Abstract
The coronavirus disease 2019 (COVID-19) pandemic, technological advancements, regulatory waivers, and user acceptance have converged to boost telehealth activities. Due to the state of emergency, regulatory waivers in the United States have made it possible for providers to deliver and bill for services across state lines for new and established patients through Health Insurance Portability and Accountability Act (HIPAA)- and non–HIPAA-compliant platforms with home as the originating site and without geographic restrictions. Platforms have been developed or purchased to perform videoconferencing, and interdisciplinary dialysis teams have adapted to perform virtual visits. Telehealth experiences and challenges encountered by dialysis providers, clinicians, nurses, and patients have exposed health care disparities in areas such as access to care, bandwidth connectivity, availability of devices to perform telehealth, and socioeconomic and language barriers. Future directions in telehealth use, quality measures, and research in telehealth use need to be explored. Telehealth during the public health emergency has changed the practice of health care, with the post–COVID-19 world unlikely to resemble the prior era. The future impact of telehealth in patient care in the United States remains to be seen, especially in the context of the Advancing American Kidney Health Initiative.
Index Words: Nephrology, end-stage kidney disease (ESRD), home dialysis, telehealth, telemedicine, remote monitoring, coronavirus disease 2019 (COVID-19), peritoneal dialysis (PD), home hemodialysis (HHD), public health emergency
The coronavirus disease 2019 (COVID-19) pandemic, new technologies, regulatory changes, and increased patient acceptance have led to accelerated evolution of telehealth in patient care settings. Telehealth delivers virtual patient care, addresses patient's medical concerns, and identifies issues that warrant in-person visits, while at the same time fostering social distancing in an effort to decrease COVID-19 transmission among health care workers and patients.1 , 2
Telehealth has benefits for clinicians, the interdisciplinary team (IDT), and patients. Due to the complexity of patients receiving dialysis, telehealth may complement but not totally replace the in-person visit3, 4, 5 and provide better oversight of care with remote monitoring.6, 7, 8 It is not surprising then that patients receiving peritoneal dialysis (PD) have responded positively to telehealth.9, 10, 11, 12, 13, 14, 15 Telehealth and home dialysis both foster greater home-based care, less travel time, and fewer trips to the clinic and leverage the principles of patient and care partner autonomy and self-care. Telehealth may also help facilitate patient education about home dialysis modalities and self-care. Telehealth has been used to manage patients with chronic kidney disease (CKD) and demonstrated equal outcomes in CKD care by either in-person or virtual visits.16 , 17 Although the opportunities for the application of technology to the betterment of the lives and care of our patients exist, implementation of these strategies continues to remain challenging. Here, members of the American Society of Nephrology COVID-19 Home Dialysis Committee review regulatory changes, use cases, implementation, and provider and patient perspectives on telehealth for home dialysis during the COVID-19 public health emergency.
Regulatory Changes Facilitating Telehealth
In the United States, the 2018 Bipartisan Budget Act extended telehealth access to home dialysis patients using home as the originating site beginning in 2019.18 In a survey of 30 PD patients conducted in August 2018, none knew of the statute allowing them to opt for telehealth from their home.19 Since 2019, nephrology and telehealth journals have informed their readers about telehealth options for home dialysis patients and how to operationalize them with little effect.15 , 20 , 21
However, during the COVID-19 pandemic, interest and need for telehealth services have increased significantly, resulting in the rapid removal of prepandemic telehealth barriers.22 In addition to removing geographic restrictions and allowing for the home to serve as the originating site, the main regulatory changes pertinent to home dialysis patients now permit: (1) billing for services across state lines, (2) delivering care to both established and new patients through telehealth, (3) use of non–Health Insurance Portability and Accountability Act (HIPAA)–compliant platforms such as Skype and FaceTime, and (4) reimbursement for audio-only visits. An additional change broadened eligibility to provide telehealth services to all health care professionals who are eligible to bill Medicare for their professional services.23 Furthermore, some but not all states have relaxed requirements for licensure as long as a provider holds a license in another state.
Telehealth Platform Considerations
Nephrology practices and dialysis providers have adopted telehealth platforms to deliver care for patients receiving dialysis. They may design platforms internally or purchase commercial products. Platforms can vary significantly in terms of cost, scalability, and technical support. Telehealth platforms range from videoconferencing alone to platforms that also allow for self-scheduling, payment collection, and physician notifications.
Both providers and patients value ease of use when comparing videoconferencing platforms. Some videoconferencing platforms can only invite patients through text message, whereas others can only email invitations.
Finally, one must consider security. Security encompasses both encryption standards and cybersecurity risk. HIPAA compliance requires data encryption, a Business Associates Agreement with the provider of the telecommunications, and the patient must be in a private physical environment. An information technology security specialist should evaluate cybersecurity and risks to network integrity to minimize potential susceptibilities of health care networks to viruses or cyber attacks.
Components of a Telemedicine Visit
The clinician performing a telehealth encounter may require several systems to operate simultaneously. The clinician may therefore need simultaneous access to a HIPAA-compliant video platform (with a high-quality camera, concurrent audio, and secure communications) and an electronic health record (to check laboratory results, write a note contemporaneously, and prescribe medications electronically).
However, the patient requires only 1 device. It must have a camera that can synchronize with the team’s platform at the predetermined appointment time. Although patients mainly use their smartphone as the primary device, options for tablets and computers (laptop and desktop with webcam) should be considered, although the device choice remains highly dependent on the specific platform.9 The staff may have to help the patient install the software application, provide instruction, test the linkage, and hold a practice session. Some patients may not have access to adequate hardware or access to technology, and this should be addressed to ensure equitable care for patients.
A visit will proceed more efficiently if, in advance of the session, the team (usually the nurse) assembles and updates the medication list, dialysis prescription, vital signs, and laboratory results and collects remote data from PD or hemodialysis (HD) flow sheets. In this regard, the use of remote patient monitoring (RPM) may be particularly useful. RPM uses digital technologies to acquire home health data, transmitting the information to health care providers.6 , 8 , 10 , 24 , 25 Existing home dialysis RPM platforms allow direct transmission of both biometric information (ie, blood pressure, blood glucose level, temperature, and weight) to providers and home HD and automated PD treatment parameters (ie, treatment completion, duration, interruptions, alarms, and ultrafiltration).26 , 27 RPM obviates the need for paper dialysis treatment logs and provides information in key domains of dialysis access, blood pressure, target weight, and ultrafiltration management while identifying treatment adherence challenges and in some cases allowing remote changes to the prescription.
Although the virtual visit lacks the traditional physical examination, a virtual “no touch” physical examination remains a possible alternative (Table 1 ). The absence of a satisfactory electronic stethoscope limits the appreciation of cardiac, pulmonary, and abdominal sounds. In a home HD patient, one cannot perceive the bruit in the arteriovenous access. Nevertheless, the no-touch examination still allows the clinician to evaluate volume status and dialysis access (catheter exit site or arteriovenous access). Volume status may be estimated by using weight, blood pressure, and ultrafiltration rate in addition to observable physical examination findings such as pedal edema. The exit site can be evaluated by electronic photograph or real-time observation.
Table 1.
The “No Touch” Physical Examination
| Parameter | Possible Findings |
|---|---|
| General | Well vs ill appearing In (no) acute distress |
| Eyes | (Non)-icteric sclera (No) droopy eyelids |
| Mouth | Dentation/oral cavity appears (ab)normal (No) thrush present Tongue (not) coated |
| Cardiac | Heart rate (ir)regular (based on patient counting the pulse out loud) Normo- vs hyper- vs hypotension (based on patient measurement of blood pressure) Tachycardia vs bradycardia vs normal pulse rate (based on patient measurement) |
| Pulmonary | Work of breathing with(out) effort (Not) speaking in full sentences (No) audible wheezing |
| Gastrointestinal | (No) tenderness when the patient presses on the abdomen |
| Genital-urologic | (No) suprapubic tenderness when the patient presses in the area superior to the pubis |
| Musculoskeletal | (No) pedal edema (No) muscle tenderness when the patient squeezes the muscle in question (No) joint swelling (No) hand twitching (No) decreased ability to turn door knob |
| Neurologic | (Not) alert and oriented (Ab)normal gait (No) localized/focal weakness (No) tremor (No) asterixis Test functional status (functional status, gross vs fine examination) |
| Psychological | Normal vs anxious mood; normal vs flat affect Good vs poor memory (Not) anxious |
| Hematologic | (No) excessive bruising or bleeding |
| PD specific | Exit site with(out) crust, drainage, or erythema |
| HD specific | AV fistula or AV graft with(out) bruit and thrill (based on patient’s assessment) (No) aneurysmal protrusion (No) purulent discharge Tunneled CVC exit site with(out) drainage or erythema |
Note: Only items that can be and are actually visualized should be documented. Some findings require the patient to elicit by tapping, squeezing, or pressing.
Abbreviations: AV, arteriovenous; CVC, central venous catheter; HD, hemodialysis; PD, peritoneal dialysis.
Other members of the IDT can perform assessments or counseling with the patient apart from the monthly visit. Studies have shown that dietitians using telemedicine platforms can successfully perform coaching programs for dietary counseling in patients with CKD, as well as diabetes management.28 , 29 A regular virtual check-in with the social worker can permit discussions around emotional distress, caregiver burnout, and medical resource acquisition and management. These interdisciplinary visits performed virtually have been successfully performed in CKD programs and can be extended into PD programs.30
A clinical presenter may assist the patient with the telehealth visit. Family members (parents for minors or a spouse or sibling for adults), caregivers, friends, or nurses can gather vital signs, assist in the physical examination, help interpret and carry out changes in care plans or orders, and be an interpreter if a language barrier exists.
As parties become facile with repeated practice and telehealth encounters, it may be possible to invite other health professionals in consultation. This act can increase patient access to care and reduce time to care, transportation time, and cost. These visits may include a virtual preoperative surgical assessment before PD catheter placement or a postoperative visit to evaluate wound healing. A PD nurse can also use telehealth to troubleshoot a PD catheter with the nephrologist or surgeon remotely. Likewise, fellows in nephrology training programs can be included in the care of these patients by using videoconferencing. This can simultaneously address training for the fellow in both PD and telehealth techniques.
The impact of such broad use of telemedicine for home dialysis care on safe and effective care, infections, hospitalizations, technique failure, and patient-reported outcomes remains to be determined. Data collected during the COVID-19 pandemic may help inform the best use of telehealth among patients receiving dialysis. Stable home dialysis patients may be one group that could easily benefit from the regular use of telehealth. However, unstable patients who experience difficulty with travel plans may also benefit from telehealth because the alternative is not to be seen at all. The collective input from patients, nephrologists, and the IDT should determine a preferred approach to plan the follow-up visit. A previsit review of patient-maintained dialysis logs, remotely monitored data metrics, laboratory trends, and a screening telephone call for patient-reported symptoms by a nurse, along with specific decision support tools, can help in triaging patients (Box 1 ).
Box 1. High-Risk Factors That Might Trigger an In-person Visit Instead of a Telemedicine Visit.
Recent events
-
•
PD- or HHD-related infection within the last 1 mo
-
•
Hospitalization or emergency department visit within the last 1 mo
-
•
New home dialysis start within the last 1 mo
Patient factors
-
•
Inability to administer ESA at home
-
•
Does not have appropriate technology for telemedicine visit
-
•
New symptoms or medical issues needing attention, eg, abdominal pain
-
•
Access-related issues
Previsit nurse, social worker, dietitian or nephrologist assessment
-
•
Concerns regarding adherence to the appropriate technique or prescribed prescription (ie, identified on remote patient monitoring)
-
•
Uncontrolled moderate-severe hypertension or significant hypotension
-
•
Patient-reported fluid imbalance not responding to prescription change or diuretics
-
•
Patient-reported new/worsening symptoms
-
•
Patient reports social isolation, severe depression, or anxiety
Abbreviations: ESA, erythropoiesis-stimulating agent; HHD, home hemodialysis; PD, peritoneal dialysis.
Challenges and Opportunities to Enhance Telehealth
Approximately 45% of dialysis units are unable to provide home dialysis, especially in rural areas where there may be less access to a nearby home dialysis unit.31 Whereas urban communities are intuitively thought to have greater access to home dialysis, local traffic patterns may affect travel time as much as distance does in rural communities. In either case, telehealth presents a solution to accessing home dialysis facilities while safely isolating at home during the COVID-19 pandemic.
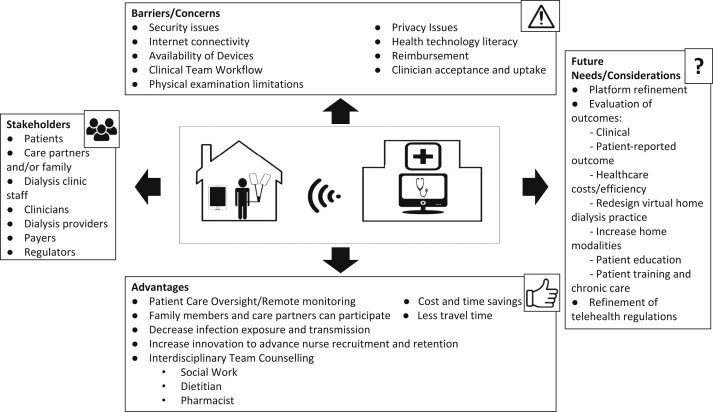
Nevertheless, challenges remain in the implementation and widespread use of telehealth (Fig 1 ). Limited patient access to capable devices continues to be a known barrier. Because of this, patients may opt to use telehealth with a friend or family member assisting if they do not have a compatible device themselves. Changes that address regulatory barriers are needed for providers to assist patients by providing connectivity devices without “inducement” concerns.32
Figure 1.
The current and future landscape of home dialysis telehealth, showing the stakeholders, advantages, barriers/concerns, and future needs/considerations of telehealth.
The regulatory waivers provided during the COVID-19 pandemic demonstrate proof of concept that solutions and compromises can be found with the end goal of better care for home dialysis patients. However, even provision of the devices themselves may not be enough. Many patients do not have access to broadband internet speeds capable of videoconferencing; in turn, disparities will worsen should audio-only options be removed after the state of emergency has been lifted.
Unfortunately, socioeconomic and language barriers persist. African Americans and Hispanics have been underrepresented in home dialysis nationwide and are underrepresented in video uptake in telehealth.33 A recent report on the use of telemedicine for chronic disease management during the COVID-19 pandemic in the primary care setting has already reported a significant decrease in the number of visits by patients older than 65 years, non–native English speakers, Medicare/Medicaid-insured patients, or patients who self-identified as a racial/ethnic minority.34
Overcoming limited access to devices and broadband internet is only the first step in addressing these challenges. Existing telemedicine platforms are unlikely to consider digital technology literacy, health literacy, age, or English language proficiency in their design.34 Moreover, most nephrologists and dialysis providers do not have systems in place to provide training to patients on how to use these tools. There exists an opportunity for telehealth technology itself to provide solutions to ensure equitable access to care. As an example, interpreter services provided within telehealth visits could help bridge both language and cultural barriers to care.
Increasing access to telehealth as described will require a concerted effort from government, regulators, payers, dialysis organizations, and nephrologists. As we move toward value-based care models, it stands to reason that removing barriers to telehealth will help increase minority representation and align with efforts to increase home dialysis use under the Advancing American Kidney Health Initiative.35
Telehealth: Implications for Dialysis Nurses
Nurses take leading roles in the startup and maintenance of many new technologies in home dialysis program while playing a primary role in coordinating all aspects of a telehealth visit to ensure optimally successful experiences for both the IDT and patient. Nurses facilitate telehealth by setting up the platform on the patient’s device and teaching them how to use it. Nurses often take the lead in coordinating and setting the stage as patient visits get underway. Although many aspects of a visit include skills that are currently used in successful home dialysis programs, the nurse has taken on the responsibility for the technical aspect of telemedicine.
Home dialysis nurse retention is a growing issue in the United States, a development that could adversely affect home growth. These telehealth-related care enhancements may provide home dialysis nursing a viable alternative career destination in a social distanced health care environment. Telehealth may overcome the challenges of creating workplaces that engage and retain nurses by incorporating flexible nurse schedules, enhancing communications with patients and colleagues, and developing collaborative relationships across the IDT. These strategic initiatives have been implemented in workplaces that have high staff engagement and retention36 , 37 and can now be experienced in the home dialysis setting. The success of these remote dialysis facilities relies on trained nurses to meet the care demands of this new and changing home dialysis experience.
Patients' Perspectives on Telehealth
Patients have had different responses to telehealth. Many of the challenges could be mitigated and improved on by having patients be involved in both the design of the technology and crafting the patient experience for a telehealth visit. One author, C.W., appreciates telehealth and reports, “I use FaceTime to interact with the doctor and nurse at a specified time. My concerns are addressed. I don’t have to travel to the dialysis unit or miss a prolonged period of time from work.” However, not all patients participate in telehealth due to lack of internet service or device.
Considerations for Telehealth in the Pediatric Population
Telehealth has also proved to be an extremely valuable resource for the pediatric dialysis community during the COVID-19 pandemic. Although 3% of all outpatient clinic visits at Children’s Mercy Kansas City before the COVID-19 pandemic were conducted through telehealth, 70% of visits during the pandemic were telehealth based (B.A.W., unpublished observation). In addition, 35 of 38 pediatric dialysis programs participating in the Standardizing Care to Improve Outcomes in Pediatric End-Stage Renal Disease (SCOPE) Collaborative reported carrying out telehealth visits during this time.
Before the COVID-19 pandemic, there was limited experience and a paucity of literature on the use of telehealth in pediatric nephrology. In a 10-year experience from Australia, telehealth was deemed to be a viable and cost-effective resource, saving an average $505 Australian dollars per consultation.38 In a review of the use of telehealth tools in pediatrics, Brophy4 emphasized the important role that telehealth could play in improving access and reducing costs related to pediatric kidney care.
The COVID-19 pandemic has stimulated an emphasis on telehealth and highlighted many of the benefits for pediatric dialysis patients and their families in terms of visit-related travel time, cost, and absence from school and work. The ability to visit with the IDT from home can also be comforting for the young child who may associate the hospital clinic with painful procedures. At the same time, ongoing challenges to be addressed include: (1) the need for accurate biometric measurements such as height and weight because growth represents a key pediatric-specific outcome parameter, and (2) the lack of privacy that may exist for a desired parentless portion of the clinic visit, particularly salient to the adolescent preparing for transfer to adult care or for a mandated routine suicide screen.
Future Directions in Telehealth Use, Quality Measures, and Research
The future landscape for advancing telehealth use in home dialysis depends on integrating technology with an efficient home program workflow, leveraging platforms that are user friendly, improving efficiency, and ensuring patient outcomes that surpass or are equal to in-person visits. Developing a balance between virtual and in-person visits for patients with high comorbidity will be critical. Change management initiatives should help the transition from traditional encounters to virtual care, especially as provider practices exit the COVID-19 pandemic.
It remains unclear as to what regulations will return when the public health emergency ends. Box 2 includes considerations that must be accounted for in any long-term planning for ongoing telehealth coverage and expansion.
Box 2. Long-term Needs for Ongoing Telehealth Coverage.
-
•
An alternative to monthly laboratory work for stable patients
-
•
Home must remain as the originating and distant sites
-
•
Allow health professional licensure across state lines
-
•
Allow health professionals to bill for services across state lines
-
•
Develop various media formats to educate patients and providers on telehealth
-
•
Formal adoption of telehealth as a preferred practice for stable home dialysis patients
-
•
Dissemination of online tools to promote modality education and training
-
•
Reduction in internet disparities
-
•
Creation of a national health information exchange
Using technology and telemedicine to educate patients will be important in a connected world while trying to decrease face-to-face interactions. Future areas of study include virtual education about CKD and dialysis modalities, virtual transplantation evaluations, and virtual training for patients and caregivers as new starts to dialysis or retraining after a prolonged hospitalization. Measuring and conducting research on the delivered quality of care and patient outcomes and comparing telehealth and standard care along with patient and health care provider satisfaction (patient-reported outcome measures) with telehealth will be important determinants of whether the virtual practice continues to grow after the COVID-19 pandemic. A large-scale study from Canada may soon provide some answers (ClinicalTrials.gov identifier NCT02670512). If telehealth use can be captured during the COVID-19 pandemic using Medicare claims data, analysis of measured quality-of-care metrics for patients such as hospitalization, readmission, emergency department visits, and transplantation wait list rates may be important to help establish the role of telemedicine as part of routine care.
Conclusion
COVID-19 has pushed telehealth to the forefront of all aspects of health care out of necessity to protect providers and patients while maintaining adequate care. Insurance coverage changes have aided in this transformation by allowing for a reasonable, sustainable, and efficient way to implement telehealth. It is reasonable to think that telehealth has reached its peak use during the pandemic and a subsequent decline may ensue when COVID-19–related waivers are lifted. However, an alternative future for telehealth could be the rapid development of supportive technologies that increase the number of patients who can be effectively cared for in their homes. Examples of such technologies include home-based point-of-care laboratory measurements based on finger sticks and home-based diagnostic tests that match accuracy with ease of use. However, it is clear that even in its current state, telehealth has forever changed the way we practice health care, and there will be no returning to a health care system devoid of it. As such, we must continue to make it better, we must continue to make it easier, and we must continue to make it available to anyone and everyone in need of care by addressing internet infrastructure, technology literacy, and socioeconomic determinants of health.
Article Information
Authors’ Full Names and Academic Degrees
Susie Q. Lew, MD, Eric L. Wallace, MD, Vesh Srivatana, MD, Bradley A. Warady, MD, Suzanne Watnick, MD, Jayson Hood, RN, David L. White, BA, Vikram Aggarwal, MD, Caroline Wilkie, BA, Mihran V. Naljayan, MD, Mary Gellens, MD, Jeffrey Perl, MD, and Martin J. Schreiber, MD.
Support
The article was written without grant or pharmaceutical funding.
Financial Disclosure
Dr Gellens is an employee of Baxter Healthcare. Dr Naljayan has received speaking honoraria from DaVita Kidney Care and served on advisory boards for DaVita Kidney Care and Baxter Healthcare. Dr Perl has received speaking honoraria from AstraZeneca, Baxter Healthcare, DaVita Healthcare Partners, Fresenius Medical Care, Dialysis Clinics Inc, and Satellite Healthcare, and has served as a consultant for Baxter Healthcare, DaVita Healthcare Partners, Fresenius Medical Care, Otsuka Canada, and LiberDi. Dr Schreiber is Chief Medical Officer, Home Modalities, DaVita Kidney Care. Dr Warady is a Consultant for Bayer, Amgen, Akebia, AstraZeneca, GlaxoSmithKline and has received research support from Baxter Healthcare and honoraria from UpToDate. Ms Wilkie has received stipends from American Society of Nephrology, University of North Carolina, and the University of Pennsylvania. Mr White is an employee of the American Society of Nephrology. Dr Wallace has received honoraria and grants from Baxter Healthcare Corp, NxStage, and Davita Healthcare Partners; has received consulting fees and grants from Sanofi-Genzyme; is a consultant for Avrobio, Freeline Therapeutics, and Amicus; and is involved in clinical trials with Idorsia, Protalix, and Freeline Therapeutics. Dr Watnick is the Chief Medical Officer of Northwest Kidney Centers. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We thank all the patients who shared their experiences and thoughts about telehealth and Kerry Leigh, BSN, RN, Project Specialist at the American Society of Nephrology, for administrative support.
Peer Review
Received July 9, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from an Associate Editor and a Deputy Editor. Accepted in revised form September 10, 2020.
Footnotes
Complete author and article information provided before references.
Collectively, the authors constitute the American Society of Nephrology (ASN) COVID-19 Home Dialysis Subcommittee
References
- 1.Chen J., Yin L., Chen X. Management of peritoneal dialysis under COVID-19: the experience in Sichuan Province People’s Hospital, China. Perit Dial Int. 2020 doi: 10.1177/0896860820935298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano G., Fontana F., Ferrari A. Peritoneal dialysis in the time of coronavirus disease 2019. Clin Kidney J. 2020;13(3):265–268. doi: 10.1093/ckj/sfaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamoto H. Telemedicine system for patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2007;27(suppl 2):S21–S26. [PubMed] [Google Scholar]
- 4.Brophy P.D. Overview on the challenges and benefits of using telehealth tools in a pediatric population. Adv Chronic Kidney Dis. 2017;24(1):17–21. doi: 10.1053/j.ackd.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Krishna V.N., Managadi K., Smith M. Telehealth in the delivery of home dialysis care: catching up with technology. Adv Chronic Kidney Dis. 2017;24(1):12–16. doi: 10.1053/j.ackd.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Nayak K.S., Ronco C., Karopadi A.N. Telemedicine and remote monitoring: supporting the patient on peritoneal dialysis. Perit Dial Int. 2016;36(4):362–366. doi: 10.3747/pdi.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosner M.H., Lew S.Q., Conway P. Perspectives from the Kidney Health Initiative on advancing technologies to facilitate remote monitoring of patient self-care in RRT. Clin J Am Soc Nephrol. 2017;12(11):1900–1909. doi: 10.2215/CJN.12781216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace E.L., Rosner M.H., Alscher M.D. Remote patient management for home dialysis patients. Kidney Int Rep. 2017;2(6):1009–1017. doi: 10.1016/j.ekir.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lew S.Q., Sikka N. Are patients prepared to use telemedicine in home peritoneal dialysis programs? Perit Dial Int. 2013;33(6):714–715. doi: 10.3747/pdi.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lew S.Q., Sikka N., Thompson C. Adoption of telehealth: remote biometric monitoring among peritoneal dialysis patients in the United States. Perit Dial Int. 2017;37(5):576–578. doi: 10.3747/pdi.2016.00272. [DOI] [PubMed] [Google Scholar]
- 11.Dey V., Jones A., Spalding E.M. Telehealth: acceptability, clinical interventions and quality of life in peritoneal dialysis. SAGE Open Med. 2016;4 doi: 10.1177/2050312116670188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnus M., Sikka N., Cherian T. Satisfaction and improvements in peritoneal dialysis outcomes associated with telehealth. Appl Clin Inform. 2017;8(1):214–225. doi: 10.4338/ACI-2016-09-RA-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallar P., Gutierrez M., Ortega O. [Telemedicine and follow up of peritoneal dialysis patients] Nefrologia. 2006;26(3):365–371. [PubMed] [Google Scholar]
- 14.Nayak A., Antony S., Nayak K.S. Remote monitoring of peritoneal dialysis in special locations. Contrib Nephrol. 2012;178:79–82. doi: 10.1159/000337816. [DOI] [PubMed] [Google Scholar]
- 15.Lew S.Q., Sikka N. Operationalizing telehealth for home dialysis patients in the United States. Am J Kidney Dis. 2019;74(1):95–100. doi: 10.1053/j.ajkd.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Tan J., Mehrotra A., Nadkarni G.N. Telenephrology: providing healthcare to remotely located patients with chronic kidney disease. Am J Nephrol. 2018;47(3):200–207. doi: 10.1159/000488004. [DOI] [PubMed] [Google Scholar]
- 17.Koraishy F.M., Rohatgi R. Telenephrology: an emerging platform for delivering renal health care. Am J Kidney Dis. 2020;76(3):417–426. doi: 10.1053/j.ajkd.2020.02.442. [DOI] [PubMed] [Google Scholar]
- 18.H.R. 1892 - Bipartisan Budget Act of 2018. https://www.congress.gov/bill/115th-congress/house-bill/1892/text
- 19.Lew S.Q., Sikka N. Telehealth awareness in a US urban peritoneal dialysis clinic: from 2018 to 2019. Perit Dial Int. 2020;40(2):227–229. doi: 10.1177/0896860819893560. [DOI] [PubMed] [Google Scholar]
- 20.Bieber S.D., Weiner D.E. Telehealth and home dialysis: a new option for patients in the United States. Clin J Am Soc Nephrol. 2018;13(8):1288–1290. doi: 10.2215/CJN.03010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lew S.Q. Telehealth in peritoneal dialysis: review of patient management. Adv Perit Dial. 2018;34(2018):32–37. [PubMed] [Google Scholar]
- 22.Kacik A. Providence goes from 700 video visits a month to 70,000 a week https://www.modernhealthcare.com/providers/providence-goes-700-video-visits-month-70000-week
- 23.Centers for Medicaid & Medicare Services Coronavirus waivers & flexibilities. https://www.cms.gov/about-cms/emergency-preparedness-response-operations/current-emergencies/coronavirus-waivers
- 24.Rosner M.H., Ronco C. Remote monitoring for continuous peritoneal dialysis. Contrib Nephrol. 2012;178:68–73. doi: 10.1159/000337812. [DOI] [PubMed] [Google Scholar]
- 25.Nayak A., Karopadi A., Antony S. Use of a peritoneal dialysis remote monitoring system in India. Perit Dial Int. 2012;32(2):200–204. doi: 10.3747/pdi.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drepper V.J., Martin P.Y., Chopard C.S. Remote patient management in automated peritoneal dialysis: a promising new tool. Perit Dial Int. 2018;38(1):76–78. doi: 10.3747/pdi.2017.00054. [DOI] [PubMed] [Google Scholar]
- 27.Remote Monitoring. https://www.amia.com/hcp/features/remote-monitoring Baxter
- 28.Kelly J.T., Conley M., Hoffmann T. A coaching program to improve dietary intake of patients with CKD: ENTICE-CKD. Clin J Am Soc Nephrol. 2020;15(3):330–340. doi: 10.2215/CJN.12341019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson G.A., Sidebottom A., Hayes J. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) telemedicine randomized controlled trial on diabetes optimal care outcomes in patients with type 2 diabetes. J Acad Nutr Diet. 2019;119(4):585–598. doi: 10.1016/j.jand.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Ishani A., Christopher J., Palmer D. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41–49. doi: 10.1053/j.ajkd.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Prakash S., Coffin R., Schold J. Travel distance and home dialysis rates in the United States. Perit Dial Int. 2014;34(1):24–32. doi: 10.3747/pdi.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.HHS Office of Inspector General Fact Sheet Notice of proposed rulemaking OIG-09336-AA10-P. October 2019. https://www.oig.hhs.gov/authorities/docs/2019/CoordinatedCare_FactSheet_October2019.pdf
- 33.Wallace E.L., Lea J., Chaudhary N.S. Home dialysis utilization among racial and ethnic minorities in the United States at the national, regional, and state level. Perit Dial Int. 2017;37(1):21–29. doi: 10.3747/pdi.2016.00025. [DOI] [PubMed] [Google Scholar]
- 34.Nouri S., Khoong E.C., Lyles C.R. Addressing equity in telemedicine for chronic disease management during the Covid-19 pandemic. NEJM Catalyst Innovations in Care Delivery. May 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.1020.0123#
- 35.Trump D. Executive Order on Advancing American Kidney Health. https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/
- 36.Koppel J., Deline M., Virkstis K. A two-pronged approach to retaining millennial nurses. J Nurs Adm. 2017;47(12):597–598. doi: 10.1097/NNA.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 37.Scruth E.A., Garcia S., Buchner L. Work life quality, healthy work environments, and nurse retention. Clin Nurse Spec. 2018;32(3):111–113. doi: 10.1097/NUR.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 38.Trnka P., White M.M., Renton W.D. A retrospective review of telehealth services for children referred to a paediatric nephrologist. BMC Nephrol. 2015;16:125. doi: 10.1186/s12882-015-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]