Abstract
In the randomized, open-label, phase 3 CheckMate 214 trial, nivolumab plus ipilimumab (nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 wk for four doses, then nivolumab 3 mg/kg every 2 wk) had superior efficacy over sunitinib (SUN; 50 mg once daily, 4 wk on, 2 wk off) in patients with untreated International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate- or poor-risk advanced renal cell carcinoma; the benefits were sustained through extended follow-up. To better characterize the association between outcomes and IMDC risk in CheckMate 214, we completed a post hoc analysis (n = 1051) of efficacy by the number of IMDC risk factors. The investigator-assessed objective response rate (ORR), overall survival (OS), and investigator-assessed progression-free survival (PFS) according to Response Evaluation Criteria in Solid Tumors v1.1 were evaluated. ORR with nivolumab plus ipilimumab was consistent across zero to six IMDC risk factors, whereas with SUN it decreased with increasing number of risk factors. Benefits of nivolumab plus ipilimumab over SUN in terms of ORR (40–44% vs 16–38%), OS (hazard ratio [HR] 0.50–0.72), and PFS (HR 0.44–0.86) were consistently observed in subgroups with one, two, three, or four to six IMDC risk factors (p < 0.05 for treatment × no. of risk factors interaction). These results demonstrate the benefit of first-line nivolumab plus ipilimumab over SUN across all intermediate-risk and poor-risk groups, regardless of the number of IMDC risk factors.
Keywords: CheckMate 214, Ipilimumab, Nivolumab, Phase 3
Patient summary
This report from the CheckMate 214 study describes a consistent efficacy benefit with first-line nivolumab plus ipilimumab over first-line sunitinib in all groups of patients with intermediate-risk or poor-risk advanced renal cell carcinoma, regardless of the number of risk factors they had before starting treatment. We conclude that there is a benefit of first-line treatment with nivolumab plus ipilimumab for all intermediate-risk patients, including those with one or two risk factors, and for all poor-risk patients, independent of the number of risk factors.
CheckMate 214 is a phase 3, randomized, open-label trial of nivolumab plus ipilimumab followed by nivolumab monotherapy versus sunitinib in previously untreated advanced renal cell carcinoma (aRCC) [1]. At the prespecified interim analysis (minimum follow-up 17.5 mo) the trial met two of three primary endpoints: nivolumab plus ipilimumab showed better overall survival (OS) and objective response rate (ORR) over sunitinib in the primary efficacy population of patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate- or poor-risk aRCC according to an independent radiology review committee [1]. Median progression-free survival (PFS) was longer with nivolumab plus ipilimumab versus sunitinib, but the difference did not meet the prespecified boundary for statistical significance (α level of p < 0.009) [1].
At minimum follow-up of 30 mo, the OS benefit with nivolumab plus ipilimumab over sunitinib was maintained (hazard ratio [HR] 0.66, 95% confidence interval [CI] 0.54–0.80; p < 0.0001), investigator-assessed ORR remained better with nivolumab plus ipilimumab over sunitinib (42% vs 29%; p = 0.0001), and a delayed investigator-assessed PFS benefit, with separation of the curves favoring nivolumab plus ipilimumab (HR 0.77, 95% CI 0.65–0.90), was observed for patients with intermediate- or poor-risk disease [2]. Investigator-assessed ORR with nivolumab plus ipilimumab was consistent across IMDC risk groups (intermediate/poor, favorable, and intent-to-treat [ITT]), whereas the proportion achieving an objective response with sunitinib declined as risk increased [2]. To better characterize the association between outcomes and IMDC risk among patients with aRCC, we present a post hoc analysis of efficacy in CheckMate 214 by the number of IMDC risk factors.
The CheckMate 214 study design has been published previously [1,2]. In brief, patients with untreated aRCC with a clear cell component were randomized 1:1 to receive either nivolumab 3 mg/kg plus ipilimumab 1 mg/kg intravenously every 3 wk for four doses followed by nivolumab 3 mg/kg every 2 wk as maintenance therapy, or sunitinib 50 mg orally once daily for 4 wk on and 2 wk off in each 6-wk cycle. Patients were stratified by region and IMDC risk group (favorable = 0 risk factors vs intermediate = 1–2 risk factors vs poor = 3–6 risk factors) using an interactive voice response system at randomization. The six IMDC components were subsequently collected via case report forms that were used to identify the specific number of risk factors present for patients with intermediate or poor risk. Patients with four to six risk factors were pooled because of small patient numbers. In this analysis, we examined OS and investigator-assessed ORR and PFS according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 by the number of IMDC risk factors. We used a multivariate Cox model to evaluate the interaction between the number of IMDC risk factors and treatment for these outcomes.
To remove potential bias with imputations, our analysis was conducted using IMDC risk information available from case report forms, and 45 ITT patients were excluded because of discrepancies between the interactive voice response system and IMDC risk categorization on case report forms; a total of 1051 patients were included in the analysis. Of these patients, 24% had none, 58% had one, 42% had two, 58% had three, 29% had four, 10% had five, and 2.9% had six IMDC risk factors. Risk factors were generally balanced between the treatment arms (Supplementary Table 1).
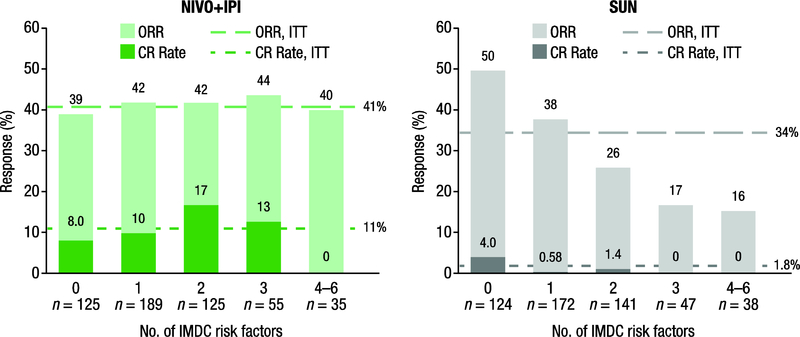
In a multivariate model, test for interaction between treatment and the number of IMDC risk factors were significant for ORR (p < 0.0001), OS (p = 0.0428), and PFS (p = 0.0033). For the complete response rate, the p value for the interaction was 0.0586. As shown in Figure 1, at minimum follow-up of 30 mo, ORR with nivolumab plus ipilimumab was consistent across zero to six risk factors (ranging from 39% to 44%) and was similar to the rate for ITT patients (41%) [2]. With sunitinib, ORR decreased as the number of risk factors increased (50–16%). Similarly, the complete response rate with nivolumab plus ipilimumab was consistent and higher than with sunitinib across zero to three risk factors (8.0–17% vs 0–4.0%; Fig. 1). No complete responses were observed in either arm among patients with the poorest risk (4–6 factors).
Fig. 1 –
Investigator-assessed ORR and best overall response per investigator by number of IMDC risk factors. CR = complete response; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium; ITT = intent-to-treat; NIVO+IPI = nivolumab + ipilimumab; ORR = objective response rate; SUN = sunitinib.
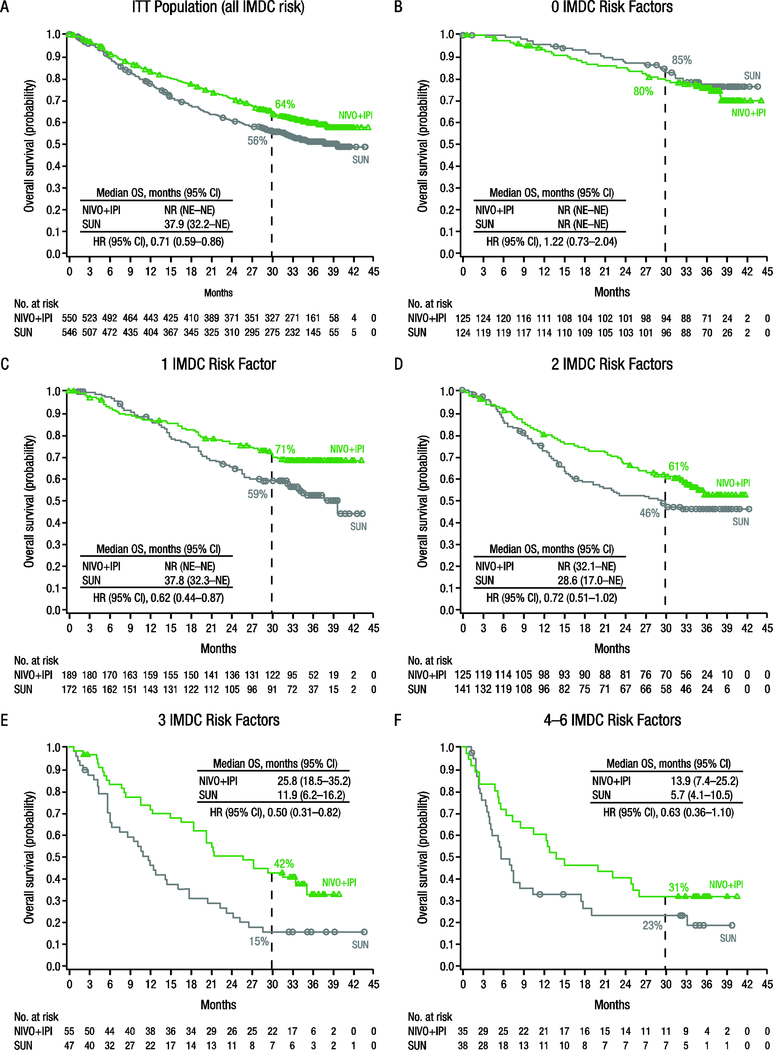
Median OS in the ITT population was longer with nivolumab plus ipilimumab than with sunitinib (HR 0.71, 95% CI 0.59–0.86; Fig. 2A) [2]. Median OS was not reached with either nivolumab plus ipilimumab or sunitinib among patients with zero risk factors (HR 1.22, 95% CI 0.73–2.04; Fig. 2B) [2]. Across one to six IMDC risk factors, OS was better with nivolumab plus ipilimumab versus sunitinib (HR 0.50–0.72; Fig. 2C–F). A benefit in investigator-assessed PFS was observed with nivolumab plus ipilimumab over sunitinib in the ITT population (HR 0.85, 95% CI 0.73–0.98; Supplementary Fig. 1A) [2]. Median PFS was longer with sunitinib versus nivolumab plus ipilimumab among patients with zero risk factors (HR 1.23, 95% CI 0.90–1.69; Supplementary Fig. 1B) [2]. Across one to six IMDC risk factors, PFS was better with nivolumab plus ipilimumab versus sunitinib (HR 0.44–0.86; Supplementary Fig. 1C–F).
Fig. 2 –
Overall survival by number of IMDC risk factors. CI = confidence interval; HR = hazard ratio; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium; ITT = intent-to-treat; NE = not estimable; NIVO+IPI = nivolumab + ipilimumab; NR = not reached; OS = overall survival; SUN = sunitinib.
These results show that the benefit of first-line nivolumab plus ipilimumab combination immunotherapy versus sunitinib is consistently observed across the number of IMDC risk factors among patients with intermediate or poor risk, including those with only one risk factor. While the 30-mo OS probability with nivolumab plus ipilimumab decreased as the number of risk factors increased, we believe that this is consistent with the expectation that patients with more risk factors have worse disease and would thus have higher mortality regardless of the treatment they receive. It is noteworthy that a multivariable analysis of individual IMDC risk factors in CheckMate 214 indicated that in the nivolumab plus ipilimumab arm, four of the six factors (anemia, neutrophilia, thrombocytosis, time from diagnosis) were not prognostic of OS, while two (Karnofsky performance status and corrected calcium) were [2]. The IMDC model, which was originally developed for tyrosine kinase inhibitors (TKIs) [3], may be more prognostic for OS in the setting of combination immunotherapy plus TKI regimens. For example, in the JAVELIN Renal 101 trial of avelumab plus axitinib versus sunitinib, PFS and ORR in both arms decreased as the number of IMDC risk factors increased [4,5]. Similarly, in KEYNOTE-426, IMDC risk appeared to be associated with objective response with pembrolizumab plus axitinib, as differences in PFS and OS between this combination and sunitinib decreased in patients with better prognoses according to IMDC risk [6]. Given these differences in efficacy with various immunotherapy regimens across IMDC risk categories, optimization of patient selection based on refined prognostic categorization may be valuable in the current era of immune checkpoint inhibitor–based therapies for aRCC. An example of this has been proposed for patients receiving TKIs, for whom significant differences in OS were observed among those treated with SUN depending on whether one, two, or more than two IMDC risk factors were present [7].
In conclusion, a consistent efficacy benefit with nivolumab plus ipilimumab over sunitinib was observed for all patients with intermediate or poor risk in CheckMate 214, regardless of the number of individual IMDC risk factors and including those with only one risk factor. Limitations of this analysis include its post hoc, exploratory nature and the smaller number of patients with zero or four to six IMDC risk factors. Future studies, including randomized clinical trials, will be necessary to better inform patient selection as the treatment landscape for aRCC evolves.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: This work was supported by Bristol-Myers Squibb. The sponsor played a role in the design and conduct of the study; collection and management of the data; and preparation and review of the manuscript.
Financial disclosures: Bernard Escudier certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Bernard Escudier reports consulting or advisory roles with Novartis, Pfizer, Bristol-Myers Squibb, Ipsen, EUSA Pharma, AVEO, and Roche/Genentech; travel expenses from Bristol-Myers Squibb, Pfizer, and Roche/Genentech; and honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Ipsen, Roche/Genentech, and EUSA Pharma. Robert J. Motzer reports consulting or advisory roles with Pfizer, Novartis, Eisai, Exelixis, Merck, Genentech/Roche, Incyte, and Lilly; and research funding from Pfizer, Bristol-Myers Squibb, Eisai, Novartis, and Genentech/Roche. Nizar M. Tannir reports consulting or advisory roles with Novartis, Exelixis, Bristol-Myers Squibb, Nektar, Pfizer, Eisai, Calithera Biosciences, Ono Pharmaceutical, and Oncorena; travel expenses from Pfizer, Novartis, Exelixis, Nektar, Bristol-Myers Squibb, Calithera Biosciences, Eisai, Onco Pharmaceuticals, and Oncorena; honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, Nektar, Calithera Biosciences, Eisai, and Ono Pharmaceutical; and research funding from Bristol-Myers Squibb, Novartis, Exelixis, Epizyme, Mirati Therapeutics, Nektar Therapeutics, and Calithera Biosciences. Camillo Porta reports consulting or advisory roles with Bristol-Myers Squibb, MSD, Pfizer, Novartis, Ipsen, EUSA Pharma, Eisai, Janssen, and GE Healthcare; speaker bureau fees from Bristol-Myers Squibb, MSD, Pfizer, Novartis, Ipsen, EUSA Pharma, Eisai, and GE Healthcare; and expert testimony fees from EUSA Pharma. Yoshihiko Tomita reports consulting or advisory roles with Novartis, Ono Pharmaceutical, and Taiho Pharmaceutical; honoraria from Astellas Medivation, Bristol-Myers Squibb, Novartis, Ono Pharmaceutical, and Pfizer; and research funding from Astellas Medivation, AstraZeneca, Ono Pharmaceutical, Pfizer, and Chugai Pharma. Matthew Maurer reports employment by and stock ownership in Bristol-Myers Squibb. M. Brent McHenry reports employment by, leadership and stock ownership in, and research funding and travel expenses from Bristol-Myers Squibb. Brian I. Rini reports consulting or advisory roles with Pfizer, Merck, Roche/Genentech, Novartis, Synthorx, Bristol-Myers Squibb, and AVEO; travel expenses from Pfizer, Bristol-Myers Squibb, and Merck; and research funding from Pfizer, Roche/Genentech, Bristol-Myers Squibb, Merck, AstraZeneca/MedImmune, Peloton Therapeutics, and AVEO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. In press. 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Motzer RJ, Campbell MT, et al. Subgroup analysis from JAVELIN Renal 101: outcomes for avelumab plus axitinib (A + Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol 2019;37(7 Suppl):544. [Google Scholar]
- 5.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 7.Iacovelli R, De Giorgi U, Galli L, et al. Is it possible to improve prognostic classification in patients affected by metastatic renal cell carcinoma with an intermediate or poor prognosis? Clin Genitourin Cancer 2018;16:355–9.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.