Abstract
Neuropathy is a common complication of long-term diabetes that impairs quality of life by producing pain, sensory loss and limb amputation. The presence of neuropathy in both insulin-deficient (type 1) and insulin resistant (type 2) diabetes along with the slowing of progression of neuropathy by improved glycemic control in type 1 diabetes has caused the majority of preclinical and clinical investigations to focus on hyperglycemia as the initiating pathogenic lesion. Studies in animal models of diabetes have identified multiple plausible mechanisms of glucotoxicity to the nervous system including post-translational modification of proteins by glucose and increased glucose metabolism by aldose reductase, glycolysis and other catabolic pathways. However, it is becoming increasingly apparent that factors not necessarily downstream of hyperglycemia can also contribute to the incidence, progression and severity of neuropathy and neuropathic pain. For example, peripheral nerve contains insulin receptors that transduce the neurotrophic and neurosupportive properties of insulin, independent of systemic glucose regulation, while the detection of neuropathy and neuropathic pain in patients with metabolic syndrome and failure of improved glycemic control to protect against neuropathy in cohorts of type 2 diabetic patients has placed a focus on the pathogenic role of dyslipidemia. This review provides an overview of current understanding of potential initiating lesions for diabetic neuropathy and the multiple downstream mechanisms identified in cell and animal models of diabetes that may contribute to the pathogenesis of diabetic neuropathy and neuropathic pain.
INTRODUCTION
Neuropathy will afflict over half of the estimated 460 million people worldwide who have diabetes[305], of whom approximately one third will also develop neuropathic pain[2]. The pathogenesis of diabetic neuropathy is uncertain and attaining and maintaining close glycemic control remains the only universal recommendation for preventing or slowing progression of the condition. While there has been considerable progress in β-cell, stem cell and whole pancreas transplantation[231] and ongoing refinement of continuous glucose monitors for maintaining consistent euglycemia[283], these advanced bioengineering solutions are unlikely to become available for the majority of the diabetic population worldwide in the foreseeable future[65]. Although the mechanisms driving degenerative neuropathy and pain are likely intertwined, the unpredictability of which patients with neuropathy also exhibit pain suggests as yet ill-defined pathways unique for pain generation. Treatment of painful diabetic neuropathy is limited to analgesics[9; 18] with efficacy of any given agent limited to unpredictable sub-populations of patients[113]. This somewhat bleak landscape has prompted extensive investigation of the pathogenic consequences of hyperglycemia and, more recently, glucose-independent neurotoxic mechanisms, as downstream sites for therapeutic intervention.
The most common presentation of diabetic neuropathy is as a distal symmetrical polyneuropathy with numbness in the distal extremities. Loss of sensation can lead to unattended wounds that, when combined with peripheral vascular disease and impaired wound healing, may lead to infection and ultimately amputation[161]. Indications of motor and autonomic nerve dysfunction may also be present. Early quantifiable features of distal symmetrical polyneuropathy (from herein termed diabetic neuropathy unless stated otherwise) include slowing of large sensory and motor fiber conduction velocity (SNCV and MNCV)[32] and depletion of small sensory nerves in the skin and cornea[276]. Peripheral nerves also exhibit resistance to ischemic conduction blockade/failure (RICB/RICF), which patients may become aware of as an ability to squat or kneel for lengthy periods of time without developing paresthesias and that can be confirmed using routine electrophysiology and a blood pressure cuff[137; 316]. Microvascular lesions similar to those reported in other organs during diabetes are also an early feature[223]. Biopsy studies have identified segmental demyelination in large fibers and axonal degeneration of all fiber classes [97] with clusters of regenerating fibers[223], but regeneration is clearly insufficient to overcome ongoing distal degenerative processes. It is now widely accepted that diabetes damages all components of the nervous system, not just peripheral nerves. Historical autopsy evidence of demyelination and neuronal degeneration in the spinal cord[330] has been supported by more recent non-invasive imaging studies[313] and there is emerging recognition of structural and functional impairments in the higher CNS[27; 314; 325].
Around a third of patients with diabetic neuropathy report intermittent or continuous paresthesias and/or pain[2]. The most frequent descriptors are of numbness, tingling, burning, pins and needles, electric shock and pain to cold[359]. Pain may develop during the pre-diabetic period[331; 338] or relatively early after diagnosis of diabetes[133] but tends to be associated with advanced degenerative neuropathy[350]. A separate and distinct pain condition, historically termed insulin neuritis, can also develop after instigation of tight glycemic control[122].
DISCUSSION
1. MECHANISTIC IMPLICATIONS FROM CLINICAL OBSERVATIONS
A number of deductions made from the clinical presentation of diabetic neuropathy have guided development of mechanistic hypotheses and both clinical and preclinical investigations:
1.1. Initiating lesion:
The bilateral presentation of diabetic neuropathy implies a systemic primary lesion, although people with diabetes are also more vulnerable to focal neuropathies[332]. The occurrence of neuropathy in both insulin-deficient (type 1) and insulin-insensitive (type 2) diabetes has driven an overwhelming experimental focus on hyperglycemia as the initiating lesion. This was encouraged by results from the DCCT study which showed that improved glycemic control beyond the accepted standard of care slowed onset and progression of diabetic complications, including peripheral neuropathy, in a large cohort of type 1 diabetic patients [227]. The association between hyperglycemia and complications, including neuropathy, is less convincing in patients with type 2 diabetes and alternative primary pathogenic mechanisms have been proposed (see below). Data from the DDCT, its follow-up (EDIC) and preclinical studies also prompted the concept of “metabolic memory” in which initial exposure to glucose makes indelible epigenetic modifications to cells that are not amenable to acute restoration of normoglycemia[372]. To date, this has proven to be more applicable to other complications of diabetes than to peripheral neuropathy[235], although there is supportive evidence associated with autonomic neuropathy[120].
1.2. Cellular targets:
As epineurial, perineurial and endoneurial blood vessels are compromised in nerves of diabetic patients with early neuropathy[223; 271], a view of diabetic neuropathy emerged as representing a secondary manifestation of microvascular disease arising from hyperglycemia-induced damage to vascular endothelial cells[218]. This aligns diabetic neuropathy with other complications of diabetes[263] and there is an association between development of neuropathy and concurrent nephropathy and retinopathy. The presence of RICB also implies an early presence of ischemic hypoxia[218], although it also suggests that nerve metabolism adapts within acceptable tolerance limits. Whether vascular insufficiency initiates peripheral neuropathy in diabetic patients or impedes the capacity of cells within the nerve to withstand direct glucotoxic or other insults remains an area of lively debate. Similarly, whether there is initial damage to Schwann cells (primary Schwannopathy) [175], to axons (primary axonopathy) [98] or independent and/or inter-dependent mechanisms of damage to each cell type[123] has also been an area of continuous investigation [351].
1.3. Degenerative neuropathy:
That numbness is usually first perceived in the toes, along with the early loss of epidermal fibers in the lower extremities, suggests a length-dependent neuropathy associated with an inability to maintain the regions of the axon that are most distant from the cell body – not unlike the travails of Napoleon when invading Russia in the winter of 1812[198]. However, reports that depletion of sensory nerves in the sub-basal plexus of the cornea is an equally sensitive marker for early neuropathy in diabetic patients indicates that distal regions of the axon are most vulnerable, irrespective of length[8; 276]. Longer axons may be particularly vulnerable to accumulation of focal lesions due to size alone, but systemic insults do not appear to discriminate based on absolute axonal length.
1.4. Painful vs painless neuropathy:
Why approximately only a third of diabetic patients with degenerative neuropathy develop pain[2] remains enigmatic. Studies have sought clinical, structural, functional or metabolic biomarkers that segregate subjects otherwise well matched in presentation of diabetes and neuropathy into those with or without pain [321; 334]. Recent examples are shown in Table 1 [30; 34; 58; 59; 76; 91; 99; 114; 115; 140; 177; 178; 196; 216; 224; 226; 242; 273–276; 285; 312; 314; 315; 320; 329; 335; 343; 350; 359; 362; 386; 416]. However, interpretation of such studies is complicated by the heterogeneity of the pain state caused by diabetes and there have been few attempts to identify biomarkers that identify specific pain sub-categories identified amongst diabetic patients[20; 350]. Perhaps the best examples to date are associations of burning pain with gain of function mutations in sub-units of the Nav1.7 ion channel[11; 29], although this represents a rare sub-group within those with painful diabetic neuropathy. Identification of features unique to subjects with pain could reveal potential pathogenic mechanisms specific for pain, with the usual caveat that any implied causality must be proven.
TABLE 1. Potential biomarkers for painful vs painless diabetic neuropathy.
Studies where no change was detected are shown in italics.
| Type | Tissue | Biomarker for painful neuropathy | Representative Publications |
|---|---|---|---|
| STRUCTURAL (PNS) | Nerve biopsy | Small fiber damage and regeneration | [34,216,224] |
| Skin biopsy | Increased regeneration and/or axonal swellings | [30,58,59,114] | |
| Cornea | Increased corneal nerve loss | [177,178,226,276] | |
| Increased corneal nerve branching | [274] | ||
| STRUCTURAL (CNS) | Cortex | Atrophy of somatosensory cortex | [314] |
| FUNCTIONAL (PNS) | Nerve | Increased epineurial blood flow | [99] |
| Skin | Impaired stimulus-evoked blood flow | [275] | |
| Skin | Increased LDI flare (small fiber function) | [196] | |
| Sensory systems | Severe hypoalgesia | [285,350] | |
| FUNCTIONAL (CNS) | Spinal cord | Impaired rate dependent depression of H wave | [226] |
| PAG | Dysfunction of descending inhibitory systems | [312] | |
| Thalamus | Hyperperfusion | [315] | |
| Anterior cingulate cortex | Hyperperfusion | [386] | |
| Limbic/striatal structures | Increased responses to stimuli | [362] | |
| OTHER | Blood | Increased CRP and slCAM-1 | [91] |
| Increased TNFα, | [242,273] | ||
| Increased iNOS | [273] | ||
| Reduced vitamin D | [320] | ||
| Reduced Glo-1 | [329] | ||
| Physiology | Sex (female) | [140,359] | |
| Increased BMI | [416] | ||
| Autonomic dysfunction | [76,115,335,343] |
1.5. Pain generator site:
It is tempting to assume that if pain is perceived in the feet, then the primary lesion site is to sensory nerves that innervate the feet. Recent studies suggesting that pain correlates with nerve regeneration markers in the skin[30; 58] have revived the old ideas that painful diabetic neuropathy may arise from instability of degenerating peripheral sensory neurons, ephaptic activation of adjacent intact peripheral fibers and/or activity of regenerating peripheral fibers[34]. Hyperactive nociceptors and recruitment of otherwise silent nociceptors have been recorded in subjects with painful diabetic neuropathy by microneurography[259] and used to pre-select subjects for clinical trial[317]. However, the “irritable nociceptor” phenotype (preserved small fiber function with hyperalgesia) formed only a small sub-set (6%) of a cohort comprising 191 subjects with painful diabetic neuropathy[350] and peripheral hyperactivity may not be the only genesis of pain. A report that onset of type 2 diabetes triggered symmetrical pain in both feet of a patient who had one leg amputated some 44 years earlier prompted the suggestion that the initiating lesion for diabetes-induced pain need not be at the site where pain is perceived[281]. In support of this, there is a growing body of data emerging from imaging studies of diabetic subjects with painful neuropathy indicating that there is CNS dysfunction and pathology in regions associated with pain processing[312; 314] while preclinical and clinical studies also suggest spinal involvement [226]. In addition, there is growing appreciation that the genesis of neuropathic pain states evolves over time, progressing from peripheral to central sites. Painful diabetic neuropathy may therefore incorporate multiple generator sites whose relative dominance waxes and wanes as concurrent degenerative pathology progresses. This complexity has implications for selecting clinical trial populations that may be more heterogenous than previously recognized[20] and result in the infrequent (NNT > 5) and unpredictable efficacy of current pain medications used to treat painful diabetic neuropathy[113].
2. GLUCOTOXIC MECHANISMS
The occurrence of neuropathy in both forms of diabetes and the success of intensive glycemic control regimens to slow progression of diabetic neuropathy in type 1 diabetes[269] naturally focused attention on hyperglycemia as the initiating event of diabetic neuropathy. Glucose enters peripheral nerve and brain via insulin-independent glucose transporters (GLUT’s). GLUT-1 is the major glucose transporter of the microvascular and perineurial components of the blood:brain and blood:nerve barriers[337], with GLUT-3 localized to peripheral neurons[340]. Insulin dependent GLUT-4 is restricted to select neuronal populations of the brain[19]. The search for detrimental consequences of hyperglycemia has focused on modifications to protein structure and function by direct glycation or enzyme-mediated glycosylation along with excess flux through glucose metabolism pathways (FIGURE 1).
Figure 1: Mechanisms of glucotoxicity in diabetic neuropathy.
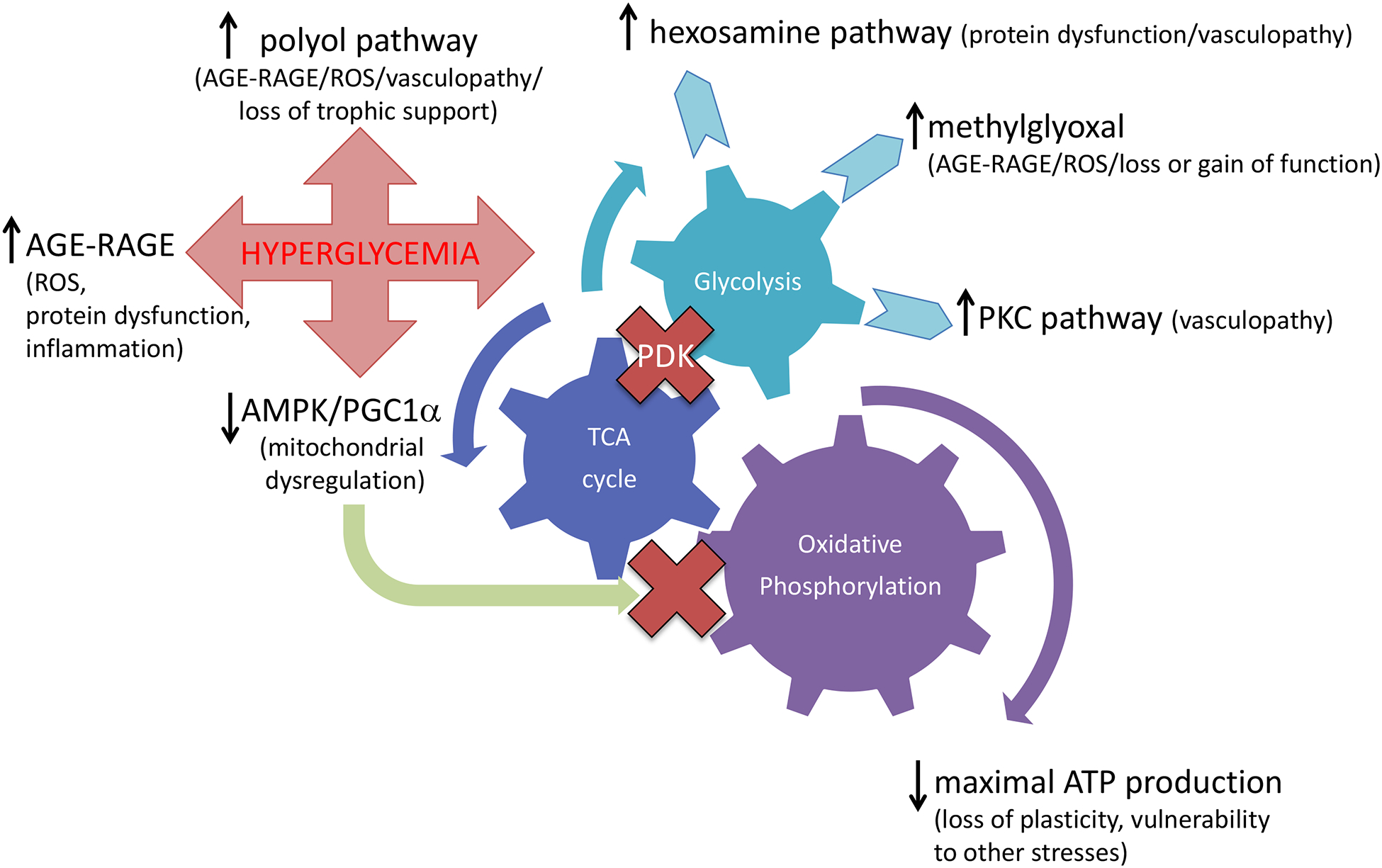
Hyperglycemia has been considered central to the pathogenesis of diabetic neuropathy and multiple mechanisms have been proposed from both preclinical studies and clinical observations (see text for details).
2.1. Models of diabetic neuropathy:
In vitro studies allow direct environmental manipulation of individual cell types and are valuable for identifying plausible pathogenic mechanisms, with the recognized caveats that these are traumatically excised tissues in artificial environments – neurons enter an axonal injury and regeneration phenotype while Schwann cells return to their non-myelinating form[118]. Perhaps the most sophisticated studies employ cells derived from adult control and diabetic animals maintained under conditions that reflect the in vivo insulin and glycemic environment from which they were derived[134] and co-culture neurons and Schwann cells to facilitate myelination[344; 366].
Of the animal models, diabetic cats exhibit nerve pathology that most closely reflects the human condition, with prominent demyelination and axonal degeneration[239]. Rats and mice are the most commonly used animal models of diabetic neuropathy and provide the majority of data that underpins current hypotheses regarding the pathogenesis of diabetic neuropathy and neuropathic pain. Both species can be used to model pre-diabetes, type 1 or type 2 diabetes using dietary, chemical or genetic initiating events. There are variations in the neuropathy phenotype and rate of progression between specific models, species, strains, laboratories and assays [26]. The provenance of new disorders identified in streptozotocin (STZ)-diabetic rodents, the most commonly used model of type 1 diabetes, should be established to address concerns about acute STZ neurotoxicity[15; 261; 296]. This can be done using concurrent 3-O-methyl glucose injection[81; 226], using insulin to reverse established disorders[226] and by validating disorders using genetic or dietary models[404]. Rodents are frequently studied over 4–12 weeks of diabetes and are best viewed as modeling early nerve dysfunction in the absence of overt pathology[380], as structural pathology in nerve trunks (FIGURE 2) is limited to reduced axonal caliber with late (4 months+) myelin thinning, occasional segmental demyelination and minor fiber loss[158; 159; 183; 270; 290; 398]. This can be viewed as a boon, as molecular and biochemical changes in nerve may be interpreted as preceding, perhaps precipitating, degenerative neuropathy. Unfortunately, it also limits any guarantees of the translation of therapies developed using these models. Recognition that diabetic rodents develop early loss of sensory nerve terminals in the epidermis of plantar skin (frequently termed intra-epidermal nerve fibers or IENF)[23] and reduced sensory nerve density in the cornea[52] that accompany indices of both sensory loss and hyperalgesia[168] to mirror the human condition[276] have revived hopes that rodent models can be used to study the early damage to small sensory neurons that is a feature of diabetic neuropathy (FIGURE 2).
Figure 2: Imaging diabetic neuropathy.
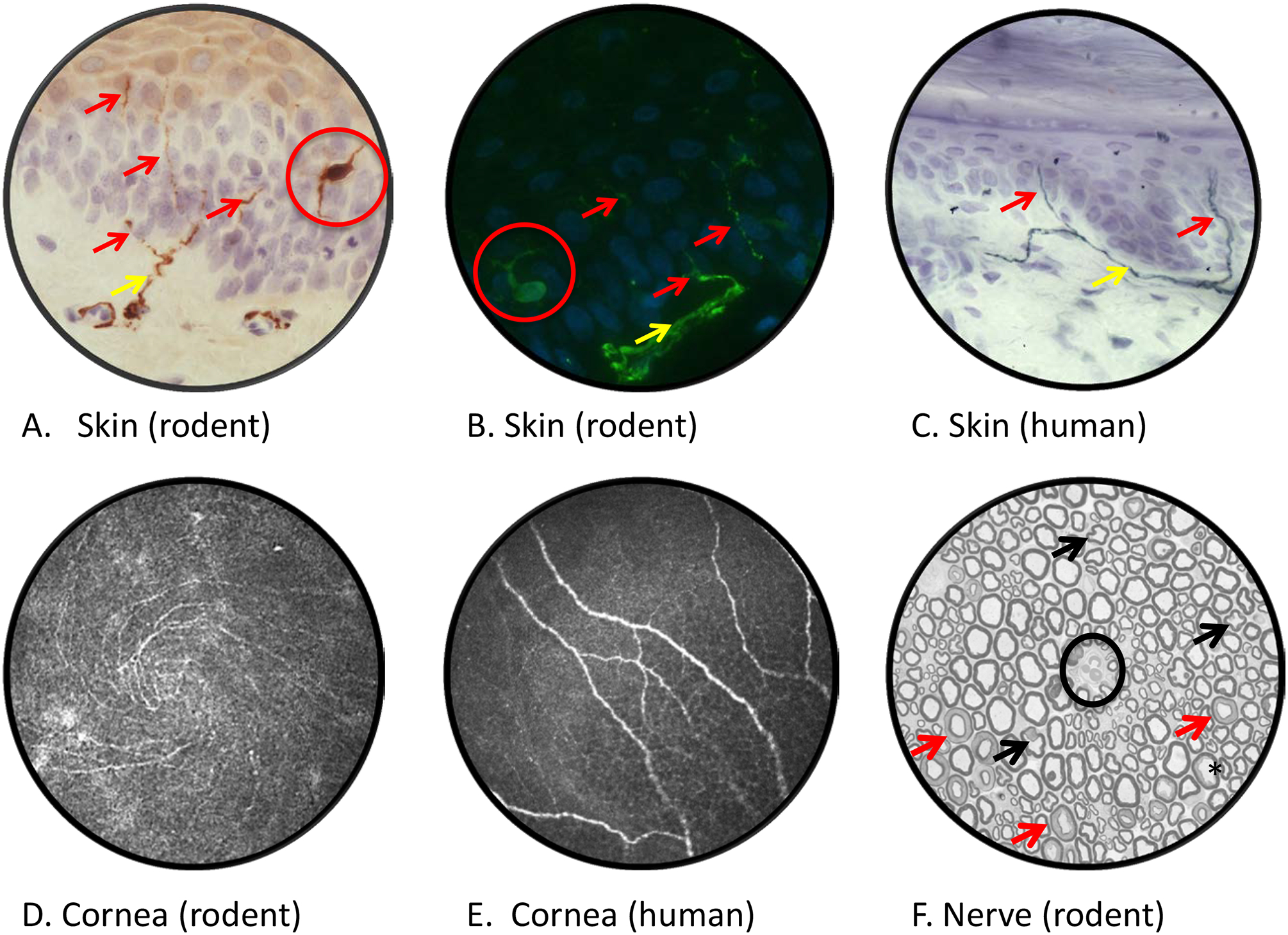
Rat plantar skin stained with antibody to PGP9.5 and viewed by bright field (panel A) or fluorescence (panel B) microscopy reveals dermal nerves (yellow arrows) projecting across the dermal:epidermal junction and into the epidermis (purple/blue counterstained nuclei) where they form profiles of intra-epidermal nerve fibers (red arrows). Note that PGP9.5 also stains epidermal Langerhans cells (red circles). Diabetic rodents and humans (panel C) show early reductions in IENF density that are associated with both sensory loss and pain [23, 276]. Confocal images of the corneal sub-basal nerve plexus of a live mouse (panel D, showing inferior whorl) and human (panel E]. Reduced corneal nerve morphometric parameters are detected within weeks of onset of diabetes in rodents [52] and in early stages of clinical diabetic neuropathy [276]. A cross section of sciatic nerve from a STZ-diabetic rat (panel F), with an endoneurial blood vessel at the centre of field (black circle), lacks overt evidence of the axonal degeneration or demyelination common in nerve biopsies from diabetic patients. Apparently mis-shapen axons (black arrows) represent normal paranodal regions of the nerve fiber while multiple myelin profiles illustrate normal Schmidt-Lanterman incisures (red arrows). Mild axonal fixation artifact is illustrated by the black star. Morphometric analysis identifies reduced mean axonal diameter in the absence of significant fiber loss, indicative of axonal atrophy or impaired maturation. Technical details of procedures used to generate these images and representative data showing the effects of diabetes are published [168]. Images by Ms. Katie Frizzi, Ms. Lucie Guernsey and Ms. Alex Marquez.
2.2. Glucose metabolism by the polyol pathway:
Early observations that tissues that contained the polyol pathway enzymes aldose reductase and sorbitol dehydrogenase were prone to diabetic complications prompted extensive research into their potential pathogenic role[96]. Within peripheral nerve, aldose reductase is localized to endothelial and Schwann cells[165] and hyperglycemia-driven flux through the polyol pathway results in accumulation of the intermediates sorbitol and fructose along with shifts in the redox balance of the associated cofactors NADPH and NADH. Downstream consequences potentially include local osmotic stress due to sorbitol accumulation[252], fructose-driven AGE formation[12] and subsequent RAGE signaling, oxidative[75] and nitrosative stress[72] and loss of neurotrophic support[237]. As reviewed elsewhere[252], pre-clinical studies showing impressive efficacy of aldose reductase inhibitors (ARI’s) against many indices of diabetic neuropathy and neuropathic pain that culminated in a number of clinical trials. To date, ARI treatment has not shown sufficiently convincing efficacy in clinical trials to support approval by most regulatory agencies, although epalrestat is approved in Japan. Whether this reflects a pathogenic mechanism that is pertinent only to diabetic rodents, sub-optimal drug properties for human use or poor clinical trial design and inappropriate endpoints remains the subject of unresolved debate[252]. Polyol pathway research is currently out of vogue but the impressive preclinical efficacy of ARI’s must either indicate a major contribution to downstream pathways that damage nerve or illustrate a disconcerting gulf between preclinical models and the human disease.
2.3. Non-enzymatic glycation and the AGE-RAGE axis:
The post-translational modification of cellular proteins caused by non-enzymatic attachment of glucose to amino acids, causing reversible progression from Schiff base to Amadori products and then irreversible formation of advanced glycation end products (AGE’s) has intermittently recurred as a mechanism of potential glucotoxicity in many organs, including nerve[352; 375]. AGE are present throughout peripheral nerve[341] and the initial focus was on modification of components of the axonal cytoskeleton that could interrupt axonal transport and axial and radial growth[230] along with modification of basement membrane and extracellular matrix proteins that could impede neuronal regeneration after injury[95; 189]. More recently, glycosylation of ion channels has been implicated in painful diabetic neuropathy[25; 258; 390], as discussed below.
Identification of a receptor for AGE (RAGE) on the surface of neurons, Schwann cells and vascular endothelial cells[377] aligned nerve with other organs prone to damage during chronic diabetes[301]. In other tissues AGE binding to RAGE activates NADPH oxidase with subsequent release of reactive oxygen species (ROS) while RAGE signaling via NF-kB modifies gene expression, promoting inflammation and dysregulation of the survival/apoptosis equilibrium. There is evidence that similar toxic events occur in nerve[358] with recent in vitro studies demonstrating that RAGE signaling potentiates TRPV1-mediated calcium signals and contribute to painful neuropathy[24; 199]. Preclinical studies of agents with anti-AGE/RAGE properties such as aminoguanidine and B vitamins show some efficacy in rodent models of diabetic neuropathy[397] and neuropathic pain[170] while a small-scale clinical trial of the vitamin B1 derivative benfotiamine suggested improvement in pain[339]. However, these agents have other potential mechanisms of action and it should also be noted that RAGE signaling is reported to have beneficial effects in nerve such as promoting neurite outgrowth[306] and that RAGE deletion attenuated indices of neuropathy in diabetic mice.[85]
2.4. Glycolysis:
Hyperglycemia-driven increases in intermediates of glycolysis have been linked to diabetic complications, including neuropathy. For example, metabolism of fructose 6-phospate by the hexosamine (glucosamine) pathway produces UDP-N-acetylglucosamine, which is highly reactive with proteins, most notably transcription factors, in a process called O-GlcNAcylation. This pathway has been particularly linked with cardiovascular complications of diabetes[265] and a recent study indicates that O-GlcNAcylation regulates remyelination of peripheral neurons after injury so that hyperglycemia-driven abnormal O-GlcNAcylation has the potential to impact nerve structure and function in diabetes[188].
In vascular tissue, glucose-derived diacylglycerol (via glyceraldehyde-3-phophate and phosphatidic acid), is a substrate for protein kinase C β (PKC β) and excess glucose drives elevated PKC β activity which in turn promotes increased vascular permeability and dysfunction[121]. The association of diabetic neuropathy with microvascular disease led to interest in the therapeutic potential of PKC β inhibitors. Following supportive preclinical studies[45], a clinical trial of ruboxistaurin in diabetic patients with neuropathy showed some improvement in skin blood flow, the NTSS-6 questionnaire, which quantified frequency and intensity of aching, burning, prickling, lancinating pain, numbness and allodynia, and in quality of life[48]. However, there was no significant effect on other measures of large and small fiber neuropathy, diminishing enthusiasm in this therapeutic approach.
There is an increasing focus on the role of the intermediate glycation product methylglyoxal in diabetic complications, including neuropathy and neuropathic pain. Methylglyoxal is formed by non-enzymatic dephosphorylation of triose phosphate intermediates of glycolysis (fructose 1,6-biphosphate, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate) and cleared by the glyoxalase pathway. Both excess glycolysis and impaired activity of glyoxalase pathway enzymes may contribute to accumulation of methylglyoxal in diabetes, which reacts with proteins to form AGE’s (see above). Mice overexpressing glyoxylase 1 do not develop indices of degenerative diabetic neuropathy[156]. A gain of function property has also been proposed from studies showing that binding of methylglyoxal to the NaV1.8 voltage gated sodium channel in sensory neurons increases excitability[25], while the potential for methylglyoxal to produce pain in diabetes may also be mediated via agonism of the TRPA1 channel in peripheral nerve[191] and spinal cord[126] and induction of the integrated stress response[21]. Recent studies have confirmed the pro-nociceptive properties of methylglyoxal in humans[93] and elevated plasma methyglyoxal has been identified as a risk factor for neuropathy in patients with type 2 diabetes[13]. Approaches to reducing methylglyoxal levels or blocking downstream consequences are in development.
2.5. Mitochondrial overdrive or idling?
It has been argued that increased substrate-driven glycolysis with subsequent Krebs’ cycle activity and oxidative phosphorylation (OXPHOS) in mitochondria will result in formation of free radicals that may not be adequately buffered so that there is oxidative damage to local structures. This hypothesis was developed in endothelial cells exposed to short periods of hyperglycemia in vitro[246]. There is little evidence that substrate driven “overdrive” of mitochondrial OXPHOS persists and is toxic to nerve. Exposing Schwann cells to acute hyperglycemia causes an increase in free radical buffering capacity and does not increase ROS production[374]. In neurons from diabetic rodents, basal respiration is unchanged and maximal mitochondrial respiratory capacity reduced, not increased[60; 410]. This is accompanied by reduced expression and activity of the mitochondrial proteome[7; 61] and dysregulation of mitochondrial biogenesis[49], with aberrant fission/fusion dynamics[303]. It has therefore been proposed that nutrient excess promotes downregulation of mitochondrial function and increasing reliance on glycolysis[106]. This metabolic shift supports normal neuronal function during hyperglycemia but may limit energy-intensive processes such as dynamic plasticity of peripheral terminals in the epidermis and the capacity to respond to other insults. Efficacy of diverse activators of the AMPK/PGC1α pathway, a nutrient sensor system that regulates mitochondrial activity and dynamics, in restoring mitochondrial respiration and preventing or reversing multiple indices of neuropathy and neuropathic pain in diabetic rodents [4; 43; 300; 402] supports this concept.
There is an emerging appreciation that not all cells within the nervous system utilize glucose for ATP production in a similar manner. For example, neurons of the CNS express high levels of pyruvate dehydrogenase (PDH), which controls entry of pyruvate into Krebs cycle and drives OXPHOS, whereas astrocytes have high levels of lactate dehydrogenase, which converts pyruvate to lactate[202]. Utilization of glycolysis-derived pyruvate in astrocytes is also limited by suppression of PDH complex activity via phosphorylation by pyruvate dehydrogenase kinase (PDK). Consequently, these relatively quiescent glial cells rely primarily on glycolysis-derived ATP and indeed may provide lactate to neurons as energy substrate[173]. In contrast, electrically active neurons keep the PDH gateway open and utilize the more efficient generation of ATP by OXPHOS[136]. The metabolic dependence of neurons on glia is highlighted by the report that selective damage to Schwann cell mitochondria results in a neuropathy with damage to both myelin and axons[370]. Diabetes has been reported to increase PDK expression and activity in peripheral neurons and glia, supporting the idea that during persistent hyperglycemia, neurons suppress entry to OXPHOS and rely on glycolysis for ATP production[278]. Conversely, PDK deficiency attenuated multiple indices of neuropathy in diabetic mice including overexpression of TRPV1 and neuropathic pain. How hyperglycemia impacts the distinct metabolic profiles of neurons and Schwann cells has yet to be widely explored but such studies may provide insight into cell-specific mechanisms of glucotoxicity.
3. IMPAIRED INSULIN SIGNALING
The correlation between glycemic control and neuropathy reported in the DCCT study is not overwhelming, while the follow up study (EDIC) failed to show reversal of established neuropathy upon instigation of improved glycemic control[227; 269]. Similar studies of glycemic control in type 2 diabetic subjects did not replicate the DDCT findings for neuropathy[291; 419] and indices of neuropathy are detected in patients with pre-diabetes (elevated fasting glucose levels and/or impaired glucose tolerance) and metabolic syndrome (representing a combination of risk factors for progression to overt diabetes – central obesity, high triacylglycerides and LDL-cholesterol, low HDL-cholesterol, hypertension and hyperglycemia)[266]. A recent study that sub-divided a cohort of 1105 recently-diagnosed diabetics into 5 groups based on multiple metabolic parameters found that peripheral neuropathy was most prevalent in those with severe insulin-deficient diabetes [407]. Taken together, these observations have driven interest in non-glucotoxic insults that may either act alone or in concert with hyperglycemia[127].
Diabetes can be viewed as a disease of impaired insulin signaling due to insulinopenia and/or insulin resistance. Insulin is structurally similar to the liver-derived peptides insulin-like growth factors 1 and 2 (IGF-1, IGF-2) and shares their growth factor-like properties. Insulin receptors are found on peripheral neurons[35], signal via Akt[129] and activation promotes growth of normal sensory[112] and motor[396] neurons. Conversely, sequestration of local insulin causes peripheral neuropathy in normal rats[35]. Schwann cells also express insulin and IGF-1 receptors and their depletion results in a peripheral neuropathy phenotype with injury to both Schwann cells and axons[135]. Loss of insulin-mediated trophic support represents a primary pathogenic mechanism of diabetic neuropathy that is independent of hyperglycemia. This applies to both type 1 and type 2 diabetes, as studies in type 2 diabetic rodents indicate that insulin signaling is impaired in peripheral nerve[128], so that the nervous system can be considered insulin resistant.
A number of preclinical studies have demonstrated a role for insulin deficiency in diabetic neuropathy and neuropathic pain. Animals injected with STZ at doses that significantly reduce insulin production but do not cause hyperglycemia, go on to develop hyperalgesia in the paw pressure test[294–296]. Insulin-resistant but normoglycemic models of pre-diabetes also develop neuropathy[81] and onset of allodynia to von Frey filaments parallels onset of insulin resistance but precedes onset of hyperglycemia in a model of type 2 diabetes[297]. Conversely, insulin-deficient diabetic rodents treated with trace amounts of systemic insulin for over 1 year to maintain body weight without impacting systemic hyperglycemia showed attenuation of large fiber conduction slowing and did not progress to paw heat hypoalgesia[37]. Most notable are studies in which trace insulin was injected into the footpad[131], infused into the spinal intrathecal space[357] or applied topically to the eye[52] of STZ-diabetic rodents. In each case, treatment prevented functional and structural indices of neuropathy without impacting hyperglycemia indicating that, provided there is adequate insulin-derived trophic support, hyperglycemia is not sufficient to induce neuropathy.
A mechanism of direct insulin action on neurons may involve mitochondria. Insulin increases mitochondrial inner membrane potential when applied direct to sensory neurons derived from normal rats in vitro[150]. Moreover, the reduced inner membrane potential, protein expression and bioenergetics profile of mitochondria from sensory neurons of STZ-diabetic rodents are restored when animals received trace insulin supplementation that also impacted functional and structural indices of diabetic neuropathy without impacting hyperglycemia[3; 61; 150]. This ability to protect against both mitochondrial dysfunction and the neuropathy phenotype also extends to IGF-1[4] with the apparent redundancy perhaps reflecting the importance of the system to cells with consistently elevated energy demands.
Insulin secretion is accompanied by equimolar release of C-peptide, the other product of pro-insulin cleavage. Although initially considered inert, there is evidence that C-peptide has biological actions in a variety of tissues, including peripheral nerve[324], possibly via an insulin-sensitizing action. As C-peptide prevents and reverses multiple indices of neuropathy[411] and neuropathic pain[180] in animal models of type 1 diabetes[171] and showed efficacy against some manifestations of diabetic neuropathy in a clinical trial[379] it should perhaps be aligned in tandem with insulin when considering primary pathogenic mechanisms of diabetic neuropathy and neuropathic pain.
4. DYSLIPIDEMIA
Major risk factors for developing diabetic neuropathy reflect exposure to impaired insulin signaling/hyperglycemia (age, duration of diabetes and long-term glycemic control) followed by vascular dysfunction (hypertension, smoking) and dyslipidemia (obesity, elevated plasma cholesterol and triacylglycerols)[31]. Clinical evidence linking changes in specific plasma lipids to neuropathy is mixed, with studies that both demonstrated, and failed to demonstrate, associations between elevated triacyglycerols, elevated LDL-cholesterol or reduced HDL-cholesterol and neuropathy. Clinical efficacy of lipid lowering agents such as statins and fibrates against neuropathy is promising, but limited[83; 245; 280; 371]. Nevertheless, there is growing interest in how dyslipidemia may damage peripheral nerves to produce degenerative and painful neuropathy that is driven by recent preclinical studies.
Indices of neuropathy and neuropathic pain are detected in rodents fed high fat diet to induce insulin resistance and dyslipidemia but not overt hyperglycemia[81; 82; 254; 373], although the neuropathy phenotype may be species and strain specific[14]. Many of these disorders are prevented or reversed by treating pre-diabetic, type 1 or type 2 diabetic rodents with diets high in n-3 polyunsaturated fatty acids (PUFA’s) to adjust the plasma ratio of n-3:n-6 PUFA [68–70; 200; 318]. Adjusting high fat diets to increase the proportion of monounsaturated fatty acids shows similar efficacy[302] and there is a growing suspicion that long chain saturated fatty acids may therefore be a pro-neuropathic entity in dyslipidemia[248]. Downstream pathogenic mechanisms may include disruption of mitochondrial function and transport in sensory neurons[303]. Dyslipidemia thus joins glucotoxicity and impaired insulin signaling as a potential driver of mitochondrial dysfunction to cause peripheral neuropathy (FIGURE 3).
Figure 3: A convergence of pathogenic mechanisms?
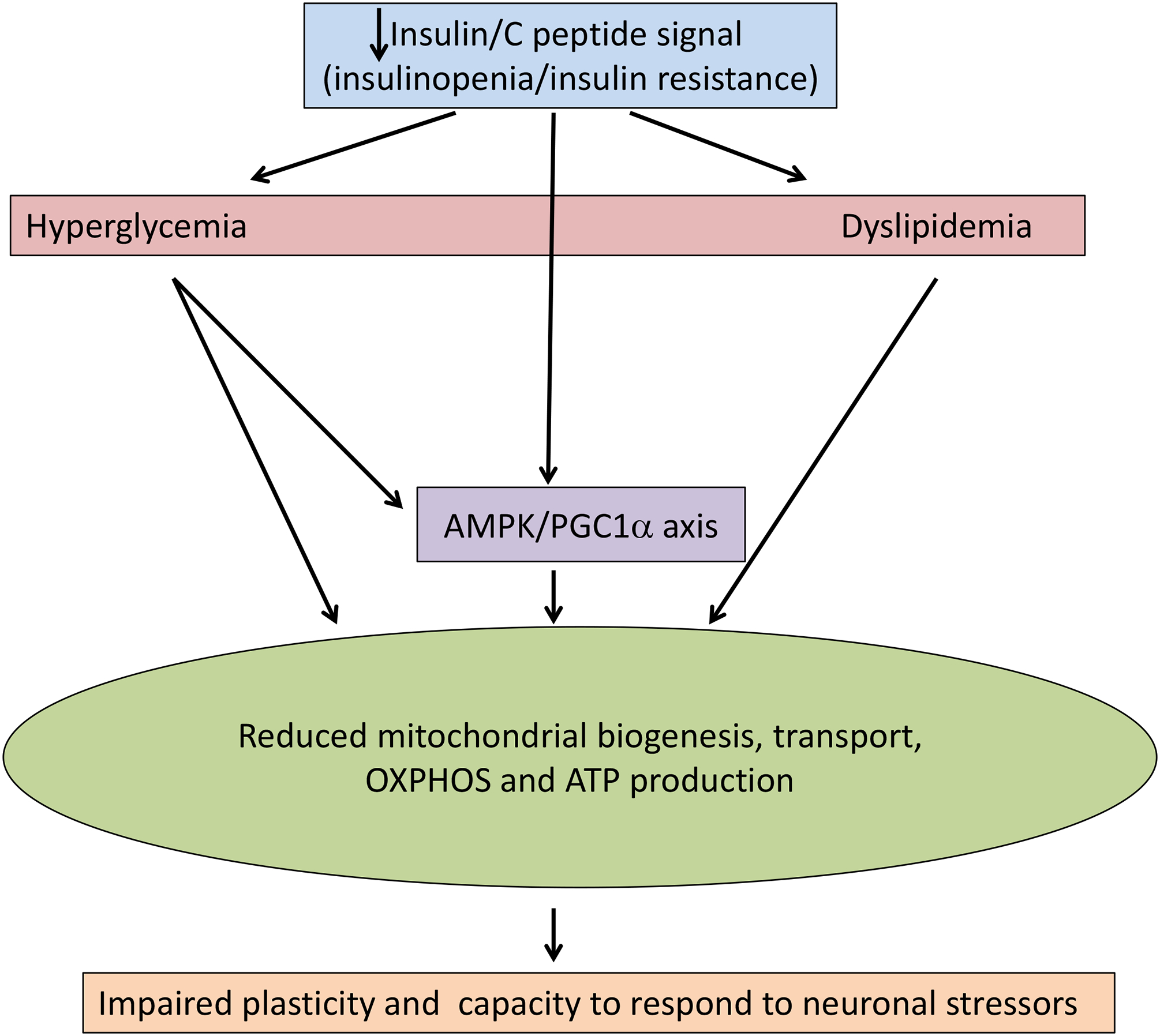
There is accumulating evidence that the primary initiators of pathogenic cascades leading to diabetic neuropathy, impaired insulin signaling, hyperglycemia and dyslipidemia share a common pathway through disruption of mitochondrial bioenergetics.
5. MOLECULAR PATHOLOGY
The preclinical literature on mechanisms of diabetic neuropathy and neuropathic pain is replete with studies describing increased/decreased activity, expression or mRNA of proteins in nerve from diabetic rodents frequently accompanied by data showing that preventing or reversing specific change can impact one or multiple indices of neuropathy and/or neuropathic pain. Studies relating such changes to the primary pathogenic mechanisms discussed above are less frequent. The advent of what are now politely termed unbiased studies has allowed a somewhat less fragmented approach, with technical and bioinformatics advances supporting analysis of large data sets and identification of clusters of differentially regulated genes for pathway analysis.
5.1. Gene expression and regulation:
Initial approaches used oligonucleotide microarrays and target amplification to identify differentially regulated genes in autonomic and sensory ganglia of type 1 diabetic rats [47; 272] and emphasized changes that preceded onset of functional and structural damage. Studies followed characterizing expression profiles of mouse models of type 1[56] and type 2 diabetes[142; 251; 262] and pre-diabetes[249], contrasting profiles of type 1 vs type 2 diabetic mice[130; 152] or diabetic rodents with or without an intervention that corrected indices of neuropathy[78; 84; 221; 401; 409] or specifically painful neuropathy[401]. Expression profiles of nerve biopsies from humans with diabetes have also been reported[153; 210; 229]. Common themes emerging from these studies largely echo the suspected pathogenic mechanisms described above such as glucose and lipid metabolism, oxidative stress and mitochondrial dysfunction. Additional abnormal gene expression clusters implicate impaired cytoskeletal organization, nerve growth and regeneration, inflammatory/immune system activity and signaling through MAPK, JAK/STAT and AMPK pathways. These studies have also driven the growing suspicion that neuropathy in type1 and type 2 diabetes has many molecular dissimilarities and the recent heightened interest in dyslipidemia as a primary pathogenic mechanism of diabetic neuropathy[101].
Both the production and degradation of mRNA and its subsequent translation are modified by interactions between the mRNA and the RNA-induced silencing complex (RISC) which consists of assorted proteins (the RNAse DICER, argonaute family proteins etc) and single stranded non-coding microRNA’s (miRNA). This mechanism of gene silencing adds additional layers of control and complexity, particularly as each gene can be silenced by many miRNA’s and each miRNA can target multiple genes. Polymorphisms of specific miRNA’s are associated with susceptibility to peripheral and autonomic neuropathy in patients with type 2 diabetes[63; 64] and miRNA have attracted recent interest both as potential contributors to the pathogenesis of diabetic neuropathy and as sites of therapeutic intervention[326]. Examples are shown in TABLE 2 [5; 53; 56; 105; 132; 148; 149; 164; 185; 215; 319; 381; 384; 394; 405; 412]. While changes in miRNA are frequently linked with such downstream pathogenic mechanisms of diabetic neuropathy as inflammation, oxidative stress and impaired neuronal growth and regeneration the primary events that trigger disruption of miRNA expression have yet to be defined. Manipulation of miRNA is an emerging therapeutic approach in which identifying nerve-specific targets or delivery systems will be critical for ensuring appropriate safety profiles that will allow translation to clinical use.
TABLE 2:
miRNA implicated in the pathogenesis of diabetic neuropathy
| miRNA species | Impact of diabetes | Model (tissue) | Effective therapeutic manipulations | Representative publications |
|---|---|---|---|---|
| multiple | Multiple upregulated | STZ-mouse (DRG) | Mimics/KO impact multiple indices of degenerative and painful neuropathy | [57] |
| Multiple downregulated | ||||
| multiple | Multiple upregulated | STZ-rat (DRG) | Not done | [132] |
| Multiple downregulated | ||||
| multiple | Multiple upregulated | STZ-rat (nerve) | Some expression changes normalized by taurine | [319] |
| Multiple downregulated | ||||
| miR-25 | downregulated | db/db-mouse (nerve) | Precursor reduced inflammation markers | [412] |
| miR-29c | upregulated | db/db mouse (DRG, nerve) | KO improved neurite growth in vitro | [164] |
| miR-34c | upregulated | STZ-mouse (trigeminal) | Antagomir improved corneal nerve growth | [148] |
| miR-96 | downregulated | HFD/STZ-rat (nerve) | Exercise increased miR, reduced Nav1.3 and thermal pain | [5] |
| miR-106a | downregulated | STZ-mouse (DRG) | Not done | [394] |
| miR-146a | upregulated | STZ-rat (nerve) | Not done | [405] |
| db/db-mouse (nerve) | Mimics improved neuropathy/pain | [215] | ||
| downregulated | STZ-rat (nerve) | Not done | [105] | |
| db/db-mouse (nerve) | Inducer improved neuropathy/pain | [381] | ||
| miR-155 | downregulated | STZ-rat (nerve) | Not done | [185] |
| upregulated | STZ-rat (nerve | Antagomir improved neuropathy | [53] | |
| miR-181a | upregulated | STZ-mouse (trigeminal) | Antagomir improved corneal nerve growth | [149] |
| miR-182 | downregulated | STZ-mouse (trigeminal) | Agomir increased corneal nerve density | [384] |
5.2. Structural proteins:
Early interest in dysfunctional axonal transport as a cause of distal degenerative neuropathy promoted interest in proteins of the axonal cytoarchitecture, with tubulin, neurofilament sub-units and associated proteins variously reported as being over or under expressed, glycated, glycosylated, polymerized and/or phosphorylated[111; 230; 311]. Proteins of the extracellular matrix also show post-translational modifications[10; 95]. Potential structural consequences include reduced axonal caliber and associated large fiber conduction slowing [238] and delayed axonal regrowth following focal lesions as reported in diabetic rodents[172] and humans[186], although fast axonal transport velocities are unchanged[1]. Altered expression or post-translational modification of myelin structural proteins such as myelin basic protein (MBP), myelin associated glycoprotein (MAG), myelin protein 0 (P0 or MPZ) and peripheral myelin protein 22 (PMP22) are also reported[102; 277; 299]. Unfortunately, appropriately fixed nerve of diabetic rodents does not display overt myelin pathology similar to that reported in animals were these proteins are selectively ablated.
5.3. Trophic factors:
The growing repertoire of trophic factors and their receptors that modulate nerve phenotype and function has been frequently accompanied by the discovery of reduced mRNA, protein, receptor and/or signaling in peripheral neurons, Schwann cells or target organs of diabetic rodents and thereafter by reports that delivery of the trophic factor gene or protein, mimetics, inducers or agonists corrects indices of neuropathy in the same. Examples include NGF[109; 141], CNTF[40; 42; 240], NT-3[110; 238], IGF-1[4; 154; 414] GDNF[62; 212], bFGF[244], HGF[181], G-CSF[187], MMP2[10], hedgehog proteins[38]. There are also reports of diabetes-induced increased expression of trophic factors, as occurs with BDNF[108] and both HIF-1 and its target gene VEGF[50; 307], that are attributed to responses to injury or compensation for loss of other trophic support systems. VEGF delivery improves indices of neuropathy in diabetic rodents [151; 292; 310], while the role of BDNF is more complex. Acute spinal delivery of BDNF causes tactile allodynia in normal rats and sequestration of endogenous spinal BDNF alleviates tactile allodynia and restores H-wave RDD (see above) in diabetic rats[206]. In contrast, chronic spinal delivery of BDNF to diabetic rats alleviated hyperalgesia[207]. In some cases, altered neurotrophic support has been linked to downstream consequences of hyperglycemia. For example, hyperglycemia-driven increased flux though the polyol pathway leads to reduced nerve levels of the Schwann cell derived factors NGF[256] and CNTF[237] and ARI treatment prevents diabetes-induced elevated Trk-C receptor mRNA expression [322]. However, the pathogenesis of most neurotrophic factor deficits in diabetic nerve remains unknown and clinical trials using trophic factors have not been promising[16; 103; 391], with the exception of early HGF trials against neuropathic pain[6] and a neurotrophic erythropoietin analog against pain and corneal nerve loss[33].
5.4. Cytokines and inflammatory pathways:
There is little evidence from human studies that diabetic neuropathy represents a typical inflammatory neuropathy. Chronic inflammatory demyelinating polyneuropathy occurs in diabetic patients but is readily distinguished from diabetic symmetrical polyneuropathy[279]. Animal models of diabetes also lack marked inflammatory infiltrates, although there are reports of transient increases in the number and activation of peripheral nerve macrophages [67; 247] and spinal microglia[363]. The absence of large increases in inflammatory cell numbers is not surprising due to the physical constraints on tissue expansion imposed by the epineurium and spinal column. Glial cells of the PNS and CNS play many roles associated with peripheral inflammatory cells following nerve injury, including release of cytokines and chemokines[92] and clearance of myelin debris[162]. As recently reviewed in detail elsewhere [289] this neuroinflammatory system is increasing recognized as being dysregulated in diabetes.
There are multiple reports of changes in both pro- and anti-inflammatory cytokines and chemokines in the nervous system of diabetic rodents. The most widely studied are increases in the pro-inflammatory cytokines IL-1β, IL-6, TNFα and interferon-γ, [420] along with changes to downstream transcription regulators such as NF-κB and Nrf2[197]. Expression of bradykinin B1 and B2 receptors by microglia and neurons is also increased in the spinal cord of diabetic rodents[46; 345] and receptor antagonists alleviate indices of pain[345]. Pathogenic consequences include induction of enzymes such as COX-2[182; 284] and isoforms of nitric oxide synthase[417] that can drive tissue damage via ischemic hypoxia and oxidative/nitrosative stress thereby contributing to both degenerative neuropathy and initiation or amplification of neuropathic pain[116]. For example, induction of COX-2 and subsequent release of prostaglandins from spinal oligodendrocytes[284] and release of pro-inflammatory cytokines[345] and BDNF[241] from activated microglia have been linked to spinally-mediated pain in diabetic rodents. Increased chemokine/receptor signaling, including CCL1/CCR8[422], CXCL12/CXCR4[160], CXCL13/CXCR5[214] and others[421] is also linked to neuropathic pain in diabetic rodents. The primary pathogenesis of many of the reported changes to the neuro-inflammatory system remains to be determined, although hyperglycemia driven flux through the polyol pathway may be involved in some aspects[157; 284; 336]. Therapeutic approaches around manipulation of the neuro-inflammatory system tested in diabetic rodents range from microglial inactivators[241; 420], COX inhibitors[268; 284], TNFα inactivators[399] or inhibitors[90], overexpression of anti-inflammatory cytokines[349], antagonists to pro-inflammatory cytokines[139], chemokine neutralizing antibodies[293] and chemokine receptor antagonists[176; 232]. Efficacy of stem cells [138; 387] and natural products [78; 167; 255] against aspects of diabetic neuropathy may also be at least partly due to their anti-inflammatory properties. To date, clinical trials have not been successful[176].
5.5. Death and Survival Pathways:
Dysregulation of cytoplasmic calcium has been implicated in many neurodegenerative diseases, given its role in triggering autophagic, necrotic and apoptotic pathways[51; 124]. Steady state cytoplasmic calcium concentrations are regulated by pumps located in mitochondria and endoplasmic reticulum and these organelles serve as calcium stores. Steady state cytoplasmic calcium concentrations are increased in sensory neurons from diabetic rodents[369] and this is associated with impaired calcium reuptake into endoplasmic reticulum by the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) pump[413]. Subsequent depletion of calcium in the sarcoplasmic reticulum produces ER stress which can precipitate cell death[308] and this mechanism has been integrated into schema of potential pathogenic mechanisms of diabetic neuropathy[250]. It is not yet known whether mitochondrial calcium pumps, such as the mitochondrial calcium uniporter (MCU) complex and voltage-dependent anion channel (VDAC) are dysfunctional in diabetes.
Reports describing marked expression of components of apoptotic death pathways in the peripheral nerve of short-term diabetic rodents[304; 309] were initially difficult to reconcile with the slowly evolving loss of neurons and axons in these models and clinical descriptions of a distal degenerative neuropathy. Later work reported that increased caspase-3 in nerve was not associated with structural features of apoptosis such as nuclear fragmentation and that DNA-repair mechanisms were activated, suggesting that peripheral nerve utilizes endogenous defence mechanisms to block progression from caspase-3 activation to apoptosis[57]. Over-activity of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) has itself been linked to a mechanism of nerve injury[219]. Elevated PARP and other survival and repair markers such as heat shock protein 27 (HSP27)[282; 418] and growth associated protein 43 (GAP43)[131] in the nerve of diabetic rodents has encouraged the view that peripheral nerve is exposed to chronic stress arising from one or more of mechanisms described above but can largely tolerate and repair metabolic injuries, paralleling to the way that it survives and repairs physical injury. Therapeutic approaches that strengthen endogenous survival and repair mechanisms include overexpression of HSP 27[193] and inhibition of HSP90 to induce HSP70, a molecular chaperone protein with multiple neuroprotective properties including protecting mitochondrial function and reducing inflammation and oxidative stress[221]. HSP90 inhibitors reverse multiple indices of neuropathy in diabetic rodents and are currently in clinical development[104]. Conversely, the emerging appreciation that there are endogenous systems that constrain nerve growth has provided opportunity to intervene and thereby promote nerve growth and regeneration pathways[94]. For example, the tumor suppressor molecule PTEN (phosphatase and tensin homolog deleted on chromosome 10) inhibits the PI3K-pAkt neuronal growth pathway[195] and is upregulated in sensory neurons from diabetic animals while knockdown of PTEN improved the otherwise impaired nerve regeneration following crush injury in STZ-diabetic mice[327].
5.6. Membrane pumps:
NCV slowing in short-term diabetic rodents that lack overt damage to axons or myelin led to interest in changes to nodal ion pumps that facilitate saltatory conduction in myelinated fibers. Chief of these was the Na+/K+ ATPase, given its role in maintaining and restoring resting membrane potential. Reduced maximal pump activity in membrane fragments associated with reduced protein expression was widely studied as a potential cause of NCV slowing and is downstream of hyperglycemia driven polyol pathway activity[125]. However, Na+/K+ ATPase pump activity is not impaired in intact endoneurial preparations from diabetic rodents[217] so the physiological relevance is unclear. Increased expression and activity of the Na+/H+ pump has also been reported in nerve of diabetic rodents and inhibition of the pump reversed functional and structural indices of neuropathy and neuropathic pain[220]. Overactivity of this pump increases cytoplasmic pH, glucose uptake and glycolysis, thereby having the potential to trigger multiple pathogenic mechanisms.
In the spinal cord, reduced expression of the potassium-chloride co-transporter 2 (KCC2) pump, which maintains the chloride gradient across neuronal membranes, has been linked with loss of GABAergic inhibitory function and neuropathic pain in diabetic rats[205]. KCC2 expression is suppressed by the neurotrophic factor BDNF and increased BDNF levels in central projections of primary afferents in diabetic rats suggest that the primary lesion may be of peripheral origin[205], although it has also been linked to activated spinal microglia[241]. The electrophysiological consequence of disrupted spinal GABAergic inhibitory tone in diabetic rodents is loss of rate dependent depression (RDD) of the H wave[206], which is secondary to impaired insulin signaling rather than hyperglycemia[226]. Loss of RDD may serve as a biomarker for identifying a sub-set of diabetic humans in whom painful neuropathy includes a contribution from spinal disinhibition[226].
5.7. TRP channels:
Transient receptor potential (TRP) channels are a family of non-selective cation permeable channels that transduce diverse extracellular stimuli into acute and chronic neuronal responses via influx of calcium[174]. TRPA, TRPV and TRPM family members are modulated by endocannabinoids, which may contribute to the analgesic properties of these substances[243]. There is substantial preclinical evidence that dysregulation or dysfunction of TRP channels may contribute to neuropathic pain in diabetes.
The TRPV1 channel, known for transducing the burning sensation of capsaicin, is activated by a range of physiological and pathological stimuli including heat, low pH, pro-inflammatory molecules and endocannabiniods. TRPV1 currents are also enhanced by insulin and IGF-1[368] and by the TRPM8 receptor[260]. An initial report [145] indicated that membrane bound TRPV1 protein is increased in DRG from diabetic rats, along with the channel phosphorylation state and both capsaicin and proton activated currents while TRPV1 protein expression increased in large sensory neurons and decreased in small sensory neurons. The pattern of TRPV1 protein expression and whole cell currents in the DRG and spinal cord of diabetic rodents paralleled progression from heat hyperalgesia to hypoalgesia[261] and increased TRPV1 expression at peripheral and central terminals of primary afferents has been implicated in allodynia to von Frey filaments[74]. Agents that reduce TRPV1 expression, antagonize TRPV1 or ablate it also alleviate thermal hyperalgesia and tactile allodynia in diabetic rats[17; 209]. Involvement in diabetic neuropathy beyond indices of pain is suggested by a report that TRPV1 agonists given to normal mice produce multiple indices of small fiber neuropathy including IENF loss[204]. Upstream events that may drive TRPV1-mediated pain include increased insulin, RAGE and protein kinase C activity[24; 368] and hypoxia[287]. Little is known about other TRPV family members, although a recent study reported that a selective TRPV4 channel antagonist blocked mechanical, but not cold, allodynia in diabetic mice[87].
Early indications of a role for the irritant sensing TRPA1 channel in diabetes-induced hyperalgesia came from studies with TRPA1 antagonists that alleviated allodynia to von Frey filaments and mechanical hyperalgesia[388; 389]. Cold allodynia in diabetic mice has also been attributed to TRPA1 activity[143]. Diabetes enhances channel activity without inducing TRPA1 protein expression in DRG of diabetic animals[288]. As discussed above, a pathogenic mechanism linking hyperglycemia with TRPA1 channel activation via methylglyoxal binding to the channel has been proposed[100; 192] and there is also a recent report linking TRPA1-mediated hyperalgesia in diabetic rodents to local hydrogen sulfide[288].
Protein for the cold/menthol sensing TRPM8 channel is elevated in the DRG of STZ-diabetic rats with concurrent cold allodynia[403]. Conversely, agonist activated TRPM8 currents are decreased in DRG of STZ-diabetic mice[260] and in normal DRG cells following exposure to methylglyoxal[66]. While this does not appear to be consistent with a role for methylglyoxal in diabetes-evoked pain (see above), it has been argued that loss of TRPM8 activity enhances other TRP channel activities associated with neuropathic pain[260]. There is clearly room for additional studies in this area.
5.8. Voltage gated calcium channels:
Gabapentin and pregabalin, widely used to treat painful neuropathy in diabetic patients[9], target the α2δ–1 sub-unit of voltage gated Ca2 channels[22]. This sub-unit regulates the trafficking and activation kinetics of pore-forming α1 sub-units and thus surface expression and activity of voltage gated Ca2 channels ([264]). The plasmalemma of sensory neurons contains multiple voltage-gated Ca2+ channels, including L-type (Cav1.2 and Cav 1.3), N-type (Cav 2.2), R type (Cav 2.3) and T-type (Cav 3.2 and Cav 3.3). Of these, the most extensively studied in the context of diabetic neuropathy is the T-type (Cav 3.2) calcium channel [354]. This channel shows altered kinetics due to post-translational modification by glycosylation under hyperglycemic conditions accompanied by enhanced gene expression and glucose-regulated trafficking[203; 390]. A role in neuropathic pain is suggested by reports that diverse interventions that target the Cav3.2 channel reverse allodynia to von Frey filaments and thermal hyperalgesia in diabetic rodents[117; 201; 233; 253]. Clinical trials of T-type calcium channel antagonists, including one that used the innovative design of using microneurography to pre-select diabetic subjects with both pain and spontaneously active C fibers[317] have yet to show efficacy[184; 415].
Increased mRNA for sub-units of the P/Q, but not N, type calcium channels have been reported in DRG of STZ-diabetic mice[365] and a P/Q and R type channel antagonist alleviated allodyina to von Frey filaments in STZ-diabetic rats and mice[77]. Reports of increased L-type currents in both primary afferent and dorsal horn neurons of STZ-diabetic rats[194; 376] have promoted studies of efficacy of the L-type channel antagonists against hyperalgesia[323] while efficacy against other indices of peripheral neuropathy were largely attributed to indirect effects via improved blood flow[28].
5.9: Voltage gated potassium channels and HCN channels:
Given the importance of potassium channels in regulating axonal excitability there are relatively few studies implicating them in the pathogenesis of diabetic neuropathy and neuropathic pain. Expression of the voltage-gated Kv channel subunits Kv1.2 and Kv1.6, but not Kv1.1, was reduced in small neuronal cell bodies of the DRG in STZ-diabetic rats coincident with reduced K+ currents and these changes were linked to enhanced C-fiber excitability and hyperalgesia[382]. Most recently, voltage gated Kv7 (KCNQ) channels, which produce a slow non-inactivating outward K+ current also called the M current due to its modulation by muscarinic antagonists[392], have been examined. There was decreased mRNA and protein for the Kv7.2, Kv7.3 and Kv7.5 channels in the DRG of STZ-diabetic rats accompanied by reduced M current density and increased neuronal excitability[406]. A Kv7 channel activator reduced neuronal excitability and alleviated allodynia to von Frey filaments and thermal hyperalgesia
Hyperpolarization-activated and cyclic nucleotide-gated channels (HCN1–4) are a distinct category of voltage-gated ion channels whose threshold potentials are regulated by cyclic nucleotides and that have been implicated in neuropathic pain states[361]. Inhibition of HCN’s1–4 or ablation of HCN2 alleviated allodynia to von Frey filaments but not thermal hypoalgesia in diabetic mice[360]. Expression of HCN1–4 protein was not altered in the peripheral nerve of diabetic mice and it was speculated that a measured increased in intracellular cAMP might activate HCN2 and thus increase primary afferent firing.
5.10. Voltage gated sodium channels:
Voltage-gated sodium channels (VGSC: NaV1.1–1.9) are critical regulators of neuronal excitability and the discovery of gain of function mutations to Nav1.7, NAv1.8 and Nav1.9 in human small fiber “channelopathy” pain states [333] has focused interest on the potential role of VGSC in painful diabetic neuropathy. Diabetes alters currents and protein expression of a variety of VGSC: Nav1.8 protein expression is consistently reported as being decreased [73; 146; 257] with a parallel increased phosphorylation or post-translational modification by methylglyoxal (see above) considered indicative of activation[25; 147]. Others, such as Nav’s 1.1, 1.2, 1.3, 1.7 and 1.9 show increased protein expression[73; 146; 147; 328], while there is disagreement over the fate of Nav1.6 [73; 146; 286]. Interestingly, around 10% of a cohort of patients with painful diabetic neuropathy expressed rare Nav1.7 variants, some of which were gain of function variants[29]. These patients tended to report more severe burning pain and increased pressure sensitivity. A gain of function variant to the β sub-unit of VGSC has also been identified in a patient with painful diabetic neuropathy[11] while a recent genome wide association study of type 2 diabetics with or without pain has drawn attention to Nav1,2 ([347]).
There have been a number of studies that have manipulated VGSC activity. Antagonists of overexpressed VGSC have been studied for their ability to block indices of neuropathic pain in both preclinical and clinical studies[353]. In diabetic rodents indices of neuropathic pain have been reduced by the non-selective VGSC blockers lidocaine[41] and mexiletine [400], by selective knockdown of NAv1.3,[346], by induction of miR-96 to reduce Nav1.3 expression[5] and by blockers of Nav1.7[385] and Nav1.8[367]. Topical lidocaine has shown efficacy against painful diabetic neuropathy [393] and is used off-label[9]. A clinical trial of a Nav1.7 blocker in subjects with painful diabetic neuropathy showed only minor effects[228], perhaps reflecting the cohort of subjects in whom pain could be due to a variety of diabetes-related mechanisms. While the pathogenesis of altered expression and/or function of VGSC is not clear, other than relatively rare gain of function mutations and a link to hyperglycemia via increased glycolysis and methlyglyoxal for modification of Nav1.8 activity[25], there is an interesting suggestion that mutations in NaV1.7 may be a primary cause of both painful diabetic neuropathy and diabetes itself due to the location of this channel on both primary sensory neurons and pancreatic β cells[144].
5.11. Neurotransmitters and receptors:
Purinergic P2X receptors are ligand (ATP)-gated non-selective cation channels location on neurons, Schwann cells and microglia[36]. There is increased P2X2R and P2X3R expression and current density in DRG of STZ-diabetic rats and mice and increased P2X4R expression by satellite glial cells[348]. Increased gene expression of P2X3R in diabetic rats has been linked to demethylation of the p2x3r gene[408]. Involvement of P2XR in pain is suggested by reports that peripheral and intrathecal delivery of antagonists alleviated tactile and thermal hyperalgesia in diabetic animals[234; 348; 395; 408]. Increased activity of receptors located at synapses and on adjacent microglia has the potential to enhance primary afferent input and spinal sensitization of sensory processing.
The spinal cord of diabetic rodents shows increased glutamate and substance P ligand binding [179; 208] and increased mRNA for subunits of glutamatergic NMDA and AMPA receptors [355]. The NMDA NR1 and NR2B subunits also show and increased phosphorylation (activation) [80; 155] secondary to elevated protein tyrosine phosphatase activity[342]. NR2B expression is also increased in a model of pre-diabetes[342]. These findings are consistent with efficacy of spinally delivered NK-1 and NMDA antagonists against allodynia and hyperalgesia in diabetic rodents [39; 71; 79; 298]. Unfortunately, side-effect free targeting of spinal excitatory receptors as a strategy to treat painful diabetic neuropathy has been largely unsuccessful to date.
Of the spinal inhibitory receptors, GABAA expression is unchanged by diabetes[169] but inhibitory function is diminished secondary to reduced KCC2 activity (see above), while GABAB expression is reduced[383]. Both basal and stimulus-evoked spinal GABA levels are increased in diabetic rats[225] and may contribute to pain via dysfunctional GABAA receptors, as GABAA antagonists alleviate allodynia and hyperalgesia[169] whereas activation of GABAB receptors shows the expected inhibitory effects[213]. Multiple serotoninergic receptor agonists have been shown to alleviate indices of pain in animal models of diabetes, including agonists or indirect activators of 5-HT1A,[163] 5HT2A/C[236] and 5HT7[364] receptors. While spinal expression of 5HT2A receptors is unchanged by diabetes[267], efficacy of duloxetine, a selective serotonin reuptake inhibitor approved for use against pain in diabetic patients[9], is via activation of these receptors in the spinal cord of diabetic rats[236]. Muscarinic M2 receptors are increased in spinal cord of diabetic rats[55] and facilitate the antinociceptive actions of cholinergic agonists operating via GABAB receptors[54].
While increased expression and/or activity of ion channels and receptors may lead to hypersensitive or destabilized primary afferents and inappropriate electrical activity, the contribution of peripherally drive to pain in diabetes may be offset by progression to a degenerative neuropathy phenotype. No matter how electrically active a primary afferent becomes, it is effectively silent if it cannot release adequate neurotransmitter at the spinal dorsal horn. For example, in diabetic rats there is an early reduction in the synthesis[89], transport[356] and stimulus-evoked spinal release of both neuropeptide[44; 119] and amino acid[225] excitatory neurotransmitters that is concurrent with enhanced pain-associated behavior in the same animals. There is also a progression from increased, to loss of, synaptic markers in the spinal cord of diabetic rats[166; 211]. Pain generator sites may evolve over time, with initial pain driven by a hypersensitive or hyperactive periphery but progressing to spinal and CNS generator/maintenance sites as primary afferent input fades with first neurochemical, then physical, degeneration. Longitudinal clinical studies in subjects with diabetic neuropathy, using such techniques as microneurography[317], RDD[226] and MRI[314] to track activity of generator sites over time may be of value.
6. SUMMARY
A PubMed search at 7pm PST on 14th February 2020 using the search phrase “diabetic neuropathy treatment” returned 17,238 hits – a lot of words for a condition with no approved therapy. It is challenging to reconcile the plethora of biochemical and molecular changes in the nervous system of diabetic rodents described above with their mildly dysfunctional neuropathy phenotype and limited nerve pathology. It is also remarkable that so many highly selective interventions against specific pathways are completely effective in preventing or reversing indices of neuropathy and pain, despite presumably continued operation of multiple other documented pathways. The sheer volume of effective interventions must raise concerns about the relevance of preclinical models and assays to the clinical condition. Experimental models of diabetic neuropathy are perhaps best viewed as hypothesis-generating tools that offer a veritable cornucopia of potential pathogenic events and plausible targets for therapeutic intervention against neurodegeneration and pain (Figure 4). Relatively few of the biochemical and molecular changes described above have been confirmed in humans and, when they are, it is not easy to determine whether altered protein expression and/or activity has physiological and pathological consequences or is itself a consequence of neuropathy.
Figure 4: A plethora of potential pain precipitating pathways?
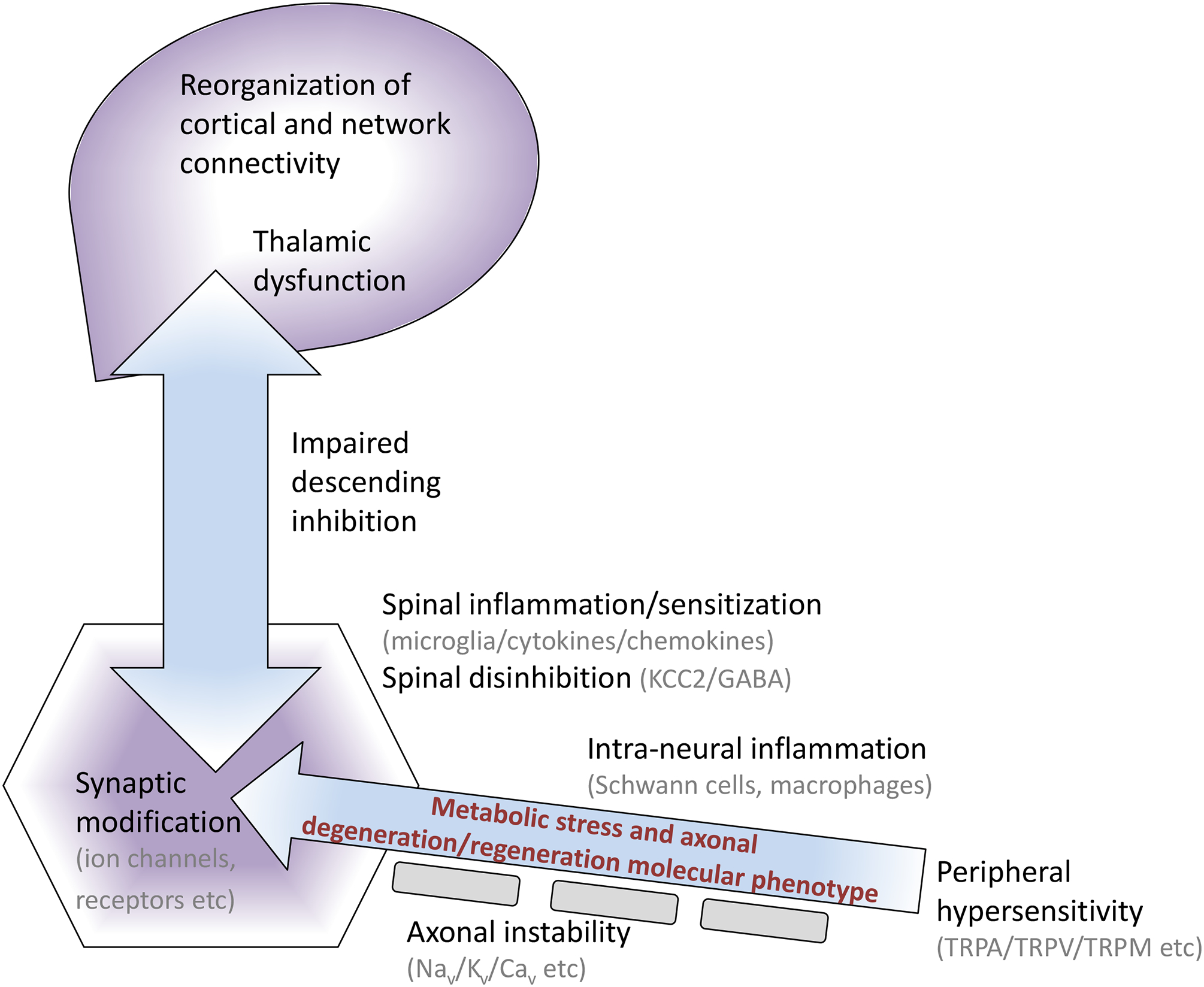
Animal models of diabetic neuropathy develop molecular and functional disorders throughout the neuraxis that have the potential to generate or amplify pain and thus serve as targets for therapeutic intervention. The majority of these disorders have yet to be validated in the human condition.
The challenge before us remains the same as it was over half a century ago following the advent of aldose reductase inhibitors[190] - namely translating mechanisms and interventions identified in preclinical models of diabetes into viable therapies to prevent and reverse diabetic neuropathy and neuropathic pain. Agents that appear promising in preclinical studies have consistently failed in clinical trials against degenerative diabetic neuropathy and there has been plenty of subsequent finger pointing, from allegations of poor drug design and unrepresentative animal models to flawed clinical trial designs and outcome measures[107; 222]. Encouragingly, recognition and analysis of prior failings [222] has prompted development of focused in vitro models to aid mechanistic and drug screening studies[118], animal models that more closely resemble the human diabetic condition [404], refinement of clinical protocols [32] and introduction of outcome measures such as nerve fiber density in the skin and cornea that highlight small fiber neuropathy[222; 276]. In contrast to degenerative neuropathy, there are a number of therapies approved by regulatory agencies to alleviate pain in diabetes, and others that are used off-label [9; 18]. However, it is notable that none were developed to target diabetes-specific mechanisms while efficacy is both unpredictable and restricted to small sub-sets of patients (NNT>5–10) [113]. Refinement of models of neuropathic pain and of assays towards those that incorporate more complex cognitive functions may improve the predictive value of preclinical studies[88] and have begun to be used in models of diabetes[378]. There is also a growing appreciation that pain in diabetic patients falls into distinct sub-types [20], potentially reflecting different dominant pathogenic mechanisms and thus responsiveness to targeted therapeutic interventions. Drugs not statistically effective against pain in an unrefined cohort have been shown to be effective in a sub-group defined by pain mechanism[86], and clinical trial designs are beginning to incorporate patient stratification based on the likely mechanism of both pain generation and the agent under investigation[317]. Together, these advances may allow the identification and development of translatable therapies that are tested against the mechanistically-appropriate population and open an encouraging gateway into the world of personalized medicine.
ACKNOWLEDGEMENTS
My thanks to Dr. Corinne Jolivalt for helpful discussions and appropriately brutal editing.
Supported by NIH award DK104512.
Footnotes
Conflict of Interest Statement: Nigel Calcutt is a paid consultant for Torray Industries Inc. and has equity interest in WinSanTor, Inc. and Cersci Inc., these being commercial entities that may potentially benefit from research and insights in this area. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
REFERENCES
- [1].Abbate SL, Atkinson MB, Breuer AC. Amount and speed of fast axonal transport in diabetes. Diabetes 1991;40(1):111–117. [DOI] [PubMed] [Google Scholar]
- [2].Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34(10):2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aghanoori MR, Smith DR, Roy Chowdhury S, Sabbir MG, Calcutt NA, Fernyhough P. Insulin prevents aberrant mitochondrial phenotype in sensory neurons of type 1 diabetic rats. Exp Neurol 2017;297:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aghanoori MR, Smith DR, Shariati-Ievari S, Ajisebutu A, Nguyen A, Desmond F, Jesus CHA, Zhou X, Calcutt NA, Aliani M, Fernyhough P. Insulin-like growth factor-1 activates AMPK to augment mitochondrial function and correct neuronal metabolism in sensory neurons in type 1 diabetes. Mol Metab 2019;20:149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aghdam AM, Shahabi P, Karimi-Sales E, Ghiasi R, Sadigh-Eteghad S, Mahmoudi J, Alipour MR. Swimming Exercise Induced Reversed Expression of miR-96 and Its Target Gene NaV1.3 in Diabetic Peripheral Neuropathy in Rats. Chin J Physiol 2018;61(2):124–129. [DOI] [PubMed] [Google Scholar]
- [6].Ajroud-Driss S, Christiansen M, Allen JA, Kessler JA. Phase 1/2 open-label dose-escalation study of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with painful diabetic peripheral neuropathy. Mol Ther 2013;21(6):1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Akude E, Zherebitskaya E, Chowdhury SK, Smith DR, Dobrowsky RT, Fernyhough P. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes 2011;60(1):288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alam U, Jeziorska M, Petropoulos IN, Asghar O, Fadavi H, Ponirakis G, Marshall A, Tavakoli M, Boulton AJM, Efron N, Malik RA. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One 2017;12(7):e0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alam U, Sloan G, Tesfaye S. Treating Pain in Diabetic Neuropathy: Current and Developmental Drugs. Drugs 2020. [DOI] [PubMed] [Google Scholar]
- [10].Ali S, Driscoll HE, Newton VL, Gardiner NJ. Matrix metalloproteinase-2 is downregulated in sciatic nerve by streptozotocin induced diabetes and/or treatment with minocycline: Implications for nerve regeneration. Exp Neurol 2014;261:654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alsaloum M, Estacion M, Almomani R, Gerrits MM, Bonhof GJ, Ziegler D, Malik R, Ferdousi M, Lauria G, Merkies IS, Faber CG, Dib-Hajj S, Waxman SG, Propane Study G. A gain-of-function sodium channel beta2-subunit mutation in painful diabetic neuropathy. Mol Pain 2019;15:1744806919849802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amani S, Fatima S. Glycation with Fructose: The Bitter Side of Nature’s Own Sweetner. Curr Diabetes Rev 2020. [DOI] [PubMed] [Google Scholar]
- [13].Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, Jensen TM, Finnerup NB, Jensen TS, Lauritzen T, Feldman EL, Callaghan BC, Charles M. Risk Factors for Incident Diabetic Polyneuropathy in a Cohort With Screen-Detected Type 2 Diabetes Followed for 13 Years: ADDITION-Denmark. Diabetes Care 2018;41(5):1068–1075. [DOI] [PubMed] [Google Scholar]
- [14].Anderson NJ, King MR, Delbruck L, Jolivalt CG. Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice. Dis Model Mech 2014;7(6):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Andersson DA, Filipovic MR, Gentry C, Eberhardt M, Vastani N, Leffler A, Reeh P, Bevan S. Streptozotocin Stimulates the Ion Channel TRPA1 Directly: INVOLVEMENT OF PEROXYNITRITE. J Biol Chem 2015;290(24):15185–15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 2002;50:393–413. [DOI] [PubMed] [Google Scholar]
- [17].Araya EI, Nones CFM, Ferreira LEN, Kopruszinski CM, Cunha JMD, Chichorro JG. Role of peripheral and central TRPV1 receptors in facial heat hyperalgesia in streptozotocin-induced diabetic rats. Brain Res 2017;1670:146–155. [DOI] [PubMed] [Google Scholar]
- [18].Ardeleanu V, Toma A, Pafili K, Papanas N, Motofei I, Diaconu CC, Rizzo M, Stoian AP. Current Pharmacological Treatment of Painful Diabetic Neuropathy: A Narrative Review. Medicina (Kaunas) 2020;56(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ashrafi G, Wu Z, Farrell RJ, Ryan TA. GLUT4 Mobilization Supports Energetic Demands of Active Synapses. Neuron 2017;93(3):606–615 e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017;158(2):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barragan-Iglesias P, Kuhn J, Vidal-Cantu GC, Salinas-Abarca AB, Granados-Soto V, Dussor GO, Campbell ZT, Price TJ. Activation of the integrated stress response in nociceptors drives methylglyoxal-induced pain. Pain 2019;160(1):160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009;29(13):4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem 2008;110(5):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bestall SM, Hulse RP, Blackley Z, Swift M, Ved N, Paton K, Beazley-Long N, Bates DO, Donaldson LF. Sensory neuronal sensitisation occurs through HMGB-1-RAGE and TRPV1 in high-glucose conditions. J Cell Sci 2018;131(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnolzer M, Lasitschka F, Neuhuber WL, Kichko TI, Konrade I, Elvert R, Mier W, Pirags V, Lukic IK, Morcos M, Dehmer T, Rabbani N, Thornalley PJ, Edelstein D, Nau C, Forbes J, Humpert PM, Schwaninger M, Ziegler D, Stern DM, Cooper ME, Haberkorn U, Brownlee M, Reeh PW, Nawroth PP. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 2012;18(6):926–933. [DOI] [PubMed] [Google Scholar]
- [26].Biessels GJ, Bril V, Calcutt NA, Cameron NE, Cotter MA, Dobrowsky R, Feldman EL, Fernyhough P, Jakobsen J, Malik RA, Mizisin AP, Oates PJ, Obrosova IG, Pop-Busui R, Russell JW, Sima AA, Stevens MJ, Schmidt RE, Tesfaye S, Veves A, Vinik AI, Wright DE, Yagihashi S, Yorek MA, Ziegler D, Zochodne DW. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab). J Peripher Nerv Syst 2014;19(2):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018;14(10):591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Biessels GJ, ter Laak MP, Hamers FP, Gispen WH. Neuronal Ca2+ disregulation in diabetes mellitus. Eur J Pharmacol 2002;447(2–3):201–209. [DOI] [PubMed] [Google Scholar]
- [29].Blesneac I, Themistocleous AC, Fratter C, Conrad LJ, Ramirez JD, Cox JJ, Tesfaye S, Shillo PR, Rice ASC, Tucker SJ, Bennett DLH. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018;159(3):469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bonhof GJ, Strom A, Puttgen S, Ringel B, Bruggemann J, Bodis K, Mussig K, Szendroedi J, Roden M, Ziegler D. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia 2017;60(12):2495–2503. [DOI] [PubMed] [Google Scholar]
- [31].Braffett BH, Gubitosi-Klug RA, Albers JW, Feldman EL, Martin CL, White NH, Orchard TJ, Lopes-Virella M, Lachin JM, Pop-Busui R, Group DES. Risk Factors for Diabetic Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bril V The Perfect Clinical Trial. Controversies in Diabetic Neuropathy 2016;127:27–41. [DOI] [PubMed] [Google Scholar]
- [33].Brines M, Dunne AN, van Velzen M, Proto PL, Ostenson CG, Kirk RI, Petropoulos IN, Javed S, Malik RA, Cerami A, Dahan A. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med 2015;20:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brown MJ, Martin JR, Asbury AK. Painful diabetic neuropathy. A morphometric study. Arch Neurol 1976;33(3):164–171. [DOI] [PubMed] [Google Scholar]
- [35].Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes 2004;53(7):1824–1830. [DOI] [PubMed] [Google Scholar]
- [36].Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol 2009(194):333–392. [DOI] [PubMed] [Google Scholar]
- [37].Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med 2004;99:55–65. [DOI] [PubMed] [Google Scholar]
- [38].Calcutt NA, Allendoerfer KL, Mizisin AP, Middlemas A, Freshwater JD, Burgers M, Ranciato R, Delcroix JD, Taylor FR, Shapiro R, Strauch K, Dudek H, Engber TM, Galdes A, Rubin LL, Tomlinson DR. Therapeutic efficacy of sonic hedgehog protein in experimental diabetic neuropathy. J Clin Invest 2003;111(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol 1997;122(7):1478–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Calcutt NA, Freshwater JD, Mizisin AP. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia 2004;47(4):718–724. [DOI] [PubMed] [Google Scholar]
- [41].Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain 1996;68(2–3):293–299. [DOI] [PubMed] [Google Scholar]
- [42].Calcutt NA, Muir D, Powell HC, Mizisin AP. Reduced ciliary neuronotrophic factor-like activity in nerves from diabetic or galactose-fed rats. Brain Res 1992;575(2):320–324. [DOI] [PubMed] [Google Scholar]
- [43].Calcutt NA, Smith DR, Frizzi K, Sabbir MG, Chowdhury SK, Mixcoatl-Zecuatl T, Saleh A, Muttalib N, Van der Ploeg R, Ochoa J, Gopaul A, Tessler L, Wess J, Jolivalt CG, Fernyhough P. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J Clin Invest 2017;127(2):608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Calcutt NA, Stiller C, Gustafsson H, Malmberg AB. Elevated substance-P-like immunoreactivity levels in spinal dialysates during the formalin test in normal and diabetic rats. Brain Res 2000;856(1–2):20–27. [DOI] [PubMed] [Google Scholar]
- [45].Cameron NE, Cotter MA. Effects of protein kinase Cbeta inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev 2002;18(4):315–323. [DOI] [PubMed] [Google Scholar]
- [46].Campos MM, Ongali B, Thibault G, Neugebauer W, Couture R. Autoradiographic distribution and alterations of kinin B(2) receptors in the brain and spinal cord of streptozotocin-diabetic rats. Synapse 2005;58(3):184–192. [DOI] [PubMed] [Google Scholar]
- [47].Carroll SL, Byer SJ, Dorsey DA, Watson MA, Schmidt RE. Ganglion-specific patterns of diabetes-modulated gene expression are established in prevertebral and paravertebral sympathetic ganglia prior to the development of neuroaxonal dystrophy. J Neuropathol Exp Neurol 2004;63(11):1144–1154. [DOI] [PubMed] [Google Scholar]
- [48].Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ 3rd, Wolka AM, Vinik AI. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care 2007;30(4):896–902. [DOI] [PubMed] [Google Scholar]
- [49].Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, Russell JW. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD(+)-dependent SIRT1-PGC-1alpha-TFAM pathway. Int Rev Neurobiol 2019;145:177–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chavez JC, Almhanna K, Berti-Mattera LN. Transient expression of hypoxia-inducible factor-1 alpha and target genes in peripheral nerves from diabetic rats. Neurosci Lett 2005;374(3):179–182. [DOI] [PubMed] [Google Scholar]
- [51].Chemaly ER, Troncone L, Lebeche D. SERCA control of cell death and survival. Cell Calcium 2018;69:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst 2013;18(4):306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen J, Li C, Liu W, Yan B, Hu X, Yang F. miRNA-155 silencing reduces sciatic nerve injury in diabetic peripheral neuropathy. J Mol Endocrinol 2019;63(3):227–238. [DOI] [PubMed] [Google Scholar]
- [54].Chen SR, Pan HL. Spinal GABAB receptors mediate antinociceptive actions of cholinergic agents in normal and diabetic rats. Brain Res 2003;965(1–2):67–74. [DOI] [PubMed] [Google Scholar]
- [55].Chen SR, Pan HL. Up-regulation of spinal muscarinic receptors and increased antinociceptive effect of intrathecal muscarine in diabetic rats. J Pharmacol Exp Ther 2003;307(2):676–681. [DOI] [PubMed] [Google Scholar]
- [56].Cheng C, Kobayashi M, Martinez JA, Ng H, Moser JJ, Wang X, Singh V, Fritzler MJ, Zochodne DW. Evidence for Epigenetic Regulation of Gene Expression and Function in Chronic Experimental Diabetic Neuropathy. J Neuropathol Exp Neurol 2015;74(8):804–817. [DOI] [PubMed] [Google Scholar]
- [57].Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes 2003;52(9):2363–2371. [DOI] [PubMed] [Google Scholar]
- [58].Cheng HT, Dauch JR, Porzio MT, Yanik BM, Hsieh W, Smith AG, Singleton JR, Feldman EL. Increased axonal regeneration and swellings in intraepidermal nerve fibers characterize painful phenotypes of diabetic neuropathy. J Pain 2013;14(9):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cheung A, Podgorny P, Martinez JA, Chan C, Toth C. Epidermal axonal swellings in painful and painless diabetic peripheral neuropathy. Muscle Nerve 2015;51(4):505–513. [DOI] [PubMed] [Google Scholar]
- [60].Chowdhury SK, Smith DR, Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis 2013;51:56–65. [DOI] [PubMed] [Google Scholar]
- [61].Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, Calcutt NA, Fernyhough P. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes 2010;59(4):1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol 2003;179(2):188–199. [DOI] [PubMed] [Google Scholar]
- [63].Ciccacci C, Latini A, Greco C, Politi C, D’Amato C, Lauro D, Novelli G, Borgiani P, Spallone V. Association between a MIR499A polymorphism and diabetic neuropathy in type 2 diabetes. J Diabetes Complicat 2018;32(1):11–17. [DOI] [PubMed] [Google Scholar]
- [64].Ciccacci C, Morganti R, Di Fusco D, D’Amato C, Cacciotti L, Greco C, Rufini S, Novelli G, Sangiuolo F, Marfia GA, Borgiani P, Spallone V. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol 2014;51(4):663–671. [DOI] [PubMed] [Google Scholar]
- [65].Cimen SG, Cimen S, Kessaris N, Kahveci E, Tuzuner A. Challenges of pancreas transplantation in developing countries, exploring the Turkey example. World J Transplant 2019;9(8):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ciobanu AC, Selescu T, Gasler I, Soltuzu L, Babes A. Glycolytic metabolite methylglyoxal inhibits cold and menthol activation of the transient receptor potential melastatin type 8 channel. J Neurosci Res 2016;94(3):282–294. [DOI] [PubMed] [Google Scholar]
- [67].Conti G, Scarpini E, Baron P, Livraghi S, Tiriticco M, Bianchi R, Vedeler C, Scarlato G. Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: a process concomitant with endoneurial induction of IL-1beta and p75NTR. J Neurol Sci 2002;195(1):35–40. [DOI] [PubMed] [Google Scholar]
- [68].Coppey L, Davidson E, Shevalye H, Obrosov A, Yorek M. Effect of Early and Late Interventions with Dietary Oils on Vascular and Neural Complications in a Type 2 Diabetic Rat Model. J Diabetes Res 2019;2019:5020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Coppey LJ, Davidson EP, Obrosov A, Yorek MA. Enriching the diet with menhaden oil improves peripheral neuropathy in streptozotocin-induced type 1 diabetic rats. J Neurophysiol 2015;113(3):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab 2012;2012:950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Coudore-Civiale M, Courteix C, Boucher M, Fialip J, Eschalier A. Evidence for an involvement of tachykinins in allodynia in streptozocin-induced diabetic rats. Eur J Pharmacol 2000;401(1):47–53. [DOI] [PubMed] [Google Scholar]
- [72].Cowell RM, Russell JW. Nitrosative injury and antioxidant therapy in the management of diabetic neuropathy. J Investig Med 2004;52(1):33–44. [DOI] [PubMed] [Google Scholar]
- [73].Craner MJ, Klein JP, Renganathan M, Black JA, Waxman SG. Changes of sodium channel expression in experimental painful diabetic neuropathy. Ann Neurol 2002;52(6):786–792. [DOI] [PubMed] [Google Scholar]
- [74].Cui YY, Xu H, Wu HH, Qi J, Shi J, Li YQ. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS One 2014;9(7):e102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cunha JM, Jolivalt CG, Ramos KM, Gregory JA, Calcutt NA, Mizisin AP. Elevated lipid peroxidation and DNA oxidation in nerve from diabetic rats: effects of aldose reductase inhibition, insulin, and neurotrophic factors. Metabolism 2008;57(7):873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].D’Amato C, Morganti R, Di Gennaro F, Greco C, Marfia GA, Spallone V. A novel association between nondipping and painful diabetic polyneuropathy. Diabetes Care 2014;37(9):2640–2642. [DOI] [PubMed] [Google Scholar]
- [77].Dalmolin GD, Silva CR, Rigo FK, Gomes GM, Cordeiro Mdo N, Richardson M, Silva MA, Prado MA, Gomez MV, Ferreira J. Antinociceptive effect of Brazilian armed spider venom toxin Tx3–3 in animal models of neuropathic pain. Pain 2011;152(10):2224–2232. [DOI] [PubMed] [Google Scholar]
- [78].Daugherty DJ, Marquez A, Calcutt NA, Schubert D. A novel curcumin derivative for the treatment of diabetic neuropathy. Neuropharmacology 2018;129:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Daulhac L, Maffre V, Mallet C, Etienne M, Privat AM, Kowalski-Chauvel A, Seva C, Fialip J, Eschalier A. Phosphorylation of spinal N-methyl-d-aspartate receptor NR1 subunits by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-induced painful neuropathy. Eur J Pain 2010. [DOI] [PubMed] [Google Scholar]
- [80].Daulhac L, Maffre V, Mallet C, Etienne M, Privat AM, Kowalski-Chauvel A, Seva C, Fialip J, Eschalier A. Phosphorylation of spinal N-methyl-d-aspartate receptor NR1 subunits by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-induced painful neuropathy. Eur J Pain 2011;15(2):169 e161–169 e112. [DOI] [PubMed] [Google Scholar]
- [81].Davidson E, Coppey L, Lu B, Arballo V, Calcutt NA, Gerard C, Yorek M. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Exp Diabetes Res 2009;2009:431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Res Rev 2010;26(4):306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2008;51(4):562–566. [DOI] [PubMed] [Google Scholar]
- [84].de Anda-Jauregui G, Guo K, McGregor BA, Feldman EL, Hur J. Pathway crosstalk perturbation network modeling for identification of connectivity changes induced by diabetic neuropathy and pioglitazone. BMC Syst Biol 2019;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].de la Hoz CL, Cheng C, Fernyhough P, Zochodne DW. A model of chronic diabetic polyneuropathy: benefits from intranasal insulin are modified by sex and RAGE deletion. Am J Physiol Endocrinol Metab 2017;312(5):E407–E419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014;155(11):2263–2273. [DOI] [PubMed] [Google Scholar]
- [87].Dias FC, Alves VS, Matias DO, Figueiredo CP, Miranda ALP, Passos GF, Costa R. The selective TRPV4 channel antagonist HC-067047 attenuates mechanical allodynia in diabetic mice. Eur J Pharmacol 2019;856:172408. [DOI] [PubMed] [Google Scholar]
- [88].Dickenson AH, Patel R. Translational issues in precision medicine in neuropathic pain. Can J Pain 2020;4(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Diemel LT, Brewster WJ, Fernyhough P, Tomlinson DR. Expression of neuropeptides in experimental diabetes; effects of treatment with nerve growth factor or brain-derived neurotrophic factor. Brain Res Mol Brain Res 1994;21(1–2):171–175. [DOI] [PubMed] [Google Scholar]
- [90].Dogrul A, Gul H, Yesilyurt O, Ulas UH, Yildiz O. Systemic and spinal administration of etanercept, a tumor necrosis factor alpha inhibitor, blocks tactile allodynia in diabetic mice. Acta Diabetol 2011;48(2):135–142. [DOI] [PubMed] [Google Scholar]
- [91].Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 2009;94(6):2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dubovy P, Jancalek R, Kubek T. Role of inflammation and cytokines in peripheral nerve regeneration. Int Rev Neurobiol 2013;108:173–206. [DOI] [PubMed] [Google Scholar]
- [93].Dull MM, Riegel K, Tappenbeck J, Ries V, Strupf M, Fleming T, Sauer SK, Namer B. Methylglyoxal causes pain and hyperalgesia in human through C-fiber activation. Pain 2019;160(11):2497–2507. [DOI] [PubMed] [Google Scholar]
- [94].Duraikannu A, Krishnan A, Chandrasekhar A, Zochodne DW. Beyond Trophic Factors: Exploiting the Intrinsic Regenerative Properties of Adult Neurons. Front Cell Neurosci 2019;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, Tomlinson DR, Gardiner NJ. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 2009;58(12):2893–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dvornik E, Simard-Duquesne N, Krami M, Sestanj K, Gabbay KH, Kinoshita JH, Varma SD, Merola LO. Polyol accumulation in galactosemic and diabetic rats: control by an aldose reductase inhibitor. Science 1973;182(4117):1146–1148. [DOI] [PubMed] [Google Scholar]
- [97].Dyck PJ, Karnes JL, Daube J, O’Brien P, Service FJ. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy. Brain 1985;108 (Pt 4):861–880. [DOI] [PubMed] [Google Scholar]
- [98].Dyck PJ, Lais A, Karnes JL, O’Brien P, Rizza R. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol 1986;19(5):425–439. [DOI] [PubMed] [Google Scholar]
- [99].Eaton SE, Harris ND, Ibrahim S, Patel KA, Selmi F, Radatz M, Ward JD, Tesfaye S. Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia 2003;46(7):934–939. [DOI] [PubMed] [Google Scholar]
- [100].Eberhardt MJ, Filipovic MR, Leffler A, de la Roche J, Kistner K, Fischer MJ, Fleming T, Zimmermann K, Ivanovic-Burmazovic I, Nawroth PP, Bierhaus A, Reeh PW, Sauer SK. Methylglyoxal Activates Nociceptors through Transient Receptor Potential Channel A1 (TRPA1) A POSSIBLE MECHANISM OF METABOLIC NEUROPATHIES. Journal of Biological Chemistry 2012;287(34):28291–28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, Fort PE. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia 2019;62(9):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Eid SA, El Massry M, Hichor M, Haddad M, Grenier J, Dia B, Barakat R, Boutary S, Chanal J, Aractingi S, Wiesel P, Szyndralewiez C, Azar ST, Boitard C, Zaatari G, Eid AA, Massaad C. Targeting the NADPH Oxidase-4 and Liver X Receptor Signaling Axis Preserve Schwann Cell Integrity in Diabetic Mice. Diabetes 2019. [DOI] [PubMed] [Google Scholar]
- [103].Eisenstein M Sangamo’s lead zinc-finger therapy flops in diabetic neuropathy. Nat Biotechnol 2012;30(2):121–123. [DOI] [PubMed] [Google Scholar]
- [104].Emery SM, Dobrowsky RT. Promoting Neuronal Tolerance of Diabetic Stress: Modulating Molecular Chaperones. Int Rev Neurobiol 2016;127:181–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Feng YH, Chen L, Luo Q, Wu M, Chen YH, Shi XH. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Dev Ther 2018;12:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fernyhough P Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep 2015;15(11):89. [DOI] [PubMed] [Google Scholar]
- [107].Fernyhough P, Calcutt NA. New Directions in Diabetic Neuropathy: Evolution or Extinction? Int Rev Neurobiol 2016;127:229–234. [DOI] [PubMed] [Google Scholar]
- [108].Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J Neurochem 1995;64(3):1231–1237. [DOI] [PubMed] [Google Scholar]
- [109].Fernyhough P, Diemel LT, Hardy J, Brewster WJ, Mohiuddin L, Tomlinson DR. Human recombinant nerve growth factor replaces deficient neurotrophic support in the diabetic rat. Eur J Neurosci 1995;7(5):1107–1110. [DOI] [PubMed] [Google Scholar]
- [110].Fernyhough P, Diemel LT, Tomlinson DR. Target tissue production and axonal transport of neurotrophin-3 are reduced in streptozotocin-diabetic rats. Diabetologia 1998;41(3):300–306. [DOI] [PubMed] [Google Scholar]
- [111].Fernyhough P, Schmidt RE. Neurofilaments in diabetic neuropathy. Int Rev Neurobiol 2002;50:115–144. [DOI] [PubMed] [Google Scholar]
- [112].Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res 1993;607(1–2):117–124. [DOI] [PubMed] [Google Scholar]
- [113].Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010;150(3):573–581. [DOI] [PubMed] [Google Scholar]
- [114].Galosi E, La Cesa S, Di Stefano G, Karlsson P, Fasolino A, Leone C, Biasiotta A, Cruccu G, Truini A. A pain in the skin. Regenerating nerve sprouts are distinctly associated with ongoing burning pain in patients with diabetes. Eur J Pain 2018;22(10):1727–1734. [DOI] [PubMed] [Google Scholar]
- [115].Gandhi RA, Marques JL, Selvarajah D, Emery CJ, Tesfaye S. Painful diabetic neuropathy is associated with greater autonomic dysfunction than painless diabetic neuropathy. Diabetes Care 2010;33(7):1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ganesh Yerra V, Negi G, Sharma SS, Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol 2013;1:394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Garcia-Caballero A, Gadotti VM, Chen L, Zamponi GW. A cell-permeant peptide corresponding to the cUBP domain of USP5 reverses inflammatory and neuropathic pain. Mol Pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gardiner NJ, Freeman OJ. Can Diabetic Neuropathy Be Modeled In Vitro? Int Rev Neurobiol 2016;127:53–87. [DOI] [PubMed] [Google Scholar]
- [119].Garrett NE, Malcangio M, Dewhurst M, Tomlinson DR. alpha-Lipoic acid corrects neuropeptide deficits in diabetic rats via induction of trophic support. Neurosci Lett 1997;222(3):191–194. [DOI] [PubMed] [Google Scholar]
- [120].Gastol J, Kapusta P, Polus A, Pitera E, Biela M, Wolkow P, Pawlinski L, Kiec-Wilk B. Epigenetic mechanism in search for the pathomechanism of diabetic neuropathy development in diabetes mellitus type 1 (T1DM). Endocrine 2020. [DOI] [PubMed] [Google Scholar]
- [121].Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 2010;106(8):1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015;138(Pt 1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol 2017;13(3):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Green DR, Llambi F. Cell Death Signaling. Cold Spring Harb Perspect Biol 2015;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Greene DA, Lattimer SA, Sima AA. Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes 1988;37(6):688–693. [DOI] [PubMed] [Google Scholar]
- [126].Griggs RB, Santos DF, Laird DE, Doolen S, Donahue RR, Wessel CR, Fu W, Sinha GP, Wang P, Zhou J, Brings S, Fleming T, Nawroth PP, Susuki K, Taylor BK. Methylglyoxal and a spinal TRPA1-AC1-Epac cascade facilitate pain in the db/db mouse model of type 2 diabetes. Neurobiol Dis 2019;127:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr Opin Endocrinol Diabetes Obes 2017;24(2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Grote CW, Groover AL, Ryals JM, Geiger PC, Feldman EL, Wright DE. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathol Commun 2013;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Grote CW, Ryals JM, Wright DE. In vivo peripheral nervous system insulin signaling. J Peripher Nerv Syst 2013;18(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gu Y, Qiu ZL, Liu DZ, Sun GL, Guan YC, Hei ZQ, Li X. Differential gene expression profiling of the sciatic nerve in type 1 and type 2 diabetic mice. Biomed Rep 2018;9(4):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Guo G, Kan M, Martinez JA, Zochodne DW. Local insulin and the rapid regrowth of diabetic epidermal axons. Neurobiol Dis 2011;43(2):414–421. [DOI] [PubMed] [Google Scholar]
- [132].Guo G, Liu Y, Ren S, Kang Y, Duscher D, Machens HG, Chen Z. Comprehensive analysis of differentially expressed microRNAs and mRNAs in dorsal root ganglia from streptozotocin-induced diabetic rats. PLoS One 2018;13(8):e0202696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Gylfadottir SS, Christensen DH, Nicolaisen SK, Andersen H, Callaghan BC, Itani M, Khan KS, Kristensen AG, Nielsen JS, Sindrup SH, Andersen NT, Jensen TS, Thomsen RW, Finnerup NB. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: a cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Habash T, Saleh A, Roy Chowdhury SK, Smith DR, Fernyhough P. The proinflammatory cytokine, interleukin-17A, augments mitochondrial function and neurite outgrowth of cultured adult sensory neurons derived from normal and diabetic rats. Exp Neurol 2015;273:177–189. [DOI] [PubMed] [Google Scholar]
- [135].Hackett AR, Strickland A, Milbrandt J. Disrupting insulin signaling in Schwann cells impairs myelination and induces a sensory neuropathy. Glia 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Halim ND, McFate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A. Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 2010;58(10):1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hampton KK, Alani SM, Wilson JI, Price DE. Resistance to ischaemic conduction failure in chronic hypoxaemia and diabetes. J Neurol Neurosurg Psychiatry 1989;52(11):1303–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Han JW, Choi D, Lee MY, Huh YH, Yoon YS. Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetic Neuropathy by Direct Modulation of Both Angiogenesis and Myelination in Peripheral Nerves. Cell Transplant 2016;25(2):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hangping Z, Ling H, Lijin J, Wenting Z, Xiaoxia L, Qi Z, Xiaoming Z, Qingchun L, Yiming L, Qian X, Ji H, Bin L, Shuo Z. The preventive effect of IL-1beta antagonist on Diabetic Peripheral Neuropathy. Endocr Metab Immune Disord Drug Targets 2019. [DOI] [PubMed] [Google Scholar]
- [140].Hebert HL, Veluchamy A, Torrance N, Smith BH. Risk factors for neuropathic pain in diabetes mellitus. Pain 2017;158(4):560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res 1990;26(2):258–267. [DOI] [PubMed] [Google Scholar]
- [142].Hinder LM, Murdock BJ, Park M, Bender DE, O’Brien PD, Rumora AE, Hur J, Feldman EL. Transcriptional networks of progressive diabetic peripheral neuropathy in the db/db mouse model of type 2 diabetes: An inflammatory story. Exp Neurol 2018;305:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hiyama H, Yano Y, So K, Imai S, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S. TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy. Mol Pain 2018;14:1744806918789812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG. Channelopathies, painful neuropathy, and diabetes: which way does the causal arrow point? Trends Mol Med 2014;20(10):544–550. [DOI] [PubMed] [Google Scholar]
- [145].Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem 2005;280(1):618–627. [DOI] [PubMed] [Google Scholar]
- [146].Hong S, Wiley JW. Altered expression and function of sodium channels in large DRG neurons and myelinated A-fibers in early diabetic neuropathy in the rat. Biochem Biophys Res Commun 2006;339(2):652–660. [DOI] [PubMed] [Google Scholar]
- [147].Hong SS, Morrow TJ, Paulson PE, Isom LL, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. Journal of Biological Chemistry 2004;279(28):29341–29350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hu J, Hu X, Kan T. MiR-34c Participates in Diabetic Corneal Neuropathy Via Regulation of Autophagy. Invest Ophthalmol Vis Sci 2019;60(1):16–25. [DOI] [PubMed] [Google Scholar]
- [149].Hu J, Huang Y, Lin Y, Lin J. Protective effect inhibiting the expression of miR-181a on the diabetic corneal nerve in a mouse model. Exp Eye Res 2020;192:107925. [DOI] [PubMed] [Google Scholar]
- [150].Huang TJ, Price SA, Chilton L, Calcutt NA, Tomlinson DR, Verkhratsky A, Fernyhough P. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes 2003;52(8):2129–2136. [DOI] [PubMed] [Google Scholar]
- [151].Hulse RP, Beazley-Long N, Ved N, Bestall SM, Riaz H, Singhal P, Ballmer Hofer K, Harper SJ, Bates DO, Donaldson LF. Vascular endothelial growth factor-A165b prevents diabetic neuropathic pain and sensory neuronal degeneration. Clin Sci (Lond) 2015;129(8):741–756. [DOI] [PubMed] [Google Scholar]
- [152].Hur J, O’Brien PD, Nair V, Hinder LM, McGregor BA, Jagadish HV, Kretzler M, Brosius FC 3rd, Feldman EL. Transcriptional networks of murine diabetic peripheral neuropathy and nephropathy: common and distinct gene expression patterns. Diabetologia 2016;59(6):1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hur J, Sullivan KA, Pande M, Hong Y, Sima AA, Jagadish HV, Kretzler M, Feldman EL. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain 2011;134(Pt 11):3222–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ishii DN. Implication of insulin-like growth factors in the pathogenesis of diabetic neuropathy. Brain Res Brain Res Rev 1995;20(1):47–67. [DOI] [PubMed] [Google Scholar]
- [155].Ismail CAN, Suppian R, Abd Aziz CB, Haris K, Long I. Increased Nociceptive Responses in Streptozotocin-Induced Diabetic Rats and the Related Expression of Spinal NR2B Subunit of N-Methyl-D-Aspartate Receptors. Diabetes Metab J 2019;43(2):222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Jack MM, Ryals JM, Wright DE. Protection from diabetes-induced peripheral sensory neuropathy--a role for elevated glyoxalase I? Exp Neurol 2012;234(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Jaiswal S, Mishra S, Torgal SS, Shengule S. Neuroprotective effect of epalrestat mediated through oxidative stress markers, cytokines and TAU protein levels in diabetic rats. Life Sci 2018;207:364–371. [DOI] [PubMed] [Google Scholar]
- [158].Jakobsen J Axonal dwindling in early experimental diabetes. I. A study of cross sectioned nerves. Diabetologia 1976;12(6):539–546. [DOI] [PubMed] [Google Scholar]
- [159].Jakobsen J Axonal dwindling in early experimental diabetes. II. A study of isolated nerve fibres. Diabetologia 1976;12(6):547–553. [DOI] [PubMed] [Google Scholar]
- [160].Jayaraj ND, Bhattacharyya BJ, Belmadani AA, Ren D, Rathwell CA, Hackelberg S, Hopkins BE, Gupta HR, Miller RJ, Menichella DM. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest 2018;128(6):2205–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018;41(4):645–652. [DOI] [PubMed] [Google Scholar]
- [162].Jessen KR, Mirsky R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front Cell Neurosci 2019;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Jesus CHA, Redivo DDB, Gasparin AT, Sotomaior BB, de Carvalho MC, Genaro K, Zuardi AW, Hallak JEC, Crippa JA, Zanoveli JM, da Cunha JM. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res 2019;1715:156–164. [DOI] [PubMed] [Google Scholar]
- [164].Jia L, Wang L, Chopp M, Li C, Zhang Y, Szalad A, Zhang ZG. MiR-29c/PRKCI Regulates Axonal Growth of Dorsal Root Ganglia Neurons Under Hyperglycemia. Mol Neurobiol 2018;55(1):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Jiang Y, Calcutt NA, Ramos KM, Mizisin AP. Novel sites of aldose reductase immunolocalization in normal and streptozotocin-diabetic rats. J Peripher Nerv Syst 2006;11(4):274–285. [DOI] [PubMed] [Google Scholar]
- [166].Jiang Y, Mizisin AP, Rearden A, Jolivalt CG. Diabetes induces changes in ILK, PINCH and components of related pathways in the spinal cord of rats. Brain Res 2010;1332:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Jimenez-Osorio AS, Gonzalez-Reyes S, Pedraza-Chaverri J. Natural Nrf2 activators in diabetes. Clin Chim Acta 2015;448:182–192. [DOI] [PubMed] [Google Scholar]
- [168].Jolivalt CG, Frizzi KE, Guernsey L, Marquez A, Ochoa J, Rodriguez M, Calcutt NA. Peripheral Neuropathy in Mouse Models of Diabetes. Curr Protoc Mouse Biol 2016;6(3):223–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain 2008;140(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Jolivalt CG, Mizisin LM, Nelson A, Cunha JM, Ramos KM, Bonke D, Calcutt NA. B vitamins alleviate indices of neuropathic pain in diabetic rats. Eur J Pharmacol 2009;612(1–3):41–47. [DOI] [PubMed] [Google Scholar]
- [171].Jolivalt CG, Rodriguez M, Wahren J, Calcutt NA. Efficacy of a long-acting C-peptide analogue against peripheral neuropathy in streptozotocin-diabetic mice. Diabetes Obes Metab 2015;17(8):781–788. [DOI] [PubMed] [Google Scholar]
- [172].Jolivalt CG, Vu Y, Mizisin LM, Mizisin AP, Calcutt NA. Impaired prosaposin secretion during nerve regeneration in diabetic rats and protection of nerve regeneration by a prosaposin-derived peptide. J Neuropathol Exp Neurol 2008;67(7):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Juaristi I, Contreras L, Gonzalez-Sanchez P, Perez-Liebana I, Gonzalez-Moreno L, Pardo B, Del Arco A, Satrustegui J. The Response to Stimulation in Neurons and Astrocytes. Neurochem Res 2019;44(10):2385–2391. [DOI] [PubMed] [Google Scholar]
- [174].Julius D TRP channels and pain. Annu Rev Cell Dev Biol 2013;29:355–384. [DOI] [PubMed] [Google Scholar]
- [175].Kalichman MW, Powell HC, Mizisin AP. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol 1998;95(1):47–56. [DOI] [PubMed] [Google Scholar]
- [176].Kalliomaki J, Attal N, Jonzon B, Bach FW, Huizar K, Ratcliffe S, Eriksson B, Janecki M, Danilov A, Bouhassira D, Group APS. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain 2013;154(5):761–767. [DOI] [PubMed] [Google Scholar]
- [177].Kalteniece A, Ferdousi M, Azmi S, Mubita WM, Marshall A, Lauria G, Faber CG, Soran H, Malik RA. Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy. Sci Rep 2020;10(1):3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kalteniece A, Ferdousi M, Petropoulos I, Azmi S, Adam S, Fadavi H, Marshall A, Boulton AJM, Efron N, Faber CG, Lauria G, Soran H, Malik RA. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep 2018;8(1):3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kamei J, Ogawa M, Kasuya Y. Development of supersensitivity to substance P in the spinal cord of the streptozotocin-induced diabetic rats. Pharmacol Biochem Behav 1990;35(2):473–475. [DOI] [PubMed] [Google Scholar]
- [180].Kamiya H, Zhang W, Ekberg K, Wahren J, Sima AA. C-Peptide reverses nociceptive neuropathy in type 1 diabetes. Diabetes 2006;55(12):3581–3587. [DOI] [PubMed] [Google Scholar]
- [181].Kato N, Nemoto K, Nakanishi K, Morishita R, Kaneda Y, Uenoyama M, Ikeda T, Fujikawa K. Nonviral gene transfer of human hepatocyte growth factor improves streptozotocin-induced diabetic neuropathy in rats. Diabetes 2005;54(3):846–854. [DOI] [PubMed] [Google Scholar]
- [182].Kellogg AP, Wiggin TD, Larkin DD, Hayes JM, Stevens MJ, Pop-Busui R. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes 2007;56(12):2997–3005. [DOI] [PubMed] [Google Scholar]
- [183].Kennedy JM, Zochodne DW. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes 2005;54(3):830–837. [DOI] [PubMed] [Google Scholar]
- [184].Kerckhove N, Pereira B, Soriot-Thomas S, Alchaar H, Deleens R, Hieng VS, Serra E, Lanteri-Minet M, Arcagni P, Picard P, Lefebvre-Kuntz D, Maindet C, Mick G, Balp L, Lucas C, Creach C, Letellier M, Martinez V, Navez M, Delbrouck D, Kuhn E, Piquet E, Bozzolo E, Brosse C, Lietar B, Marcaillou F, Hamdani A, Leroux-Bromberg N, Perier Y, Vergne-Salle P, Gov C, Delage N, Gillet D, Romettino S, Richard D, Mallet C, Bernard L, Lambert C, Dubray C, Duale C, Eschalier A. Efficacy and safety of a T-type calcium channel blocker in patients with neuropathic pain: A proof-of-concept, randomized, double-blind and controlled trial. Eur J Pain 2018;22(7):1321–1330. [DOI] [PubMed] [Google Scholar]
- [185].Khamaneh AM, Alipour MR, Sheikhzadeh Hesari F, Ghadiri Soufi F. A signature of microRNA-155 in the pathogenesis of diabetic complications. J Physiol Biochem 2015;71(2):301–309. [DOI] [PubMed] [Google Scholar]
- [186].Khoshnoodi M, Truelove S, Polydefkis M. Effect of diabetes type on long-term outcome of epidermal axon regeneration. Ann Clin Transl Neurol 2019;6(10):2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kim KS, Song YS, Jin J, Joe JH, So BI, Park JY, Fang CH, Kim MJ, Cho YH, Hwang S, Ro YS, Kim H, Ahn YH, Sung HJ, Sung JJ, Park SH, Lipton SA. Granulocyte-colony stimulating factor as a treatment for diabetic neuropathy in rat. Mol Cell Endocrinol 2015;414:64–72. [DOI] [PubMed] [Google Scholar]
- [188].Kim S, Maynard JC, Strickland A, Burlingame AL, Milbrandt J. Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN. Proc Natl Acad Sci U S A 2018;115(31):8019–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].King RH, Llewelyn JG, Thomas PK, Gilbey SG, Watkins PJ. Diabetic neuropathy: abnormalities of Schwann cell and perineurial basal laminae. Implications for diabetic vasculopathy. Neuropathol Appl Neurobiol 1989;15(4):339–355. [DOI] [PubMed] [Google Scholar]
- [190].Kinoshita JH, Dvornik D, Kraml M, Gabbay KH. The effect of an aldose reductase inhibitor on the galactose-exposed rabbit lens. Biochim Biophys Acta 1968;158(3):472–475. [DOI] [PubMed] [Google Scholar]
- [191].Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, Viisanen H, Akerman KE, Lindstedt K, Pertovaara A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res 2012;65(1):149–158. [DOI] [PubMed] [Google Scholar]
- [192].Koivisto A, Pertovaara A. Transient receptor potential ankyrin 1 (TRPA1) ion channel in the pathophysiology of peripheral diabetic neuropathy. Scand J Pain 2013;4(3):129–136. [DOI] [PubMed] [Google Scholar]
- [193].Korngut L, Ma CH, Martinez JA, Toth CC, Guo GF, Singh V, Woolf CJ, Zochodne DW. Overexpression of human HSP27 protects sensory neurons from diabetes. Neurobiol Dis 2012;47(3):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kostyuk E, Voitenko N, Kruglikov I, Shmigol A, Shishkin V, Efimov A, Kostyuk P. Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons. Diabetologia 2001;44(10):1302–1309. [DOI] [PubMed] [Google Scholar]
- [195].Krishnan A, Zochodne DW. Is Cytoplasmic PTEN a Specific Target for Neuronal Survival? Mol Neurobiol 2015;52(3):1758–1764. [DOI] [PubMed] [Google Scholar]
- [196].Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Abnormal LDIflare but normal quantitative sensory testing and dermal nerve fiber density in patients with painful diabetic neuropathy. Diabetes Care 2009;32(3):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology 2017;25(4):393–402. [DOI] [PubMed] [Google Scholar]
- [198].Kushchayev SV, Belykh E, Fishchenko Y, Salei A, Teytelboym OM, Shabaturov L, Cruse M, Preul MC. Two bullets to the head and an early winter: fate permits Kutuzov to defeat Napoleon at Moscow. Neurosurg Focus 2015;39(1):E3. [DOI] [PubMed] [Google Scholar]
- [199].Lam D, Momeni Z, Theaker M, Jagadeeshan S, Yamamoto Y, Ianowski JP, Campanucci VA. RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS One 2018;13(2):e0193312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Lamping KG, Nuno DW, Coppey LJ, Holmes AJ, Hu S, Oltman CL, Norris AW, Yorek MA. Modification of high saturated fat diet with n-3 polyunsaturated fat improves glucose intolerance and vascular dysfunction. Diabetes Obes Metab 2013;15(2):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Latham JR, Pathirathna S, Jagodic MM, Choe WJ, Levin ME, Nelson MT, Lee WY, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes 2009;58(11):2656–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Laughton JD, Bittar P, Charnay Y, Pellerin L, Kovari E, Magistretti PJ, Bouras C. Metabolic compartmentalization in the human cortex and hippocampus: evidence for a cell- and region-specific localization of lactate dehydrogenase 5 and pyruvate dehydrogenase. BMC Neurosci 2007;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lazniewska J, Rzhepetskyy Y, Zhang FX, Zamponi GW, Weiss N. Cooperative roles of glucose and asparagine-linked glycosylation in T-type calcium channel expression. Pflugers Arch 2016;468(11–12):1837–1851. [DOI] [PubMed] [Google Scholar]
- [204].Lee YC, Lu SC, Hsieh YL. Establishing a Mouse Model of a Pure Small Fiber Neuropathy with the Ultrapotent Agonist of Transient Receptor Potential Vanilloid Type 1. J Vis Exp 2018(132). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Lee-Kubli C, Marshall AG, Malik RA, Calcutt NA. The H-Reflex as a Biomarker for Spinal Disinhibition in Painful Diabetic Neuropathy. Curr Diab Rep 2018;18(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lee-Kubli CA, Calcutt NA. Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. Pain 2014;155(2):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Li L, Yu T, Yu L, Li H, Liu Y, Wang D. Exogenous brain-derived neurotrophic factor relieves pain symptoms of diabetic rats by reducing excitability of dorsal root ganglion neurons. Int J Neurosci 2016;126(8):749–758. [DOI] [PubMed] [Google Scholar]
- [208].Li N, Young MM, Bailey CJ, Smith ME. NMDA and AMPA glutamate receptor subtypes in the thoracic spinal cord in lean and obese-diabetic ob/ob mice. Brain Res 1999;849(1–2):34–44. [DOI] [PubMed] [Google Scholar]
- [209].Li P, Xiong DL, Sun WP, Xu SY. Effects of baicalin on diabetic neuropathic pain involving transient receptor potential vanilloid 1 in the dorsal root ganglia of rats. Neuroreport 2018;29(17):1492–1498. [DOI] [PubMed] [Google Scholar]
- [210].Li Y, Ma WG, Xie CQ, Zhang M, Yin XH, Wang FF, Xu J, Shi BY. Identification of genes and signaling pathways associated with diabetic neuropathy using a weighted correlation network analysis A consort study. Medicine 2016;95(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Lin JY, Huang XL, Chen J, Yang ZW, Lin J, Huang S, Peng B. Stereological study on the number of synapses in the rat spinal dorsal horn with painful diabetic neuropathy induced by streptozotocin. Neuroreport 2017;28(6):319–324. [DOI] [PubMed] [Google Scholar]
- [212].Liu GS, Shi JY, Lai CL, Hong YR, Shin SJ, Huang HT, Lam HC, Wen ZH, Hsu KS, Chen CH, Howng SL, Tai MH. Peripheral gene transfer of glial cell-derived neurotrophic factor ameliorates neuropathic deficits in diabetic rats. Hum Gene Ther 2009;20(7):715–727. [DOI] [PubMed] [Google Scholar]
- [213].Liu P, Yuan HB, Zhao S, Liu FF, Jiang YQ, Guo YX, Wang XL. Activation of GABAB Receptor Suppresses Diabetic Neuropathic Pain through Toll-Like Receptor 4 Signaling Pathway in the Spinal Dorsal Horn. Mediators Inflamm 2018;2018:6016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Liu S, Liu X, Xiong H, Wang W, Liu Y, Yin L, Tu C, Wang H, Xiang X, Xu J, Duan B, Tao A, Zhao Z, Mei Z. CXCL13/CXCR5 signaling contributes to diabetes-induced tactile allodynia via activating pERK, pSTAT3, pAKT pathways and pro-inflammatory cytokines production in the spinal cord of male mice. Brain Behav Immun 2019;80:711–724. [DOI] [PubMed] [Google Scholar]
- [215].Liu XS, Fan B, Szalad A, Jia L, Wang L, Wang X, Pan W, Zhang L, Zhang R, Hu J, Zhang XM, Chopp M, Zhang ZG. MicroRNA-146a Mimics Reduce the Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes 2017;66(12):3111–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Llewelyn JG, Gilbey SG, Thomas PK, King RH, Muddle JR, Watkins PJ. Sural nerve morphometry in diabetic autonomic and painful sensory neuropathy. A clinicopathological study. Brain 1991;114 (Pt 2):867–892. [DOI] [PubMed] [Google Scholar]
- [217].Lockett MJ, Tomlinson DR. The effects of dietary treatment with essential fatty acids on sciatic nerve conduction and activity of the Na+/K+ pump in streptozotocin-diabetic rats. Br J Pharmacol 1992;105(2):355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Low PA. Recent advances in the pathogenesis of diabetic neuropathy. Muscle Nerve 1987;10(2):121–128. [DOI] [PubMed] [Google Scholar]
- [219].Lupachyk S, Shevalye H, Maksimchyk Y, Drel VR, Obrosova IG. PARP inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: correlation with peripheral nerve function. Free Radic Biol Med 2011;50(10):1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lupachyk S, Watcho P, Shevalye H, Vareniuk I, Obrosov A, Obrosova IG, Yorek MA. Na+/H+ exchanger 1 inhibition reverses manifestation of peripheral diabetic neuropathy in type 1 diabetic rats. Am J Physiol Endocrinol Metab 2013;305(3):E396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Ma J, Pan P, Anyika M, Blagg BS, Dobrowsky RT. Modulating Molecular Chaperones Improves Mitochondrial Bioenergetics and Decreases the Inflammatory Transcriptome in Diabetic Sensory Neurons. ACS Chem Neurosci 2015;6(9):1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Malik RA. Wherefore Art Thou, O Treatment for Diabetic Neuropathy? Int Rev Neurobiol 2016;127:287–317. [DOI] [PubMed] [Google Scholar]
- [223].Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, Ward JD. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005;48(3):578–585. [DOI] [PubMed] [Google Scholar]
- [224].Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ. Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol 2001;101(4):367–374. [DOI] [PubMed] [Google Scholar]
- [225].Malmberg AB, O’Connor WT, Glennon JC, Cesena R, Calcutt NA. Impaired formalin-evoked changes of spinal amino acid levels in diabetic rats. Brain Res 2006;1115(1):48–53. [DOI] [PubMed] [Google Scholar]
- [226].Marshall AG, Lee-Kubli C, Azmi S, Zhang M, Ferdousi M, Mixcoatl-Zecuatl T, Petropoulos IN, Ponirakis G, Fineman MS, Fadavi H, Frizzi K, Tavakoli M, Jeziorska M, Jolivalt CG, Boulton AJM, Efron N, Calcutt NA, Malik RA. Spinal Disinhibition in Experimental and Clinical Painful Diabetic Neuropathy. Diabetes 2017;66(5):1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Martin CL, Albers JW, Pop-Busui R, Group DER. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].McDonnell A, Collins S, Ali Z, Iavarone L, Surujbally R, Kirby S, Butt RP. Efficacy of the Nav1.7 blocker PF-05089771 in a randomised, placebo-controlled, double-blind clinical study in subjects with painful diabetic peripheral neuropathy. Pain 2018;159(8):1465–1476. [DOI] [PubMed] [Google Scholar]
- [229].McGregor BA, Eid S, Rumora AE, Murdock B, Guo K, de Anda-Jauregui G, Porter JE, Feldman EL, Hur J. Conserved Transcriptional Signatures in Human and Murine Diabetic Peripheral Neuropathy. Sci Rep-Uk 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].McLean WG. The role of axonal cytoskeleton in diabetic neuropathy. Neurochem Res 1997;22(8):951–956. [DOI] [PubMed] [Google Scholar]
- [231].Memon B, Abdelalim EM. Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors. Cells 2020;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Menichella DM, Abdelhak B, Ren D, Shum A, Frietag C, Miller RJ. CXCR4 chemokine receptor signaling mediates pain in diabetic neuropathy. Mol Pain 2014;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Messinger RB, Naik AK, Jagodic MM, Nelson MT, Lee WY, Choe WJ, Orestes P, Latham JR, Todorovic SM, Jevtovic-Todorovic V. In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain 2009;145(1–2):184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Migita K, Moriyama T, Koguchi M, Honda K, Katsuragi T, Takano Y, Ueno S. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett 2009;452(2):200–203. [DOI] [PubMed] [Google Scholar]
- [235].Misra A, Bloomgarden Z. Metabolic memory: Evolving concepts. J Diabetes 2018;10(3):186–187. [DOI] [PubMed] [Google Scholar]
- [236].Mixcoatl-Zecuatl T, Jolivalt CG. A spinal mechanism of action for duloxetine in a rat model of painful diabetic neuropathy. Br J Pharmacol 2011;164(1):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Mizisin AP, Calcutt NA, DiStefano PS, Acheson A, Longo FM. Aldose reductase inhibition increases CNTF-like bioactivity and protein in sciatic nerves from galactose-fed and normal rats. Diabetes 1997;46(4):647–652. [DOI] [PubMed] [Google Scholar]
- [238].Mizisin AP, Calcutt NA, Tomlinson DR, Gallagher A, Fernyhough P. Neurotrophin-3 reverses nerve conduction velocity deficits in streptozotocin-diabetic rats. J Peripher Nerv Syst 1999;4(3–4):211–221. [PubMed] [Google Scholar]
- [239].Mizisin AP, Nelson RW, Sturges BK, Vernau KM, Lecouteur RA, Williams DC, Burgers ML, Shelton GD. Comparable myelinated nerve pathology in feline and human diabetes mellitus. Acta Neuropathol 2007;113(4):431–442. [DOI] [PubMed] [Google Scholar]
- [240].Mizisin AP, Vu Y, Shuff M, Calcutt NA. Ciliary neurotrophic factor improves nerve conduction and ameliorates regeneration deficits in diabetic rats. Diabetes 2004;53(7):1807–1812. [DOI] [PubMed] [Google Scholar]
- [241].Morgado C, Pereira-Terra P, Cruz CD, Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes Metab 2011;13(2):150–159. [DOI] [PubMed] [Google Scholar]
- [242].Mu ZP, Wang YG, Li CQ, Lv WS, Wang B, Jing ZH, Song XJ, Lun Y, Qiu MY, Ma XL. Association Between Tumor Necrosis Factor-alpha and Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: a Meta-Analysis. Mol Neurobiol 2017;54(2):983–996. [DOI] [PubMed] [Google Scholar]
- [243].Muller C, Morales P, Reggio PH. Cannabinoid Ligands Targeting TRP Channels. Front Mol Neurosci 2018;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Nakae M, Kamiya H, Naruse K, Horio N, Ito Y, Mizubayashi R, Hamada Y, Nakashima E, Akiyama N, Kobayashi Y, Watarai A, Kimura N, Horiguchi M, Tabata Y, Oiso Y, Nakamura J. Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes 2006;55(5):1470–1477. [DOI] [PubMed] [Google Scholar]
- [245].Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol 2014;2(11):894–900. [DOI] [PubMed] [Google Scholar]
- [246].Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404(6779):787–790. [DOI] [PubMed] [Google Scholar]
- [247].Nukada H, McMorran PD, Baba M, Ogasawara S, Yagihashi S. Increased susceptibility to ischemia and macrophage activation in STZ-diabetic rat nerve. Brain Res 2011;1373:172–182. [DOI] [PubMed] [Google Scholar]
- [248].O’Brien PD, Guo K, Eid SA, Rumora AE, Hinder LM, Hayes JM, Mendelson FE, Hur J, Feldman EL. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Model Mech 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].O’Brien PD, Guo K, Eid SA, Rumora AE, Hinder LM, Hayes JM, Mendelson FE, Hur J, Feldman EL. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Model Mech 2020;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].O’Brien PD, Hinder LM, Sakowski SA, Feldman EL. ER stress in diabetic peripheral neuropathy: A new therapeutic target. Antioxid Redox Signal 2014;21(4):621–633. [DOI] [PubMed] [Google Scholar]
- [251].O’Brien PD, Hur J, Hayes JM, Backus C, Sakowski SA, Feldman EL. BTBR ob/ob mice as a novel diabetic neuropathy model: Neurological characterization and gene expression analyses. Neurobiol Dis 2015;73:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets 2008;9(1):14–36. [DOI] [PubMed] [Google Scholar]
- [253].Obradovic A, Hwang SM, Scarpa J, Hong SJ, Todorovic SM, Jevtovic-Todorovic V. CaV3.2 T-type calcium channels in peripheral sensory neurons are important for mibefradil-induced reversal of hyperalgesia and allodynia in rats with painful diabetic neuropathy. PLoS One 2014;9(4):e91467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes 2007;56(10):2598–2608. [DOI] [PubMed] [Google Scholar]
- [255].Oh YS. Bioactive Compounds and Their Neuroprotective Effects in Diabetic Complications. Nutrients 2016;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Ohi T, Saita K, Furukawa S, Ohta M, Hayashi K, Matsukura S. Therapeutic effects of aldose reductase inhibitor on experimental diabetic neuropathy through synthesis/secretion of nerve growth factor. Exp Neurol 1998;151(2):215–220. [DOI] [PubMed] [Google Scholar]
- [257].Okuse K, Chaplan SR, McMahon SB, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci 1997;10(3–4):196–207. [DOI] [PubMed] [Google Scholar]
- [258].Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, Poiro N, Digruccio MR, Krishnan K, Covey DF, Lee JH, Barrett PQ, Jevtovic-Todorovic V, Todorovic SM. Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels. Diabetes 2013;62(11):3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Orstavik K, Jorum E. Microneurographic findings of relevance to pain in patients with erythromelalgia and patients with diabetic neuropathy. Neurosci Lett 2010;470(3):180–184. [DOI] [PubMed] [Google Scholar]
- [260].Pabbidi MR, Premkumar LS. Role of Transient Receptor Potential Channels Trpv1 and Trpm8 in Diabetic Peripheral Neuropathy. J Diabetes Treat 2017;2017(4). [PMC free article] [PubMed] [Google Scholar]
- [261].Pabbidi RM, Cao DS, Parihar A, Pauza ME, Premkumar LS. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol Pharmacol 2008;73(3):995–1004. [DOI] [PubMed] [Google Scholar]
- [262].Pande M, Hur J, Hong Y, Backus C, Hayes JM, Oh SS, Kretzler M, Feldman EL. Transcriptional profiling of diabetic neuropathy in the BKS db/db mouse: a model of type 2 diabetes. Diabetes 2011;60(7):1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Park S, Kang HJ, Jeon JH, Kim MJ, Lee IK. Recent advances in the pathogenesis of microvascular complications in diabetes. Arch Pharm Res 2019;42(3):252–262. [DOI] [PubMed] [Google Scholar]
- [264].Patel R, Dickenson AH. Mechanisms of the gabapentinoids and alpha 2 delta-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect 2016;4(2):e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Peterson SB, Hart GW. New insights: A role for O-GlcNAcylation in diabetic complications. Crit Rev Biochem Mol Biol 2016;51(3):150–161. [DOI] [PubMed] [Google Scholar]
- [266].Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing Diabetic Neuropathy: Something Old, Something New. Diabetes Metab J 2018;42(4):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Pichon X, Wattiez AS, Becamel C, Ehrlich I, Bockaert J, Eschalier A, Marin P, Courteix C. Disrupting 5-HT(2A) receptor/PDZ protein interactions reduces hyperalgesia and enhances SSRI efficacy in neuropathic pain. Mol Ther 2010;18(8):1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Pop-Busui R, Marinescu V, Van Huysen C, Li F, Sullivan K, Greene DA, Larkin D, Stevens MJ. Dissection of metabolic, vascular, and nerve conduction interrelationships in experimental diabetic neuropathy by cyclooxygenase inhibition and acetyl-L-carnitine administration. Diabetes 2002;51(8):2619–2628. [DOI] [PubMed] [Google Scholar]
- [269].Pop-Busui R, Martin C. Neuropathy in the DCCT/EDIC-What Was Done Then and What We Would Do Better Now. Int Rev Neurobiol 2016;127:9–25. [DOI] [PubMed] [Google Scholar]
- [270].Powell HC, Myers RR. Axonopathy and microangiopathy in chronic alloxan diabetes. Acta Neuropathol 1984;65(2):128–137. [DOI] [PubMed] [Google Scholar]
- [271].Powell HC, Rosoff J, Myers RR. Microangiopathy in human diabetic neuropathy. Acta Neuropathol 1985;68(4):295–305. [DOI] [PubMed] [Google Scholar]
- [272].Price SA, Zeef LA, Wardleworth L, Hayes A, Tomlinson DR. Identification of changes in gene expression in dorsal root ganglia in diabetic neuropathy: correlation with functional deficits. J Neuropathol Exp Neurol 2006;65(7):722–732. [DOI] [PubMed] [Google Scholar]
- [273].Purwata TE. High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res 2011;4:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Puttgen S, Bonhof GJ, Strom A, Mussig K, Szendroedi J, Roden M, Ziegler D. Augmented Corneal Nerve Fiber Branching in Painful Compared With Painless Diabetic Neuropathy. J Clin Endocrinol Metab 2019;104(12):6220–6228. [DOI] [PubMed] [Google Scholar]
- [275].Quattrini C, Harris ND, Malik RA, Tesfaye S. Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care 2007;30(3):655–659. [DOI] [PubMed] [Google Scholar]
- [276].Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007;56(8):2148–2154. [DOI] [PubMed] [Google Scholar]
- [277].Rachana KS, Manu MS, Advirao GM. Insulin influenced expression of myelin proteins in diabetic peripheral neuropathy. Neurosci Lett 2016;629:110–115. [DOI] [PubMed] [Google Scholar]
- [278].Rahman MH, Jha MK, Kim JH, Nam Y, Lee MG, Go Y, Harris RA, Park DH, Kook H, Lee IK, Suk K. Pyruvate Dehydrogenase Kinase-mediated Glycolytic Metabolic Shift in the Dorsal Root Ganglion Drives Painful Diabetic Neuropathy. J Biol Chem 2016;291(11):6011–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Rajabally YA, Stettner M, Kieseier BC, Hartung HP, Malik RA. CIDP and other inflammatory neuropathies in diabetes - diagnosis and management. Nat Rev Neurol 2017;13(10):599–611. [DOI] [PubMed] [Google Scholar]
- [280].Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D’Emden MC, Laakso M, Baker JR, Keech AC, investigators Fs. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet 2009;373(9677):1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Rajbhandari SM, Jarratt JA, Griffiths PD, Ward JD. Diabetic neuropathic pain in a leg amputated 44 years previously. Pain 1999;83(3):627–629. [DOI] [PubMed] [Google Scholar]
- [282].Ramji N, Toth C, Kennedy J, Zochodne DW. Does diabetes mellitus target motor neurons? Neurobiol Dis 2007;26(2):301–311. [DOI] [PubMed] [Google Scholar]
- [283].Ramli R, Reddy M, Oliver N. Artificial Pancreas: Current Progress and Future Outlook in the Treatment of Type 1 Diabetes. Drugs 2019;79(10):1089–1101. [DOI] [PubMed] [Google Scholar]
- [284].Ramos KM, Jiang Y, Svensson CI, Calcutt NA. Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes 2007;56(6):1569–1576. [DOI] [PubMed] [Google Scholar]
- [285].Raputova J, Srotova I, Vlckova E, Sommer C, Uceyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, Forer L, Belobradkova J, Olsovsky J, Weber P, Dusek L, Jarkovsky J, Bednarik J. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain 2017;158(12):2340–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Ren YS, Qian NS, Tang Y, Liao YH, Yang YL, Dou KF, Toi M. Sodium channel Nav1.6 is up-regulated in the dorsal root ganglia in a mouse model of type 2 diabetes. Brain Res Bull 2012;87(2–3):244–249. [DOI] [PubMed] [Google Scholar]
- [287].Ristoiu V, Shibasaki K, Uchida K, Zhou Y, Ton BH, Flonta ML, Tominaga M. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain 2011;152(4):936–945. [DOI] [PubMed] [Google Scholar]
- [288].Roa-Coria JE, Pineda-Farias JB, Barragan-Iglesias P, Quinonez-Bastidas GN, Zuniga-Romero A, Huerta-Cruz JC, Reyes-Garcia JG, Flores-Murrieta FJ, Granados-Soto V, Rocha-Gonzalez HI. Possible involvement of peripheral TRP channels in the hydrogen sulfide-induced hyperalgesia in diabetic rats. BMC Neurosci 2019;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Robb JL, Morrissey NA, Weightman Potter PG, Smithers HE, Beall C, Ellacott KLJ. Immunometabolic Changes in Glia - A Potential Role in the Pathophysiology of Obesity and Diabetes. Neuroscience 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Robertson DM, Sima AA. Diabetic neuropathy in the mutant mouse [C57BL/ks(db/db)]: a morphometric study. Diabetes 1980;29(1):60–67. [DOI] [PubMed] [Google Scholar]
- [291].Rodriguez-Gutierrez R, Montori VM. Glycemic Control for Patients With Type 2 Diabetes Mellitus: Our Evolving Faith in the Face of Evidence. Circ Cardiovasc Qual Outcomes 2016;9(5):504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Rojas DR, Tegeder I, Kuner R, Agarwal N. Hypoxia-inducible factor 1alpha protects peripheral sensory neurons from diabetic peripheral neuropathy by suppressing accumulation of reactive oxygen species. J Mol Med (Berl) 2018;96(12):1395–1405. [DOI] [PubMed] [Google Scholar]
- [293].Rojewska E, Zychowska M, Piotrowska A, Kreiner G, Nalepa I, Mika J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front Immunol 2018;9:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Romanovsky D, Cruz NF, Dienel GA, Dobretsov M. Mechanical hyperalgesia correlates with insulin deficiency in normoglycemic streptozotocin-treated rats. Neurobiol Dis 2006;24(2):384–394. [DOI] [PubMed] [Google Scholar]
- [295].Romanovsky D, Dobretsov M. Pressure-induced pain: early sign of diabetes-associated impairment of insulin production in rats. Neurosci Lett 2010;483(2):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Romanovsky D, Hastings SL, Stimers JR, Dobretsov M. Relevance of hyperglycemia to early mechanical hyperalgesia in streptozotocin-induced diabetes. J Peripher Nerv Syst 2004;9(2):62–69. [DOI] [PubMed] [Google Scholar]
- [297].Romanovsky D, Walker JC, Dobretsov M. Pressure pain precedes development of type 2 disease in Zucker rat model of diabetes. Neurosci Lett 2008;445(3):220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Rondon LJ, Privat AM, Daulhac L, Davin N, Mazur A, Fialip J, Eschalier A, Courteix C. Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. J Physiol 2010;588(Pt 21):4205–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Rowe-Rendleman CL, Eichberg J. P0 phosphorylation in nerves from normal and diabetic rats: role of protein kinase C and turnover of phosphate groups. Neurochem Res 1994;19(8):1023–1031. [DOI] [PubMed] [Google Scholar]
- [300].Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, Akude E, Morrow D, Calcutt NA, Fernyhough P. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain 2012;135(Pt 6):1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Ruiz HH, Ramasamy R, Schmidt AM. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2020;161(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Rumora AE, LoGrasso G, Hayes JM, Mendelson FE, Tabbey MA, Haidar JA, Lentz SI, Feldman EL. The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. J Neurosci 2019;39(19):3770–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Rumora AE, Savelieff MG, Sakowski SA, Feldman EL. Disorders of mitochondrial dynamics in peripheral neuropathy: Clues from hereditary neuropathy and diabetes. Int Rev Neurobiol 2019;145:127–176. [DOI] [PubMed] [Google Scholar]
- [304].Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis 1999;6(5):347–363. [DOI] [PubMed] [Google Scholar]
- [305].Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, Committee IDFDA. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- [306].Saleh A, Smith DR, Tessler L, Mateo AR, Martens C, Schartner E, Van der Ploeg R, Toth C, Zochodne DW, Fernyhough P. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol 2013;249:149–159. [DOI] [PubMed] [Google Scholar]
- [307].Samii A, Unger J, Lange W. Vascular endothelial growth factor expression in peripheral nerves and dorsal root ganglia in diabetic neuropathy in rats. Neurosci Lett 1999;262(3):159–162. [DOI] [PubMed] [Google Scholar]
- [308].Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013;1833(12):3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes 2003;52(1):165–171. [DOI] [PubMed] [Google Scholar]
- [310].Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, Silver M, Krainin JG, Weinberg DH, Ropper AH, Isner JM. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest 2001;107(9):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Scott JN, Clark AW, Zochodne DW. Neurofilament and tubulin gene expression in progressive experimental diabetes: failure of synthesis and export by sensory neurons. Brain 1999;122 (Pt 11):2109–2118. [DOI] [PubMed] [Google Scholar]
- [312].Segerdahl AR, Themistocleous AC, Fido D, Bennett DL, Tracey I. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain 2018;141(2):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Selvarajah D, Wilkinson ID, Emery CJ, Harris ND, Shaw PJ, Witte DR, Griffiths PD, Tesfaye S. Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care 2006;29(12):2664–2669. [DOI] [PubMed] [Google Scholar]
- [314].Selvarajah D, Wilkinson ID, Fang F, Sankar A, Davies J, Boland E, Harding J, Rao G, Gandhi R, Tracey I, Tesfaye S. Structural and Functional Abnormalities of the Primary Somatosensory Cortex in Diabetic Peripheral Neuropathy: A Multimodal MRI Study. Diabetes 2019;68(4):796–806. [DOI] [PubMed] [Google Scholar]
- [315].Selvarajah D, Wilkinson ID, Gandhi R, Griffiths PD, Tesfaye S. Microvascular perfusion abnormalities of the Thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care 2011;34(3):718–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Seneviratne KN, Peiris OA. The effect of ischaemia on the excitability of human sensory nerve. J Neurol Neurosurg Psychiatry 1968;31(4):338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Serra J, Duan WR, Locke C, Sola R, Liu W, Nothaft W. Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in C-nociceptors in patients with painful diabetic neuropathy: a randomized controlled trial. Pain 2015;156(11):2175–2183. [DOI] [PubMed] [Google Scholar]
- [318].Shevalye H, Yorek MS, Coppey LJ, Holmes A, Harper MM, Kardon RH, Yorek MA. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J Neurophysiol 2015;114(1):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Shi X, Qiu Z, Inam UL, Zhang M, Li K, Wu P, Suleman R, Aadil RM, Piao F. The microRNAs Expression Profile in Sciatic Nerves of Diabetic Neuropathy Rats After Taurine Treatment by Sequencing. Adv Exp Med Biol 2019;1155:935–947. [DOI] [PubMed] [Google Scholar]
- [320].Shillo P, Selvarajah D, Greig M, Gandhi R, Rao G, Wilkinson ID, Anand P, Tesfaye S. Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet Med 2019;36(1):44–51. [DOI] [PubMed] [Google Scholar]
- [321].Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, Gandhi R, Wilkinson ID, Tesfaye S. Painful and Painless Diabetic Neuropathies: What Is the Difference? Curr Diab Rep 2019;19(6):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Shimoshige Y, Enomoto R, Aoki T, Matsuoka N, Kaneko S. The involvement of aldose reductase in alterations to neurotrophin receptors and neuronal cytoskeletal protein mRNA levels in the dorsal root ganglion of streptozotocin-induced diabetic rats. Biol Pharm Bull 2010;33(1):67–71. [DOI] [PubMed] [Google Scholar]
- [323].Shutov L, Kruglikov I, Gryshchenko O, Khomula E, Viatchenko-Karpinski V, Belan P, Voitenko N. The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats. Cell Mol Neurobiol 2006;26(7–8):1541–1557. [DOI] [PubMed] [Google Scholar]
- [324].Sima AA. C-peptide and diabetic neuropathy. Expert Opin Investig Drugs 2003;12(9):1471–1488. [DOI] [PubMed] [Google Scholar]
- [325].Sima AA. Encephalopathies: the emerging diabetic complications. Acta Diabetol 2010;47(4):279–293. [DOI] [PubMed] [Google Scholar]
- [326].Simeoli R, Fierabracci A. Insights into the Role of MicroRNAs in the Onset and Development of Diabetic Neuropathy. Int J Mol Sci 2019;20(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Singh B, Singh V, Krishnan A, Koshy K, Martinez JA, Cheng C, Almquist C, Zochodne DW. Regeneration of diabetic axons is enhanced by selective knockdown of the PTEN gene. Brain 2014;137(Pt 4):1051–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Singh J, Yousuf MS, Jones KE, Shelemey PTM, Joy T, Macandili H, Kerr BJ, Zochodne DW, Sauve Y, Ballanyi K, Webber CA. Characterization of the Nile Grass Rat as a Unique Model for Type 2 Diabetic Polyneuropathy. J Neuropathol Exp Neurol 2018;77(6):469–478. [DOI] [PubMed] [Google Scholar]
- [329].Skapare E, Konrade I, Liepinsh E, Strele I, Makrecka M, Bierhaus A, Lejnieks A, Pirags V, Dambrova M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J Diabetes Complications 2013;27(3):262–267. [DOI] [PubMed] [Google Scholar]
- [330].Slager UT. Diabetic myelopathy. Arch Pathol Lab Med 1978;102(9):467–469. [PubMed] [Google Scholar]
- [331].Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006;29(6):1294–1299. [DOI] [PubMed] [Google Scholar]
- [332].Smith BE. Focal and entrapment neuropathies. Handb Clin Neurol 2014;126:31–43. [DOI] [PubMed] [Google Scholar]
- [333].Sopacua M, Hoeijmakers JGJ, Merkies ISJ, Lauria G, Waxman SG, Faber CG. Small-fiber neuropathy: Expanding the clinical pain universe. J Peripher Nerv Syst 2019;24(1):19–33. [DOI] [PubMed] [Google Scholar]
- [334].Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Curr Diab Rep 2013;13(4):533–549. [DOI] [PubMed] [Google Scholar]
- [335].Spallone V, Morganti R, D’Amato C, Cacciotti L, Fedele T, Maiello MR, Marfia G. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain 2011;15(2):153–160. [DOI] [PubMed] [Google Scholar]
- [336].Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ, Srivastava S, Ramana KV. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact 2011;191(1–3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Stark B, Carlstedt T, Cullheim S, Risling M. Developmental and lesion-induced changes in the distribution of the glucose transporter Glut-1 in the central and peripheral nervous system. Exp Brain Res 2000;131(1):74–84. [DOI] [PubMed] [Google Scholar]
- [338].Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig 2017;8(5):646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes 2008;116(10):600–605. [DOI] [PubMed] [Google Scholar]
- [340].Stuart CA, Wen G, Peng BH, Popov VL, Hudnall SD, Campbell GA. GLUT-3 expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2000;279(4):E855–861. [DOI] [PubMed] [Google Scholar]
- [341].Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des 2008;14(10):953–961. [DOI] [PubMed] [Google Scholar]
- [342].Suo M, Wang P, Zhang M. Role of Fyn-mediated NMDA receptor function in prediabetic neuropathy in mice. J Neurophysiol 2016;116(2):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Tack CJ, van Gurp PJ, Holmes C, Goldstein DS. Local sympathetic denervation in painful diabetic neuropathy. Diabetes 2002;51(12):3545–3553. [DOI] [PubMed] [Google Scholar]
- [344].Takaku S, Yako H, Niimi N, Akamine T, Kawanami D, Utsunomiya K, Sango K. Establishment of a myelinating co-culture system with a motor neuron-like cell line NSC-34 and an adult rat Schwann cell line IFRS1. Histochem Cell Biol 2018;149(5):537–543. [DOI] [PubMed] [Google Scholar]
- [345].Talbot S, Chahmi E, Dias JP, Couture R. Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J Neuroinflammation 2010;7(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Tan AM, Samad OA, Dib-Hajj SD, Waxman SG. Virus-Mediated Knockdown of Nav1.3 in Dorsal Root Ganglia of STZ-Induced Diabetic Rats Alleviates Tactile Allodynia. Mol Med 2015;21:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Tang Y, Lenzini PA, Pop-Busui R, Ray PR, Campbell H, Perkins BA, Callaghan B, Wagner MJ, Motsinger-Reif AA, Buse JB, Price TJ, Mychaleckyj JC, Cresci S, Shah H, Doria A. A Genetic Locus on Chromosome 2q24 Predicting Peripheral Neuropathy Risk in Type 2 Diabetes: Results From the ACCORD and BARI 2D Studies. Diabetes 2019;68(8):1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Teixeira JM, Dos Santos GG, Neves AF, Athie MCP, Bonet IJM, Nishijima CM, Farias FH, Figueiredo JG, Hernandez-Olmos V, Alshaibani S, Tambeli CH, Muller CE, Parada CA. Diabetes-induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia. Neuroscience 2019;398:158–170. [DOI] [PubMed] [Google Scholar]
- [349].Thakur V, Gonzalez M, Pennington K, Chattopadhyay M. Viral vector mediated continuous expression of interleukin-10 in DRG alleviates pain in type 1 diabetic animals. Mol Cell Neurosci 2016;72:46–53. [DOI] [PubMed] [Google Scholar]
- [350].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice AS, Bennett DL. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016;157(5):1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Thomas PK. The morphological basis for alterations in nerve conduction in peripheral neuropathy. Proc R Soc Med 1971;64(3):295–298. [PMC free article] [PubMed] [Google Scholar]
- [352].Thornalley PJ. Glycation in diabetic neuropathy: characteristics, consequences, causes, and therapeutic options. Int Rev Neurobiol 2002;50:37–57. [DOI] [PubMed] [Google Scholar]
- [353].Tibbs GR, Posson DJ, Goldstein PA. Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol Sci 2016;37(7):522–542. [DOI] [PubMed] [Google Scholar]
- [354].Todorovic SM. Painful Diabetic Neuropathy: Prevention or Suppression? Int Rev Neurobiol 2016;127:211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res 2005;136(1–2):275–281. [DOI] [PubMed] [Google Scholar]
- [356].Tomlinson DR, Robinson JP, Willars GB, Keen P. Deficient axonal transport of substance P in streptozocin-induced diabetic rats. Effects of sorbinil and insulin. Diabetes 1988;37(4):488–493. [DOI] [PubMed] [Google Scholar]
- [357].Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia 2006;49(5):1081–1088. [DOI] [PubMed] [Google Scholar]
- [358].Toth C, Martinez J, Zochodne DW. RAGE, diabetes, and the nervous system. Curr Mol Med 2007;7(8):766–776. [DOI] [PubMed] [Google Scholar]
- [359].Truini A, Spallone V, Morganti R, Tamburin S, Zanette G, Schenone A, De Michelis C, Tugnoli V, Simioni V, Manganelli F, Dubbioso R, Lauria G, Lombardi R, Jann S, De Toni Franceschini L, Tesfaye S, Fiorelli M, Spagnoli A, Cruccu G. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain 2018;159(12):2658–2666. [DOI] [PubMed] [Google Scholar]
- [360].Tsantoulas C, Lainez S, Wong S, Mehta I, Vilar B, McNaughton PA. Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci Transl Med 2017;9(409):eaam6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Tsantoulas C, Mooney ER, McNaughton PA. HCN2 ion channels: basic science opens up possibilities for therapeutic intervention in neuropathic pain. Biochem J 2016;473(18):2717–2736. [DOI] [PubMed] [Google Scholar]
- [362].Tseng MT, Chiang MC, Chao CC, Tseng WY, Hsieh ST. fMRI evidence of degeneration-induced neuropathic pain in diabetes: enhanced limbic and striatal activations. Hum Brain Mapp 2013;34(10):2733–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia 2008;56(4):378–386. [DOI] [PubMed] [Google Scholar]
- [364].Ulugol A, Oltulu C, Gunduz O, Citak C, Carrara R, Shaqaqi MR, Sanchez AM, Dogrul A. 5-HT7 receptor activation attenuates thermal hyperalgesia in streptozocin-induced diabetic mice. Pharmacol Biochem Behav 2012;102(2):344–348. [DOI] [PubMed] [Google Scholar]
- [365].Umeda M, Ohkubo T, Ono J, Fukuizumi T, Kitamura K. Molecular and immunohistochemical studies in expression of voltage-dependent Ca2+ channels in dorsal root ganglia from streptozotocin-induced diabetic mice. Life Sci 2006;79(21):1995–2000. [DOI] [PubMed] [Google Scholar]
- [366].Urban MJ, Li C, Yu C, Lu Y, Krise JM, McIntosh MP, Rajewski RA, Blagg BS, Dobrowsky RT. Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro 2010;2(4):e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Urru M, Muzzi M, Coppi E, Ranieri G, Buonvicino D, Camaioni E, Coppini R, Pugliese AM, Tanaka B, Estacion M, Waxman SG, Dib-Hajj SD, Chiarugi A. Dexpramipexole blocks Nav1.8 sodium channels and provides analgesia in multiple nociceptive and neuropathic pain models. Pain 2019. [DOI] [PubMed] [Google Scholar]
- [368].Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain 2005;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Verkhratsky A, Fernyhough P. Calcium signalling in sensory neurones and peripheral glia in the context of diabetic neuropathies. Cell Calcium 2014;56(5):362–371. [DOI] [PubMed] [Google Scholar]
- [370].Viader A, Golden JP, Baloh RH, Schmidt RE, Hunter DA, Milbrandt J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J Neurosci 2011;31(28):10128–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Villegas-Rivera G, Roman-Pintos LM, Cardona-Munoz EG, Arias-Carvajal O, Rodriguez-Carrizalez AD, Troyo-Sanroman R, Pacheco-Moises FP, Moreno-Ulloa A, Miranda-Diaz AG. Effects of Ezetimibe/Simvastatin and Rosuvastatin on Oxidative Stress in Diabetic Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Oxid Med Cell Longev 2015;2015:756294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Villeneuve LM, Natarajan R. Epigenetics of diabetic complications. Expert Rev Endocrinol Metab 2010;5(1):137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 2009;58(10):2376–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal 2009;11(3):425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Vlassara H, Brownlee M, Cerami A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc Natl Acad Sci U S A 1981;78(8):5190–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Voitenko NV, Kruglikov IA, Kostyuk EP, Kostyuk PG. Effect of streptozotocin-induced diabetes on the activity of calcium channels in rat dorsal horn neurons. Neuroscience 2000;95(2):519–524. [DOI] [PubMed] [Google Scholar]
- [377].Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci 2005;1043:598–604. [DOI] [PubMed] [Google Scholar]
- [378].Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017;21(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Wahren J, Foyt H, Daniels M, Arezzo JC. Long-Acting C-Peptide and Neuropathy in Type 1 Diabetes: A 12-Month Clinical Trial. Diabetes Care 2016;39(4):596–602. [DOI] [PubMed] [Google Scholar]
- [380].Walker D, Carrington A, Cannan SA, Sawicki D, Sredy J, Boulton AJ, Malik RA. Structural abnormalities do not explain the early functional abnormalities in the peripheral nerves of the streptozotocin diabetic rat. J Anat 1999;195 (Pt 3):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Wang L, Chopp M, Lu X, Szalad A, Jia L, Liu XS, Wu KH, Lu M, Zhang ZG. miR-146a mediates thymosin beta4 induced neurovascular remodeling of diabetic peripheral neuropathy in type-II diabetic mice. Brain Res 2019;1707:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Wang XC, Wang S, Zhang M, Gao F, Yin C, Li H, Zhang Y, Hu SJ, Duan JH. Alpha-Dendrotoxin-sensitive Kv1 channels contribute to conduction failure of polymodal nociceptive C-fibers from rat coccygeal nerve. J Neurophysiol 2016;115(2):947–957. [DOI] [PubMed] [Google Scholar]
- [383].Wang XL, Zhang Q, Zhang YZ, Liu YT, Dong R, Wang QJ, Guo YX. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett 2011;490(2):112–115. [DOI] [PubMed] [Google Scholar]
- [384].Wang Y, Zhao X, Wu X, Dai Y, Chen P, Xie L. microRNA-182 Mediates Sirt1-Induced Diabetic Corneal Nerve Regeneration. Diabetes 2016;65(7):2020–2031. [DOI] [PubMed] [Google Scholar]
- [385].Wang-Fischer Y, Garyantes T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. J Diabetes Res 2018;2018:8054073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Watanabe K, Hirano S, Kojima K, Nagashima K, Mukai H, Sato T, Takemoto M, Matsumoto K, Iimori T, Isose S, Omori S, Shibuya K, Sekiguchi Y, Beppu M, Amino H, Suichi T, Yokote K, Uno T, Kuwabara S, Misawa S. Altered cerebral blood flow in the anterior cingulate cortex is associated with neuropathic pain. J Neurol Neurosurg Psychiatry 2018;89(10):1082–1087. [DOI] [PubMed] [Google Scholar]
- [387].Waterman RS, Morgenweck J, Nossaman BD, Scandurro AE, Scandurro SA, Betancourt AM. Anti-inflammatory mesenchymal stem cells (MSC2) attenuate symptoms of painful diabetic peripheral neuropathy. Stem Cells Transl Med 2012;1(7):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Wei H, Chapman H, Saarnilehto M, Kuokkanen K, Koivisto A, Pertovaara A. Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil-treated non-diabetic rat. Neuropharmacology 2010;58(3):578–584. [DOI] [PubMed] [Google Scholar]
- [389].Wei H, Viisanen H, Amorim D, Koivisto A, Pertovaara A. Dissociated modulation of conditioned place-preference and mechanical hypersensitivity by a TRPA1 channel antagonist in peripheral neuropathy. Pharmacol Biochem Behav 2013;104:90–96. [DOI] [PubMed] [Google Scholar]
- [390].Weiss N, Black SA, Bladen C, Chen L, Zamponi GW. Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch 2013;465(8):1159–1170. [DOI] [PubMed] [Google Scholar]
- [391].Wellmer A, Misra VP, Sharief MK, Kopelman PG, Anand P. A double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhBDNF) in diabetic polyneuropathy. J Peripher Nerv Syst 2001;6(4):204–210. [DOI] [PubMed] [Google Scholar]
- [392].Wickenden AD, McNaughton-Smith G. Kv7 channels as targets for the treatment of pain. Curr Pharm Des 2009;15(15):1773–1798. [DOI] [PubMed] [Google Scholar]
- [393].Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine-medicated plaster vs other relevant interventions and placebo for post-herpetic neuralgia (PHN): a systematic review. Acta Neurol Scand 2011;123(5):295–309. [DOI] [PubMed] [Google Scholar]
- [394].Wu YB, Xu DD, Zhu X, Yang GW, Ren MS. MiR-106a Associated with Diabetic Peripheral Neuropathy Through the Regulation of 12/15-LOX-meidiated Oxidative/Nitrative Stress. Curr Neurovasc Res 2017;14(2):117–124. [DOI] [PubMed] [Google Scholar]
- [395].Xu GY, Li GW, Liu NG, Huang LYM. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Molecular Pain 2011;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. Insulin as an in vivo growth factor. Exp Neurol 2004;188(1):43–51. [DOI] [PubMed] [Google Scholar]
- [397].Yagihashi S, Kamijo M, Baba M, Yagihashi N, Nagai K. Effect of aminoguanidine on functional and structural abnormalities in peripheral nerve of STZ-induced diabetic rats. Diabetes 1992;41(1):47–52. [DOI] [PubMed] [Google Scholar]
- [398].Yagihashi S, Nishihira M, Baba M. Morphometrical analysis of the peripheral nerve lesions in experimental diabetes rats. Tohoku J Exp Med 1979;129(2):139–149. [DOI] [PubMed] [Google Scholar]
- [399].Yamakawa I, Kojima H, Terashima T, Katagi M, Oi J, Urabe H, Sanada M, Kawai H, Chan L, Yasuda H, Maegawa H, Kimura H. Inactivation of TNF-alpha ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab 2011;301(5):E844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Yamamoto H, Shimoshige Y, Yamaji T, Murai N, Aoki T, Matsuoka N. Pharmacological characterization of standard analgesics on mechanical allodynia in streptozotocin-induced diabetic rats. Neuropharmacology 2009;57(4):403–408. [DOI] [PubMed] [Google Scholar]
- [401].Yamazaki S, Yamaji T, Murai N, Yamamoto H, Matsuda T, Price RD, Matsuoka N. FK1706, a novel non-immunosuppressive immunophilin ligand, modifies gene expression in the dorsal root ganglia during painful diabetic neuropathy. Neurol Res 2012;34(5):469–477. [DOI] [PubMed] [Google Scholar]
- [402].Yerra VG, Areti A, Kumar A. Adenosine Monophosphate-Activated Protein Kinase Abates Hyperglycaemia-Induced Neuronal Injury in Experimental Models of Diabetic Neuropathy: Effects on Mitochondrial Biogenesis, Autophagy and Neuroinflammation. Mol Neurobiol 2017;54(3):2301–2312. [DOI] [PubMed] [Google Scholar]
- [403].Yoon H, Thakur V, Isham D, Fayad M, Chattopadhyay M. Moderate exercise training attenuates inflammatory mediators in DRG of Type 1 diabetic rats. Exp Neurol 2015;267:107–114. [DOI] [PubMed] [Google Scholar]
- [404].Yorek MA. Alternatives to the Streptozotocin-Diabetic Rodent. Int Rev Neurobiol 2016;127:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Yousefzadeh N, Alipour MR, Soufi FG. Deregulation of NF-small ka, CyrillicB-miR-146a negative feedback loop may be involved in the pathogenesis of diabetic neuropathy. J Physiol Biochem 2015;71(1):51–58. [DOI] [PubMed] [Google Scholar]
- [406].Yu T, Li L, Liu H, Li H, Liu Z, Li Z. KCNQ2/3/5 channels in dorsal root ganglion neurons can be therapeutic targets of neuropathic pain in diabetic rats. Mol Pain 2018;14:1744806918793229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Zaharia OP, Kuss O, Strassburger K, Burkart V, Szendroedi J, Roden M. Diabetes clusters and risk of diabetes-associated diseases - Authors’ reply. Lancet Diabetes Endocrinol 2019;7(11):828–829. [DOI] [PubMed] [Google Scholar]
- [408].Zhang HH, Hu J, Zhou YL, Qin X, Song ZY, Yang PP, Hu SF, Jiang XH, Xu GY. Promoted Interaction of Nuclear Factor-kappa B With Demethylated Purinergic P2X3 Receptor Gene Contributes to Neuropathic Pain in Rats With Diabetes. Diabetes 2015;64(12):4272–4284. [DOI] [PubMed] [Google Scholar]
- [409].Zhang L, Qu S, Liang A, Jiang H, Wang H. Gene expression microarray analysis of the sciatic nerve of mice with diabetic neuropathy. Int J Mol Med 2015;35(2):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Zhang L, Zhao H, Blagg BS, Dobrowsky RT. C-terminal heat shock protein 90 inhibitor decreases hyperglycemia-induced oxidative stress and improves mitochondrial bioenergetics in sensory neurons. J Proteome Res 2012;11(4):2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Zhang W, Kamiya H, Ekberg K, Wahren J, Sima AA. C-peptide improves neuropathy in type 1 diabetic BB/Wor-rats. Diabetes Metab Res Rev 2007;23(1):63–70. [DOI] [PubMed] [Google Scholar]
- [412].Zhang Y, Song C, Liu J, Bi Y, Li H. Inhibition of miR-25 aggravates diabetic peripheral neuropathy. Neuroreport 2018;29(11):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [413].Zherebitskaya E, Schapansky J, Akude E, Smith DR, Van der Ploeg R, Solovyova N, Verkhratsky A, Fernyhough P. Sensory neurons derived from diabetic rats have diminished internal Ca2+ stores linked to impaired re-uptake by the endoplasmic reticulum. ASN Neuro 2012;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Zhuang HX, Wuarin L, Fei ZJ, Ishii DN. Insulin-like growth factor (IGF) gene expression is reduced in neural tissues and liver from rats with non-insulin-dependent diabetes mellitus, and IGF treatment ameliorates diabetic neuropathy. J Pharmacol Exp Ther 1997;283(1):366–374. [PubMed] [Google Scholar]
- [415].Ziegler D, Duan WR, An G, Thomas JW, Nothaft W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain 2015;156(10):2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Ziegler D, Landgraf R, Lobmann R, Reiners K, Rett K, Schnell O, Strom A. Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract 2018;139:147–154. [DOI] [PubMed] [Google Scholar]
- [417].Zochodne DW, Verge VM, Cheng C, Hoke A, Jolley C, Thomsen K, Rubin I, Lauritzen M. Nitric oxide synthase activity and expression in experimental diabetic neuropathy. J Neuropathol Exp Neurol 2000;59(9):798–807. [DOI] [PubMed] [Google Scholar]
- [418].Zochodne DW, Verge VM, Cheng C, Sun H, Johnston J. Does diabetes target ganglion neurones? Progressive sensory neurone involvement in long-term experimental diabetes. Brain 2001;124(Pt 11):2319–2334. [DOI] [PubMed] [Google Scholar]
- [419].Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, Hayward RA, Craven T, Coleman RL, Chalmers J, Collaborators on Trials of Lowering Glucose g. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017;5(6):431–437. [DOI] [PubMed] [Google Scholar]
- [420].Zychowska M, Rojewska E, Kreiner G, Nalepa I, Przewlocka B, Mika J. Minocycline influences the anti-inflammatory interleukins and enhances the effectiveness of morphine under mice diabetic neuropathy. J Neuroimmunol 2013;262(1–2):35–45. [DOI] [PubMed] [Google Scholar]
- [421].Zychowska M, Rojewska E, Pilat D, Mika J. The role of some chemokines from the CXC subfamily in a mouse model of diabetic neuropathy. J Diabetes Res 2015;2015:750182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Nalepa I, Mika J. Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target - Evidence from a mouse diabetic neuropathy model. Int Immunopharmacol 2017;52:261–271. [DOI] [PubMed] [Google Scholar]


