Abstract
Background
Coronavirus disease-19 (COVID-19) has been associated with overt and subclinical myocardial dysfunction. We observed a recurring pattern of reduced basal left ventricular (LV) longitudinal strain on speckle-tracking echocardiography in hospitalized patients with COVID-19 and subsequently aimed to identify characteristics of affected patients. We hypothesized that patients with COVID-19 with reduced basal LV strain would demonstrate elevated cardiac biomarkers.
Methods and Result
Eighty-one consecutive patients with COVID-19 underwent speckle-tracking echocardiography. Those with poor quality speckle-tracking echocardiography (n = 2) or a known LV ejection fraction of <50% (n = 4) were excluded. Patients with an absolute value basal longitudinal strain of <13.9% (2 standard deviations below normal) were designated as cases (n = 39); those with a basal longitudinal strain of ≥13.9% were designated as controls (n = 36). Demographics and clinical variables were compared. Of 75 included patients (mean age 62 ± 14 years, 41% women), 52% had reduced basal strain. Cases had higher body mass index (median 34.1; interquartile range 26.5–37.9 kg/m2 vs median 26.9, interquartile range, 24.8–30.0 kg/m2, P = .009), and greater proportions of Black (74% vs 36%, P = .0009), hypertensive (79% vs 56%, P = .026), and diabetic patients (44% vs 19%, P = .025) compared with controls. Troponin and N-terminal pro-brain natriuretic peptide levels trended higher in cases, but were not significantly different.
Conclusions
Reduced basal LV strain is common in patients with COVID-19. Patients with hypertension, diabetes, obesity, and Black race were more likely to have reduced basal strain. Further investigation into the significance of this strain pattern is warranted.
Key Words: Echocardiography; myocardial dysfunction; COVID-19, strain
Coronavirus disease 2019 (COVID-19), resulting from infection with severe acute respiratory syndrome coronavirus 2, has caused a worldwide pandemic with high morbidity and mortality. Although the respiratory system sees the major impact of the illness, increasing reports describe important cardiac manifestations, which are in turn associated with poor outcomes.1, 2, 3, 4 Various forms of myocardial injury, defined by elevated cardiac biomarkers and/or abnormalities on transthoracic echocardiography (TTE) and cardiac magnetic resonance imaging have been reported.2 , 5, 6, 7, 8, 9 TTE can be particularly useful in the inpatient setting to quickly and inexpensively evaluate regional myocardial injury; however, owing to the infectious risk to medical staff, routine echocardiography for all patients with COVID-19 should be avoided.10
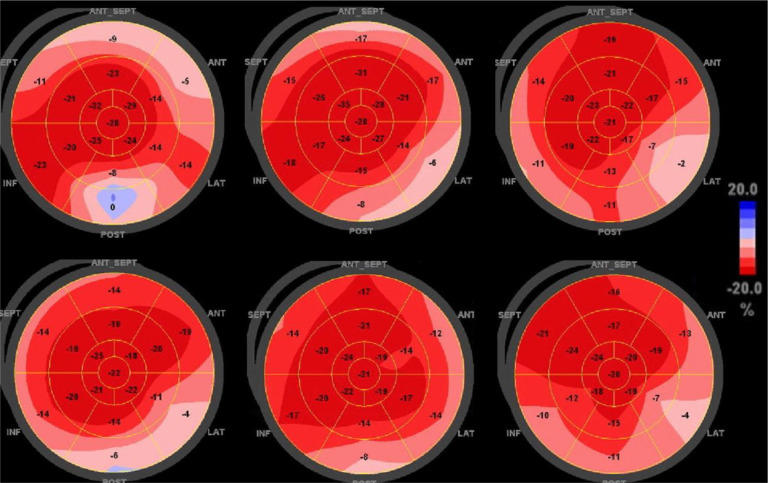
A technique easily combined with standard echocardiographic measurements is 2-dimensional speckle-tracking echocardiography (STE), which tracks unique speckle pathways during the cardiac cycle to determine myocardial deformation and strain. Global longitudinal strain (GLS) has been shown to be more sensitive in detecting left ventricular (LV) dysfunction compared with the LV ejection fraction (LVEF).11 Regional strain can be analyzed based on the American Heart Association 17-segment model of the LV, represented as a “bull's-eye” polar map with the LV apex in the center and each segment color-coded according to level of strain (Figure ). Segmental longitudinal strain (LS) patterns can be helpful in identifying cardiac diseases when a characteristic abnormality is seen in conjunction with clinical data, such as the pathognomonic apical-sparing strain reduction seen in cardiac amyloidosis.12
Figure.
Polar maps displaying regional strain pattern on TTE in patients with COVID-19. Composite strain polar map display of 6 representative patients with COVID-19 found to have a recurring pattern of reduced LS in the basal segments of the left ventricle
A recurring pattern of reduced basal LV LS on STE was observed in hospitalized patients with COVID-19 (Figure). This study aimed to characterize the COVID-19 patient population with this strain pattern. We hypothesized that cardiac biomarker levels would be elevated in patients with reduced basal LS.
Methods
This study was approved by and conducted according to the Johns Hopkins Institutional Review Board. This was a single-center study of patients admitted to the Johns Hopkins Hospital from March 25, 2020, to May 14, 2020, with positive COVID-19 nasopharyngeal polymerase chain reaction testing and who underwent clinically indicated transthoracic STE.
STE was performed by an experienced cardiac sonographer using Vivid E9 ultrasound systems (General Electric Vingmed Ultrasound, Milwaukee, WI) at a median of 4 days after presentation. Images were analyzed by two independent reviewers on a dedicated workstation using EchoPAC software (version 202, GE ultrasound). Within this article, strain is referred to in absolute values.
Hospitalized patients with COVID-19 who underwent STE during admission were assessed for regional strain. Patients with a history of an LVEF of <50%, arrhythmia at the time of TTE, or insufficient quality to ascertain regional strain were excluded. Polar maps based on the American Heart Association 17-segment model were analyzed for LS at basal, mid, and apical levels. Mean basal LS in healthy patients has been reported as –18.3% ± 2.2%; therefore, patients with an average absolute value basal LS of <13.9% (2 standard deviations from normal) were designated as cases.13 Otherwise, patients were considered controls. Clinical and demographic variables were collected from the electronic medical record and deidentified. Normality was assessed with Shapiro–Wilk testing. Comparisons between groups were performed using Welch's t test for normally distributed continuous variables that passed Levene's test for homogeneity of variance, the Mann–Whitney U test for skewed continuous variables or those with unequal variance, and the χ2 or Fisher's exact test, as appropriate, for categorical variables. Laboratory data were recorded at peak level. Analyses were performed using GraphPad Prism v.8.4.2. A P value of <.05 was considered statistically significant.
Results
In total, 81 patients with COVID-19 underwent STE, and 75 were included in the analysis; 2 were excluded for poor quality, and 4 were excluded for a prior LVEF of <50%. The mean patient age was 61.9 ± 13.5 years, and 41% were women. There were high overall incidences of intensive care unit admission (73%), mechanical ventilation (61%), shock (47%), and death (17%). Of these patients, 52% had a reduced basal strain on STE (basal LS 10.0 ± 2.9% vs 16.9 ± 2.3%, P < .001). The LVEF was similar between groups; however, the GLS was significantly lower in cases compared with controls (13.9 ± 4.1% vs 18.8 ± 2.7%, P < .001). Additional echocardiographic variables representing LV structure, diastolic function, and right ventricular function were not different between the groups (Table ). Cases had higher body mass index (median 31.4 kg/m2, interquartile range 26.5–37.9 kg/m2vs 26.9 kg/m2, interquartile range 24.8–30.0 kg/m2, P = .009), and prevalence of hypertension (79% vs 56%, P = .026), and diabetes mellitus (44% vs 19%, P = .025). There was a higher proportion of Black patients among cases (74% vs 36%, P = .0009). Levels of troponin I and N-terminal pro-brain natriuretic peptide trended higher in cases, but were not statistically different from controls. Inflammatory markers and clinical outcomes were similar between the groups (Table).
Table.
Characteristics of Patients With COVID-19 Based on Basal LV Strain
| Characteristics | Total Cohort (n = 75) | Normal basal strain (n = 36) | Reduced basal strain (n = 39) | P Value |
|---|---|---|---|---|
| Age, years | 61.9 ± 13.5 | 61.4 ± 14.2 | 62.4 ± 13.1 | .92 |
| Female | 31 (41) | 12 (33) | 19 (49) | .019 |
| Race | ||||
| White | 13 (17) | 9 (25) | 4 (10) | .13 |
| African American | 42 (56) | 13 (36) | 29 (74) |
.0009 OR (95% CI): 5.1 (1.7–15.6) |
| Hispanic | 14 (19) | 9 (25) | 5 (13) | .24 |
| Other | 7 (9) | 5 (14) | 2 (5) | .25 |
| Body mass index, kg/m2 | 28.7 [25.9–34.7] | 26.9 [24.8–30.0] | 31.4 [26.5–37.9] | .009 |
| Comorbidities | ||||
| Hypertension | 51 (68) | 20 (56) | 31 (79) |
.026 OR (95% CI): 3.1 (1.01–9.9) |
| Diabetes mellitus | 24 (32) | 7 (19) | 17 (44) |
.025 OR (95% CI): 3.2 [1.02–10.7] |
| Hyperlipidemia | 37 (49) | 17 (47) | 20 (51) | .73 |
| Coronary artery disease | 7 (9) | 3 (8) | 4 (10) | 1.0 |
| HFpEF | 7 (9) | 1 (3) | 6 (15) | .11 |
| Serum biomarkers | ||||
| Troponin I, ng/mL | 0.05 [0.03–0.23] | 0.04 [0.03–0.16] | 0.05 [0.03–0.31] | .62 |
| NT-proBNP, pg/mL | 372 [140–2273] (n = 65) | 254 [115–724] (n = 33) | 464 [153–4300] (n = 32) | .20 |
| C-reactive protein, mg/dL | 18.2 [7.7–34.0] | 19.3 [10.7–33.8] | 20.3 [9.3–39.2] | .76 |
| IL-6, pg/mL | 122 [38.3–410] (n = 50) | 92.8 [37.4–287] (n = 25) | 151.4 [42.6–653] (n = 26) | .44 |
| Ferritin, ng/mL | 1182 [781–2042] | 1330 [820–1779] | 1118 [708–3313] | .98 |
| Clinical events | ||||
| ICU admission | 55 (73) | 28 (78) | 30 (68) | .34 |
| Shock | 35 (47) | 13 (36) | 24 (55) | .10 |
| Atrial arrhythmia | 22 (29) | 9 (25) | 15 (34) | .38 |
| VTE | 15 (20) | 6 (17) | 9 (20) | .19 |
| Mechanical ventilation | 46 (61) | 23 (64) | 25 (57) | .76 |
| Death | 13 (17) | 8 (22) | 6 (14) | .31 |
| Echo parameters | ||||
| TAPSE | 1.9 ± 0.39 | 1.9 ± 0.40 | 1.8 ± 0.37 | .20 |
| Mitral E-wave velocity, cm/s | 0.64 [0.52–0.77] | 0.62 [0.56–0.81] | 0.65 [0.51–0.83] | .98 |
| Mitral A-wave velocity, cm/s | 0.74 [0.54–0.83] | 0.73 [0.49–0.80] | 0.76 [0.59–0.85] | .22 |
| Mitral E/A ratio | 0.92 [0.76–1.1] | 0.94 [0.82–1.3] | 0.88 [0.70–1.0] | .16 |
| Mitral E’, cm/s | 0.08 [0.06–0.09] | 0.08 [0.07–0.09] | 0.07 [0.06–0.09] | .13 |
| LVEDV, mL | 78.0 [60.0–92.8] | 81.0 [66.5–93.5] (n = 29) | 74.0 [59.8–89.3] (n = 34) | .36 |
| LVEF, % | 62.0 [55.0–62.5] | 62.5 [55.0–64.4] | 57.5 [47.5–62.5] | .11 |
| GLS (absolute value), % | 16.4 ± 4.1 | 18.8 ± 2.7 | 13.9 ± 4.1 | <.0001 |
| Avg basal LV strain (absolute value), % | 13.4 ± 4.4 | 16.9 ± 2.3 | 10.0 ± 2.9 | <.0001 |
Values are mean ± standard deviation, number (%) or median [IQR]. Boldface entries indicate statistical significance. Avg, average; CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; ICU, intensive care unit; IQR, interquartile range; NT-proBNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio; LVEDV, LV end diastolic volume; LVESV, LV end systolic volume; TAPSE, tricuspid annular plane systolic excursion; VTE, venous thromboembolism.
Discussion
This study presents a novel observation of reduced basal LV LS in 52% in hospitalized patients with COVID-19 undergoing STE. Compared with patients with a normal basal LS, those with a reduced basal LS were more likely to have concomitant obesity, hypertension, and diabetes, which are known risk factors for worse outcomes in COVID-19.14 The reduced basal LS group additionally had a greater proportion of Black patients, which is notable given reports of COVID-19 disproportionately affecting Black populations in America.15 These findings support studies showing LV GLS as a more sensitive marker of early myocardial dysfunction than LVEF in a variety of etiologies.11 Additionally, this finding suggests that bedside STE provides important information regarding cardiac involvement early in the course of COVID-19.
Complementing our findings, a recently published study describing a reduced basal segmental LV strain in more than one-half of evaluated patients with COVID-19 has suggested that this parameter may be an early marker of myocardial involvement.9 Abnormalities in RV strain and LV GLS have also been reported and indicate subclinical myocardial injury.5 , 6 Importantly, cardiac magnetic resonance imaging in a significant proportion of recovered patients with COVID-19 revealed fibrosis and edema predominantly in the basal and mid LV segments, corresponding with our STE findings.8 Case reports and series of patients with various forms of myocarditis, including influenza myocarditis, have described a similar pattern of reduced basal strain on STE.16 , 17 Abnormal basal LS has also been seen in infiltrative cardiomyopathies including, Anderson–Fabry disease.18 One study of cardiac magnetic resonance imaging in patients with Anderson–Fabry disease suggested that basal inferolateral segments represent the area of basal LV with the greatest mobility throughout the cardiac cycle and thus may be more susceptible to junctional stresses.19 These prior findings, along with our current work, raise the possibility that this basal injury pattern reflects the susceptibility of certain myocardial regions to inflammatory or systemic stressors rather than a geographic predilection specific to severe acute respiratory syndrome coronavirus 2. Further supporting this theory are studies showing apical-sparing LV dysfunction in patients with acute brain hemorrhage.20 , 21 The associated catecholamine surge in these clinical scenarios may also contribute to cardiac injury in COVID-19.
Another plausible hypothesis, more specific to COVID-19, involves the viral receptor, angiotensin-converting enzyme 2 (ACE2). This membrane-bound enzyme is responsible for production of angiotensin (1–7), leading to well-described anti-inflammatory and anti-thrombotic effects.22 ACE2 is highly expressed in fat, and epicardial adipose tissue (EAT) is more prominent in the atrioventricular groove and lateral LV wall, closer to the basal segments.23 Loss of ACE2 has been shown to result in heart failure with preserved ejection fraction, mediated in part by EAT inflammation. Angiotensin (1–7) decreases obesity-associated cardiac dysfunction predominantly via its role in adiponectin expression and attenuation of EAT inflammation.22 Thus, severe acute respiratory syndrome coronavirus 2 binding of ACE2 may occur more prominently in areas of high EAT, such as the basal LV, and cause subclinical dysfunction via inflammatory downstream effects, perhaps more readily in overweight and obese patients.
Our study also presents some limitations. The modest sample size may lead to type II errors in cardiac biomarker and clinical comparisons. Furthermore, the proportion of women was higher in the low basal strain group, which is unexpected considering that men are more likely to contract COVID-19 and suffer worse outcomes.24 This sex difference could also blunt differences in biomarkers and outcomes. Although the reduced basal LV strain was found during admission for COVID-19, whether these abnormalities were previously present and thus unrelated to the infection is unknown. We excluded patients with a prior LVEF of <50%, who are likely to have baseline abnormal strain. No patients had prior STE data available. The prevalence of hypertension, which can affect LV strain, was high in this group, although hypertensive patients commonly display septal rather than generalized basal strain reduction.12 A significant number of TTEs were of insufficient quality to analyze strain. Given that patients most difficult to obtain high-quality TTE images from (obese, intubated, proned) represent a considerable portion of individuals affected by COVID-19, this factor may introduce bias. Last, given that this was an exploratory study with several comparisons, there is a risk for a type I error. Larger studies with preplanned hypotheses are needed to confirm our findings.
Conclusions
We report a pattern of reduced LV basal strain seen in more than one-half of studied patients with COVID-19 and occurring more frequently in patients with high-risk cardiovascular comorbidities and Black race. Although this study was not powered to detect differences in hard clinical outcomes such as death, it is known that Black patients and those with obesity, hypertension, and diabetes have worse overall outcomes with COVID-19. This strain pattern often occurs despite preserved LVEF and may herald early subclinical myocardial injury. Further studies in larger cohorts of patients with COVID-19 are warranted to determine prognostic significance.
Disclosures
None.
Dr. Goerlich is supported by the Ruth L. Kirschstein Institutional National Research Service Award; T32HL007227-Pathophysiology of Myocardial Disease. Dr. Minhas is supported by the National Heart, Lung, and Blood Institute training grant T32HL007024. Dr. Hays is supported by the Magic that Matters Fund of Johns Hopkins Medicine and NIH/NHLBI 1R01HL147660. Dr. Cingolani is supported by the Magic that Matters Fund, and the Michel Mirowski Discovery Award, Johns Hopkins Medicine.
Footnotes
See page 103 for disclosure information.
References
- 1.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janus S.E., Hajjari J., Karnib M., Tashtish N., Al-Kindi S.G, Hoit B.D. Prognostic value of left ventricular global longitudinal strain in COVID-19. Am J Cardiol. 2020;131:134–136. doi: 10.1016/j.amjcard.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Li H., Zhu S., Xie Y., Wang B., He L., et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;28:S1936. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q., Yang K., Wang W., Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.004. May 12 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stobe S., Richter S., Seige M., Stehr S., Laufs U, Hagendorff A. Echocardiographic characteristics of patients with SARS-CoV-2 infection. Clin Res Cardiol. 2020;14:1–18. doi: 10.1007/s00392-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood D.A., Mahmud E., Thourani V.H., Sathananthan J., Virani A., Poppas A., et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: guidance from North American Societies. Can J Cardiol. 2020;36:971–976. doi: 10.1016/j.cjca.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potter E, Marwick T.H. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–274. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Zito C., Longobardo L., Citro R., Galderisi M., Oreto L., Carerj M.L., et al. Ten years of 2D longitudinal strain for early myocardial dysfunction detection: a clinical overview. Biomed Res Int. 2018;2018 doi: 10.1155/2018/8979407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata Y., Wu V.C., Otsuji Y, Takeuchi M. Normal range of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kass D.A., Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes L., Jr., Enwere M., Williams J., Ogundele B., Chavan P., Piccoli T., et al. Black-White risk differentials in COVID-19 (SARS-COV2) transmission, mortality and case fatality in the United States: translational epidemiologic perspective and challenges. Int J Environ Res Public Health. 2020;17:4322. doi: 10.3390/ijerph17124322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha S.J., Woo J.S., Kwon S.H., Oh C.H., Kim K.S., et al. Acute regional myocarditis with normal ventricular wall motion diagnosed by two-dimensional speckle tracking imaging. Korean J Intern Med. 2013;28:732–735. doi: 10.3904/kjim.2013.28.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawwa N., Popovic Z.B, Isma'eel H.A. Discordant electrocardiogram left ventricular wall thickness and strain findings in influenza myocarditis. Echocardiography. 2015;32:1880–1884. doi: 10.1111/echo.13024. [DOI] [PubMed] [Google Scholar]
- 18.Esposito R., Galderisi M., Santoro C., Imbriaco M., Riccio E., Maria Pellegrino A., et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment-naive Anderson-Fabry disease patients. Eur Heart J Cardiovasc Imaging. 2019;20:438–445. doi: 10.1093/ehjci/jey108. [DOI] [PubMed] [Google Scholar]
- 19.Deva D.P., Hanneman K., Li Q., Ng M.Y., Wasim S., Morel C., et al. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-Fabry disease. J Cardiovasc Magn Reson. 2016;18:14. doi: 10.1186/s12968-016-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M., Oh J.H., Lee K.B., Kang G.H., Park Y.H., Jang W.J., et al. Clinical and echocardiographic characteristics of acute cardiac dysfunction associated with acute brain hemorrhage- difference from takotsubo cardiomyopathy. Circ J. 2016;80:2026–2032. doi: 10.1253/circj.CJ-16-0395. [DOI] [PubMed] [Google Scholar]
- 21.Kagiyama N., Sugahara M., Crago E.A., Qi Z., Lagattuta T.F., Yousef K.M., et al. Neurocardiac injury assessed by strain imaging is associated with in-hospital mortality in patients with subarachnoid hemorrhage. JACC Cardiovasc Imaging. 2020;13:535–546. doi: 10.1016/j.jcmg.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Patel V.B., Mori J., McLean B.A., Basu R., Das S.K., Ramprasath T., et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65:85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbara S., Desai J.C., Cury R.C., Butler J., Nieman K, Reddy V. Mapping epicardial fat with multi-detector computed tomography to facilitate percutaneous transepicardial arrhythmia ablation. Eur J Radiol. 2006;57:417–422. doi: 10.1016/j.ejrad.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]