Abstract
Patient: Male, 29-year-old
Final Diagnosis: COVID provoked thromboembolism
Symptoms: Abdominal pain
Medication:—
Clinical Procedure: —
Specialty: Infectious Diseases • Radiology
Objective:
Rare co-existance of disease or pathology
Background:
In corona virus disease 2019 (COVID-19), which emerged in December 2019 and is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), most case presentations have been related to the respiratory tract. Several recent studies reveal that angiotensin-converting enzyme 2 (ACE2), which was found in the target cells of the virus, is highly expressed in the lungs, small bowel, and vasculature.
Case Report:
A 29-year-old male construction worker from India presented with left-sided colicky abdominal pain. He tested positive for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reverse transcription-polymerase chain reaction (RT-PCR). Isolated superior mesenteric vein thrombosis was diagnosed by CT (computed tomography) scan. He was managed by anti-coagulants and clinically improved.
Conclusions:
This case report indicates that isolated venous thrombosis of the abdominal vessels without concurrent arterial thrombosis can be a complication of the hyper-coagulability state in COVID-19 patients. Hence, early evaluation of abdominal vessels in covid-19 patients who present with any abdominal symptoms should be considered, especially when found to have an elevated D-dimer level, as early treatment of thrombosis with low-molecular-weight heparin can have a significant impact on the therapeutic outcome.
MeSH Keywords: COVID-19, SARS Virus, Venous Thrombosis
Background
Corona virus disease 2019 (Covid-19) is caused by the severe acute respiratory syndrome (SARS-CoV-2) virus, a beta-coronavirus, which emerged in Wuhan, Hubei, China in Dec 2019. The most common symptoms of the disease initially reported were related to the respiratory tract [1]. Venous thromboembolism has emerged as an important complication in hospitalized patients, and recent studies have shown that severe COVID-19 is associated with arterial and venous thromboembolic disease, which confers a poor prognosis. It was also found that the virus targets endothelial cells, which results in endothelial dysfunction [2].
Angiotensin-converting enzyme 2 (ACE2), which is the target of SARS-CoV-2, demonstrates high expression in the lungs, small bowel, and vasculature. The disease is now known to affect the cardiovascular and gastrointestinal systems, and is probably a multi-systemic disorder. There have been several case reports and case series on bowel ischemia secondary to mesenteric artery thrombosis, and 1 report described a combination of visceral arterial and venous thrombosis. Patients were usually older, and some had underlying medical conditions. We report a case of isolated acute thrombosis of the superior mesenteric vein in the absence of splanchnic arterial thrombosis, in a young COVID-19 patient who had no co-morbidities and was relatively healthy, with presentation as isolated abdominal discomfort.
Case Report
A 29-year-old male construction worker from India presented with left-sided colicky abdominal pain associated with nausea, vomiting, and decreased appetite. He did not have any acute respiratory symptoms.
His vitals were stable and he was febrile on admission. His blood pressure was 114/65 mmHg, heart rate 58/min, respiratory rate 15/min, temperature 37.1°C, and oxygen saturation 100% on room air. On examination, the abdomen was soft with mild tenderness over the left periumbilical region, without guarding.
Investigations
An initial abdominal radiograph did not reveal any renal calculi or signs of intestinal obstruction (Figure 1).
Figure 1.
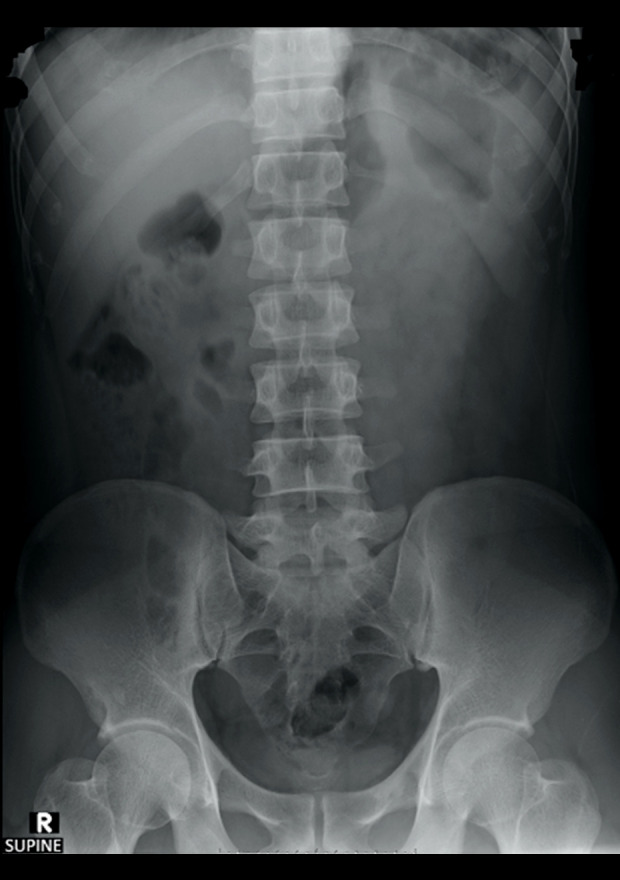
The abdominal radiograph on presentation was unremarkable.
Blood tests revealed elevated C-reactive protein (59.5 mg/L; reference range 0–5 mg/L), lactate dehydrogenase (275U/L; reference range 125–220 U/L), ferritin (279.1 ng/ml; reference range 21.8–275.0 ng/ml), and lactate (1.5 mmol/L; reference range 0.5–2.2 mmol/L). The full blood count, renal panel, liver function test, amylase, and bone metabolism panel were within normal limits.
Due to an outbreak of COVID-19 in the patient’s dormitory, SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) was performed on a nasopharyngeal swab using the A*STAR FORTITUDE KIT 2.0 COVID-19 Real-Time RT-PCR test. A chest radiograph was obtained, which revealed air-space opacities in the left lower zone (Figure 2). The COVID-19 tests returned positive for SARS-CoV-2 (RT-PCR) on the following day and the patient was isolated according to an established protocol.
Figure 2.

A chest radiograph revealed left lower-zone consolidation (arrow).
Due to his worsened abdominal pain and persistent vomiting, a CT scan of the abdomen and pelvis was requested and performed. The study was performed on a Siemens AS plus 64-slice scanner, which is our dedicated scanner for emergency and suspected covid-19 cases. Eighty ml of Omnipaque at a rate of 2 mL/s was used as intravenous contrast.
A long-segment filling defect was demonstrated in the superior mesenteric vein (SMV) (Figure 3). The superior mesenteric artery and other splanchnic vessels showed normal contrast opacification. There was also diffuse small-bowel wall thickening involving the jejunal loops, with adjacent mesenteric fat stranding secondary to mesenteric venous congestion (Figure 4). Minor ascites was also noted. The CT lung window of the bases demonstrated left basal peripheral consolidation (Figure 5) [3]. No bacterial infection was documented.
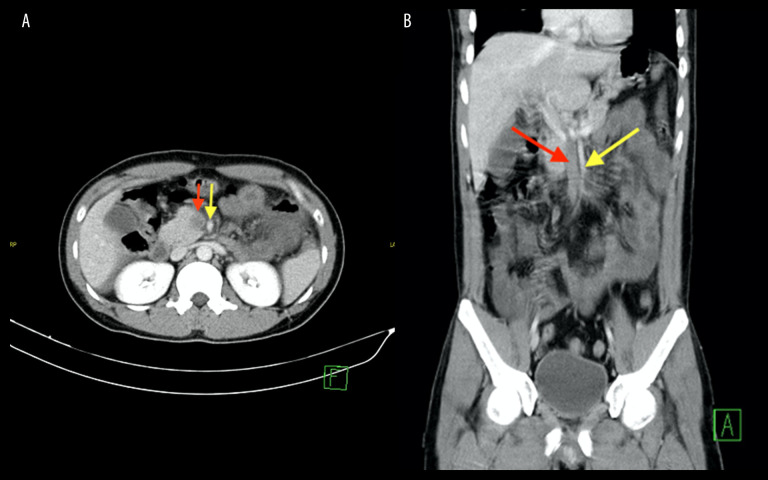
Figure 3.
The axial (A) and coronal (B) images of the abdomen reveal a large filling defect in the superior mesenteric vein (red arrow), in keeping with thrombosis. The superior mesenteric artery was noted to be patent (yellow arrow).
Figure 4.

An axial CT scan showed diffuse mural thickening of the small-bowel loops (arrow), with normal contrast enhancement, in keeping with inflammatory changes.
Figure 5.
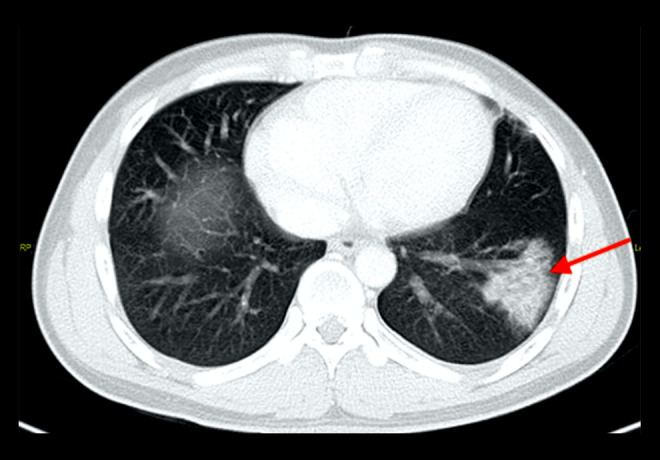
Axial CT scan of the lung base, confirming left lower-lobe consolidation (arrow), as seen on the chest radiograph.
The small-bowel wall thickening was due to mural edema and venous congestion secondary to back pressure from the SMV thrombosis and uninterrupted arterial supply. There was no bowel wall ischemia, as the arterial supply was patent.
A coagulation panel obtained after the diagnosis of SMV thrombosis revealed a D-dimer (>20.00 microg/ml; reference range: <0.5 microg/ml) and fibrinogen (4.65 g/L; reference range 1.95–4.51 g/L). Activated partial thromboplastin time (APTT) and prothrombin time (PT) were not prolonged, but lupus anticoagulant was present.
Management and outcome
The patient was then started on a therapeutic dose of enoxaparin, a low-molecular-weight heparin, 1 mg/kg twice a day by subcutaneous injection [4]. His abdominal pain gradually subsided as his inflammatory markers improved. He resumed a normal diet by day 6 of hospitalization. He did not require any supplemental oxygen.
Discussion
Hospitalized COVID-19 patients have high rates of vascular thromboembolism (VTE), particularly those who are critically ill [2]. In a Dutch study of 184 critically ill COVID-19 patients in the ICU, 31% developed thrombotic complications, with 27% developing pulmonary embolism and 3.7% developing ischemic stroke [5]. The thrombogenic state of COVID-19 patients is postulated to be due to a combination of direct viral infection of endothelial cells, which results in endothelial dysfunction, marked abnormalities in markers of hypercoagulability, including elevated levels of D-dimer, fibrinogen, Von Willebrand factor (vWF) activity, and vWF antigen (vWF: Ag), as well as factor VIII [2,6]. In addition, lupus anticoagulant is reported to be frequently found in patients with COVID-19, which may confer additional thrombotic risks [7]. Abdominal venous thrombosis, such as SMV thrombosis, is rarely reported [8,9].
Gastrointestinal symptoms in COVID-19 patients are largely non-specific, including abdominal pain, diarrhea, and vomiting [10]. Bhayana et al. reported that 17% of inpatients with abdominal symptoms went on to undergo cross-sectional imaging, usually associated with increasing age and intensive care unit admission [11]. Bowel wall abnormalities were shown on 31% of the CT scans, such as bowel wall thickening and pneumatosis. Signs of late ischemia were seen in 20% of CT scans in ICU patients (2.7% of ICU patients), for which the established etiology also was small-vessel arterial thrombosis.
Conclusions
This case indicates that isolated venous thrombosis of the abdominal vessels without concurrent arterial thrombosis can be a complication of hypercoagulability state in COVID-19 patients [12]. Therefore, early evaluation of abdominal vessels in covid-19 patients who present with abdominal symptoms should be considered, especially when there is an elevated D-dimer level, as early treatment of thrombosis with low-molecular-weight heparin can have a significant impact on the therapeutic outcome [13].
Footnotes
Department and Institution where work was done
Department of Radiology, Ng Teng Fong General Hospital, National University Health System (NUHS), Singapore, Singapore
Conflicts of interest
None.
References:
- 1.Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020;7:CD013665. doi: 10.1002/14651858.CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–98. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carotti M, Salaffi F, Sarzi-Puttini P, et al. Chest CT features of coronavirus disease 2019 (COVID-19) pneumonia: key points for radiologists. Radiol Med. 2020;125(7):636–46. doi: 10.1007/s11547-020-01237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–99. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–47. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azouz E, Yang S, Monnier-Cholley L, Arrivé L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med. 2020;46(7):1464–65. doi: 10.1007/s00134-020-06079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18(8):2064–65. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sellevoll HB, Saeed U, Young VS, et al. Acute abdomen as an early symptom of COVID-19. Covid-19 med akutte magesmerter som debutsymptom. Tidsskr Nor Laegeforen. 2020;140(7):tidsskr.20.0262. doi: 10.4045/tidsskr.20.0262. [DOI] [PubMed] [Google Scholar]
- 9.de Barry OD, Mekki A, Diffre C, et al. Arterial and venous abdominal thrombosis in a 79-year-old woman with covid-19 pneumonia. Radiol Case Rep. 2020;15(7):1054–57. doi: 10.1016/j.radcr.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong SH, Lui RNS, Sung JJY. Covid-19 and the digestive system. Gastroenterol Hepatol. 2020;35(5):744–48. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 11.Bhayana R, Som A, Li MD, et al. Abdominal imaging findings in COVID-19: Preliminary observations. Radiology. 2020 doi: 10.1148/radiol.2020201908. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchandot B, Trimaille A, Curtiaud A, et al. Thromboprophylaxis: balancing evidence and experience during the COVID-19 pandemic. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02231-3. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schunemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]