Abstract
Phosphorus reduction can prevent against vascular calcification (VC) in chronic kidney disease (CKD), but the mechanisms underlying its actions remain unclear. The aim of the present study was to determine the effect of a fortified phosphorus-lowing treatment on VC in CKD. Serum levels of creatinine, blood urea nitrogen (BUN), fibroblast growth factor 23 (FGF23), calcium and phosphorus, and the plasma levels of parathyroid hormone (PTH) were determined in an animal model of CKD treated with or without lanthanum. Haematoxylin and eosin (H&E) staining was performed to examine the structure of kidney tissues. Western blot analysis was performed to compare the levels of total- (t-) extracellular signal-related kinase (ERK) and phospho- (p-)ERK among the different experimental groups to investigate the effect of FGF23 on p-ERK expression. In the animal model, administration of adenine increased the serum levels of creatinine, BUN, FGF23 and phosphorus but decreased the serum levels of calcium. In addition, adenine treatment increased the plasma levels of PTH. H&E staining showed that lanthanum treatment did not alter the severity of renal cortex injury. Furthermore, the levels of t-ERK levels did not notably differ between the Adenine-free, Adenine-vehicle and Adenine-lanthanum groups, whereas the levels of p-ERK and aortic calcium in the Adenine-vehicle group were significantly upregulated. In addition, ectopic overexpression of FGF23 increased the levels of p-ERK, Msx2 and Osx in a dose-dependent manner. Furthermore, a total of 48 patients were enrolled in the present study. In the fortified group, the serum levels of FGF23, phosphorus and PTH were significantly reduced, whereas the serum levels of calcium were significantly increased, indicating an enhanced preventative effect in the fortified group. The results of the present study suggest that FGF23 may be used as a therapeutic target in the management and prevention of VC in CKD.
Keywords: phosphorus, lanthanum, FGF23, vascular calcification, chronic kidney disease
Introduction
Chronic kidney disease (CKD), which is defined by the estimated glomerular filtration rate (eGFR) and/or albuminuria, is recognized as a global health issue, the incidence, morbidity and mortality rates of which are increasing over the past two decades, and affects 10-16% of adults worldwide (1). Several patients with CKD also suffer from cardiovascular complications, which are major contributors to mortality in patients with late-stage CKD (2). Therefore, timely prevention, diagnosis and treatment of CKD, including the treatment of medical conditions and diseases that lead to CKD, are critical (1). Increased vascular calcification (VC) in patients with CKD resulted in poor overall survival as well as cardiovascular mortality and morbidity (3). In addition, hyperphosphatemia, (high levels of phosphorus in the blood), may severely damage the kidneys, bone, and heart (4). As calcium is leached from bones in hyperphosphatemia, it can result in weakened bones as well as calcium deposition in other tissues. In particular, calcium deposition in the vasculature may increase the risk of VC and CKD (4).
As a hormone that is primarily secreted by osteocytes, fibroblast growth factor 23 (FGF23) regulates the metabolism of vitamin D and phosphate. In particular, the serum levels of phosphorus are regulated by FGF23, which also regulates the production and secretion of parathyroid hormone (5). A previous study reported that both Klotho and canonical FGF receptors are required during FGF23 signalling (6). In addition, the serum levels of FGF23 gradually increases as kidney function declines (7). For example, the serum levels of FGF23 are only 2-5 times the physiological levels during the early stages of CKD, but may be >200 times the normal levels during the later stages of CKD (8). In addition, clinical studies have shown a positive correlation between the concentration of FGF23 and the levels of VC, cardiovascular risk and mortality in patients with CKD (8,9).
It has been shown that that the expression of both extracellular signal-related kinase (ERK) and AKT are increased by FGF23 (10,11). Furthermore, the activation of ERK accelerates VC, whereas the activation of both AKT and ERK inhibits VC (12,13). In addition, FGF23 activates ERK 1/2 in the parathyroid, kidney and Klotho-expressing cells (6). It is hypothesized that Klotho regulates FGF23 signalling through FGFR1 activation, which in-turn increases the phosphorylation of ERK1/2 and the transcription of the early growth response-1 gene in Klotho-expressing tissues, such as the parathyroid gland, pituitary gland and kidney (10). However, tissues which do not express Klotho are not affected by FGF23 (for example, skeletal muscle, adrenal gland, thymus, small intestine, stomach, spleen, lung, heart and liver) (6).
In the present study, an animal model of renal failure was established, which was then treated with a phosphorus lowering agent to evaluate its effect on VC. Furthermore, the effects of different phosphorus reducing treatments in an animal model of VC were assessed.
Materials and methods
Animal experiments
Male Wistar rats aged 12 weeks (72 rats, with an average weight of 400 g) were used in the present study; 24 rats were continuously fed the same diet and were used as non-uremic controls (Adenine-free group), and the remaining 48 rats received 0.75% adenine in addition to the same diet. All rats were treated for 4 weeks to initiate uraemia.
After oral administration and metabolism of adenine, it becomes 2,8-dihydroxyadenine, which can form tubular crystals and damage renal tissues (11). The 48 rats in the two treatment groups were administered either vehicle (Adenine-vehicle group, n=24) or lanthanum (Adenine-lanthanum group, n=24) within 24 h of application of adenine. Lanthanum was dissolved in a solution of 0.85% NaCl, 10 mM succinic acid and 0.9% benzyl alcohol (pH 4.5), and was subsequently given via subcutaneous (SC) injection at a dose of 0.3 mg/kg. The SC injection was given once a day for a total of 4 weeks. Furthermore, the 24 rats on the adenine diet were administered empty vehicles by SC once every day for a total of 4 weeks. All animal studies were performed in strict compliance with the Guide for the Care and Use of Laboratory Animals (14) and ethical approval was granted by the Ethics Committee of Dongguan People's Hospital Affiliated to Southern Medical University. No rats were lost during the study. The animals were sacrificed by a lethal intravenous dose of sodium pentobarbital (100 mg/kg) after completion of the experiment. Death was confirmed by lack of a pulse, breathing, corneal reflex, response to toe pinch and a lack of respiratory sounds and heartbeat. In addition, percutaneous cardiac puncture can be performed after the animal is unconscious. If the needle and attached syringe do not move after insertion into the heart (aspiration of blood provides evidence of correct location), this suggests indicates lack of cardiac muscle movement and thus, death.
Serum and plasma analysis
A final blood sample of 2 ml was obtained from the left jugular vein of each subject. The plasma levels of PTH were measured using a PTH ELISA kit (cat. no. 60-2305; Immunotopics, Inc.). In addition, serum levels of FGF23 and PTH were measured using a PTH-FGF23 magnetic bead assay (EMD Millipore) according to the manufacturer's protocol. The serum levels of creatinine were measured using a creatinine enzymatic assay (cat. no. c7548; Pointe Scientific, Inc.). The levels of total calcium, blood urea nitrogen (BUN), and phosphorus were measured using a blood chemistry analyser (Olympus AU 400; Olympus Corporation).
Histology of the kidneys
After 30 days post-operation, the animals were anaesthetized using a mixture of ketamine (35 mg/kg), xylazine (5 mg/kg), and acepromazine (1 mg/kg) and the kidney cortex tissue samples were removed and fixed in 10% neutral buffered formalin (NBF) for 48 h at room temperature, embedded in paraffin, sliced into 4 µm sections, and stained with haematoxylin and eosin. Animals were sacrificed using a lethal intravenous dose of sodium pentobarbital (100 mg/kg). Kidney tubulointerstitial damage was evaluated semi-quantitatively under a light microscope, and a score of 0-5 was assigned to each sample based on the magnitude of tubulointerstitial damage in the renal cortex: 0, normal; grade 1, <10%; grade 2, 10-25%; grade 3, 25-50%; grade 4, 50-75%; and grade 5, 75-100%.
Histology of aortic tissues
After 30 days post-operation, the animals were anaesthetized and the aortic arch containing 1 cm of descending aortic tissue was harvested from each animal and fixed in 10% NBF. Subsequently, paraffin-embedded longitudinal sections (4-µm in thickness) were prepared and subjected to von Kossa staining (15) to assess the condition of calcified lesions. Semi-quantitative histological scoring of aortic wall mineralization was performed based on the following criteria: Grade 1, minimal = detectible patchy focal vascular mineralization of the tunica media; grade 2, mild=<10% of the entire aortic sample was mineralized; grade 3, moderate=10-50% of the entire aortic sample was mineralized; and grade 4, significant >50% of the entire sample was mineralized.
Human subjects
A total of 48 subjects with a median age of 52.8 years (range, 46-50 years) were enrolled in the present study and observed for 24 weeks. All subjects were aged 18 years or older, and the inclusion criteria were as follows: i) Subjects have been treated with haemodialysis for ≥3 months; ii) plasma concentration of intact PTH (iPTH) >300 pg/ml; iii) plasma concentration of bio-iPTH (biPTH) >160 pg/ml; iv) in the case of iPTH and biPTH concentrations of 150-300 and 80-160 pg/ml, respectively, the albumin-corrected value for the calcium-phosphorus product was >50 mg2/dl2 during the treatment with vitamin D sterols; and v) albumin-corrected serum calcium ≥8.4 mg/dl. The exclusion criteria were as follows: i) Subjects that had been previously treated with lanthanum carbonate or calcium-free phosphate-binding substances, including aluminium hydroxide, lanthanum carbonate, or sevelamer hydrochloride within 30 days prior to enrolment; ii) subjects that were previously treated with bisphosphonates, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors and other cholesterol reducing drugs within 30 days prior to enrolment; iii) subjects with a thoracic aortic aneurysm, cardiac transplantation, aortic/cardiac valve replacement, coronary artery stent, coronary artery bypass grafting, or active atrial fibrillation; iv) subjects who had undergone parathyroidectomy within the past 3 months or were anticipating parathyroidectomy in the following 6 months; v) subjects scheduled for kidney transplantation; vi) subjects with digestive disorders that may affect their ability to receive oral medications; and vii) subjects with known allergies to lanthanum carbonate. The present study was approved by the Institutional Ethics Review Committee of Dongguan People's Hospital Affiliated to Southern Medical University. The characteristics of all participants are presented in Table I. Written informed consent was obtained from each subject prior to the start of this study. This study was performed in accordance with the principles of the Declaration of Helsinki (12).
Table I.
Patient characteristics.
| Characteristics | Regular group, n=40 | Fortified group, n=40 | P-value |
|---|---|---|---|
| Age, years | 52.5±5.3 | 53.1±6.2 | 0.61 |
| Sex, male, n (%) | 27 (67.5) | 28 (70.0) | 0.84 |
| CKD etiology, n (%) | 0.81 | ||
| Diabetes | 5 (12.5) | 6 (15.0) | |
| Hypertension | 5 (12.5) | 5 (12.5) | |
| IgAN | 19 (47.5) | 20 (50.0) | |
| Chronic GN (excluding IgAN) | 3 (7.5) | 3 (7.5) | |
| Others | 8 (20.0) | 6 (15.0) | |
| Systolic BP, mmHg | 120.8±10.4 | 125.8±13.5 | 0.73 |
| Diastolic BP, mmHg | 72.3±3.9 | 74.3±5.6 | 0.64 |
CKD, chronic kidney disease; IgAN, Immunoglobulin A nephropathy; GN, glomerulonephritis; BP, blood pressure.
Design of the human study
Subjects were randomly divided into two groups, the regular phosphorus treatment group (regular group, n=24) and the fortified phosphorus treatment group (fortified group, n=24). The subjects in both groups were exclusively treated with lanthanum carbonate to minimize possible confounding effects caused by other phosphate-binding drugs. In the regular group, the subjects were treated with 750 mg/day lanthanum carbonate. In the fortified group, the dose of lanthanum carbonate started at 750 mg/day, and increased sequentially to 1,500, 2,250 and 3,000 mg/day in 1-week intervals. The flow chart of the design of the human study is shown in Fig. 1. During the study period, no subjects dropped out of the research. Therefore, all 48 subjects were included for analysis.
Figure 1.
Flow diagram of participant enrolment performed according to the guidelines recommended by Consolidated Standards of Reporting Trials. CDK, chronic kidney disease; PO4, phosphate; MSCT, multi-slice spiral computed tomography; FGF23, fibroblast growth factor 23.
Evaluation of human subjects
Serum samples were collected from all subjects, and the levels of FGF23, calcium, phosphorus and PTH were determined using a blood chemistry analyser. The status of vascular calcification was evaluated and scored using multi-slice spiral computed tomography (MSCT) as described previously (13).
Western blot analysis
To evaluate the effect of FGF23 on the levels of phospho- (p-)ERK in humans and rats, VSMCs were treated as follows: i) 5 and 10 ng FGF23 and evaluated at 30 min; ii) 5 ng FGF23 and evaluated after 0, 15 and 30 min. Tissue and cell samples were first frozen in liquid nitrogen and then lysed using a lysis buffer (Vazyme Biotech Co., Ltd.). Subsequently, the samples were centrifuged at 241.49 x g for 15 min at 4°C to remove debris. The supernatant from each sample was collected, and its protein concentration was measured using a bicinchoninic acid kit (Beyotime Institute of Biotechnology). An appropriate quantity of protein sample was then mixed with 2X SDS loading buffer and boiled at 100°C for 5 min. Subsequently, 50 µg protein sample was loaded per lane onto a 10% SDS gel and resolved by SDS-PAGE, transferred to a PVDF membrane, which was then blocked with 5% skimmed milk for 1 h at room temperature and incubated overnight at 4°C with diluted (1:100) primary antibodies against total- (t-)ERK (cat. no. 4695, 1:5,000; Cell Signalling Technology), p-ERK (cat. no. 4370, 1:5,000; Cell Signalling Technology), Msx2, Osx (cat. no. sc-393325, 1:5,000; Santa Cruz Biotechnology) or β-actin (cat. no. 3700, 1:5,000; Cell Signalling Technology). Subsequently, the membrane was washed 3 times with Tris(-HCl)-buffered saline + Tween-20, 0.1%, and incubated at room temperature for 1 h with a goat-anti-rabbit horseradish peroxidase-conjugated secondary antibody (cat. no. ab6721; 1:12,000; Abcam). The membrane was then soaked in an enhanced chemiluminescence reagent (Biomiga, Inc.). After the removal of excess liquid, signals were visualized using X-ray film. The ratio between the mean optical density (OD) value of the target protein and the mean OD value of β-actin was calculated as the relative expression of the p-ERK and t-ERK. Each experiment was performed three times. Densitometry analysis was performed using Quantity One (version 4.6.6; Bio-Rad Laboratories, Inc.) software.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (IBM, Corp.). Data are shown as the mean ± standard deviation. The differences between two groups were compared using a Student's t-test, whereas the differences among several groups were compared using a one-way ANOVA, with a post-hoc Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Serum levels of creatinine, BUN, FGF23, calcium, phos- phorus and the plasma levels of PTH were compared among the different animal groups
As shown in Fig. 2, the initial serum levels of creatinine (Fig. 2A) and BUN (Fig. 2B) were comparable among the Adenine-free, Adenine-vehicle and Adenine-lanthanum groups. After 2 weeks of adenine administration, the serum levels of creatinine (Fig. 2A) and BUN (Fig. 2B) in the Adenine-vehicle and Adenine-lanthanum groups were significantly increased. After 4 weeks of adenine administration, the levels of serum creatinine (Fig. 2A) and BUN (Fig. 2B) in all rats on the adenine diet were significantly higher compared with the values after 2 weeks. Furthermore, the levels of serum creatinine (Fig. 2A) and BUN (Fig. 2B) in the Adenine-lanthanum group were significantly lower compared with the Adenine-vehicle group, suggesting that lanthanum treatment alleviated uraemia. As shown in Fig. 2C, both uremic groups displayed significantly higher levels of plasma PTH compared with those of the non-uremic controls in week 4. Furthermore, the Adenine-lanthanum group exhibited significantly lower levels of plasma PTH compared with the Adenine-vehicle group in week 4, when the serum levels of FGF23 in uremic rats was significantly higher compared with the non-uremic rats (Fig. 2D). In addition, the serum levels of FGF23 were significantly reduced in the Adenine-lanthanum group compared with the Adenine-vehicle group, and the serum levels of total calcium in the Adenine-lanthanum group was significantly reduced in week 4 (Fig. 2E). Finally, in both weeks 2 and 4, the serum levels of phosphorus in uremic rats was significantly higher compared with the non-uremic rats, although the serum phosphorus levels were the highest in the Adenine-vehicle group (Fig. 2F).
Figure 2.
Serum levels of creatinine, BUN, FGF23, calcium and phosphorus, and the plasma levels of PTH were compared among the different groups. (A) Serum levels of creatinine, (B) BUN, (C) FGF23, (D) calcium and (E) phosphorus were compared among the Adenine-free, Adenine-vehicle and Adenine-lanthanum groups. (F) Plasma levels of PTH were compared among the Adenine-free, Adenine-vehicle and Adenine-lanthanum groups. BUN, blood urea nitrogen; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone. *P<0.05, **P<0.05 vs. Adenine-lanthanum group.
Kidney histology among the four animal groups
The damage to kidney tissues presented as tubular necrosis, degeneration, dilation, intratubular cellular debris, epithelial attenuation, and crystal deposition. The kidney tissues collected from the rats fed the adenine diet (Fig. 3B and C) exhibited a similar pattern of renal cortex damage and moderate-to-severe diffuse tubulointerstitial injuries (Fig. 3A). The interstitium in all groups was expanded and infiltrated by fibroblasts and inflammatory cells. Treatment with lanthanum failed to reduce the severity and incidence of kidney injuries.
Figure 3.
Histological staining of the three animal groups. (A) Rats on a non-adenine diet exhibited normal kidney histology results. (B) Rats in the Adenine-vehicle group showed damaged kidney tissues. (C) Rats in the Adenine-lanthanum group showed kidney damage similar to that of rats in the Adenine-vehicle group. Arrows highlight the kidney tubulointerstitium.
Levels of t-ERK, p-ERK and aortic calcium among the different animal groups
The protein levels of t-ERK in kidney cortex tissues collected from the Adenine-free, Adenine-vehicle and Adenine-lanthanum groups were comparable (Fig. 4A). The levels of p-ERK (Fig. 4A) and aortic calcium (Fig. 4C) in the Adenine-vehicle group were significantly higher compared with the Adenine-lanthanum group. In addition, the levels of p-ERK in the Adenine-lanthanum group was slightly but not significantly higher compared with the Adenine-free group.
Figure 4.
Protein expression levels of t-ERK, p-ERK and aortic calcium content in the different animal groups. (A) t-ERK levels were similar among Adenine-free, Adenine-vehicle and Adenine-lanthanum groups, whereas p-ERK levels were higher in the Adenine-lanthanum group compared with the Adenine-vehicle group. (B) Aortic calcium content was significantly higher in the Adenine-lanthanum group compared with the Adenine-vehicle group. (C) Aortic calcium content was significantly higher in the Adenine-lanthanum group compared with the Adenine-vehicle group. p-, phospho-; t-, total-; ERK, extracellular signal-related kinase.
Effect of FGF23 on ERK phosphorylation and osteoblast differentiation
The effects of FGF23 on ERK phosphorylation in human and rat vascular smooth muscle cells (VSMCs) were evaluated by treating the cells with different doses of FGF23 (5 or 10 ng). In addition, the effects of FGF23 on the levels of p-ERK in human and rat VSMCs were evaluated at different time points (0, 15 and 30 min) by treating the cells with 5 ng of FGF23. As shown in Figs. 5A and 6A, FGF23 increased the protein expression levels of p-ERK in a dose-dependent manner in both human (Fig. 5A) and rat (Fig. 6A) VSMCs. In addition, FGF23 treatment significantly increased the protein expression levels of p-ERK in both human (Fig. 5B) and rat (Fig. 6B) VSMCs in a time-dependent manner. To further determine whether FGF23 affected the levels of osteoblastic markers, the cells were treated with different doses of FGF23 and the levels of Msx2 and Osx were measured. The protein expression levels of Msx2 (Figs. 5C and 6C) and Osx (Figs. 5D and 6D) were increased in both human and rat VSMCs in a dose-dependent manner.
Figure 5.
Effects of different doses of FGF23 on ERK phosphorylation and osteoblastic differentiation in human VSMCs. FGF23 increased the protein expression levels of p-ERK in a (A) dose- and (B) time-dependent manner. FGF23 increased the protein expression levels of (C) Msx2 and (D) Osx2 in a dose-dependent manner. *P<0.05 vs. 0 ng/ml FGF23 treatment. FGF23, fibroblast growth factor 23; ERK, extracellular signal-related kinase; VSMC, vascular smooth muscle cell; p-, phospho-; t-, total-.
Figure 6.
Effects of different doses of FGF23 on ERK phosphorylation and osteoblastic differentiation in rat VSMCs. FGF23 increased the protein expression levels of p-ERK in a (A) dose- and (B) time-dependent manner. FGF23 increased the protein expression levels of (C) Msx2 and (D) Osx2 in a dose-dependent manner. *P<0.05 vs. 0 ng/ml FGF23 treatment. FGF23, fibroblast growth factor 23; ERK, extracellular signal-related kinase; VSMC, vascular smooth muscle cell; p-, phospho-; t-, total-.
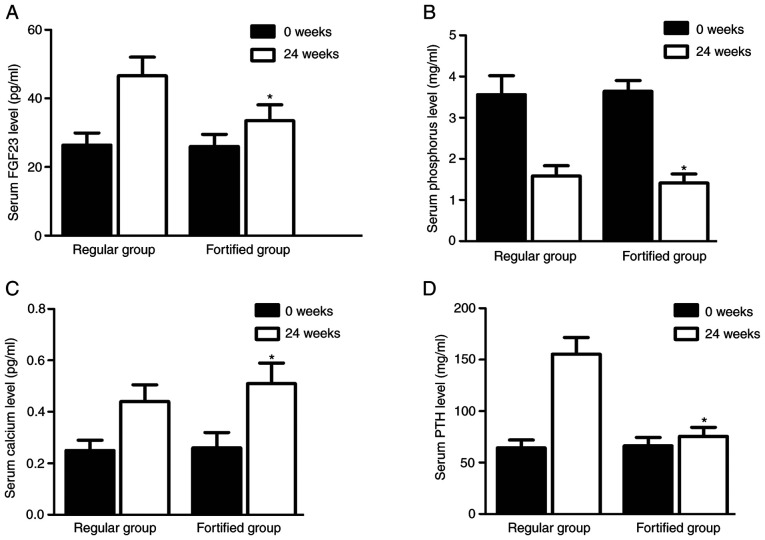
Serum levels of FGF23, calcium, phosphorus and PTH were different between the two groups
Serum levels of FGF23 (Fig. 7A), calcium (Fig. 7B), phosphorus (Fig. 7C) and PTH (Fig. 7D) in the regular group were similar to those in the fortified group at the beginning of the experiment. However, the serum levels of FGF23 (Fig. 7A), phosphorus (Fig. 7B) and PTH (Fig. 7D) in the fortified group were significantly lower compared with the regular group 24 weeks later, whereas the serum levels of calcium (Fig. 7C) in the fortified group were significantly higher compared with the regular group.
Figure 7.
Serum levels of FGF23, calcium, phosphorus and PTH differed in the different patient groups. After 2 weeks of phosphorus reduction treatment, (A) the serum levels of FGF23 in the fortified group were significantly lower compared with the regular group; (B) the serum levels of calcium in the fortified group were significantly higher compared with the regular group; (C) the serum levels of phosphorus in the fortified group were significantly lower compared with the regular group; and (D) the serum levels of PTH in the fortified group were significantly lower compared with the regular group. *P<0.05 vs. regular group at 0 weeks. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone.
Effect of phosphorus-lowering treatment on vascular calci- fication
The levels of vascular calcification were evaluated by MSCT and scored accordingly. The preventative effect of fortified phosphorus-lowering treatment was significantly greater compared with the regular phosphorus-lowering treatment (Fig. 8).
Figure 8.
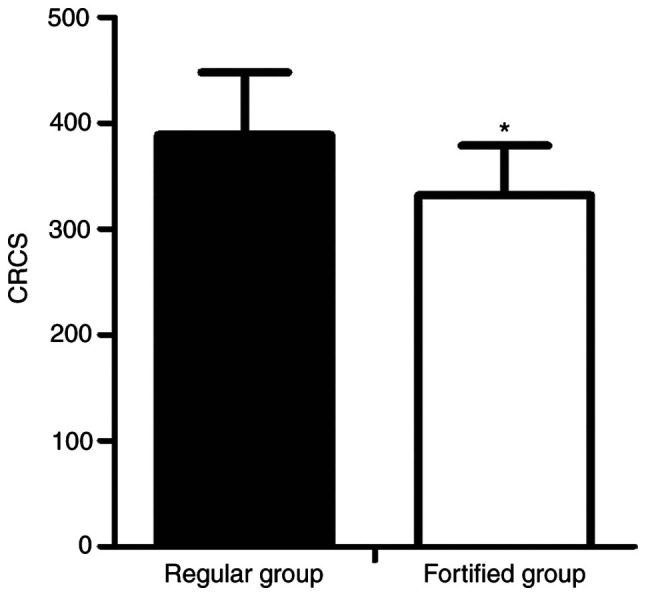
Preventative effect of fortified phosphorus-lowering treatment was significantly improved compared with regular phosphorus-lowering treatment. *P<0.05 vs. 0 ng/ml FGF23 treatment. CRCS, coronary artery calcium score.
Discussion
VC is characterized by arterial wall thickening and loss of elasticity (16). VC associated with CKD is an active and complex pathological process. Inhibitors and promoters of VC are regulated by a wide range of cellular processes (17). Although patients with CKD are more likely to suffer from medial calcification, VC is not a rare occurrence in CKD patients (17). In particular, as CKD progresses, the incidence of VC increases due to decreased kidney functions (18). As VC has several adverse effects, including ischaemic cardiac diseases, it poses a significant risk to the health of patients with CKD (19). During CKD, decreased phosphorus elimination by renal filtration may result in an increased phosphorus load during the early phase of CKD, although symptoms of hyperphosphatemia are usually not apparent until the later stages (20). In addition, an increased level of phosphorus during CKD initiates a wide range of different endocrine responses, such as increased secretion of FGF23, which causes secondary hyperparathyroidism, cardiovascular diseases, renal failure, ectopic calcification and metabolic bone diseases (21). In addition, hyperphosphatemia results in increased mortality rates among patients undergoing dialysis (22). However, the restriction on dietary phosphorus intake is no longer recommended as dietary phosphorus is positively correlated with the nutritional status of end-stage renal disease patients, and a reduction in phosphorus intake benefits survival (23). Therefore, drugs that are capable of reducing the blood levels of phosphorus are continuously used as the first-line treatment for hyperphosphatemia (24).
FGF23 is a 32 kDa protein primarily expressed and synthesized by osteocytes and osteoblasts (25). It has been shown that elevated levels of FGF23 results in hereditary hypophosphatemic diseases, such as autosomal dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets and X-linked hypophosphatemia, as well as acquired hypophosphatemic disorders, including tumour-induced osteomalacia. In addition, inhibition of FGF23 results in hyperphosphatemia and tumoral calcinosis, which manifests as increased levels of soft tissue calcifications and 1,25(OH)2D (26). Furthermore, targeting FGFR 1, 3 and 4 receptor complexes, transmembrane α-Kl and β glucuronidase suppresses the re-absorption of phosphate in the kidneys by reducing the number of Na+-dependent co-transporters (27). In addition, the inhibition of Cyp27b1, an enzyme that converts 25(OH)D to 1,25(OH)2D, and the stimulation of 1,25(OH)2D catabolism both reduce the quantity of circulating 1,25(OH)2D by increasing the activity of 24-hydroxylase (Cyp24) (28). FGF23 enhances the activity of several signal transduction pathways by interacting with the Klotho-FGF receptor complex (29). Furthermore, the levels of serum FGF23 are increased during early stage CKD before any measurable changes in serum levels of PTH and phosphate can be detected (30). By regulating the phosphate flux mediated by sodium/phosphate co-transporters 2a and 2c, Klotho protects cells against apoptosis and senescence (31). In particular, it was shown that Klotho reduces the severity of VC (32). Increased FGF23 expression and Klotho deficiency induced arterial stiffness and VC in animal experiments (10).
In the present study, the serum levels of creatinine, BUN FGF23, calcium and phosphorus as well as the plasma levels of PTH among three animal groups, the Adenine-free, Adenine-vehicle and Adenine-lanthanum groups were measured. The serum levels of creatinine, BUN, FGF23, calcium and phosphorus, and the plasma levels of PTH were different among the different groups.
It was previously shown both in vitro and in vivo that FGF23 induced ERK1/2 phosphorylation to subsequently enhance the activity of MAPK signalling and the synthesis of early growth response 1 proteins (6,27). As a member of the MAPK family, ERK is activated by a wide range of inflammatory cytokines and environmental stressors (33-36). Upon activation, ERK participates in the regulation of various cellular responses and intracellular signalling pathways as well as the pathogenesis of several diseases, such as rheumatoid arthritis (36). For example, in hereditary osteoarthritis, activation of discoidin domain receptor 2 by type II collagen increases the expression of MMP-13 through p38 and Ras/Raf/MEK/ERK signalling (37). In the present study, western blot analysis was performed to compare the levels of t-ERK and p-ERK among the different groups. The results showed that the levels of t-ERK were comparable among the groups. However, the levels of p-ERK and aortic calcium in the Adenine-vehicle group were significantly higher compared with the Adenine-lanthanum group.
The process of VC is similar to the process of bone mineralization, and the pathogenesis of VC also involves the synthesis of bone-related proteins, including type-I collagen and osteopontin, by VSMCs (38). It was also shown that through the ERK pathway and oxidative stress, VC is induced by advanced oxidation protein products (AOPPs) via the promotion of osteoblast differentiation of VSMCs (39). In the present study, FGF23 increased the levels of p-ERK in a dose-dependent manner, and that the effects of FGF23 increased in time-dependent manner. Furthermore, FGF23 also increased the levels of Msx2 and Osx in a dose-dependent manner in human and rat VSMCs. In 48 subjects stratified into two groups based on treatment, a regular group and a fortified group, serum levels of FGF23, phosphorus and PTH in the fortified group were reduced, whereas the serum levels of calcium in the fortified group was significantly increased. Thus, it was hypothesized that the changes in the serum levels of FGF23 were positively correlated with the changes in the serum levels of calcium in the fortified group but not in the regular group.
However, in the animal experiment, lanthanum treatment failed to ameliorate the severity and incidence of kidney injuries, but the treatment did decrease the levels of Scr and BUN. These paradoxical results may be attributed to the fact that several other factors other than Scr and BUN may influence kidney injuries. Therefore, in future studies, the involvement of additional mechanisms will be further investigated. One limitation of the present study was that the cohort recruited was limited in ethnicity. Thus, in future studies, larger, more diverse cohorts should be investigated.
In summary, it was shown that the reduction in phosphorus by lanthanum reduced the levels of FGF23 and the severity of VC in CKD. In addition, upregulation of FGF23 expression increased the serum levels of phosphorus. Furthermore, increased serum phosphorus concentrations were associated with progression of VC and increased risk of death in patients undergoing dialysis. These results suggest that targeting FGF23 may be beneficial in the management and prevention of VC in patients with CKD.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JJ and GL designed the study. YL and DZ collected the references. JJ, YL, DZ, ZW and HZ collected and analysed the data. JJ and GL wrote the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal studies were performed in strict compliance with the Guide for the Care and Use of Laboratory Animals and ethical approval was granted by the Ethics Committee of Dongguan People's Hospital Affiliated to Southern Medical University. Use of patient samples was approved by the Institutional Ethics Review Committee of Dongguan People's Hospital Affiliated to Southern Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kohl LP, Shroff GR, Herzog CA. Understanding risks associated with chronic kidney disease: Translating observational data to patient care. Clin Chem. 2013;59:876–879. doi: 10.1373/clinchem.2012.201012. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Invest. 2006;36(Suppl 2):51–62. doi: 10.1111/j.1365-2362.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 3.Massy ZA, Mazière C, Kamel S, Brazier M, Choukroun G, Tribouilloy C, Slama M, Andrejak M, Maziere JC. Impact of inflammation and oxidative stress on vascular calcifications in chronic kidney disease. Pediatr Nephrol. 2005;20:380–382. doi: 10.1007/s00467-004-1623-9. [DOI] [PubMed] [Google Scholar]
- 4.Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. 2013;98:6–15. doi: 10.3945/ajcn.112.053934. [DOI] [PubMed] [Google Scholar]
- 5.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism-unique biological characteristics of FGF23. Bone. 2007;40:1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki M, Ozono K, Okada T, Tachikawa K, Kondou H, Ohata Y, Michigami T. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J Cell Biochem. 2010;111:1210–1221. doi: 10.1002/jcb.22842. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Peripheral vascular calcification in long-haemodialysis patients: Associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 10.Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014;85:1103–1111. doi: 10.1038/ki.2013.332. [DOI] [PubMed] [Google Scholar]
- 11.Asadur R, Daisuke Y, Abu S, Kento K, Hirofumi H, Daisuke N, Akira N. A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS One. 2018;13:e0192531. doi: 10.1371/journal.pone.0192531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th edition. National Academies Press (US); Washington, DC: 2011. [Google Scholar]
- 15.Shima WN, Ali AM, Subramani T, Mohamed Alitheen NB, Hamid M, Samsudin AR, Yeap SK. Rapid growth and osteogenic differentiation of mesenchymal stem cells isolated from human bone marrow. Exp Ther Med. 2015;9:2202–2206. doi: 10.3892/etm.2015.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B, et al. Bone formation in carotid plaques: A clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.STR.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 17.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 19.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 20.Prié D, Torres PU, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–889. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 21.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: Evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770–779. doi: 10.1097/01.ASN.0000113243.24155.2F. [DOI] [PubMed] [Google Scholar]
- 23.Lynch KE, Lynch R, Curhan GC, Brunelli SM. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:620–629. doi: 10.2215/CJN.04620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kestenbaum B. Phosphate metabolism in the setting of chronic kidney disease: Significance and recommendations for treatment. Semin Dial. 2007;20:286–294. doi: 10.1111/j.1525-139X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 26.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–8472. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 29.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E. Mineral metabolism parameters throughout chronic kidney disease stages 1-5-achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 31.Dërmaku-Sopjani M, Sopjani M, Saxena A, Shojaiefard M, Bogatikov E, Alesutan I, Eichenmüller M, Lang F. Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of Klotho. Cell Physiol Biochem. 2011;28:251–258. doi: 10.1159/000331737. [DOI] [PubMed] [Google Scholar]
- 32.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 33.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 35.Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 2010;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 36.Thiel MJ, Schaefer CJ, Lesch ME, Mobley JL, Dudley DT, Tecle H, Barrett SD, Schrier DJ, Flory CM. Central role of the MEK/ERK MAP kinase pathway in a mouse model of rheumatoid arthritis: Potential proinflammatory mechanisms. Arthritis Rheum. 2007;56:3347–3357. doi: 10.1002/art.22869. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 38.Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 39.You H, Yang H, Zhu Q, Li M, Xue J, Gu Y, Lin S, Ding F. Advanced oxidation protein products induce vascular calcification by promoting osteoblastic trans-differentiation of smooth muscle cells via oxidative stress and ERK pathway. Ren Fail. 2009;31:313–319. doi: 10.1080/08860220902875182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.