristics of included studies.
| Authors | Study Design | Setting | Samples | Age | Male (%) | Obesity | DM(%) | Hypertension (%) | Measured Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Argenziano, 2020 [29] | Observational retrospective | US | 850 (236 vs 614) | N/A (62 vs.64) Median | 60.1 (66.9 vs 57.5) | 38 (45.7 vs. 39.5) | 26 (42.8 vs 37.8) | 50.7 (66.9 vs. 59.8) | ICU Admission |
| Dreher M, 2020 [30] | Observational prospective | Germany | 50 (24 vs. 26) | 65 (62 vs. 68) Median | 66 (62 vs. 69) | 34 (46 vs. 23) | 58 (63 vs. 54) | 70 (67 vs. 73) | ARDS |
| Gregoriano, 2020 [31] | Observational retrospective | Switzerland | 99 (35 vs. 64) | 67 (69 vs. 63.5) Median | 63 (80 vs. 53) | 27 (34 vs. 23) | 22 (23 vs. 22) | 57 (54 vs. 58) | Severe COVID-19 |
| Hur K, 2020 [32] | Observational Prospective | US | 486 (138 vs. 348) | 59 (65 vs. 57) Median | 55.8 (63.8 vs. 52.6) | 34 (46 vs. 23) | 58 (63 vs. 54) | 70 (67 vs. 73) | Mechanical Ventilation |
| Kalligeros, 2020 [33] | Observational retrospective | US | 103 (44 vs. 59) | 60 (61.5 vs 57) Median | 61.2 (65.9 vs. 57.6) | 47.5 (56.8 vs. 40.6) | 36.8 (47.7 vs 28.8) | 64 (70.4 vs 59.3) | ICU Admission |
| Ortis-Brizuela, 2020 [34] | Observational Prospective | Mexico | 309 (140 vs. 169) | 43 (49 vs. 39) Median | 59.2 (60.7 vs. 58.0) | 39.6 (39.7 vs. 39.5) | 13.3 (22.9 vs. 5.3) | 19.7 (32.1 vs. 9.5) | Hospital Admission |
| Ortis-Brizuela, 2020 [34] | Observational Prospective | Mexico | 140 (29 vs. 111) | 43 (53 vs. 48) Median | 60.8 (69 vs. 58.9) | 35.7 (51.7 vs. 36.1) | 22.8 (41.4 vs. 18) | 32.1 (34.5 vs. 31.5) | ICU Admission |
| Petrilli, 2020 [35] | Observational Prospective | US | 2729 (990 vs. 1739) | 63 (68 vs. 60) median | 61.3 (66.3 vs. 58.4) | 39.6 (37.8 vs. 40.6) | 34.7 (39.3 vs. 32) | 62.0 (68.7 vs. 58.3) | ICU Admission |
| Simmonet A, 2020 [36] | Observational retrospective | French | 124 (85 vs. 39) | 60 (60 vs. 60) median | 73 (75 vs. 67) | N/A | 23 (27 vs. 13) | 49 (56 vs. 32) | Mechanical Ventilation |
| Suleyman, 2020 [37] | Observational retrospective | US | 463 (355 vs. 108) | 57.5 (61.4 vs. 44.8) Mean | 44.1 (46.5 vs. 36.1) | 76.8 (80.5 vs. 61.2) | 38.4 (43.4 vs 20.4) | 63.7 (72.7 vs 34.3) | Hospital Admission |
| Yu T, 2020 [38] | Observational retrospective | China | 95 (24 vs. 71) | 33.4 (45.92 vs. 35.73) Mean | 55.7 (58.3 vs. 54.9) | N/A | N/A | N/A | ARDS |
| Cao J, 2020 [39] | Observational Prospective | China | 102 (17 vs. 85) | 54 (72 vs. 53) Median | 52 (76.5 vs 47.1) | N/A | 10.8 (35.3 vs. 5.9) | 27.5 (64.7 vs. 20) | Mortality |
| Chen Q, 2020 [40] | Observational retrospective | China | 145 (43 vs. 102) | 47.5 (52.8 vs. 45.3) 47.5 median | 54.4 (53.5 vs. 54.9) | N/A | 9.6 (16.3 vs. 6.9) | 15.1 (20.9 vs. 12.7) | Severe COVID-19 |
| Li X, 2020 [41] | Observational Prospective | China | 548 (269 vs. 279) | 60 (65 vs. 56) Median | 50.9 (56.9 vs. 45.2) | N/A | 15.1 (19.3 vs. 11.1) | 30.3 (38.7 vs. 22.2 | Severe COVID-19 |
| Hu L, 2020 [42] | Observational retrospective | China | 323 (172 vs. 151) | 61 (N/A) | 51.4 (52.9 vs. 49.7) | 14.5 | 14.6 (19.1 vs. 9.3) | 32.5 (38.3 vs. 25.8) | Severe COVID-19 |
| Stavros, 2020 [43] | Observational retrospective | US | 124 (60 vs. 64) | 64.5 (61.4 vs 67.6) Mean | 61.2 (68.3 vs 54) | N/A | 29.8 (30 vs. 29.6 | 58 (50 vs. 65.6) | ICU Admission |
Data compared between poor outcome (+) and outcome (−) group. DM: Diabetes Mellitus type 2; US: United States ICU: Intensive Care Unit; ARDS: Acute Respiratory Distress Syndrome; N/A: Not available.
3.3. Body mass index (BMI) and related poor outcome
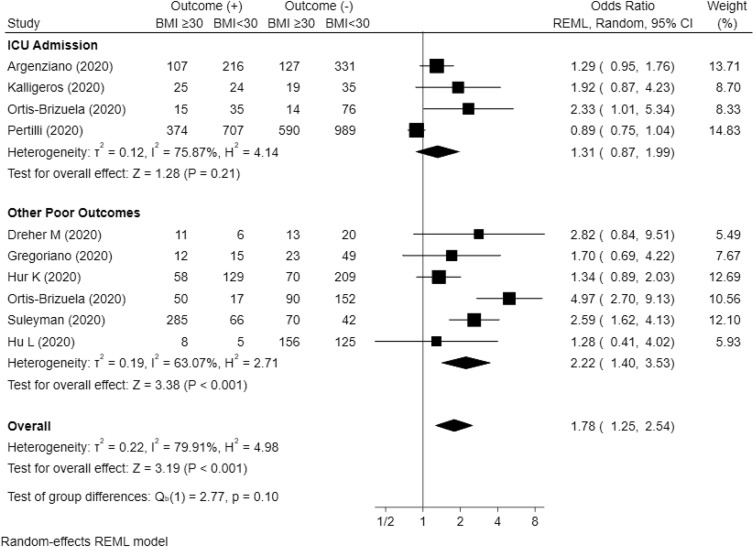
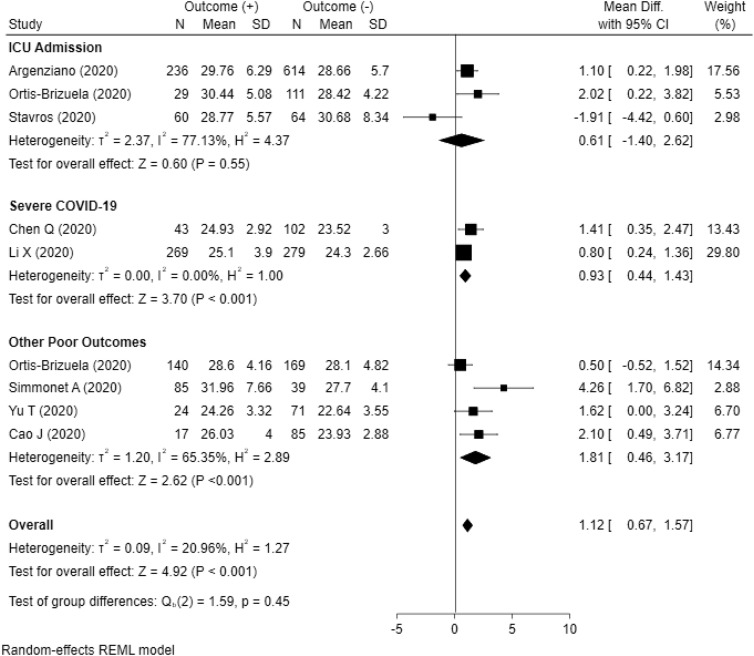
Meta-analysis conducted from ten studies showed Obesity was associated with related poor outcome with OR = 1.78 (95% CI, 1.25–2.54, P < 0.001) with heterogeneity (I [2]) = 79.91%. Subgroup analysis showed obesity was associated with other poor outcome (ARDS, severe COVID-19, use of mechanical ventilation, and hospital admission) with OR = 2.22 (95% CI, 1.40–3.53, P < 0.001) but not associated with ICU admission with OR = 1.31 (95% CI, 0.87–1.99, P = 0.21). Meta-analysis conducted from nine studies presented BMI as continuous data (kg/m2) showed higher BMI was associated with related poor outcome with mean difference = 1.12 (95% CI, 0.67–1.57, P < 0.001) with heterogeneity (I [2]) = 20.96%. Subgroup analysis showed higher BMI was associated with other poor outcome (Mortality, ARDS, use of mechanical ventilation, and hospital admission) with mean difference = 1.81 (95% CI, 0.46–3.17, P < 0.001) and severe COVID-19 with mean difference = 0.93 (95% CI, 0.44–1.43, P < 0.001). However, higher BMI was not associated with ICU admission with mean difference = 0.61 (95% CI, −1.40 to 2.62, P = 0.55). The results of meta-analysis are summarized in Fig. 2 and Fig. 3 .
Fig. 2.
Forest plot presented BMI as dichotomous outcome with cut-off ≥30 kg/m2. Subgroup analysis showed obesity was not associated with ICU admission (p = 0.21) but associated with other poor outcomes (p < 0.001). Overall, obesity was associated with composite poor outcomes (P < 0.001).
Fig. 3.
Forest plot presented with BMI as continuous outcome. Subgroup analysis showed ICU admission was not associated with BMI (p = 0.55), but higher BMI was associated with severe COVID-19 (p < 0.001) and other poor outcomes (p < 0.001). Overall, higher BMI was associated with composite poor outcomes (P < 0.001).
3.4. Meta-regression
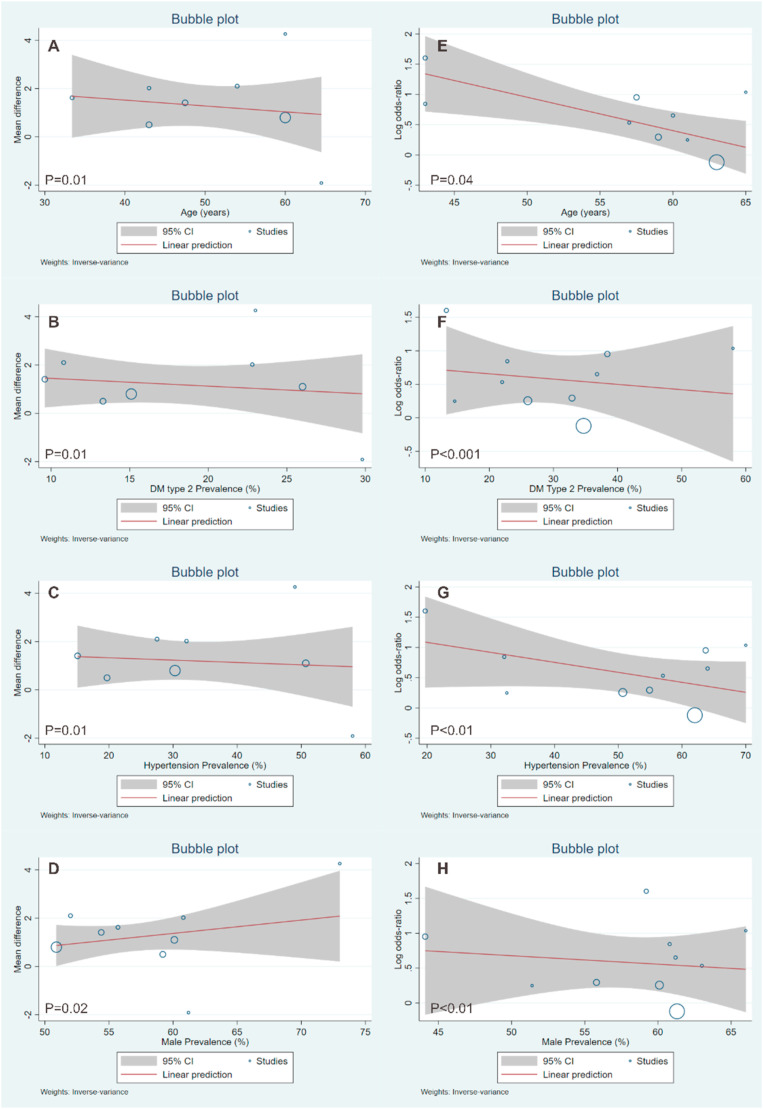
Meta-regression showed the association of obesity and related poor outcome was affected by age (P = 0.04), DM type 2 (P < 0.001), hypertension (P < 0.01), and gender (P < 0.01). Meta-regression showed the association of BMI and related poor outcome was affected by age (P = 0.01), DM type 2 (P = 0.01), hypertension (P = 0.01), and gender (P = 0.02). Other comorbids were not included in meta-regression due to limited data in available studies. Meta-regression analysis were shown in Fig. 4 .
Fig. 4.
Meta-regression for increased BMI (A–D) and obesity (E–H) showed both the association of higher BMI and obesity were affected by age, DM type 2, hypertension, and gender.
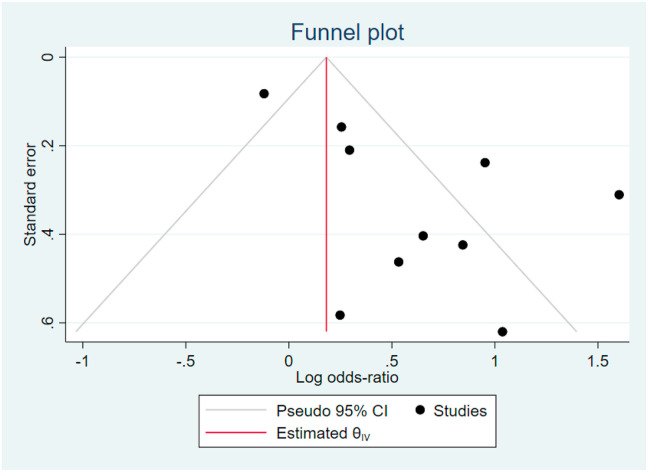
3.5. Publication bias
The funnel plot graph for obesity showed asymmetrical non-inverted funnel that may indicate the presence of publication bias (see Fig. 5). Thus, a more formal evaluation of small study effect using Harbord’s test was conducted. Harbord’s test showed P = 0.204 indicated that there were no evidence of small-study effects.
Fig. 5.
Funnel Plot showed asymmetrical non-inverted funnel. Harbord’s test was conducted with p = 0.204.
4. Discussion
Body Mass Index (BMI) is used to classify obesity. BMI is calculated as the ratio of weight in kilograms to the square of height in meters, expressed in units of kg/m2. According to WHO, BMI was classified into six groups: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), pre-obesity (25–29.9 kg/m2), obesity class I (30–34.9 kg/m2), obesity class II (35–39.9 kg/m2), and obesity class III (>40 kg/m2) [7]. The worldwide prevalence of overweight (BMI ≥ 25 kg/m2) and obesity has increased significantly and extent that nearly a third of the world population. The trend in obesity has increased in both adults and children of all ages with The American and The European were two regions with the highest prevalence of obesity [8,9]. Although China has similar incidence compared to US [10]. Obesity can be seen as a pandemic. According to Centers for Disease Control and Prevention (CDC), the age-adjusted prevalence of obesity was 42.4% and 9.4% for severe obesity [11].
This meta-analysis study showed that both higher BMI and obesity were associated with related poor outcomes (ICU admission, ARDS, severe COVID-19, use of mechanical ventilation, hospital admission, and mortality) in COVID-19 adult patients. Furthermore, subgroup analysis showed that both higher BMI and obesity were not associated with ICU admission but still associated with other poor outcomes. ICU admission criteria may be difficult to define clearly. Since it addressed not only administrative guideline but also ethical and medico-legal aspect of patient care. Thus, the interpretation results should be viewed carefully. Meta-regression showed age, DM type 2, and hypertension were inversely proportional with effect of higher BMI and obesity on related poor outcome. This means the effect of higher BMI and obesity was less in older, DM type 2, and hypertensive patients. It is not certain why the meta-regression analysis results showed a smaller effect of obesity and it should be addressed by further existing literature. While many studies had proven the association of both DM and hypertension on poor outcome, effect of gender (male) should be noted [12,13]. This meta-analysis also showed the effect of higher BMI on poor outcome was stronger in male patients. In contrast, effect of obesity was less in male patients. SARS-CoV-2 uses angiotensin-converting enzyme-2 (ACE2) expressed in the lungs and other tissues, as the main entry point into the cells [14]. Sex hormone may have a role in ACE-2 receptor expression. Healthy young male have higher activity of ACE-2 [15]. In addition, decreased oestrogen in post-menopausal women decrease ACE-2 expression [16]. This uncertainty should be addressed by the existing literature in the future.
Previous data showed that obesity was present in nearly one-third of hospitalizations and fatal cases during the 2009H1N1 pandemic and recognized as an independent risk factor for severity, hospitalization, increased risk of transmission, and mortality of influenza during the H1N1 pandemic in 2009 [17,18]. Therefore, it is not surprising that higher BMI and obesity as is also associated with poor outcome in SARS-CoV-2 infection as well.
Obesity has been identified as a significant risk factor for severe disease following lower respiratory tract infections. The mechanical effects of obesity can cause airway narrowing and increased resistance. In addition, airway narrowing in obesity correlates with airway closure and airway hyperresponsiveness (AHR) [5]. Obesity appears to be associated with a higher chance to developing respiratory complication and need for ICU admission and mechanical ventilation [19]. COVID-19 can lead to potential airways threatening and result in ARDS and respiratory failure [18]. Respiratory muscle strength decreases and oxygen demand increase more than three-fold due to increased airway resistance and chest wall mechanics [20]. Increased oxygen consumption can lead to respiratory failure and predispose the need for more oxygen support. In addition, due to anatomical factor obese patients have a higher incidence difficult mask ventilation compared to non-obese patients [21].
Other factor that probably explains the association between obesity and poor outcome of COVID-19 is that obesity has a negative effect on the immune system and host defense mechanism [22]. Adipose tissue involved in many physiologic and metabolic processes as well as a reservoir for T lymphocytes and macrophages. Excess body fat reduces the response to antiviral agents through poor T cell and macrophage functions [23]. In other hand, obesity is also associated with chronic activation of the innate immune system related to local and systemic inflammation [22] Adipose tissues secrete adipokines differentially expressed between obese and lean subject. Patients who are obese experience chronic alterations in circulating inflammatory mediators derived from adipokines [24]. Other inflammatory mediators that are increased in obesity include tumor necrosis factor alpha (TNF-α), interleukin (IL-) 8 and IL-6, high-sensitivity C-reactive protein (hs-CRP) and monocyte chemoattractant protein-1 (MCP-1) [6]. Increased inflammatory cytokines leading to cytokine storm was also reported in some studies [25]. As proposed by Siddiqi, a minority of COVID-19 patients will transition into the third and most severe stage of illness, which manifests as an extra-pulmonary systemic hyper inflammation syndrome. In this stage, markers of systemic inflammation appear to be elevated. COVID-19 infection results in a decrease in helper, suppressor and regulatory T cell counts. Inflammatory cytokines and biomarkers such as interleukin (IL)-2, IL-6, IL-7, granulocyte-colony stimulating factor, macrophage inflammatory protein 1-α, tumor necrosis factor-α, C reactive protein, ferritin, and D-dimer are significantly elevated in those patients with more severe disease [26]. It can be assumed that obese COVID-19 patients more likely to fall to this stage.
At present, there is no definitive therapy for COVID-19 treatment. Prevention of disease transmission remain most important concern. The fact that prevalence of overweight and obesity extent nearly a third of the world population, seems like we have to face two pandemics simultaneously. A healthy lifestyle, particularly achieving ideal body weight, is very important in preventing and reducing composite poor outcome including mortality if a person contracted to COVID-19. Stakeholders have an obligation to encourage the community to implement a healthy lifestyle to reduce the prevalence of overweight and obesity especially during COVID-19 pandemic.
4.1. Limitations
The measured outcomes (ICU admission, severe COVID-19, ARDS) definition presented in this meta-analysis might be different between each study. This may be due to the use of different guidelines by each hospital across the world complicated by the decision from the physicians in charge. Effect size for BMI as continuous outcome was calculated using mean difference. However, Some studies reported median and interquartile-range (IQR) instead of mean and standard deviation. In this case, we use approximation formula from Wan et al. for sample size<50 and Cochrane for sample size>50 [27,28].
5. Conclusion
Obesity is a risk factor of composite poor outcome of COVID-19. BMI should always be assessed in all COVID-19 patient and special attention should be given to patients with obesity.
Consent for publication
The authors declare that this manuscript is original, has not been published before and is not currently being considered for publication.
Funding statement
The author(s) received no funding for this work.
Ethics approval
Not applicable. No ethical approval will be needed because data from previous published studies in which informed consent was obtained by primary investigators will be retrieved and analysed.
Authors’ contributions
AYS, NNS, and AP conceived and designed the study. PS, HS, IDK, FF acquire the data. AYS and AP performed data extraction and interpreted the data. AYS and AP performed the statistical analysis. All authors contributed to the writing of the manuscript.
Declaration of competing interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgment
Not applicable.
References
- 1.WHO. Coronavirus disease 2019 (COVID-19) situation report – 209. 2020. [Google Scholar]
- 2.Kruglikov I.L., Scherer P.E. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity. 2020;28(7):1187–1190. doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T., et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020:1–9. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon A.E., Peters U. The effect of obesity on lung function. Expet Rev Respir Med. 2018;12(9):755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO 2020. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi Available from:
- 8.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metab Clin Exp. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Michalakis K., Goulis D.G., Vazaiou A., Mintziori G., Polymeris A., Abrahamian-Michalakis A. Obesity in the ageing man. Metab Clin Exp. 2013;62(10):1341–1349. doi: 10.1016/j.metabol.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Hu C., Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67(1):3–11. doi: 10.2337/dbi17-0013. [DOI] [PubMed] [Google Scholar]
- 11.Colaneri M., Sacchi P., Zuccaro V., Biscarini S., Sachs M., Roda S., et al. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia. Euro Surveill Bull Eur Sur Les Maladies Transmissibles = Eur Commun Dis Bull. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000460. North Italy, 21 to 28 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes & metabolic syndrome. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapater P., Novalbos J., Gallego-Sandín S., Hernández F.T., Abad-Santos F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J Cardiovasc Pharmacol. 2004;43(5):737–744. doi: 10.1097/00005344-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Komukai K., Mochizuki S., Yoshimura M. Gender and the renin–angiotensin–aldosterone system. Fund Clin Pharmacol. 2010;24(6):687–698. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 17.Moser J.S., Galindo-Fraga A., Ortiz-Hernandez A.A., Gu W., Hunsberger S., Galan-Herrera J.F., et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Respir Viruses. 2019;13(1):3–9. doi: 10.1111/irv.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honce R., Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severin R., Arena R., Lavie C.J., Bond S., Phillips S.A. Respiratory muscle performance screening for infectious disease management following COVID-19: a highly pressurized situation. Am J Med. 2020;133(9):1025–1032. doi: 10.1016/j.amjmed.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood A. Altered resting and exercise respiratory physiology in obesity. Clin Chest Med. 2009;30(3):445–454. doi: 10.1016/j.ccm.2009.05.003. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon T.S., Fox P.E., Somasundaram A., Minhajuddin A., Gonzales M.X., Pak T.J., et al. The influence of morbid obesity on difficult intubation and difficult mask ventilation. J Anesth. 2019;33(1):96–102. doi: 10.1007/s00540-018-2592-7. [DOI] [PubMed] [Google Scholar]
- 22.Frasca D., McElhaney J. Influence of obesity on pneumococcus infection risk in the elderly. Front Endocrinol. 2019;10:71. doi: 10.3389/fendo.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coelho M., Oliveira T., Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci : AMS. 2013;9(2):191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbert K., Rice M., Malhotra A. Obesity and ARDS. Chest. 2012;142(3):785–790. doi: 10.1378/chest.12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X., Wu K., Wang D., Yue X., Song D., Zhu Y., et al. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-κB. Virology. 2007;365(2):324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant Off Publ Int Soc Heart Transplantation. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J., Wells G. Wiley Online Library; 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 29.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Deutsches Arzteblatt Int. 2020;117(16):271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregoriano C., Koch D., Haubitz S., Conen A., Fux C.A., Mueller B., et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020;150:w20316. doi: 10.4414/smw.2020.20316. [DOI] [PubMed] [Google Scholar]
- 32.Hur K., Price C.P.E., Gray E.L., Gulati R.K., Maksimoski M., Racette S.D., et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2020;163(1):170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz-Brizuela E., Villanueva-Reza M., González-Lara M.F., Tamez-Torres K.M., Román-Montes C.M., Díaz-Mejía B.A., et al. Clinical and epidemiological characteristics OF patients diagnosed with COVID-19 IN a tertiary care center IN Mexico city: a prospective cohort study. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 2020;72(3):165–177. doi: 10.24875/RIC.20000211. [DOI] [PubMed] [Google Scholar]
- 35.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory Syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A., et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA network open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu T., Cai S., Zheng Z., Cai X., Liu Y., Yin S., et al. Association between clinical manifestations and prognosis in patients with COVID-19. Clin Therapeut. 2020;42(6):964–972. doi: 10.1016/j.clinthera.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao J., Tu W.-J., Cheng W., Yu L., Liu Y.-K., Hu X., et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clinical Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q., Zheng Z., Zhang C., Zhang X., Wu H., Wang J., et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020:1–9. doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basta G., Del Turco S., Caselli C., Melani L., Vianello A. It’s world war at CoViD-19. The first battle on the front of the viral invasion against the exitus for interstitial pneumonia was decisive. Recenti Prog Med. 2020;111(4):238–252. doi: 10.1701/3347.33187. [DOI] [PubMed] [Google Scholar]
- 42.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.M., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clinical Infect Dis Official Publ Infect Dise Soc Am. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Memtsoudis S.G., Ivascu N.S., Pryor K.O., Goldstein P.A. Obesity as a risk factor for poor outcome in COVID-19-induced lung injury: the potential role of undiagnosed obstructive sleep apnoea. Br J Addiction: Br J Anaesth. 2020;125(2):e262–e263. doi: 10.1016/j.bja.2020.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]