Abstract
Background
The outbreak of COVID-19 caused by SARS-CoV-2 has been a pandemic. The objective of our study was to explore the association between sex and clinical outcomes in patients with COVID-19.
Methods
Detailed clinical data including clinical characteristics, laboratory tests, imaging features and treatments of 1190 cases of adult patients with confirmed COVID-19 were retrospectively analyzed. Associations between sex and clinical outcomes were identified by multivariable Cox regression analysis.
Results
There were 635 (53.4%) male and 555 (46.6%) female patients in this study. Higher rates of acute kidney injury (5.5% vs. 2.9%, p = 0.026), acute cardiac injury (9.1% vs. 4.3%, p = 0.001), and disseminated intravascular coagulation (2.5% vs. 0.7%, P = 0.024) were observed in males. Compared with female patients, male patients with COVID-19 had a higher inhospital mortality rate (15.7% vs. 10.3%, p = 0.005). However, Cox regression analysis showed that sex did not influence inhospital mortality of COVID-19 patients.
Conclusions
Male sex was associated with a worse prognosis of COVID-19, but it seems not to be an independent prognostic factor.
Keywords: COVID-19, Sex, Organ failure, Mortality
Abbreviation
- COVID-19
corona virus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- ACE2
angiotensin-converting enzyme 2
- SOFA
sequential organ failure assessment
- APACHE II
acute physiology and chronic health evaluation II
- ARDS
acute respiratory distress syndrome
- COPD
chronic obstructive pulmonary disease
- AKI
acute kidney injury
- AHI
acute hepatic injury
- ACI
acute cardiac injury
- DIC
disseminated intravascular coagulation
- MV
mechanical ventilation
- FDPs
fibrin/fibrinogen degradation products
- PT
prothrombin time
- APTT
Activated-partial thromboplastin time
- TT
thrombin time
- TBIL
total bilirubin
- ALT
alanine-aminotransferase
- AST
aspartate-aminotransferase
- BUN
blood urea nitrogen
- SCr
serum creatinine
- CK-MB
creatine kinase isoenzyme
- hsCRP
hyper-sensitive C-reactive protein
- PCT
procalcitonin
- SAA
serum amyloid protein A
- IL
interleukins
1. Background
The outbreak of novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], has rapidly turn into a pandemic. To 26 July 2020, it has affected more than 15,700,000 cases and claimed more than 640,000 lives (https://covid19.who.int). Although the disease is now better contained in China, the prevalence in other parts of the world remains serious. Researches on underlying mechanism of COVID-19 outcomes become urgent worldwide.
Published studies from multiple countries on clinical characteristics of the disease have indicated that males appear with higher incidence and mortality of COVID-19 than females [[2], [3], [4], [5]]. One study of 44672 patients with confirmed COVID-19 in Mainland China, carried out by the Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, found the male-to-female ratio in China overall was 1.06:1, case fatality rate for males was 2.8% and for females was 1.7% [3]. In a study of 1099 patients with COVID-19 from 552 hospitals in 30 provinces in China, the male-to-female ratio was 1.39:1 [4]. Italian epidemiologic data showed the male-to-female ratio was up to 3:1 for SARS-CoV-2 infection [5].
Given the sex-based disparity in incidence and hospitalization, as well as in mortality, the clinical characteristics of the disease need to be stratified by sex. In the present study, we analyzed 1190 adult patients with confirmed COVID-19 in Wuhan Infectious Disease Hospital, to explore the association between sex and clinical outcomes of COVID-19 in Chinese population.
2. Methods
2.1. Patient population
This study was approved by the Medicine Institutional Review Board of Wuhan Infectious Disease Hospital (KY-2020-03.01). Patients between the ages of 18 and 94 years with confirmed COVID-19 admitted to Wuhan Infectious Disease Hospital from December 29, 2019 to February 28, 2020 were enrolled. The clinical outcomes were monitored up to March 2, 2020, the final date of follow-up.
All patients enrolled in this study were diagnosed according to World Health Organization interim guidance [6]. Nasal and pharyngeal-swab specimens from patients with history of epidemiology and characteristics of virus pneumonia in chest computed tomographic (CT) or X-ray were obtained. Patients with positive results from high-throughput sequencing or real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens were confirmed with COVID-19.
2.2. Data collection
Epidemiological, demographic, clinical, laboratory and radiological data, treatments and outcomes data were extracted from medical records. The data were reviewed by a trained team of physicians. Information collected included age, sex, comorbidities, exposure history, laboratory findings, chest CT and x-ray scans, respiratory support during hospitalization, therapeutic strategy and outcomes.
Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [7]. Acute kidney injury (AKI) was identified according to the Kidney Disease: Improving Global Outcomes definition [8]. Acute cardiac injury (ACI) was defined if the serum levels of cardiac biomarkers were above the 99th percentile upper reference limit or new abnormalities were shown in electrocardiography and echocardiography [9]. Acute hepatic injury (AHI) was identified as total bilirubin increase by 51.3 μmol/L and alanine aminotransferase increase to five times the upper reference limit. Platelet counts, prothrombin time test, and the levels of fibrin/fibrinogen degradation products (FDPs) were components in the major sets of diagnostic criteria of disseminated intravascular coagulation (DIC) [10]. The sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE II) scores were determined on admission.
2.3. Statistical analysis
Continuous variables were described as mean (95% confidence interval), or as median (interquartile range 25–75% [IQR]), and compared using Student's t-test or Mann-Whitney U test. Categorical variables were described as number (percentage), and compared using chi-square test or Fisher exact test, as appropriate. The association between sex and inhospital mortality in COVID-19 patients were explored by Cox proportional hazard regression model and displayed as hazard ratios (HR) and 95% confidence intervals (CI). The model was adjusted on priori–decided baseline variables of clinical interest and on those with a p value of less than 0.05 in the univariate analysis. The included variables were: age, sex, comorbidity, SOFA sore, lymphocyte, platelet, D-dimer, glucocorticoid and antiviral drugs. A two-sided p value less than 0.05 was defined as statistically significant for all the analyses.
All statistical analyses were performed using SPSS (version 24.0, SPSS Inc., Chicago, IL, USA) and SAS (version 9.4, SAS Institute, Cary, NC).
3. Results
3.1. Demographic and clinical characteristics
1190 adult patients with confirmed COVID-19 were recorded in Wuhan Infectious Disease Hospital during the study period. Of these 1190 patients, 635 (53.4%) were male and 555 (46.6%) were female. Clinical characteristics for males versus females are shown in Table 1 . The age distribution as well as clinical symptoms and vital signs on admission had no difference between the two groups. The frequencies of smoking (8.8% vs. 0%, p < 0.001) and drinking (8.8% vs. 0.2%, p < 0.001) were much higher in males. Besides hypertension (males 28.6% vs. females 23.3%, p = 0.037), the comorbidities were similar in the two groups. More female health care workers were infected through occupational exposure than males (2.2% vs. 0.7%, p = 0.025).
Table 1.
Clinical characteristics of patients with COVID-19 by gender category.
| All patients (n = 1190) | Males (n = 635) | Females (n = 555) | P value | |
|---|---|---|---|---|
| Age, Median (IQR)-yr | 57 (47,67) | 56 (46,67) | 58 (48,67) | 0.176 |
| Distribution-n (%) | 0.106 | |||
| <45 yr | 240 (20.2) | 144 (22.7) | 96 (17.3) | |
| 45~54 yr | 266 (22.3) | 142 (22.3) | 124 (22.3) | |
| 55~64 yr | 305 (25.6) | 146 (23.0) | 159 (28.7) | |
| >65 yr | 379 (31.9) | 203 (32.0) | 176 (31.7) | |
| Smoking-n (%) | 45 (3.8) | 45 (7.1) | 0 (0) | <0.001 |
| Drinking-n (%) | 48 (4.0) | 47 (7.4) | 1 (0.2) | <0.001 |
| Comorbidity-n (%) | ||||
| COPD | 22 (1.9) | 16 (2.6) | 6 (1.1) | 0.051 |
| Diabetes | 144 (12.2) | 80 (12.7) | 64 (11.6) | 0.564 |
| Hypertension | 308 (26.1) | 180 (28.6) | 128 (23.3) | 0.037 |
| Chronic cardiac disease | 86 (7.3) | 51 (8.1) | 35 (6.4) | 0.247 |
| Chronic kidney disease | 30 (2.6) | 20 (3.2) | 10 (1.8) | 0.140 |
| Chronic liver disease | 40 (3.4) | 20 (3.2) | 20 (3.6) | 0.670 |
| Stroke | 39 (3.3) | 23 (3.7) | 16 (2.9) | 0.481 |
| Malignancy | 34 (2.9) | 16 (2.6) | 18 (3.3) | 0.452 |
| Exposure history-n (%) | ||||
| Huanan seafood market | 131 (11.4) | 89 (14.6) | 42 (7.8) | <0.001 |
| Medical staff | 16 (1.4) | 4 (0.7) | 12 (2.2) | 0.025 |
| SOFA | 3 (1,5) | 3 (1,5) | 2 (1,5) | 0.071 |
| APACHEⅡ | 3 (1,6) | 3 (1,6) | 3 (2,6) | 0.850 |
Abbreviation: chronic obstructive pulmonary disease, COPD; sequential organ failure assessment, SOFA; acute physiology and chronic health evaluation II, APACHE II.
3.2. Laboratory and radiologic findings and treatments
There were numerous differences in laboratory findings between male and female patients (Table 2 ), including higher leucocyte and neutrophil counts, lower lymphocyte and platelet counts, lower CD4/CD8 ratio, longer PT and APTT, higher levels of organ function parameters like TBIL, ALT, AST, BUN, SCr and CK-MB, as well as higher levels of infection or inflammation markers like hsCRP, PCT, serum amyloid A, serum ferritin and IL-6 in males. Stratification by age group revealed that males of all ages had higher levels of organ and coagulation function parameters (e.g. TBIL, ALT, AST, SCr, CK-MB and PT) than those in the age-matched females (Fig. 1 ).
Table 2.
Laboratory findings of patients with COVID-19 by gender category.
| All patients (n = 1190) | Males (n = 635) | Females (n = 555) | P value | |
|---|---|---|---|---|
| Laboratory findings, Mean (95%CI) | ||||
| Leucocytes(10^9/L) | 8.0 (7.7,8.4) | 8.7 (8.2,9.2) | 7.3 (6.7,8.0) | <0.001 |
| Neutrophils (10^9/L) | 6.9 (6.4,7.4) | 7.8 (7.0,8.7) | 5.8 (5.2,6.4) | <0.001 |
| Lymphocytes (10^9/L) | 1.5 (1.3,1.7) | 1.4 (1.2,1.6) | 1.7 (1.3,2.1) | 0.124 |
| Platelets (10^9/L) | 199 (194,205) | 192 (185,200) | 207 (200,215) | 0.005 |
| CD4 (/uL) | 456 (409,502) | 420 (365,474) | 513 (430,596) | 0.052 |
| CD8 (/uL) | 265 (240,291) | 269 (237,301) | 260 (217,303) | 0.742 |
| CD4/CD8 | 2.0 (1.7,2.4) | 1.8 (1.6,1.9) | 2.4 (1.6,3.3) | 0.049 |
| PT (s) | 13.3 (12.6,14.1) | 13.5 (12.6,14.3) | 13.1 (11.8,14.4) | 0.612 |
| APTT (s) | 29.3 (28.8,29.9) | 30.6 (29.7,31.5) | 27.9 (27.2,28.5) | <0.001 |
| TT (s) | 19.6 (19.2,20.1) | 19.8 (19.1,20.4) | 19.5 (18.9,20.1) | 0.546 |
| D-dimer (ug/mL) | 7.5 (5.3,9.7) | 8.5 (5.5,11.5) | 6.4 (3.1,9.7) | 0.374 |
| TBIL (umol/L) | 16.3 (15.4,17.1) | 17.7 (16.6,18.7) | 14.6 (13.4,15.9) | <0.001 |
| ALT (U/L) | 62 (55,68) | 76 (68,84) | 46 (36,55) | <0.001 |
| AST (U/L) | 56 (47,64) | 61 (51,70) | 50 (35,66) | 0.242 |
| BUN (mmol/L) | 7.9 (6.6,9.1) | 8.7 (6.9,10.5) | 6.9 (5.2,8.6) | 0.146 |
| SCr (umol/L) | 89.1 (84.3,94.0) | 100.5 (94.3106.8) | 76.2 (68.8,83.7) | <0.001 |
| CK-MB (U/L) | 24.3 (14.5,34.2) | 30.1 (12.1,48.1) | 17.7 (13.5,22.0) | <0.001 |
| hsCRP (mg/L) | 57.6 (53.3,61.9) | 67.6 (61.2,73.9) | 46.2 (40.5,51.9) | <0.001 |
| PCT (ng/mL) | 0.7 (0.4,1.0) | 0.7 (0.3,1.1) | 0.6 (0.1,1.1) | 0.008 |
| SAA (mg/L) | 170.6 (157.4183.9) | 184.4 (174.5194.3) | 155.1 (129.3180.9) | 0.030 |
| Serum ferritin (ng/mL) | 619.2 (553.9684.5) | 792.2 (682.8901.7) | 405.3 (358.8451.9) | <0.001 |
| IL-6 (pg/mL) | 309.1 (275.6342.7) | 414.7 (360.2469.2) | 189.4 (156.6222.3) | <0.001 |
Abbreviation: prothrombin time, PT; Activated-partial thromboplastin time, APTT; thrombin time, TT; total bilirubin, TBIL; alanine-aminotransferase, ALT; aspartate-aminotransferase, AST; blood urea nitrogen, BUN; serum creatinine, SCr; creatine kinase isoenzyme, CKMB; hyper-sensitive C-reactive protein, hsCRP; procalcitonin, PCT; serum amyloid protein A, SAA; interleukin, IL; mechanical ventilation, MV; extracorporeal membrane oxygenation, ECMO.
Fig. 1.
Comparison of organ and coagulation function parameters in patients with COVID-19 by sex and age group.
*P < 0.05 for males vs. age-matched females. Abbreviation: total bilirubin, TBIL; alanine-aminotransferase, ALT; aspartate-aminotransferase, AST; serum creatinine, SCr; creatine kinase isoenzyme, CK-MB; prothrombin time, PT; Activated-partial thromboplastin time, APTT.
We also analyzed sex differences in chest radiological findings and therapies during hospitalization. The radiological findings were similar in males and females. However, male patients were more likely to receive glucocorticoids during hospitalization than female patients (30.7% vs. 20.6%, p < 0.001).
3.3. Clinical outcomes
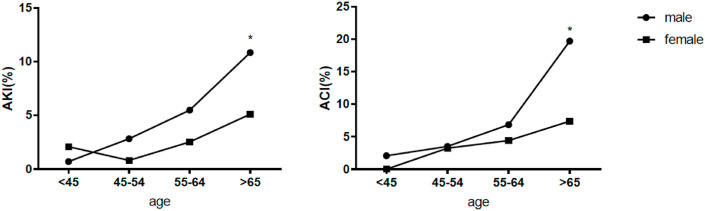
Calculations of clinical outcomes were stratified by sex and were summarized in Table 3 . Among the 1190 patients, there were 100 male deaths accounted for 63.7% of all deaths, and 57 female deaths accounted for 36.3% of all deaths. The inhospital mortality rate of male patients was significantly higher than that of female patients (15.7% vs. 10.3%, p = 0.005). In male patients, we found significantly higher rates of acute kidney injury (5.5% vs. 2.9%, p = 0.026), acute cardiac injury (9.1% vs. 4.3%, p = 0.001) and DIC (2.5% vs. 0.7%, p = 0.024) than in female patients. However, there were no sex differences in incidence of ARDS, acute hepatic injury or shock. The median length of hospitalization for male patients was 11 days, while that for female patients was 10 days (p = 0.003). Considering that age represents an important issue on clinical outcomes, we compared organ failure and inhospital mortality between males and females in different age groups. As shown in Fig. 2, Fig. 3 , the rates of organ failure occurrence and mortality of males and females both increased with age. In patients older than 65 years, male patients had significantly higher incidences of organ failure and inhospital mortality over female patients.
Table 3.
Clinical outcomes of patients with COVID-19 by gender category.
| All patients (n = 1190) | Males (n = 635) | Females (n = 555) | P value | |
|---|---|---|---|---|
| Outcomes | ||||
| ARDS-n (%) | 228 (19.2) | 133 (20.9) | 95 (17.1) | 0.094 |
| AKI-n (%) | 51 (4.3) | 35 (5.5) | 16 (2.9) | 0.026 |
| AHI-n (%) | 115 (9.7) | 71 (11.2) | 44 (7.9) | 0.058 |
| ACI-n (%) | 82 (6.9) | 58 (9.1) | 24 (4.3) | 0.001 |
| DIC-n (%) | 19 (1.7) | 15 (2.5) | 4 (0.7) | 0.024 |
| Shock-n (%) | 69 (5.8) | 41 (6.5) | 28 (5.1) | 0.299 |
| Duration of MV, Median (IQR)-days | 5 (2,8) | 5 (2,8) | 5 (2,10) | 0.919 |
| Duration of in-hospital stay, Median (IQR)-days | 11 (7,14) | 11 (8,15) | 10 (7,14) | 0.002 |
| In-hospital mortality-n (%) | 157 (13.2) | 100 (15.7) | 57 (10.3) | 0.005 |
Abbreviation: acute respiratory distress syndrome, ARDS; acute kidney injury, AKI; acute hepatic injury, AHI; acute cardiac injury, ACI; disseminated intravascular coagulation, DIC.
Fig. 2.
Comparison of acute kidney injury (AKI) and acute cardiac injury (ACI) in patients with COVID-19 by sex and age group.
*P < 0.05 for males vs. age-matched females.
Fig. 3.
Comparison of in-hospital mortality in patients with COVID-19 by sex and age group.
*P < 0.05 for males vs. age-matched females.
3.4. Multivariable cox regression of inhospital mortality
Our multivariable Cox regression model revealed no relation between sex and inhospital mortality (Table 4 ). SOFA score on admission (HR 1.057, 95% CI 1.028–1.087, p < 0.001), lymphocytopenia (HR 1.249, 95% CI 1.054–1.479, p = 0.010), thrombocytopenia (HR 1.600, 95% CI 1.142–2.247, p = 0.006) and elevated D-dimer (HR 1.156, 95% CI 1.065–1.255, p < 0.001) were correlated with increased mortality risk during hospitalization. In addition, glucocorticoid and antiviral treatment was negatively associated with inhospital mortality (HR 0.618, 95% CI 0.521–0.735, p < 0.001 and HR 0.747, 95% CI 0.648–0.862, p < 0.001, respectively).
Table 4.
Multivariate Analysis of Risk Factors associated with in-hospital Mortality in patients with COVID-19.
| Variables | Cox regression model HR 95% CI P value | ||
|---|---|---|---|
| Age (<45yr as reference) | |||
| 45~54 yr | 0.950 | 0.777, 1.161 | 0.615 |
| 55~64 yr | 0.888 | 0.726, 1.085 | 0.246 |
| >65 yr | 1.034 | 0.834, 1.282 | 0.760 |
| Male sex | 1.020 | 0.889, 1.171 | 0.779 |
| Comorbidity | 0.994 | 0.854, 1.157 | 0.936 |
| SOFA | 1.057 | 1.028, 1.087 | <0.001 |
| Lymphocytopenia | 1.249 | 1.054, 1.479 | 0.010 |
| Thrombocytopenia | 1.600 | 1.142, 2.247 | 0.006 |
| Elevated D-dimer | 1.156 | 1.065, 1.255 | <0.001 |
| Glucocorticoid | 0.618 | 0.521, 0.735 | <0.001 |
| Antiviral drugs | 0.747 | 0.648, 0.862 | <0.001 |
Abbreviation: sequential organ failure assessment, SOFA.
4. Discussion
With the intensification of the adverse impact of COVID-19 pandemic to the world, lots of studies on biological and clinical characteristics of the disease have been published. Official Chinese statistics showed that the male-to-female ratio was 1.06:1 in China and the fatality rate for males was 2.8% versus 1.7% for females [3]. Given the sex differences in case fatality rate, the clinical characteristics of the disease need to be stratified by sex.
In our study, male patients showed significantly increased incidence of acute kidney injury, acute cardiac injury and DIC, as well as higher inhospital mortality. Angiotensin-converting enzyme 2 (ACE2), the main binding site for the entry of SARS-CoV-2 into host cells, is predominantly expressed in heart, kidney, and testis [[11], [12], [13]]. This may explain the vulnerability of cardiovascular system and kidney to SARS-CoV-2 infection. However, after stratification by age group, significant gender differences in organ failure and mortality were observed only in patients older than 65 years. Interestingly, males of all ages showed higher levels of organ and coagulation function parameters than that in age-matched female patients. Biological sex, age, as well as gender-associated lifestyle behaviors and comorbidities might contribute to the disparity between men and women in COVID-19 outcomes.
Unhealthy lifestyle behaviors like smoking and drinking are prevalent in males. Alcohol and pathogenic constituents of tobacco smoke including nitric oxide, carbon monoxide and phenolic free radicals has proved proinflammatory [[14], [15], [16]]. What's more, active smoking has been shown to raise ACE2 receptor expression in the lungs, which increases SARS-CoV-2 attachment and entry into alveolar epithelial cells [17,18].
Comorbidities that are associated with fatal COVID-19 cases in the study, include diabetes, hypertension, heart disease, stock and chronic obstructive pulmonary disease (Supplementary Table 1). Among these comorbidities, hypertension occurs more frequently in male patients. Obesity, hypertension and diabetes in the context of metabolic syndrome is considered to provide a permissive inflammatory environment that intensifies a rapid development of cytokine storm which is associated with COVID-19 severity [19,20].
Previous studies confirmed that estrogen have a protective effect on vascular endothelial cells [[21], [22], [23]]. Endothelial dysfunction may contribute to acute kidney injury and acute cardiac injury in critically ill patients [24,25]. However, in patients with COVID-19, mechanisms behind the gender differences might not only due to the levels of endogenous hormones, given that laboratory findings in postmenopausal elderly female patients were still superior to their male peers.
Robust immune systems in females might play another positive role in the gender disparity. Firstly, the bi-allelic expressions of several immune regulatory genes which located on the X chromosome (e.g. TLR7, TLR8, FOXP3, CXCR3 and CD40L) contribute to stronger immune response against viruses in women [[26], [27], [28]]. Secondly, a comparison of adaptive immune responses between males and females demonstrates that females generally have higher levels of CD4/CD8 ratio [29]. The sex-based differences in CD4/CD8 ratio also exist in our patients with COVID-19. In addition, several antiviral genes expressed by cytotoxic T cells carry estrogen receptor elements in their promoter and contribute to stronger cytotoxic response in females [30,31]. Thus, females exhibit robust innate and adaptive immune responses to viral infections.
In spite of the marked sex differences in organ function and mortality in the univariate analysis, we found no relation between sex and hospital deaths in multivariable analysis, which showed that SOFA score, lymphocytopenia, thrombocytopenia and elevated D-dimer were all independent prognostic factors. We conclude that the sex underlying mechanisms are not strong enough to significantly alter such a complex and multifactorial endpoint inhospital mortality. Clinical outcomes are not only related to patients’ conditions and disease severity, but also closely related to treatment including respiratory support, glucocorticoid and antiviral therapy. In the study, more males were treated with glucocorticoid due to severe disease. COX proportional regression analysis showed that glucocorticoid therapy reduced the inhospital mortality. Although glucocorticoid therapy of COVID-19 remains controversial and might impact the inhospital mortality, but given comparing men vs. women, the main aim of this study, the bias may be minimized.
4.1. Limitations
This study had some limitations. Firstly, although our research was based on a large population, this was a retrospective single-center study with all patients enrolled from Wuhan Infectious Disease Hospital at the early stages of COVID-19 outbreak. The interpretation of our findings might be limited by the sample size. In addition, in the early stages of COVID-19 outbreak, the disease treatment including respiratory support, glucocorticoid and antiviral therapy mainly relied on clinical judgment and experience of the doctors due to lack of evidence-based guidelines, which might impact the inhospital mortality. However, comparing men vs. women, the main aim of this study, the bias may be minimized. Secondly, not all laboratory tests were performed on all patients, which might introduce some biases albeit the use of multiple analytical strategies to increase the robustness of our estimates. Thirdly, we did not have data on menopausal status of female patients, although no significant relationship was identified when we searched the interaction based on the average age of menopause. Finally, there was no long-term follow-up.
5. Conclusion
In summary, male sex was associated with a worse prognosis of COVID-19, but it seems not to be an independent prognostic factor.
CRediT authorship contribution statement
Jiao Liu: drafted the manuscript. Lidi Zhang: drafted the manuscript. Yizhu Chen: collected the clinical data. Zhixiong Wu: collected the clinical data. Xuan Dong: collected the clinical data. Jean-Louis Teboul: drafted the manuscript. Sheng Zhang: did statistical analysis. Xiaofei Ye: did statistical analysis. Yongan Liu: summarized all the collected data. Tao Wang: summarized all the collected data. Hangxiang Du: summarized all the collected data. Wenzhe Li: summarized all the collected data. Dechang Chen: revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the staffs in Wuhan Infectious Disease Hospital to take care of COVID-19 patients, and all the patients and their families included in the current study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2020.106159.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D., et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarty D., Nair S.S., Hammouda N., Ratnani P., Gharib Y., Wagaskar V., et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol. 2020;3(1):374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China. China CDC Weekly. 2020;2(8):113–122. https://doi: 10.46234/ccdcw2020.032 [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Stadio A., Ricci G., Greco A., de Vincentiis M., Ralli M. Mortality rate and gender differences in COVID-19 patients dying in Italy: a comparison with other countries. Eur. Rev. Med. Pharmacol. Sci. 2020;24(8):4066–4067. doi: 10.26355/eurrev_202004_20980. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance, 28 January 2020. https://apps.who.int/iris/handle/10665/330893
- 7.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin definition. J. Am. Med. Assoc. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.M.D. Okusa, A. Davenport. Reading between the (guide)lines--the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int.. 85(1):39-48, 10.1038/ki.2013.378. [DOI] [PMC free article] [PubMed]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levi M., Sivapalaratnam S. Disseminated intravascular coagulation: an update on pathogenesis and diagnosis. Expet Rev. Hematol. 2018;11(8):663–672. doi: 10.1080/17474086.2018.1500173. [DOI] [PubMed] [Google Scholar]
- 11.Yan R., Zhang Y.Y., Li Y.N., Xia L., Guo Y.Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Li X.J., Chen M.Q., Feng Y., Xiong C.L. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh P., Woodward M., Rumley A., Lowe G. Associations of plasma pro-inflammatory cytokines, fibrinogen, viscosity and C-reactive protein with cardiovascular risk factors and social deprivation: the fourth Glasgow MONICA study. Br. J. Haematol. 2008;141(6):852–861. doi: 10.1111/j.1365-2141.2008.07133.x. [DOI] [PubMed] [Google Scholar]
- 15.Watts R.N., Ponka P., Richardson D.R. Effects of nitrogen monoxide and carbon monoxide on molecular and cellular iron metabolism: mirror-image effector molecules that target iron. Biochem. J. 2003;369(Pt 3):429–440. doi: 10.1042/BJ20021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berg R., Haenen G.R., van den Berg H., Bast A. Nuclear factor-kappaB activation is higher in peripheral blood mononuclear cells of male smokers. Environ. Toxicol. Pharmacol. 2001;9(4):147–151. doi: 10.1016/s1382-6689(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 17.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardavas C.I., Nikitar K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowlton A.A., Lee A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012;135(1):54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu B.Y., Huang S.Y., Yin L.H. The cytokine storm and COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montt-Guevara M.M., Palla G., Spina S., Bernacchi G., Cecchi E., Campelo A.E., et al. Regulatory effects of estetrol on the endothelial plasminogen pathway and endothelial cell migration. Maturitas. 2017;99:1–9. doi: 10.1016/j.maturitas.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Gohar E.Y., Pollock D.M. Sex-specific contributions of endothelin to hypertension. Curr. Hypertens. Rep. 2018;20(7):58. doi: 10.1007/s11906-018-0856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrier R.W., Wang W. Acute renal failure and sepsis. N. Engl. J. Med. 2004;351(2):159–169. doi: 10.1089/sur.2017.261. [DOI] [PubMed] [Google Scholar]
- 25.Gomez H., Ince C., Backer D.D., Pickkers P., Payen D., Hotchkiss J., et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 27.Kritas S.K., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34(1) doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 28.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 29.Khan D., Ahmed S.A. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2016;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34(12):1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewagama A., Patel D., Yarlagadda S., Strickland F.M., Richardson B.C. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Gene Immun. 2009;10(5):509–516. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.