Abstract
Early risk stratification for complications and death related to Coronavirus disease 2019 (COVID-19) infection is needed. Because many patients with COVID-19 who developed acute respiratory distress syndrome have diffuse alveolar inflammatory damage associated with microvessel thrombosis, we aimed to investigate a common clinical tool, the CHA(2)DS(2)-VASc, to aid in the prognostication of outcomes for COVID-19 patients. We analyzed consecutive patients from the multicenter observational CORACLE registry, which contains data of patients hospitalized for COVID-19 infection in 4 regions of Italy, according to data-driven tertiles of CHA(2)DS(2)-VASc score. The primary outcomes were inpatient death and a composite of inpatient death or invasive ventilation. Of 1045 patients in the registry, 864 (82.7%) had data available to calculate CHA(2)DS(2)-VASc score and were included in the analysis. Of these, 167 (19.3%) died, 123 (14.2%) received invasive ventilation, and 249 (28.8%) had the composite outcome. Stratification by CHA(2)DS(2)-VASc tertiles (T1: ≤1; T2: 2 to 3; T3: ≥4) revealed increases in both death (8.1%, 24.3%, 33.3%, respectively; p <0.001) and the composite end point (18.6%, 31.9%, 43.5%, respectively; p <0.001). The odds ratios for mortality and the composite end point for T2 patients versus T1 CHA(2)DS(2)-VASc score were 3.62 (95% CI:2.29 to 5.73,p <0.001) and 2.04 (95% CI:1.42 to 2.93, p <0.001), respectively. Similarly, the odds ratios for mortality and the composite end point for T3 patients versus T1 were 5.65 (95% CI:3.54 to 9.01, p <0.001) and 3.36 (95% CI:2.30 to 4.90,p <0.001), respectively. In conclusion, among Italian patients hospitalized for COVID-19 infection, the CHA(2)DS(2)-VASc risk score for thromboembolic events enhanced the ability to achieve risk stratification for complications and death.
Coronavirus disease 2019 (COVID-19) is a viral infection causing acute respiratory distress syndrome (ARDS) and was initially observed in December 2019 in Wuhan, China.1 One of the concerning features of COVID-19 patients is the development of severe coagulopathy. Indeed, a recent report from Wuhan revealed that among patients who died from COVID-19, 71.4% met criteria for disseminated intravascular coagulation. Some researchers argue that thrombotic derangement is related to multiorgan damage disease or that it is a direct effect of infection on hepatic function. Accordingly, recent studies found a high incidence (25%) of venous thromboembolism in COVID-19 patients.2 , 3 Additionally, elevated levels of D-dimer and fibrinogen have been reported in COVID-19 patients, which appear to be associated with negative outcomes.4 Further, others have demonstrated that COVID-19 patients treated with tissue plasminogen activator and heparin experienced more favorable outcomes compared to untreated patients, had improvements in respiratory compliance (expressed by PaO2/FiO2 ratio), and had reductions in D-dimer serum levels.5 , 6 These findings may support the hypothesis that the disseminated intravascular coagulation was of a thrombotic origin instead of bleeding diathesis or multiorgan damage.2 However recent data of COVID-19 patients’ autopsies reveal extensive areas of inflammatory infiltration associated with interstitial oedema and thrombotic lesions in microvessels and in some cases, even massive pulmonary embolism.7, 8, 9 On the basis of these findings, we sought to investigate thromboembolic risk in COVID-19 patients by utilizing CHA(2)DS(2)-VASc. Specifically, in this case series, we explored the relationship between CHA(2)DS(2)-VASc score and the need for mechanical ventilation and/or inpatient mortality.
Methods
We used the CORACLE registry, which include relevant data on COVID-19 patients hospitalized in 4 regions of Italy, to perform this analysis. All patients were at least 18 years old, were admitted to the hospital on or after February 22, 2020, and had COVID-19 infection, confirmed using nose or throat swab testing with real-time reverse transcription polymerase chain reaction. All patients received at least 2 venous administrations of low molecular weight heparin as thromboembolism prophylaxis. We chose to restrict this analysis to patients having data available on admission to calculate CHA(2)DS(2)-VASc score, as well as having a known inpatient mortality status (i.e., discharged alive or died in the hospital) at the time of analysis. At hospital admission, all patients gave their written consent to data collection anonymously. The study was conducted according to the principles outlined in the Declaration of Helsinki. This work was approved by the ethical committee of Turin (CORACLE registry: epidemiology clinical characteristics and therapy in real life patients affected by Sars-Cov-2).
The CHA(2)DS(2)-VASc score was calculated as follows: 1 or 2 points in each category as follows: age within 65 to 74 years = 1, age ≥75 years = 2, female = 1, presence of hypertension = 1, presence of diabetes = 1, previous myocardial infarct or peripheral artery disease = 1, previous stroke = 2, diagnosis of congestive heart failure (HF) = 1. Hence, CHA(2)DS(2)-VASc scores range from 0 to 9 points and a score ≥2 is associated with thromboembolic risk, indicating the need for anticoagulation in atrial fibrillation patients.10 We analyzed patients according to data-driven tertiles of CHA(2)DS(2)-VASc scores.
Age and CHA(2)DS(2)-VASc scores were skewed and are presented as median [25th percentile to 75th percentile]. In order to more clearly evaluate risk, we categorized CHA(2)DS(2)-VASc according to data-driven tertiles. Categorical variables are reported as counts and proportions. Differences in patient characteristics across CHA(2)DS(2)-VASc tertiles were assessed via the Kruskal-Wallis Test and Chi-Square test, as appropriate. Receiver operating characteristic (ROC) curve analysis was employed to quantify the prognostic power of CHA(2)DS(2)-VASc score for death and also for the composite end point (death and/or receiving invasive ventilation). We additionally examined crude odds ratios (OR) for death for individual CHA(2)DS(2)-VASc components: age category, gender, hypertension, diabetes mellitus, ischemic heart disease, stroke, and HF. Analyses were performed using SPSS version 20.0.
Results
We collected data from 1045 patients in the CORACLE registry. Of these patients, 864 (82.7%) had data required to calculate CHA(2)DS(2)-VASc score and were included in this analysis. Our sample had a median age of 65 [53 to 76] years and a median CHA(2)DS(2)-VASc score of 2 [1 to 3]. Males were more prevalent than females (62.2%). The rates of comorbidities were as follows: hypertension 48.6%, diabetes 15.7%, ischemic heart disease 11.2%, chronic obstructive pulmonary disease 9.4%, stroke 7.6% and HF 6.1%. Data-driven tertiles of CHA(2)DS(2)-VASc scores were as follows T1: ≤1, T2: 2 to 3, T3: ≥4. Patient characteristics according to CHA(2)DS(2)-VASc tertiles are shown in Table 1 .
Table 1.
Clinical characteristics of patients hospitalized for COVID-19 infection in the Italian CORACLE registry according to thromboembolic risk quantified by CHA(2)DS(2)-VASc Score
| CHA(2)DS(2)-VASc scores |
|||||
|---|---|---|---|---|---|
| Variable | All patients (n = 864) | ≤1 (n = 381) | 2 – 3 (n = 276) | ≥4 (n = 207) | p value |
| Age (years) | 65 [53-76] | 53 [45-59] | 71 [65-78] | 80 [74-85] | <0.001 |
| Men | 537 (62.2%) | 281 (73.8%) | 160 (58%) | 96 (46.4%) | <0.001 |
| Hypertension | 420 (48.6%) | 58 (15.2%) | 175 (63.4%) | 187 (90.3%) | <0.001 |
| Diabetes mellitus | 136 (15.7%) | 9 (2.4%) | 45 (16.3%) | 82 (39.6%) | <0.001 |
| Chronic obstructive pulmonary disease1 | 81 (9.4%) | 11 (2.9%) | 32 (11.6%) | 38 (18.4%) | <0.001 |
| Heart failure | 53 (6.1%) | 2 (0.5%) | 5 (1.8%) | 46 (22.2%) | <0.001 |
| Ischemic heart disease/PAD | 107 (12.4%) | 0 | 25 (9.15) | 82 (39.6%) | <0.001 |
| Stroke | 66 (7.6%) | 0 | 11 (4%) | 55 (26.6%) | <0.001 |
| Smoker167 | 0.47 | ||||
| Current | 65 (9.3%) | 23 (7.8%) | 22 (9.8%) | 20 (11.2%) | |
| Former | 48 (6.9%) | 17 (5.8%) | 15 (6.7%) | 16 (8.9%) | |
| Chronic Kidney Disease543 Atrial Fibrillation464 |
77/321(24%) 39/400(9.5%) |
18/172(10%) 5/179(2.8%) |
33/123(27%) 12/140(8.5%) |
26/103(25%) 22/115(19%) |
<0.001 <0.001 |
| Therapy | |||||
| ACEi1 | 156 (18.1%) | 24 (6.3%) | 68 (24.7%) | 64 (30.9%) | <0.001 |
| ARB1 | 127 (14.7%) | 16 (4.2%) | 58 (21.1%) | 53 (25.6%) | <0.001 |
| Beta-blockers1 | 168 (19.4%) | 20 (5.2%) | 60 (21.8%) | 88 (42.5%) | <0.001 |
| Calcium channel blockers1 | 152 (17.6%) | 22 (5.8%) | 70 (25.5%) | 60 (29%) | <0.001 |
| Thiazid diuretics49 | 107 (13.1%) | 10 (2.8%) | 34 (13.4%) | 63 (31.3%) | <0.001 |
| Loop diuretics268 | 93 (15.6%) | 11 (5.0%) | 27 (13.6%) | 55 (30.9%) | <0.001 |
| Acetil salicilic acid178 Peripheral oxygen saturation (%)487 Respiratory rate (n)717 D-dimer (ng/ml)690 Troponin (ng/ml)690 |
105 (15.3%) 95 [91-97] 26 [19-28] 610 [122-1361] 19 [9-51] |
11 (3.4%) 96 [94-98] 25 [20-27] 609 [71-1535] 11 [7-28] |
41 (19.1%) 95 [91-97] 24 [18-30] 609 [181-1078] 22 [9-51] |
53 (34.9%) 93[89-96] 27 [20-30] 620 [175-1400] 50[13-115] |
<0.001 <0.001 0.25 0.90 <0.001 |
ACEi = angiotensin II converting-enzyme inhibitor; ARB = aldosterone receptor blocker; PAD = peripheral artery disease (2.5% of total population).
Continuous variables: median [quartile 1, quartile 3].
Superscripts indicate missing data.
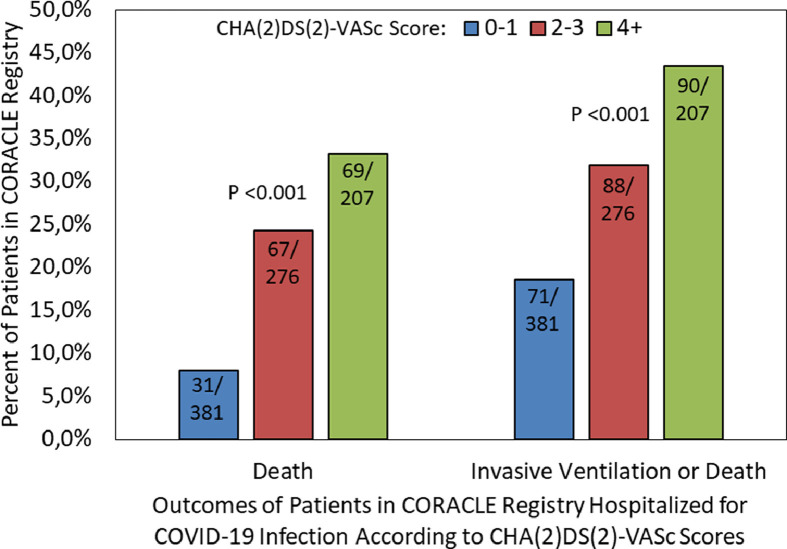
A total of 167 patients (19.3%) died and 123 (14.2%) received invasive ventilation. There were 41 (33.3%) of the ventilated patients who died, whereas 126 (17%) of the 741 nonventilated patients died. The composite outcome of death and/or receiving invasive ventilation was observed in 249 (28.8%) patients. We observed a statistically significant increasing percentage of death (8.1%, 24.3%, 33.3%, respectively; p <0.001) and composite end point (18.6%, 31.9%, 43.5%, respectively; p <0.001) according to tertiles (Figure 1 ). The ORs for mortality and the composite end point for patients with the second versus first tertile of CHA(2)DS(2)-VASc score were 3.62 (95% CI: 2.29 to 5.73, p <0.001) and 2.04 (95% CI: 1.42 to 2.93, p <0.001), respectively. Similarly, the ORs for mortality and the composite end point for patients with the third versus first tertile were 5.65 (95% CI: 3.54 to 9.01, p <0.001) and 3.36 (95% CI: 2.30 to 4.90, p <0.001), respectively. ROC curve analysis confirmed that CHA(2)DS(2)-VASc was significantly able to prognosticate both mortality (area under the ROC curve [AUC] = 0.69, 95% CI: 0.65 to 0.73, p <0.001) and the composite end point (AUC = 0.64, 95% CI: 0.60 to 0.68, p <0.001) (Figure 2 ).
Figure 1.
Rates of death and composite end point (death or invasive ventilation) according to tertiles of CHA(2)DS(2)-VASc scores (Differences in adverse events rate across CHA(2)DS(2)-VASc tertiles were assessed via the chi-square test).
Figure 2.
Receiver Operating Characteristic curves for death and the composite end point of death or invasive ventilation for the predictor of CHA(2)DS(2)-VASc score (ROC curve analysis was employed to quantify the prognostic power of CHA(2)DS(2)-VASc score for death and also for the composite end point (death and/or receiving invasive ventilation).
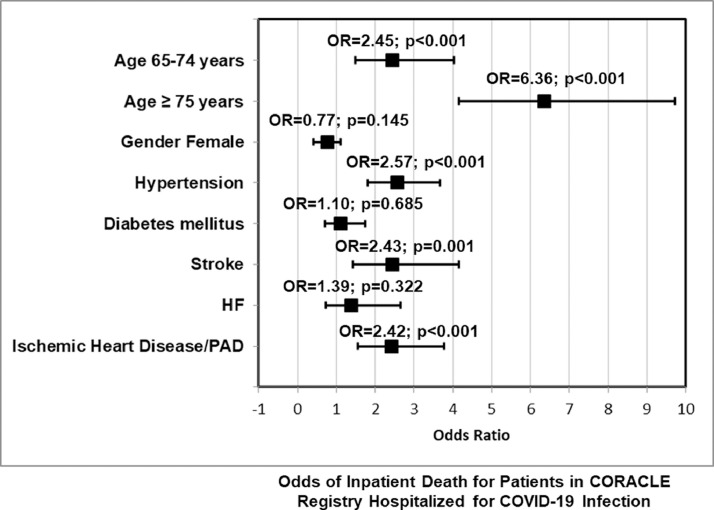
Moreover, we analysed the crude OR of individual CHA(2)DS(2)-VASc components. Gender did not significantly alter the risk of death (female versus male OR: 0.77, 95% CI: 0.42 to 1.10, p = 0.145). Similarly, neither diabetes mellitus (OR: 1.10, 95% CI: 0.70 to 1.73, p = 0.685) nor HF (OR: 1.39, 95% CI: 0.72 to 2.66, p = 0.322) were significantly associated to mortality. Conversely, age demonstrated a strong association with mortality (65 to 74 vs <65 years OR: 2.45, 95% CI: 1.49 to 4.02, p <0.001; 75+ vs <65 years OR: 6.36, 95% CI: 4.16 to 9.71, p <0.001). Further, hypertension (OR: 2.57, 95% CI: 1.80 to 3.67, p <0.001), stroke (OR: 2.43, 95% CI: 1.42 to 4.16, p = 0.001) and ischemic heart disease and/or peripheral artery disease (OR: 2.42, 95% CI: 1.56 to 3.77, p <0.001) were all significantly associated with death (Figure 3 ).
Figure 3.
Forest plot of odds ratios for mortality of individual CHA(2)DS(2)-VASc components (crude OR for death for individual CHA(2)DS(2)-VASc components: age category, gender, hypertension, diabetes mellitus, ischemic heart disease, stroke, and heart failure).
Discussion
To our knowledge, this is the first study to stratify COVID-19 patients according to CHA(2)DS(2)-VASc score. Our findings indicate that in this Italian population, patients with higher CHA(2)DS(2)-VASc scores had higher likelihoods of adverse outcomes. Patients with CHA(2)DS(2)-VASc score = 2 to 3 and CHA(2)DS(2)-VASc score ≥ 4 had a higher rate of adverse events in terms of both mortality and the composite end point of invasive ventilation and/or mortality than those with CHA(2)DS(2)-VASc score ≤1. ROC curve analysis confirmed the prognostic ability of CHA(2)DS(2)-VASc score. Additionally, individual components of the CHA(2)DS(2)-VASc score, such as age, hypertension, stroke, and ischemic heart disease and/or peripheral artery disease impacted patient outcomes. In this hospitalized sample, approximately 12% presented with severe respiratory distress or sudden oxygen desaturation requiring invasive ventilation and intensive care unit (ICU) admission. Although many clinical variables included in CHA(2)DS(2)-VASc such as age, hypertension, and Cardiovascular (CV) diseases, were demonstrated to increase the risk in this setting, until now a precise scale and weight of each variable were lacking. Indeed several studies demonstrated that patients with high CV risk and history of CAD, ictus, HF experienced a worse prognosis. Whereas female gender that is included in CHA(2)DS(2)-VASc appears to be a protective factor in COVID patients.
The most commonly recognized features characterizing COVID infection are low oxygen saturation and high respiratory rate. Both clinical manifestations are likely suggestive of extensive lung infection diffusion, bilateral involvement, and increased alveolar permeability with severe tissue damage. In this scenario, patients admitted to the ICU undergoing to noninvasive or invasive mechanic ventilation, often died.11 A John Hopkins hospital report including 1,441,128 COVID-19 cases showed that about 20% of patients need ICU admission with invasive respiratory management. Among severe ARDS cases, alterations in blood-clotting have been observed, with possible microthrombi occurring within the tiny blood vessels interacting with the lung alveoli, preventing capillaries from filling, and leading to impaired gas exchange.12 Therefore, there is an unmet need for the early recognition of these severe and/or critical patients before ARDS occurs.
Notably, others have attempted to build prediction models in order to better risk stratify COVID-19 patients. Two Chinese reports identified the following the following variables as being related to a poorer prognosis: advanced age, high C-Reactive Protein levels, and large comorbidity burden.13 Similarly, a retrospective analysis of 487 patients revealed that older age, male gender, and hypertension were all independent predictors of severe COVID-19 disease requiring hospital admission.14 Further, a retrospective, multicentre cohort study of 191 Chinese COVID-19 patients demonstrated that older age, higher Sequential Organ Failure Assessment score, and D-dimer >1 μg/ml were independently associated with in-hospital death, revealing significant power to detect high risk subjects.4 Similarly, other reports have demonstrated that increased levels of D-dimer and fibrinogen, as well as lower levels of platelets, are indicative of severe infection.2 , 15, 16, 17, 18 These findings have prompted the use of tissue plasminogen activator and heparin, which have been associated with decreased COVID-19 disease severity.5 , 6 Other laboratory markers such as PAI-I, platelet counts, interleukins, have been analysed in order to initially identify patients with higher risk of complications. Unfortunately, none of these variables are recognized in most common scores used for thromboembolic events prediction. Our approach using the CHA(2)DS(2)-VASc score has the advantages over these prior attempts of risk stratification in that it is simple, can be done upon admission, and is not dependent on or confounded by laboratory or other measurements. However, we cannot assert that current score may be applicable in all COVID populations presenting with different clinical characteristics, CV risk, and respiratory disease involvement. Accordingly, a larger sample size from the UK showed some discrepancies in terms of baseline risk profile and mortality rate compared to our sample size, and CHA(2)DS(2)-VASc should be tested in a larger population before being systematically applied.19 However, comparing ventilated patients with those of the cited study, the mortality risk appears quite similar.20 This percentage reflects the mortality rate of the larger Italian analysis of ICU patients in Lombardy (1,591 patients). Indeed, Grasselli et al, reported a 26% mortality rate among ICU patients. Patients who did not receive invasive ventilation may have died due to a contraindication of invasive ventilation, resulting in respiratory deterioration.21
It should be biologically plausible that the CHA(2)DS(2)-VASc score would predict outcomes for patients with COVID-19 infection. Some reports demonstrated that sudden clinical deterioration is often linked to increased intravascular coagulation, and several concerns were recently raised regarding the possible relation between COVID-19 infection and blood-clotting alteration.11 However, no data exist regarding the prophylactic use of anticoagulant drugs, although COVID-19 patients are prone to thromboembolic events and DIC.2 , 3 , 22, 23, 24 The mechanisms underlying infection, inflammation and coagulopathy in COVID-19 disease are poorly understood, but likely reflect the course of similar viral infections, such as SARS and ebola.2 In normal conditions, the coagulation cascade activation recognizes 3 different pathways: the antithrombin system, the endothelial protein C system, and tissue factor pathway inhibitor. In acute sepsis, all these pathways are inhibited due to of impaired synthesis, ongoing consumption and proteolytic degradation leading to a complete derangement of endothelial function in peripheral vessels and capillary district. Overexpression of tissue factor, activation of C protein system, and the inhibition of physiological fibrinolysis processes represent the key mechanisms involved in this coagulopathy. Indeed, viral infection and subsequent immune response mediated by macrophages and T lymphocytes generate an overexpression of immune mediators such as cytokines and chemokines (interleukin (IL)-1,IL-6,IL-8, IL-21, TNF-β and Monocyte chemoattractant protein-1). which activate endothelium. Therefore, Tumor necrosis factor, IL-6 and IL-1 amplify the procoagulant activity stimulating both thrombi formation and increased vascular permeability due to its endothelial effects.25, 26, 27, 28 In the lung, excretion of fibrin is related to alveolar damage through hyaline membrane deposition, which lead to alveolar collapse. Together with alveolar collapse, microvascular thrombi impair gas exchange, which results in ARDS and acute lung injury. Although, the CHA(2)DS(2)-VASc is primary designed for embolic risk prevention in atrial fibrillation, the prothrombotic state related to COVID-19 infection likely involves both micro and macro vascular pulmonary district reflecting diffuse endothelial dysfunction. This procoagulant activity may also be systematically present in several organs, and it could also facilitate the risk of large vessel thrombo-embolic disease.29 Therefore, the clinical scenario includes an initial viral infection of the respiratory tract experiencing the first host immune response. In specific conditions in which inflammation is associated with the cytokine storm and the immune response upregulation, a pro-coagulation state prevails.29
Our study has all the limitations of retrospective studies of prospectively collected data. A detailed laboratory analysis contemporarily investigating D-dimer, fibrinogen, and plasminogen levels was not available for study. Accordingly, a more detailed score including both clinical and laboratory variables deserve a specific analysis and validation and it could become much more appropriate for risk assessment definition in this setting. CHA(2)DS(2)-VASc could reasonably forecast thromboembolic complications, but other CV complications such as acute coronary syndrome, acute HF, myocarditis, and arrhythmic event, could be underestimated. Our population did not include any data regarding baseline physical activity, cardiorespiratory fitness and body mass index. Moreover, this analysis lacks of some data about clinical data, laboratory analysis variables, comorbidities and therapy. Moreover, external validation of our analysis in a separate cohort is required to better understand the model's ability to prognosticate outcomes for COVID-19 patients. Our results have been validated in hospitalized patients in Italy with bed positioning and cannot be generalized to nonhospitalized patients with less severe conditions in other countries. Finally, we do not know whether additional heparin treatment or other anticoagulant drugs could potentially prevent hypercoagulation.
In conclusion, patients hospitalized for COVID-19 infection are at high risk for systemic and pulmonary embolization. We found that in this case series of Italian patients, those with higher CHA(2)DS(2)-VASc scores had higher rates of mechanical ventilation or death; CHA(2)DS(2)-VASc scores could be easily calculated at admission in order to initially discern patients with increased thromboembolic risk. It is plausible that clinicians may wish to choose a specific anticoagulant treatment for such high risk patients. At present, whether patients hospitalized with COVID-19 with higher CHA(2)DS(2)-VASc scores should be considered for a specific anticoagulant treatment as well as other hypotheses generated by these descriptive data, require direct testing in analytic studies designed a priori to do so.
Authors Contribution
Gaetano Ruocco: Conceptualization, Methodology, Writing- Original draft preparation, Reviewing and Editing. Peter A. McCullough, Kristen M. Tecson: Writing- Original draft preparation, Reviewing and Editing. Massimo Mancone, Gaetano M. De Ferrari, Fabrizio D'Ascenzo, Francesco G. De Rosa: Visualization, Investigation, Reviewing and Editing. Anita Paggi, Giovanni Forleo, Gioel G. Secco, Gianfranco Pistis, Silvia Monticone, Marco Vicenzi, Irene Rota, Francesco Blasi, Francesco Pugliese; Visualization, Investigation. Francesco Fedele: Visualization, Investigation, Reviewing and Editing. Alberto Palazzuoli: Conceptualization, Methodology, Writing- Original draft preparation, Reviewing and Editing.
Acknowledgment
The authors are indebted to the following individuals for their contribution to this work: Anna G Palazzo, MD (Infectious Disease, Department of Medical Sciences, University of Torino, AOU Città della salute e della Scienza, 10126 Torino, Italy); Maurizio Landolina, MD, Erika Taravelli, MD (Cardiology Division, Ospedale Maggiore of Crema, 26013 Crema, Italy); Francesco Mojoli, MD, Guido Tavazzi, MD (Intensive Care University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy); Alex Di Nizio, MD, (Department of Medicine, Surgery and Neuroscience, Anesthesiology and Intensive Care, University Hospital of Siena, Siena, Italy); Francesca Montagnani, MD (Hospital Department of Specialized and Internal Medicine, Infectious Diseases Unit, University Hospital of Siena, Siena, Italy; Department of Medical Biotechnologies, University of Siena, Italy); Marco Schiavone, MD, Gianfranco Mitacchione, MD (Azienda Ospedaliera - Polo Universitario - "Luigi Sacco" Milano Italy); Gabriella D'Ettorre, MD, Giancarlo Ceccarelli, MD (Department of public health and infectious diseases, Sapienza, University of Rome, Italy); Paolo Severino, MD, Lucia Ilaria Birtolo, MD, Fabio Infusino, MD, Carlo Lavalle, MD (Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Sapienza, University of Rome, Italy); Franco Ruberto, MD, Francesco Alessandri, MD (Department of General Surgery, Surgical Specialities "Paride Stefanini" Rome Italy); Maria Chiara Colaiacomo, MD, Gioacchino Galardo, MD (Department of Emergency, Sapienza University of Rome, Italy); Alessio Mattei, MD (Division of Pneumology University of Torino, AOU Città della salute e della Scienza, Torino, Italy); Monica Andriani, MD (Division of Cardiology University of Torino, AOU Città della salute e della Scienza, Torino, Italy).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
The study design and data collection are taken by the Italian registry CORACLE including patients of north Italian regions and being and observational Trial it does not require financial resources.
References
- 1.Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, Huang J, He N, Yu H, Lin X, Wei S, Wu T. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1–9. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue Plasminogen Activator (tPA) Treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Li QR, Huang X, Cui Y, Li XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 8.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in african american patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;2600:30243–30245. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carsana L, Sonzogni A, Nasr A, Rossi R, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. Lancet Infect Dis. 2020;3099:30434–30435. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–865. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]
- 11.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, Nishimura M, Koh Y, Du B, Asian Critical Care Clinical Trials Group Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Available at:https://exclusive.multibriefs.com/content/treating-covid-19-related-respiratory-failure-with-an-anticoagulant-a-compa/medical-allied-healthcare. Accessed April 9, 2020.
- 13.Wynants L, Van Calster B, Bonten MMJ, Collins GS, Debray TPA, De Vos M, Haller MC, Heinze G, Moons KGM, Riley RD, Schuit E, Smits LJM, Snell KIE, Steyerberg EW, Wallisch C, van Smeden M. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;80:656–665. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Zhang Z, Tian J, Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann Palliat Med. 2020;9:428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 18.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG, ISARIC4C investigators Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, COVID-19 Lombardy ICU Network. Nailescu A, Corona A, Zangrillo A, Protti A, Albertin A, Forastieri Molinari A, Lombardo A, Pezzi A, Benini A, Scandroglio AM, Malara A, Castelli A, Coluccello A, Micucci A, Pesenti A, Sala A, Alborghetti A, Antonini B, Capra C, Troiano C, Roscitano C, Radrizzani D, Chiumello D, Coppini D, Guzzon D, Costantini E, Malpetti E, Zoia E, Catena E, Agosteo E, Barbara E, Beretta E, Boselli E, Storti E, Harizay F, Della Mura F, Lorini FL, Donato Sigurtà F, Marino F, Mojoli F, Rasulo F, Grasselli G, Casella G, De Filippi G, Castelli G, Aldegheri G, Gallioli G, Lotti G, Albano G, Landoni G, Marino G, Vitale G, Battista Perego G, Evasi G, Citerio G, Foti G, Natalini G, Merli G, Sforzini I, Bianciardi L, Carnevale L, Grazioli L, Cabrini L, Guatteri L, Salvi L, Dei Poli M, Galletti M, Gemma M, Ranucci M, Riccio M, Borelli M, Zambon M, Subert M, Cecconi M, Mazzoni MG, Raimondi M, Panigada M, Belliato M, Bronzini N, Latronico N, Petrucci N, Belgiorno N, Tagliabue P, Cortellazzi P, Gnesin P, Grosso P, Gritti P, Perazzo P, Severgnini P, Ruggeri P, Sebastiano P, Covello RD, Fernandez-Olmos R, Fumagalli R, Keim R, Rona R, Valsecchi R, Cattaneo S, Colombo S, Cirri S, Bonazzi S, Greco S, Muttini S, Langer T, Alaimo V, Viola U. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wan J, Liu P, Elalamy I, Wang C, Prevention Treatment of VTE Associated with COVID-19 Infection Consensus Statement Group. Pulmonary Embolism Pulmonary Vascular Diseases Group of the Chinese Thoracic Society. Pulmonary Embolism Pulmonary Vascular Disease Working Committee of Chinese Association of Chest Physicians. National Cooperation Group on Prevention Treatment of Pulmonary Embolism Pulmonary Vascular Disease. National Program Office for Prevention Treatment of Pulmonary Embolism Deep Vein Thrombosis. China Grade Center. Evidence-based Medicine Center of School of Basic Medical Sciences of Lanzhou University Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120:937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin Thromb Hemost. 2015;41:650–658. doi: 10.1055/s-0035-1556730. [DOI] [PubMed] [Google Scholar]
- 28.Evans CE., Zhao Y-Y. Impact of thrombosis on pulmonary endothelial injury and repair following sepsis. Am J Physiol Lung Cell Mol Physiol. 2017;312:L441–L451. doi: 10.1152/ajplung.00441.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa106. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]