Abstract
Background & Aims
Liver injury due to coronavirus disease 2019 (COVID-19) is being increasingly recognized. Abnormal liver chemistry tests of varying severities occur in a majority of patients. However, there is a dearth of accompanying liver histologic studies in these patients.
Methods
The current report details the clinical courses of 2 patients having severe COVID-19 hepatitis. Liver biopsies were analyzed under light microscopy, portions of liver tissue were hybridized with a target probe to the severe acute respiratory syndrome coronavirus-2 S gene, and small sections from formalin-fixed paraffin-embedded liver tissue were processed for electron microscopy.
Results
The liver histology of both cases showed a mixed inflammatory infiltrate with prominent bile duct damage, endotheliitis, and many apoptotic bodies. In situ hybridization and electron microscopy suggest the intrahepatic presence of severe acute respiratory syndrome coronavirus-2, the findings of which may indicate the possibility of direct cell injury.
Conclusions
On the basis of the abundant apoptosis and severe cholangiocyte injury, these histopathologic changes suggest a direct cytopathic injury. Furthermore, some of the histopathologic changes may resemble acute cellular rejection occurring after liver transplantation. These 2 cases demonstrate that severe COVID-19 hepatitis can occur even in the absence of significant involvement of other organs.
Keywords: COVID-19, Non-hepatotropic Virus, Hepatitis, Liver Biopsy, SARS-CoV-2, Liver Injury
Abbreviations used in this paper: ACE2, angiotensin-converting enzyme receptors; ACR, acute cellular rejection; COVID-19, coronavirus disease 2019; DILI, drug-induced liver injury; EM, electron microscopy; HCV, hepatitis C virus; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2
Summary.
Liver injury from COVID-19 infection is increasingly being identified, with a dearth of corresponding liver biopsy data to date. The unique liver histology of 2 patients who both recovered with severe COVID-19 hepatitis is presented. Liver involvement may be the only manifestation of COVID-19 infection.
Coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome CoV-2 (SARS-CoV-2), is a global pandemic of unprecedented proportions.1 During the months of March and April 2020, New York City became the epicenter of the COVID-19 pandemic, and Mount Sinai Hospital was one of the major hospitals in the region that took care of several thousand COVID-19 patients. In the midst of the pandemic, ongoing liver transplantation (LT) and referrals of patients with severe liver disease continued unabated.2
Initial reports focused on COVID-19 as mainly involving the lungs and being the main cause of morbidity and mortality.3 However, neurologic, cardiac, renal, hepatobiliary, and gastrointestinal tract involvements are increasingly being recognized.3 The reported incidence of liver injury in patients with COVID-19 ranges from 14% to 53%.4, 5, 6 Those studies are mainly based on abnormal liver enzyme elevations in the absence of tissue examination. In one study, a significant number of cases were noted to have elevation of liver enzymes occurring at approximately the tenth day of hospitalization and associated with lopinavir/ritonavir treatment.7 The authors also found that prolonged hospital stays were associated with abnormal liver enzymes noted at the time of initial admission.7 Risk factors included underlying chronic liver disease such as viral hepatitis and nonalcoholic fatty liver disease (NAFLD).4 In a study involving 252 COVID-19 patients, the investigators found that patients having NAFLD had a significantly higher risk of disease progression, a higher likelihood of developing abnormal liver tests during hospitalization, and longer viral shedding times as compared with patients without NAFLD.6
In a previous study of postmortem examinations, liver histology showed lobular lymphocytic inflammation and centrilobular sinusoidal dilatation, with only a few cases showing parenchymal necrosis.8 Furthermore, in another autopsy study of a 51-year-old man, microvesicular steatosis and mild lobular and portal inflammation were noted.9 At the present time, no detailed histologic, immunohistochemical, and ultrastructural findings have been reported in the literature regarding infection of the liver by COVID-19. Herein we report 2 patients who presented with high aminotransferases and underwent liver biopsy that showed acute hepatitis. A detailed histologic analysis along with in situ hybridization and electron microscopy (EM) were performed on liver tissue to suggest direct hepatic involvement by the novel coronavirus SARS-CoV-2.
Clinical Histories
Case 1
The patient is a 63-year-old man who underwent LT in 2017 for hepatitis C (HCV) and alcohol-related liver disease. He received a genotype 1b HCV (+) donor with a pre-perfusion liver biopsy showing no underlying fibrosis and mild portal inflammation. He underwent antiviral therapy with sofosbuvir/ledipasvir starting 3 months after LT and achieved a sustained virologic response. He had developed dialysis-dependent chronic renal failure related to calcineurin inhibitor nephrotoxicity, hypertension, and insulin-dependent diabetes mellitus but always had normal liver chemistry tests and never previously had an episode of acute cellular rejection (ACR). The patient was stable for 2 years after LT until he suffered a stroke in January 2020. Laboratory tests on discharge from the transplant center after his stroke were normal with aspartate aminotransferase 12 U/L, alanine aminotransferase 14 U/L, alkaline phosphatase 68 U/L, and normal serum bilirubin. The tacrolimus dose was stable with a level of 4–6 ng/mL. He was discharged to a rehabilitation facility on regular doses of his medications, which included aminosalicylic acid 81 mg, atorvastatin, calcium acetate, dorzolamide drops, famotidine, insulin, labetalol, nifedipine, ropinirole, sodium bicarbonate, tacrolimus 2 mg twice a day, and tamsulosin. While residing at the rehabilitation facility, the patient developed constitutional symptoms and was found to be COVID-19 (+) via nasopharyngeal swab testing and referred to a local hospital where the liver enzymes were noted to be abnormal (Table 1). The patient was started on N-acetyl cysteine and acyclovir empirically; however, follow-up laboratory tests showed rising liver enzymes.
Table 1.
Liver Enzyme Values of Case 1 and Case 2
| AST, U/L (1–35) | ALT, U/L (1–45) | Alk Phos, U/L (38–126) | T bili, mg/dL (0.1–1.2) | D-dimer, μg/mL (0–0.5) | Ferritin, ng/mL (30–400) | Procalcitonin, ng/mL (<0.49) | CRP, mg/L (0–5) | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | ||||||||
| HD 1 | 1083 | 1035 | 824 | 1.1 | ||||
| HD 2 | 2074 | 1761 | 907 | 3.1 | ||||
| HD 3 | 1691 | 1578 | 915 | 3.9 | 1.84 | 4677 | 5.24 | 24.5 |
| HD 6 | 769 | 994 | 1363 | 7.1 | 1.38 | 2863 | 3.57 | 11.3 |
| HD 9 | 541 | 776 | 1568 | 9.3 | 0.77 | 2520 | 1.16 | 7.0 |
| Case 2 | ||||||||
| HD 1 | 2786 | 2909 | 210 | 13.8 | 827 | 0.35 | 8.4 | |
| HD 8 | 1700 | 1856 | 132 | 20.6 | 1290 | 1558 | 0.26 | 0.7 |
| HD 15 | 591 | 650 | 184 | 31.5 | 172 | 0.9 | ||
| HD 18 | 506 | 616 | 241 | 33.5 | 0.27 | 224 | 0.28 | 0.7 |
| HD 24 | 200 | 221 | 166 | 10.7 |
Alk Phos, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; HD, hospital day; T bili, total bilirubin.
He was transferred to Mount Sinai Hospital for further management. Reverse transcription polymerase chain reaction (PCR) on a repeat nasopharyngeal swab specimen confirmed SARS-CoV-2 infection, and the tacrolimus dose was increased to 3 mg twice a day in the setting of a trough level of 3.0 ng/mL. On presentation, the aminotransferases and alkaline phosphatase levels were elevated, as was the total serum bilirubin. Doppler ultrasonography showed no vascular or biliary abnormalities. The following additional lab results were obtained: undetectable PCR for herpes simplex 1 and 2, HCV, hepatitis B virus, Epstein-Barr virus, and cytomegalovirus, undetectable antibody to hepatitis E, and undetectable immunoglobulin M for hepatitis A virus. The triglyceride level was 483 mg/dL (0–150). Inflammatory markers were significantly elevated (Table 1) including lactate dehydrogenase of 702 U/L (reference range, 100–220). The platelet count was 132 × 103, and the white blood cell count was 3.9 × 103. The patient’s clinical and serologic features supported the diagnosis of COVID-19 infection.
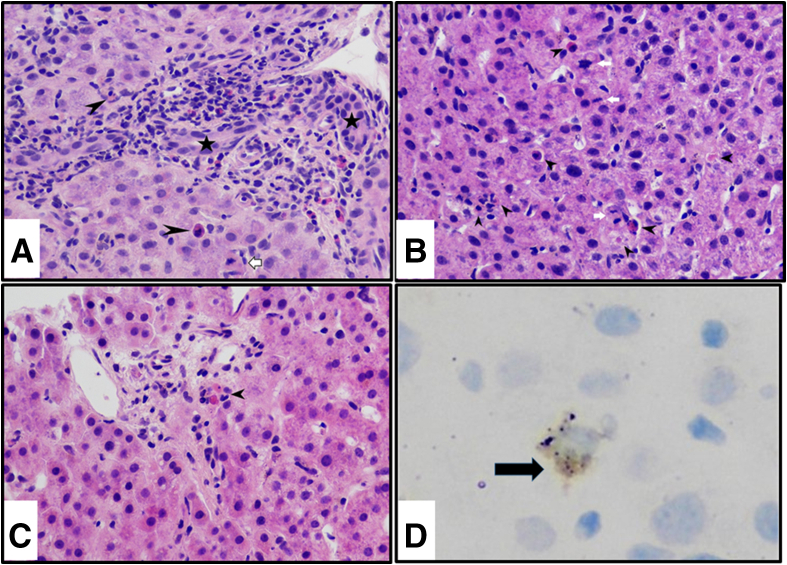
A liver biopsy was performed 9 days after the initial presentation and on hospital day 6. The histologic changes are highlighted in Figure 1. The majority of the portal tracts uniformly showed a mixed inflammatory infiltrate with a few eosinophils, prominent bile duct damage, and endotheliitis (Figure 1a). The lobule showed disarray with many apoptotic bodies, foci of necrosis, and abundant mitotic figures (Figure 1b). Focal central venulitis was noted. C4d immunostain was positive in a few endothelial cells lining the portal and central venules. CD61 immunostain to determine the presence of fibrin thrombi was negative. In rare portal tracts, severe bile duct damage with cholangiocytes undergoing apoptosis was seen (Figure 1c). A diagnosis of ACR and concomitant severe acute hepatitis was rendered.
Figure 1.
Liver biopsy findings of case 1. (a) Portal tract showing mixed inflammatory infiltrate consisting of lymphocytes, blast-like cells, and eosinophils, along with mild endotheliitis and severe bile duct damage. Bile duct is barely unrecognizable, and in its place are apoptotic cholangiocytes (arrowheads). Open arrowhead indicates accompanying hepatic arteriole. H&E, original magnification ×40. Black stars indicate a damaged bile duct. (b) Lobule shows disarray with many hepatocytes undergoing apoptosis in various stages (black arrowheads), while there is also concomitant increased mitotic activity (white arrows). H&E, original magnification ×40. (c) A few portal tracts are devoid of inflammation. Because of paucity of inflammation in this particular portal tract, one can visualize the cholangiocytes undergoing apoptosis (arrow). H&E, original magnification ×40. (d) RNA localization of COV-S protein using RNAScope showing dot-like particles (black arrow) in cytoplasm of an infected endothelial cell.
Slides for RNAScope for SARS-CoV-2 spike protein showed positive staining in rare cells and were seen as cytoplasmic dotted signals. In the absence of double immunostaining, the type of cells with positive staining could not be identified with certainty (Figure 1d). Throughout the entire 3.2-cm length of the liver biopsy, only 8 such cells were found to be positive. No cells were positive for the SARS-CoV-2 spike protein anti-sense strand probe, indicating that no active replication could be detected.
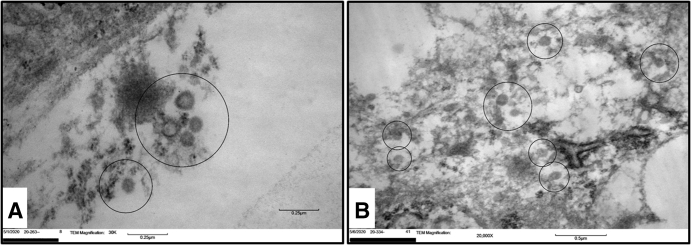
EM revealed the presence of viral-like particles displaying a double membrane electron-dense periphery with characteristic outward projecting processes consistent with a peplomer-like arrangement and measuring 100 nm on average, consistent with members of the Coronaviridae family10, 11, 12 (Figure 2a). The virions were mostly located within intracytoplasmic vesicles in close proximity to the endoplasmic reticulum. After the biopsy, the tacrolimus level was optimized to achieve a trough level of approximately 10. Infectious diseases department was consulted and recommended that the patient did not warrant medical treatment for COVID-19 infection because he was oxygenating normally and had a normal chest x-ray. Under observation, the patient’s liver chemistry tests slowly down trended, as did all of the inflammatory markers (Table 1) until discharge.
Figure 2.
Electron microscopy photos of case 1 and case 2. (a) EM showing presence of viral-like particles (encircled) displaying a double membrane electron-dense periphery with characteristic outward projecting processes consistent with a peplomer-like arrangement and measuring 100 nm on average. (b) Viral-like particles (encircled) measuring approximately 100 nm on average, with noticeable surface (peplomeric) projections appearing to be within sinusoidal endothelial cells.
Case 2
The patient is a 36-year-old previously healthy woman who presented to a local hospital with 6 days of progressive nausea, vomiting, and scleral icterus. She also had anorexia, myalgias, and fevers 1 week before her presentation, taking approximately 1.5 g acetaminophen and no other prescription or over-the-counter medications. Initial laboratory tests at presentation on April 6, 2020 showed markedly elevated aminotransferases, total serum bilirubin, and a mildly elevated alkaline phosphatase (Table 1). The international normalized ratio was 1.3. Additional serologic markers included antinuclear antibodies + 1:160, liver-kidney microsomal antibody negative, anti-smooth muscle antibody negative, cytomegalovirus immunoglobulin M negative, Epstein-Barr virus PCR negative, herpes simplex virus (–), human immunodeficiency virus (–), and negative acute serologies for hepatitis A virus, hepatitis B virus, and HCV. Serologic tests are outlined in Table 1. She had normal magnetic resonance imaging of the liver; a nasopharyngeal swab was (+) for COVID-19. The patient was started on an infusion of N-acetyl cysteine, and a liver biopsy was performed on hospital day 7. She was thereafter transferred to Mount Sinai Hospital.
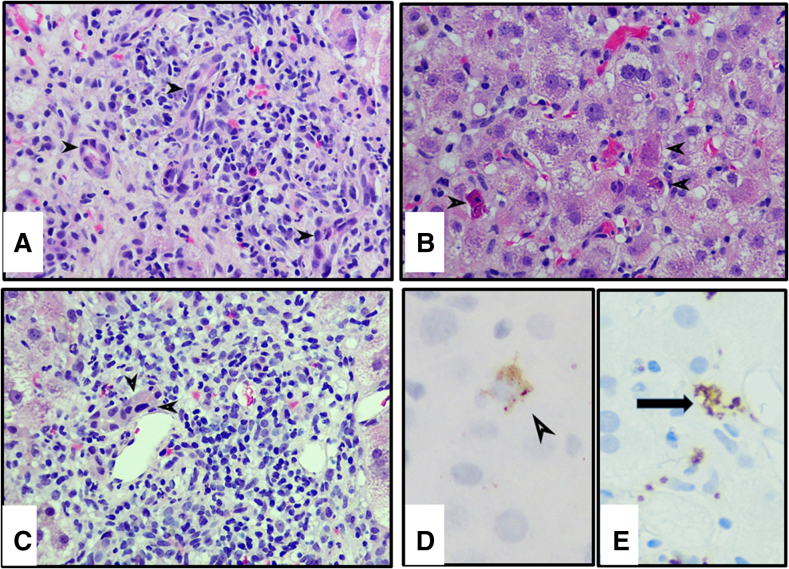
Review of the liver biopsy (Figure 3) showed severe acute hepatitis with marked lobular disarray characterized by inflammatory infiltrates, many acidophilic bodies, ballooned hepatocytes, scattered ceroid-laden macrophages, Kupffer cell hyperplasia, and activated sinusoidal lining cells. Portal areas showed mild to moderate mixed inflammation composed of lymphocytes, few plasma cells, scattered eosinophils and neutrophils, accompanied by mild interface hepatitis and mild ductular reaction (Figure 3a and b). Centrilobular hepatocyte necrosis with dropout and focal confluent necrosis were seen. Mild endotheliitis was noted, and most of the interlobular bile ducts were severely damaged. The severe damage was characterized by cholangiocytes having eosinophilic cytoplasm and densely pyknotic nuclei indicative of apoptosis (Figure 3c). CD61 immunostaining showed granular positive staining along the sinusoidal endothelial lining and in some of the terminal hepatic venules, indicative of the presence of non-occlusive fibrin thrombi (Figure 3d).
Figure 3.
Liver biopsy findings of case 2. (a) Portal tract with mixed inflammation, including eosinophils, activated lymphocytes, and rare plasma cells. Bile ducts are severely damaged (arrowheads), with some cholangiocytes undergoing acidophilic change and apoptosis. Detached necrotic cholangiocytes are seen in the lumen (bottom right). H&E, original magnification ×40. (b) High power view of lobule showing scattered hepatocytes undergoing apoptosis (arrowheads), with rest of the lobule showing ballooning degeneration of hepatocytes and foci of necroinflammation. H&E, original magnification ×40. (c) Portal tract containing dense mixed inflammatory infiltrate with bile duct showing cholangiocytes undergoing acidophilic change (arrowheads). H&E, original magnification ×40. (d) Immunostain for CD61 shows granular lace-like pattern of staining decorating the endothelial lining of the sinusoids as well as terminal hepatic venule (arrowhead). Original magnification ×40. (e) In situ hybridization showing hepatocyte with intracytoplasmic granular staining for COV-Spike protein (arrow). Original magnification ×40.
Similar to what was seen in case 1, RNAScope for SARS-CoV-2 spike protein was positive in rare cells (Figure 3e). A total of 6 positive cells throughout the biopsy length of 3.8 cm were identified; no cells were positive for viral replication by spike protein anti-sense strand probe. Transmission electron microscopy showed viral-like particles measuring approximately 100 nm on average, with noticeable surface (peplomeric) projections. Ultrastructurally, these virions appeared to be within sinusoidal endothelial cells10, 11, 12 (Figure 2b).
The patient was deemed not to require treatment for COVID-19 because she was oxygenating well and had no radiographic evidence of pneumonia. She was also found to have thyroid-stimulating hormone of .008 and was diagnosed with thyroiditis, which has recently been reported in COVID-19 infection,13 and she was started on cholestyramine and ursodiol. She had repeat negative PCR testing for SARS CoV-2 on hospital days 15, 18, and 24 at Mount Sinai. Because she was feeling much better she was discharged, with lactate dehydrogenase of 209 and D-dimer <0.27. Laboratory testing 1 week later showed an ongoing decrease in her liver enzymes, although the bilirubin was still elevated to 10.7 mg/dL with an indirect fraction of 7.8 mg/dL.
Discussion
The cases presented in the current report had markedly elevated liver enzymes at presentation, with both fitting the clinical course timeline as well as the histologic features of severe COVID-19 hepatitis. RNA localization of SARS-CoV-2 S protein using in situ hybridization and EM findings suggest that SARS-CoV-2 may have played a major role in the liver damage incurred by these 2 patients. Despite the initial presentation of severe liver damage, both patients recovered from COVID-19 hepatitis.
Currently, it is known that the development of abnormal liver chemistry tests and various forms of liver injury may result from COVID-19,14 with the majority of cases having mildly elevated liver enzymes.4 In a series of 99 COVID-19 patients, only 1 case presented with severe liver injury with markedly elevated liver enzymes, whereas 43 of 99 showed mild alanine aminotransferase and aspartate aminotransferase elevations (in 28% and 35%, respectively).15 In patients with significantly increased liver enzymes, there were longer hospital stays, and most patients were taking medications such as lopinavir/ritonavir, with the latter raising the possibility of drug-induced liver injury (DILI).7 A recent review concluded that significantly elevated aminotransferases are only found in severe cases of COVID-19.16 However, to date there are only case reports or small case series detailing the liver histologic findings found in patients with COVID-19 infection.4 Microvesicular steatosis and the presence of mild lymphocytic inflammation in the lobules and rarely in portal tracts are the main findings reported.4 Furthermore, there is a dearth of reported liver biopsies in COVID-19 infection of solid organ recipients.17 Aside from one post-LT pediatric case wherein a liver biopsy was performed several days after LT, no other cases have been reported.18
Liver biopsies from both patients in this study showed prominent mitoses of hepatocytes along with acidophilic bodies, ballooning degeneration, and mild inflammation. Histology also showed mixed inflammatory infiltrate in portal tracts, endotheliitis, and severe bile duct damage. Although these features are seen in acute T-cell–mediated rejection, which might conceivably explain the portal tract findings in patient 1, patient 2 did not have LT and yet showed a similar histology. Lagana et al18 reported the presence of increased mitotic activity and the presence of numerous apoptotic bodies in their post-LT case. A case series from the SARS epidemic in 2003 described the histology of 3 patients. These patients all had elevated liver enzymes in the 300–400 range, and liver histology showed prominent mitoses.19 The authors surmised that the prominent mitoses were likely due to a hyperproliferative state and cell cycle arrest. They further described ballooning degeneration and mild to moderate lobular inflammation. The authors ascribed these findings to what was previously reported in avian coronavirus and transmissible gastroenteritis coronavirus infections in which the coronavirus had exerted extensive cytopathic effect through the induction of apoptosis of host cells by activation of caspase.19, 20, 21 The 2 cases presented here appear to have similar cytopathic effect from SARS-CoV-2 due to the increased number of apoptotic bodies noted on both liver biopsies.
The proposed mechanism of liver injury from SARS-CoV-2 includes severe inflammatory responses and direct cytotoxicity due to active viral replication with angiotensin-converting enzyme receptors (ACE2) being abundant in the liver, particularly in cholangiocytes and endothelial cells.22 It has been shown that SARS-CoV-2 interacts with host receptors in liver cells. Gene expression of ACE2 transmembrane serine protease 2 (TMPRSS2) and paired basic amino acid cleaving enzyme (FURIN) has been shown. All 3 receptors are abundant in cholangiocytes and hepatocytes as well as in endothelial cells. Because these 3 receptors are present in various liver cells, SARS-CoV-2 may cause direct injury via a cytopathic effect, either by lysis and/or by inducing necrosis and apoptosis.22, 23, 24 Therefore, the ballooning degeneration and apoptosis as well as the striking bile duct damage may possibly be due to direct viral injury, with in situ hybridization and EM demonstrating viral particles within the liver. Previous case reports have failed to demonstrate the detection of intrahepatic viral particles. SARS-CoV-2 can also infect the gastrointestinal tract because of the abundant ACE2 receptors present.24,25 Therefore, the possibility that SARS-CoV-2 may enter the liver via the portal vein (portal venous viremia) can also be entertained.23 Varga et al26 have shown SARS-CoV-2 can directly infect endothelial cells across vascular beds of different organs, although not specifically in the liver. Widespread endothelial cell dysfunction in heart, kidney, lung, and small intestine resulted in apoptosis and prominent endotheliitis of submucosal vessels.26 Another mechanism believed to be the underlying cause of liver injury is ischemic changes in those with severe COVID-19 and DILI.4,27,28 In addition to the direct damage, translocation, and DILI, immune-mediated liver injury is another mechanism to consider. This is especially important in light of the increased inflammatory markers noted on serologic testing.16 Although SARS-CoV-2 virus is a new virus and our understanding of its tissue and cellular localization and replication is very limited, the sparse distribution of the virus as seen in the liver in the 2 current cases is not surprising because of the comparable sporadic and occasional cellular distribution described with the first SARS-CoV coronavirus in the early and mid-2000s.29,30 Despite the fact that we could not prove that the virus is replicating within the liver tissue because we did not see positive signals for the anti-sense strand of the S gene of the virus, we could not rule out such a possibility because the viral particles are sparsely distributed, and there is a limited amount of liver tissue in these core needle biopsies.
The clinical picture of the 2 patients is that of a spontaneously resolving COVID-19 infection with the markedly elevated liver tests and inflammatory markers decreasing concurrently. Although the patients did not have severe pulmonary, cardiac, or neurologic manifestations of COVID-19 infection that warranted treatment, significant liver chemistry abnormalities developed that correlated with the histologic liver damage noted. Neither patient had known underlying intrinsic liver disease, but if they did in light of the severe histologic liver damage noted, it is possible that they might have developed acute-on-chronic liver failure due to COVID-19.14,31
Some of the histologic findings seen in Case 1 are also typically seen in ACR, such as mixed inflammatory infiltrate in portal tracts, endotheliitis, and severe bile duct damage. In addition, there was histologic evidence of acute hepatitis, likely because of COVID-19, ie, COVID-19 hepatitis. Lagana et al18 showed similar findings in their case that occurred 7 days after transplantation. Although it is possible that this patient had both mild ACR and concurrent COVID-19 hepatitis, it is more likely that the histologic features are due to COVID-19 because the magnitude of the aminotransferase elevation is not typical of mild ACR, and the substantive decline in liver tests with only a minimal increase in tacrolimus dose is not typical of ACR.
Although both of the reported post-LT cases may be episodes of ACR that were triggered by COVID-19, as can occur with other viral infections,32 it is more likely that these histologic features are all related to COVID-19 infection because case 2 in the current report had very similar features. The endotheliitis demonstrated in these 2 patients appears similar to that reported in other organs.26,33 The histology thus suggests both a direct viral cytopathic effect and an immunologic liver injury involving cholangiocytes, hepatocytes, and liver sinusoidal endothelial cells. The significant bile duct damage and apoptosis of cholangiocytes are very prominent in these cases. In the absence of intrinsic liver disease, resolution of the direct cytopathologic injury may occur with clearance of SARS-CoV-2, yet it will remain to be seen whether the immunologic component of the liver injury will as well. Thus, close follow-up of such patients is warranted.
In conclusion, the current report details the liver histologic findings of acute COVID-19 infection in 2 patients, which are supported by the use of in situ hybridization and EM studies. Apoptosis, especially of cholangiocytes, abundant mitoses, mixed inflammatory infiltrate in portal tracts, endotheliitis, and severe bile duct damage are typical. This histology suggests a direct cytopathic viral injury most notably in sinusoidal endothelial cells and other liver cells, with concurrent immunologic features. Such histologic changes can resemble ACR, so LT physicians must consider this possibility in post-LT patients with COVID-19 infection. These cases also demonstrate that patients can have severe liver injury in the absence of significant involvement of other organs by COVID-19.
Materials and Methods
Histopathology
Transjugular needle liver biopsy was performed for patient 1, and a percutaneous liver biopsy was performed for patient 2. Core biopsy specimens were immediately placed in formalin and fixed for a minimum of 3 hours. Routine tissue processing through graded alcohols was performed. Thereafter, the liver biopsy tissues were embedded in paraffin (FFPE). Three 4-μm-thick sections were cut for H&E stain to assess different levels and one 4-μm section for Masson trichrome stain. H&E and trichrome-stained slides were analyzed by routine light microscopy. Adequacy of the specimen was determined including the linear length of tissue and the number of complete portal tracts. Morphologic evaluation was performed under light microscopy. Histopathologic features analyzed included degree of portal and lobular inflammation, parenchymal damage including apoptosis, presence of fibrosis, bile duct injury, and endothelial damage.
In Situ Hybridization
Sections at 3 μm in thickness were taken from FFPE for RNA in situ hybridization using the RNAscope HPV kit (Advanced Cell Diagnostics, Inc, Hayward, CA) according to the manufacturer’s instructions on a Leica Bond III automated stainer (Leica Biosystems, Buffalo Grove, IL). Tissue sections were hybridized separately with a target probe to the SARS-CoV-2 S gene encoding the spike protein (catalogue #848561) to detect viral proteins within the cells to indicate infected cells and SARS-CoV-2 antisense strand of the S gene (catalogue #845701) to detect active viral replication within the cells. Positive controls for the SARS-CoV-2 S and antisense strand of the genes were prepared in-house from SARS-CoV-2-infected Vero cell lines (cell lines gift from Dr Florian Krammer, Icahn School of Medicine at Mount Sinai, New York, NY). To assess for the RNA integrity, probes for the endogenous housekeeping gene UBC (catalogue #RS7760) were used. The preamplifier, amplifier, and horseradish peroxidase–labeled probes were then hybridized sequentially, followed by color development with DAB. Specific staining signals were identified as brown, punctate dots present in the cytoplasm and/or nucleus.
Transmission Electron Microscopy
The tissue processed for EM was received in paraffin blocks. Designated portions of the specimen were dissected out with a single edge razor blade and placed into xylene overnight to dissolve the paraffin. The tissue was then brought to water through decreasing concentrations of ethanol and then fixed with 3% glutaraldehyde in a 0.2 sodium cacodylate buffer at pH 7.4.The specimen was then post-fixed with 1% osmium tetroxide tissues and embedded in Epon 812. One micrometer plastic sections were cut and stained with methyl blue and azure II for light microscopic orientation and to select smaller representative areas. Ultrathin 60-nm sections were collected into 200 mesh copper grids and stained with lanthanum and lead citrate. Sections were examined in a Hitachi 7650 (Tokyo, Japan) transmission electron microscope at 80 kV.
All authors had access to the study data and reviewed and approved the final manuscript.
Acknowledgments
CRediT Authorship Contributions
Maria Isabel Fiel, MD (Conceptualization: Lead; Data curation: Lead; Methodology: Equal; Writing – original draft: Lead)
Siraj M. El Jamal, MD (Methodology: Supporting; Writing – original draft: Supporting)
Alberto Paniz-Mondolfi, MD (Methodology: Supporting; Writing – original draft: Supporting)
Ronald E. Gordon, PhD (Methodology: Supporting; Writing – original draft: Supporting)
Jason Reidy, PhD (Methodology: Supporting)
Jela Bandovic, MD (Methodology: Supporting; Visualization: Supporting)
Rashmi Advani, MD (Data curation: Supporting)
Saikiran Kilaru, MD (Data curation: Supporting)
Kamron Pourmand, MD (Data curation: Supporting)
Stephen Ward, MD, PhD (Formal analysis: Supporting)
Swan N. Thung, MD (Formal analysis: Supporting)
Thomas D. Schiano, MD (Conceptualization: Equal; Formal analysis: Equal; Writing – original draft: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Coronavirus disease 2019 (COVID-19) situation report-99. [Google Scholar]
- 2.Hygiene NDoHaM. COVID-19 data. 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page Available from:
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Sun J., Aghemo A., Forner A., Valenti L. COVID-19 and liver disease. Liver Int. 2020 doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Z., Chen L., Li J., Cheng X., Jingmao Y., Tian C., Zhang Y., Huang S., Liu Z., Cheng J. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., Xiao S.Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ML PEaM . CRC Press; Boca de Raton, FL: 1988. Electron microscopy in viral diagnosis. [Google Scholar]
- 11.Oshiro L.S., Schieble J.H., Lennette E.H. Electron microscopic studies of coronavirus. J Gen Virol. 1971;12:161–168. doi: 10.1099/0022-1317-12-2-161. [DOI] [PubMed] [Google Scholar]
- 12.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020 doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brancatella A., Ricci D., Viola N., Sgrò D., Santini F., Latrofa F. Subacute thyroiditis after SARS-CoV-2 infection. J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targher G., Mantovani A., Byrne C.D., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., Zheng K.I., Chen Y.P., Eslam M., George J., Zheng M.H. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020 doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 15.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zippi M., Fiorino S., Occhigrossi G., Hong W. Hypertransaminasemia in the course of infection with SARS-CoV-2: incidence and pathogenetic hypothesis. World J Clin Cases. 2020;8:1385–1390. doi: 10.12998/wjcc.v8.i8.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., Arcasoy S., Aversa M.M., Benvenuto L.J., Dadhani D., Kapur S., Dove L.M., Brown R.S., Rosenblatt R.E., Samstein B., Uriel N., Farr M.A., Satlin M., Small C.B., Walsh T., Kodiyanplakkal R.P., Miko B.A., Aaron J.G., Tsapepas D.S., Emond J.C., Verna E.C. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020 doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagana S.M., De Michele S., Lee M.J., Emond J.C., Griesemer A.D., Tulin-Silver S.A., Verna E.C., Martinez M., Lefkowitch J.H. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0186-SA. [DOI] [PubMed] [Google Scholar]
- 19.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C., Choi K.W., Tso Y.K., Lau T., Lai S.T., Lai C.L. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C., Kokuho T., Kubota T., Watanabe S., Inumaru S., Yokomizo Y., Onodera T. A serodiagnostic ELISA using recombinant antigen of swine transmissible gastroenteritis virus nucleoprotein. J Vet Med Sci. 2001;63:1253–1256. doi: 10.1292/jvms.63.1253. [DOI] [PubMed] [Google Scholar]
- 21.Eléouët J.F., Druesne N., Chilmonczyk S., Monge D., Dorson M., Delmas B. Comparative study of in-situ cell death induced by the viruses of viral haemorrhagic septicaemia (VHS) and infectious pancreatic necrosis (IPN) in rainbow trout. J Comp Pathol. 2001;124:300–307. doi: 10.1053/jcpa.2001.0467. [DOI] [PubMed] [Google Scholar]
- 22.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A., van der Voort P.H.J., Mulder D.J., van Goor H. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020 doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirola C.J., Sookoian S. COVID-19 and ACE2 in the liver and gastrointestinal tract: putative biological explanations of sexual dimorphism. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong S.H., Lui R.N., Sung J.J. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 25.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Fan J.G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.To K.F., Lo A.W. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) J Pathol. 2004;203:740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M., Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cakaloglu Y., Devlin J., O'Grady J., Sutherland S., Portmann B.C., Heaton N., Tan K.C., Williams R. Importance of concomitant viral infection during late acute liver allograft rejection. Transplantation. 1995;59:40–45. doi: 10.1097/00007890-199501150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]