Abstract
The blood brain barrier (BBB) poses a significant challenge for drug delivery of macromolecules into the brain. Convection-enhanced delivery (CED) circumvents the BBB through direct intracerebral infusion using a hydrostatic pressure gradient to transfer therapeutic compounds. The efficacy of CED is dependent on the distribution of the therapeutic agent to the targeted region. Here we present a review of convection enhanced delivery of macromolecules, emphasizing the role of tracers in enabling effective delivery and discuss current challenges in the field.
Keywords: Convection enhanced delivery, drug delivery system, glioblastoma, surrogate markers, tracers
1. INTRODUCTION
Despite recent advances in state-of-the-art surgical resection, radiotherapy, and chemotherapy, glioblastoma multiforme (GBM) continues to have a poor prognosis [1, 2]. The ability to deliver therapeutic agents to a GBM tumor is a significant hurdle and accounts for some of the difficulty in treating this disease [3]. Since Paul Ehrlich first described the blood brain barrier in 1885 [4]_ENREF_3, it has been widely accepted that systemically applied drugs exhibit limited penetration into the central nervous system. Over 100 years later, treatment delivery options for intracerebral malignancies continue to be limited and, in their current form of non-targeted systemic or intrathecal delivery approaches, are ultimately confounded by side effects of toxicity, injury to surrounding tissues and suboptimal drug delivery to the tumor site. In addition to the challenges of navigating past the blood brain barrier, the highly invasive nature of malignant brain tumors further hinders our ability to effectively target these diseased tissues using conventional surgical resection and radiation therapy [5].
Convection-enhanced delivery (CED) targets therapies directly into the tumor thus circumventing the blood brain barrier. This is accomplished through direct intracerebral catheter placement and often a pump that is the source of flow of fluid carrying the therapeutic agent. The fluid carries, or advects, the agents throughout the interstitial spaces [6]. If CED is applied after surgical resection of a brain tumor, then the microinfusion catheters placed under stereotactic guidance target the peritumoral region with bulk flow, supplementing intrinsic diffusivity of both small and large therapeutic compounds to greatly enhance distribution [6]. In addition, the theoretical benefit of directly applying active molecules intracerebrally is the fact that an intact blood brain barrier would conversely limit egress of therapeutic agent to extracerebral tissues, effectively enhancing drug delivery while reducing the potential risk of systemic toxicity [7]. Furthermore, because CED depends on fluid flow to carry the agent, it is possible to achieve a relatively constant concentration of drug spanning a predictable distance from the site of infusion before the drop-off [7], which provides superior localization of the therapy to the tumor upon accurate catheter placement [7, 8].
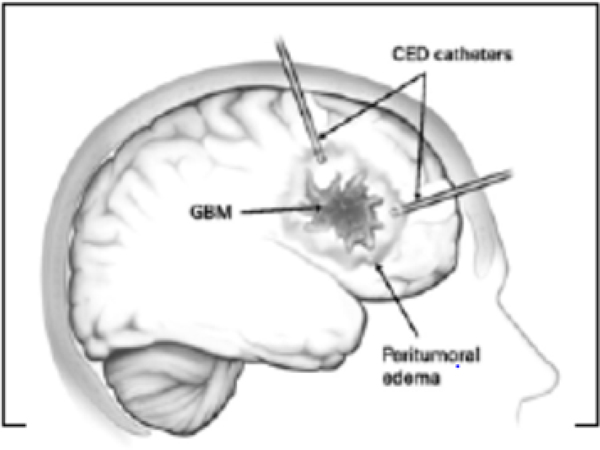
Given that the efficacy of any drug delivered by CED will be dependent on the ability to achieve sufficient concentrations of a therapeutic agent to a targeted region, much attention has been placed on the factors that dictate optimal catheter placement prior to infusion (Fig. 1) [8, 9]. If catheters are inaccurately placed, leakage of infusate into the intraventricular spaces and subarachnoid will result in poor drug delivery and distribution [9]. Currently, catheters generate a certain amount of backflow where the fluid flow in a tissue-free region created by fluid pressure along the outer surface of the catheter [9–11]. In addition, any large (millimeter sized) bubbles may redirect the flow away from the catheter tip in unpredictable ways. Thus optimization of devices and of various protocols constitute an important part of improving the delivery of CED [12]. However, in this review, we shall focus on the role of imaging, and of technologies to help determine the distribution of CED-infused drug into the brain using differing imaging modalities, in improving CED. Although there have been many imaging studies, most have been in animal models that fail to replicate the scale of drug distribution required in humans [9, 13–15]_ENREF_11.In fact, despite the rigorous use of standardized guidelines, multiple Phase III clinical trials examining CED as a delivery technique have failed to demonstrate improvement over existing therapies [16–20]. The PRECISE (Phase III Randomized Evaluation of CED of IL13-PE38QQR with Survival Endpoint) study (NeoPharm, Inc.) compared patient survival following treatment with local implantation of carmustine-impregnated wafers and direct intracerebral infusion of a Pseudomonas-based exotoxin, cintredekinbesudotox. A retrospective analysis of this study showed that while the original investigators reported no significant difference in overall survival between groups, suboptimal delivery may have accounted for the disappointing results [7, 8]. The catheter positioning score (hazard ratio 0.93, p = 0.043) and the number of optimally positioned catheters (hazard ratio 0.72, p = 0.038) had a significant effect on progression-free survival [21]. Thus, technologies that improve our ability to predict optimal catheter placement and provide imaging for the delivery of therapeutic agents in real-time represent technologies that will further our understanding of CED and its significance in the treatment of intracerebral malignancy.
Fig. (1) –
Diagram of CED Catheter
2. IN VIVO IMAGING OF CED INFUSATE DISTRIBUTION
Currently, there are relatively few methods that have been developed to reliably image CED infusate distribution. Some estimate that over a 1000 patients, a majority of whom have malignant gliomas, have been treated with CED infusions without convincing evidence of efficiency [22]. Historically, mathematical models have been used to predict drug transport as a function of patient-specific parameters, though such models must be validated using techniques that allow measurement of drug localization and concentration in vivo. To date, approaches that have been used to track CED-infusates in vivo rely primarily on radiographic alterations that arise from the presence of fluid, drug-induced effects on the target tissue, or tracer molecules, but these are only indirect measures that in theory do not precisely recapitulate therapeutic infusate coverage. Recently, there have been multiple advances in our understanding with regards to our ability to radiographically validate CED-infused drug distribution to a target region, a brief review of which follows.
2.1. Radiolabels
Early attempts to validate the enhanced drug delivery offered by CED, were performed by co-infusing iodine 123-labeled human serum albumin (123I-HSA)with the therapeutic agent. [23–25] The radiolabeled albumin replaced the unlabeled albumin which is usually the carrier protein for the agent. Single-photon emission computed tomography (SPECT) is used to determine the volume of distribution. However, this method has its set of draw backs- appropriate radiotracers are often difficult to develop and access and SPECT imaging is a relatively low resolution creating a paucity of anatomical information [26].
2.2. Iopanoic Acid
Iopanic acid is a nonionic contrast agent, organically bound to Iodine. It has been shown to have many favorable characteristics in validating CED-infused drug distribution including reliable imaging by CT scanning, a good safety profile even in patients intolerant to Iodine derivatives [27] and homogeneity [28]. Also, from a practical standpoint, CT scanning as an imaging modality is available in most clinical centers. While, Iopanic acid has been shown to be effective in monitoring the delivery of various low [29] and high molecular weight drugs [28], it has its limitations. Some of these are poor prediction of the distribution of small, lipophilic, or reactive drugs and concerns for adverse reactions between it and coinfused therapeutic agents.
2.3. Magnetic Resonance Imaging (MRI)T2 Imaging Changes
Preclinical models using diffusion weighted MRI for early tissue characterization have suggested that MRI signal changes may be used to estimate distribution of CED infused drug [14, 30, 31]. More recently, clinical evidence withT2-weighted MRI has been shown to accurately reflect the increase in water content in the interstitium following administration with CED. Sampson et al. demonstrated that this method could be used in combination with SPECT imaging to predict intraparenchymal drug coverage, showing discernable alterations following infusion even in patients with preexisting hyperintense signals on T2-weighted MRI [13].
2.4. Gadolinium Conjugated Agents
Gadolinium is a rare-earth element. Chelation with diethylenetriaminepentaacetic acid (DPTA)results in a paramagnetic metal ion used in MRI [32]. Due to its small molecular weight, gadolinium is used as a surrogate marker for infusate distribution and has been bound to albumin and liposomal constructs or used independently to reflect the coverage area of a macromolecular infusate.
2.4.1. Albumin
Albumin-linked tracers have been used in a number of studies as a surrogate marker for macromolecular distribution via CED in the primate brain. CED-infused albumin tracers can be linked to Gd-DTPA [14, 33–36], which can then be visualized with conventional imaging modalities like MRI.
2.4.2. Gadolinium-based Liposome Constructs
Liposomes are phospholipid nanoparticles used to carry therapeutic agents. Gadolinium–diethylenetriaminepentaacetic acid (Gd-DTPA) has been used as a surrogate tracer given that is widely available as an MRI contrast agent. Some have attempted to incorporate gadolinium-based molecules as a surrogate tracer into drug impregnated liposomes [37]. However, liposome constructs may be prohibitively difficult to manufacture and thus present a less feasible approach.
2.4.3. Small molecule Gadolinium Infusate
People have used small molecules containing Gadolinium-DTPA in various proprietary formulations known as Magnevist, Omniscan, or Prohance in a large number of animal studies. Usually the tracer distribution is indicated by a threshold on the intensity of a T1-weighted image resulting in a “volume of distribution.” More recently, a quantitative assessment of the tracer concentration has been developed [11, 38]. In conjunction with previous studies, concentration of radio-labeled tracers can now be measured [11, 14, 39, 40].
2.5. Other Tracers
Preclinical data using CED of monodispersedmaghemite nanoparticles (MNP) and MRI imaging to assess effective administration has been published [41]. This agent promises to enable the use of larger drugs and carriers previously thought inappropriate for CED and may increase the half-life of the therapeutic agent due to slow release.
2.6. Comparisons Between Tracers
2.6.1. T2 MRI Versus I123 Albumin Tracer
In an attempt to provide a quantitative comparison between MR signal change and drug distribution, Sampson et al. [13] co-infused a surrogate tracer 123I-HSAand compared with T2 changes [42], revealing inherent limitations with distribution elucidated by T2-weighted MRI. First, while minor changes in hyperintense signals on T2-weighted MRI following infusion were discernable in patients with preexisting peritumoral edema, these changes were ultimately indistinguishable from edema secondary to malignant glioma [13]. In addition, T2-weighted MRI was not sufficient to assess hyperintense signal in gray matter structures due to the much lower expandability of the interstitium in such cytoarchitectures. While T2-weighted MRI signals might be useful for determining the infusate distribution to extraparenchymal regions including the interventricular space or subarachnoid space, it may be insufficient when assessing the exact location of the infusate in the context of preexisting edema, or when visualization of distribution throughout gray matter is required.
More recently, Lonser and colleagues found that real – time T2 and diffusion weighted MRI significantly underestimated tissue volume of distribution during CED over a wide range of molecular sizes [43].
2.6.2. Gd-DTPA Versus 124I Albumin Tracer
Our group has recently demonstrated the ability to define the distribution of larger molecules (in this case 124I-HSA) by Gd-DTPA which is a much smaller molecule [44]. We simultaneously infused patients presenting with supratentorial recurrent malignant gliomas using an EGFRvIII-targeted immunotoxin in combination with 124I-HSA (to permit PET imaging) and Gd-DTPA. As assessed by MRI and PET/CT scans, Gd-DTPA infusions provided anatomically and volume\trically accurate information about infusate distribution as well as extraparenchymal leak into CSF spaces and resection cavities.
2.6.3. Gd-DTPA Versus Gd-Albumin Tracer
Gd-DTPA’s small molecular weight (938 Dalton in the Magnevist ™ formulation) initially raised concern for its ability to accurately predict the distribution of larger therapeutic molecules. Gadolinium-bound albumin (MW 72,000 D) has been recently compared in distribution with Gd-DTPA (MW 590 D) at pial and ependymal boundaries in primate models [45]. While this study demonstrated with FLAIR MR imaging that both small and large molecular weighted molecules exhibited similar distribution, this result is inconsistent with previous reports that size plays a role in the ability for drug to be distributed to a given region [46].
3. AGENTS
Although a large number of therapeutic options have been examined for CED, only a few agents have been formally studied in clinical trials. Some promising animal studies include utilization of CED for boron neutron capture therapy [47] with boronated epidermal growth factor [48], liposomal topotecan [49], adeno-associated virus thymidine kinase [50], liposomal irinotecan [49], carboplatin, and gemcitabine [51].
Some of the more prominent clinical trials studying CED have included: TP-38, cintredekinbesudotox, IL-4 pseudomonas exotoxin, paclitaxel, AP 12009, and cotara.Sampson et al. used CED infusion of TP-38 which is a chimeric protein that fuses TGF-α with a genetically modified Pseudomonas exotoxin. Overall survival was 28 weeks with a subset survival of 33 weeks in the 20 patients with no residual disease and 20.1 weeks in patients with residual disease [39]. There was one patient that at the time of publication had a survival of 260 weeks. The toxicities with TP-38 included 5 patients with seizures who had previous seizure disorders. Grade 3 or 4 toxicity was seen in 2 patients [24].
Cintredekinbesudotox was tested in 51 patients over the series of 3 phase I clinical trials for recurrent high grade gliomas. There was an overall survival rate of 45.9 weeks post-treatment, with 9 patients exhibiting progression free survival for 1 year and seven patients with progression free survival of 2 years [52–54]. There were grade 3 or 4 toxicities reported with headache, convulsion, and hemiparesis; six patients developed hemiparesis [54]. A phase III trial was completed for cintredekinbesudotox that was randomized against Gliadel wafers for recurrent GBM. The median survival was comparable between CED and Gliadel wafers at 36.4 weeks and 35.3 weeks respectively. Despite no statistical significance in improved survival for CED-administered therapy, inadequate drug distribution and improper catheter placement that might have explained the results [55, 56].
A recombinant cytotoxin that is a fusion between IL-4 and Pseudomonas exotoxin was applied via CED in a phase I clinical trial for recurrent gliomas [19, 57, 58]. When comparing the recombinant cytotoxin to resection alone the overall median survival of recurrent GBM was 5.8 months and 5.3 months, respectively [57]. There were no noted systemic toxicities, and the most common adverse events were cerebral edema, seizures, and headaches.
On infusion of paclitaxel for recurrent grade III or IV gliomas, Lidar et al. demonstrated an overall median survival of 7.5 months. However, there was a significant number of treatment related adverse events including transient neurological deterioration from cerebral edema (20%), bacterial infections (15%), and chemical meningitis (30%) [31].
AP 12009, an antisense oligonucleotide that binds to TGF-β2 mRNA to inhibit translation [59], was studied through three phase I/II clinical trials for CED that demonstrated a median overall survival of 44 weeks for GBM and 146.6 weeks for patients with anaplastic astrocytoma [60]. There were 29 treatment-related toxicities, but these were mostly minor and limited to grade 2 and 3 toxicities.
Cotara, an immunotoxin with an antibody specific for histone H1-DNA complex that was labeled with 131I, was studied in 51 patients enrolled in phase I and II studies. This treatment achieved a median survival in GBM patients of 37.9 weeks [18]. Eighteen patients had a grade 3 or 4 neurological toxicity, while 4 patients had grade 3 systemic toxicities [18].
These clinical trials have demonstrated the promise of convection enhanced delivery as a therapeutic option for patients with recurrent high grade gliomas with a relatively low toxicity profile associated with therapy. Further investigation using a phase 3 clinical trial and image guided therapies is needed in the future of CED clinical trials.
4. PLANNING CED
We now turn briefly to the second aspect of technologies to improve CED delivery. In analogy to the planning of radiation therapy, CED would benefit from the use of reliable computer modeling as well as the real-time monitoring of the catheter placement and infusion processes. We have reported on the performance of an early version of a computer planning system [25]. A detailed report on the algorithms employed, and performance in animals is forthcoming [61]_ENREF_52. As mentioned, costly failures in clinical trials involving CED may in part be attributable to failures in understanding tissue physiology (e.g., the inhomogeneities in fluid pathways in brain) and in employing such information to target agent delivery. In fact, radiation therapy became mainstream only when therapists could plan the treatment before it starts. It is important that we move towards the goal of testing the therapy, and not the delivery. We feel that only then will CED acquire success in clinical trials and become an approved procedure for drug delivery. In conjunction with this, improvements in the efficacy of the navigation and placement of the catheters are also useful. Recent renewed interest in interventional MR, together with advances in software to control the scanner to obtain rapid imaging of catheter placement during the procedure, will help CED as well as other interventional procedures.
CONCLUSION
The infusion of therapies to the intracerebal compartment offers many advantages and is a promising platform for treatment in patients with GBM. Presently, there are few established techniques for in vivo validation of mathematical models that predict the distribution of infusate following CED. As mentioned herein, what has previously been considered disappointing results for CED in clinical trials may reflect suboptimal catheter placement. In vivo imaging for CED will also weigh heavily on our ability to monitor infusate distribution and evaluate CED, as well as potential impact on outcomes, with greater precision and fidelity.
ACKNOWLEDGEMENT
None declared.
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- [1].Kanu OO, Mehta A, Di C, et al. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets 2009; 13(6): 701–18. [DOI] [PubMed] [Google Scholar]
- [2].Galanis E, Buckner J. Chemotherapy for high-grade gliomas. Br J Cancer 2000; 82(8): 1371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reardon DA, Rich JN, Friedman HS, et al. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol 2006; 24(8): 1253–65. [DOI] [PubMed] [Google Scholar]
- [4].Ehrlich P. Address in Pathology, ON CHEMOTHERAPY: Delivered before the Seventeenth International Congress of Medicine. Br Med J 1913; 2(2746): 353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol 2007; 25(16): 2295–305. [DOI] [PubMed] [Google Scholar]
- [6].Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 1994; 91(6): 2076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ding D, Kanaly CW, Bigner DD, et al. Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1–1. J Neurooncol 2010; 98(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sampson JH, Archer G, Pedain C, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 2010; 113(2): 301–9. [DOI] [PubMed] [Google Scholar]
- [9].Sampson JH, Brady ML, Petry NA, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007; 60(2 Suppl 1): ONS89–98; discussion ONS-9. [DOI] [PubMed] [Google Scholar]
- [10].Tanner PG, Holtmannspotter M, Tonn JC, et al. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery 2007; 61(4): E880–2; discussion E2. [DOI] [PubMed] [Google Scholar]
- [11].Raghavan R, Mikaelian S, Brady M, et al. Fluid infusions from catheters into elastic tissue: I. Azimuthally symmetric backflow in homogeneous media. Phys Med Biol 2010; 7; 55(1): 281–304. [DOI] [PubMed] [Google Scholar]
- [12].Raghavan R. Intraparenchymal delivery and its discontents. Drug delivery to the central nervous system 2010; 1: 85–135. [Google Scholar]
- [13].Sampson JH, Raghavan R, Provenzale JM, et al. Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am J Roentgenol 2007; 188(3): 703–9. [DOI] [PubMed] [Google Scholar]
- [14].Mardor Y, Rahav O, Zauberman Y, et al. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res 2005; 65(15): 6858–63. [DOI] [PubMed] [Google Scholar]
- [15].Raghavan R, Brady ML, Rodriguez-Ponce MI, et al. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 2006; 20(4): E12. [DOI] [PubMed] [Google Scholar]
- [16].Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 2007; 25(7): 837–44. [DOI] [PubMed] [Google Scholar]
- [17].Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 1997; 3(12): 1362–8. [DOI] [PubMed] [Google Scholar]
- [18].Patel SJ, Shapiro WR, Laske DW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery 2005; 56(6): 1243–52; discussion 52–3. [DOI] [PubMed] [Google Scholar]
- [19].Rand RW, Kreitman RJ, Patronas N, et al. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res 2000; 6(6): 2157–65. [PubMed] [Google Scholar]
- [20].Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol 2003; 65(1): 27–35. [DOI] [PubMed] [Google Scholar]
- [21].Sampson JH, Archer G, Pedain C, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. Journal of neurosurgery 2010; 113(2): 301–9. [DOI] [PubMed] [Google Scholar]
- [22].Rogawski MA. Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 2009; 6(2): 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sampson J, Reardon DA, Akabani G. A phase I study of intratumoral infusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin (TP-38) for the treatment of malignant brain tumors [Abstract]. Proc Am Assoc Cancer Res 2002; 43(746). [Google Scholar]
- [24].Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol 2008; 10(3): 320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sampson JH, Raghavan R, Brady ML, et al. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro Oncol 2007; 9(3): 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bidros DS, Liu JK, Vogelbaum MA. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol 2010; 6(1): 117–25. [DOI] [PubMed] [Google Scholar]
- [27].Ebersold MJ, Houser OW, Quast LM. Iopamidol myelography: morbidity in patients with previous intolerance to iodine derivatives. J Neurosurg 1991; 74(1): 60–3. [DOI] [PubMed] [Google Scholar]
- [28].Croteau D, Walbridge S, Morrison PF, et al. Real-time in vivo imaging of the convective distribution of a low-molecular-weight tracer. J Neurosurg 2005; 102(1): 90–7. [DOI] [PubMed] [Google Scholar]
- [29].Nguyen TT, Pannu YS, Sung C, et al. Convective distribution of macromolecules in the primate brain demonstrated using computerized tomography and magnetic resonance imaging. J Neurosurg 2003; 98(3): 584–90. [DOI] [PubMed] [Google Scholar]
- [30].Mardor Y, Roth Y, Lidar Z, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res 2001; 61(13): 4971–3. [PubMed] [Google Scholar]
- [31].Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 2004; 100(3): 472–9. [DOI] [PubMed] [Google Scholar]
- [32].Weinmann HJ, Brasch RC, Press WR, et al. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol 1984; 142(3): 619–24. [DOI] [PubMed] [Google Scholar]
- [33].Lonser RR, Schiffman R, Robison RA, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology 2007; 68(4): 254–61. [DOI] [PubMed] [Google Scholar]
- [34].Murad GJ, Walbridge S, Morrison PF, et al. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg 2007; 106(2): 351–6. [DOI] [PubMed] [Google Scholar]
- [35].Wang SC, White DL, Pope JM, et al. Magnetic resonance imaging contrast enhancement versus tissue gadolinium concentration. Invest Radiol 1990; 25 Suppl 1: S44–5. [DOI] [PubMed] [Google Scholar]
- [36].Lonser RR, Walbridge S, Garmestani K, et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg 2002; 97(4): 905–13. [DOI] [PubMed] [Google Scholar]
- [37].Saito R, Bringas JR, McKnight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res 2004; 64(7): 2572–9. [DOI] [PubMed] [Google Scholar]
- [38].Raghavan R, Brady M, Chen Z, et al. Quantifying Fluid Infusions and Tissue Expansion in Brain. IEEE Trans Biomed Eng 2011. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [39].Sampson JH, Reardon DA, Friedman AH, et al. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro Oncol 2005; 7(1) : 90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weber F, Bauer E, Brady M, et al. , editors. Assessing drug disposition in convection enhanced drug delivery using Gadolinium DTPA and computerized modeling. Society of Neurooncology; 2003. [Google Scholar]
- [41].Perlstein B, Ram Z, Daniels D, et al. Convection-enhanced delivery of maghemite nanoparticles: Increased efficacy and MRI monitoring. Neuro Oncol 2008; 10(2): 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jackson MT, Sampson J, Prichard HM. Platinum and palladium variations through the urban environment: evidence from 11 sample types from Sheffield, UK. Sci Total Environ 2007; 385(1–3): 117–31. [DOI] [PubMed] [Google Scholar]
- [43].Iyer RR, Butman JA, Walbridge S, Gai ND, Heiss JD, Lonser RR. Tracking accuracy of T2- and diffusion-weighted magnetic resonance imaging for infusate distribution by convection-enhanced delivery. J Neurosurg 2011; 115(3): 474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sampson JH, Brady M, Raghavan R, et al. Co-localization of gadolinium-DTPA with high molecular weight molecules after intracerebral convection-enhanced delivery in man. Neurosurgery 2011; 69(3): 668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jagannathan J, Walbridge S, Butman JA, Oldfield EH, Lonser RR. Effect of ependymal and pial surfaces on convection-enhanced delivery. J Neurosurg 2008; 109(3): 547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Szerlip NJ, Walbridge S, Yang L, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg 2007; 107(3): 560–7. [DOI] [PubMed] [Google Scholar]
- [47].Barth RF, Soloway AH, Fairchild RG. Boron neutron capture therapy of cancer. Cancer Res 1990; 50(4): 1061–70. [PubMed] [Google Scholar]
- [48].Yang W, Barth RF, Adams DM, et al. Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res 2002; 62(22): 6552–8. [PubMed] [Google Scholar]
- [49].Saito R, Krauze MT, Noble CO, et al. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro Oncol 2006; 8(3): 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cunningham J, Oiwa Y, Nagy D, et al. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant 2000; 9(5): 585–94. [DOI] [PubMed] [Google Scholar]
- [51].Degen JW, Walbridge S, Vortmeyer AO, et al. Safety and efficacy of convection-enhanced delivery of gemcitabine or carboplatin in a malignant glioma model in rats. J Neurosurg 2003; 99(5): 893–8. [DOI] [PubMed] [Google Scholar]
- [52].Debinski W, Obiri NI, Powers SK, et al. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res 1995; 1(11): 1253–8. [PubMed] [Google Scholar]
- [53].Debinski W, Gibo DM, Hulet SW, et al. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res 1999; 5(5): 985–90. [PubMed] [Google Scholar]
- [54].Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 2007; 25(7): 837–44. [DOI] [PubMed] [Google Scholar]
- [55].Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery 2007; 61(5): 1031–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- [56].Sawyer AJ, Piepmeier JM, Saltzman WM. New methods for direct delivery of chemotherapy for treating brain tumors. Yale J Biol Med 2006; 79(3–4): 141–52. [PMC free article] [PubMed] [Google Scholar]
- [57].Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol 2003; 64(1–2): 125–37. [DOI] [PubMed] [Google Scholar]
- [58].Rainov NG, Heidecke V. Long term survival in a patient with recurrent malignant glioma treated with intratumoral infusion of an IL4-targeted toxin (NBI-3001). J Neurooncol 2004; 66(1–2): 197–201. [DOI] [PubMed] [Google Scholar]
- [59].Schlingensiepen R, Goldbrunner M, Szyrach MN, et al. Intracerebral and intrathecal infusion of the TGF-beta 2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides 2005; 15(2): 94–104. [DOI] [PubMed] [Google Scholar]
- [60].Hau P, Jachimczak P, Schlingensiepen R, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 2007; 17(2): 201–12. [DOI] [PubMed] [Google Scholar]
- [61].Raghavan R, Brady M. Predictive models of pressure-driven infusions into brain parenchyma Phys Med Biol 2011; 56(19): 6179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]