Abstract
As a member of the calpain protein family, calpain6 (CAPN6) is highly expressed mainly in the placenta and embryos. It plays a number of important roles in cellular processes, such as the stabilization of microtubules, the main-tenance of cell stability, the control of cell movement and the inhibition of apoptosis. In recent years, various studies have found that CAPN6 is one of the contributing factors associated with the tumorigenesis of uterine tumors and osteosarcoma, and that CAPN6 participates in the development of tumors by promoting cell proliferation and angiogenesis, and by inhibiting apoptosis, which is mainly regulated by the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) pathway. Due to its abnormal cellular expression, CAPN6 has also been found to be associated with a number of diseases, such as white matter damage and muscular dystrophy. Therefore, CAPN6 may be a novel therapeutic target for these diseases. In the present review, the role of CAPN6 in disease and its possible use as a target in various therapies are discussed.
Keywords: calpain6, cell proliferation, apoptosis, therapeutic target
1. Overview of CAPN6
Discovery
Calpain is a Ca2+-dependent cysteine protease (1). There have been 16 calpain genes identified to date (2). In 1997, Dear et al discovered 2 new calpain genes in vertebrates, one of these being calpain6 (CAPN6) (3), which is also known as CANPX and calpamodulin (4). CAPN6 is located on chromosome Xq23 (5). The mRNA expression of CAPN6 has been identified to be high in the placental chorionic plate, particularly during embryogenesis with a stronger expression signaling the somite, the mandibular tissue of the first branchial arch, developing skeletal muscle, the myocardium, epithelial cells bordering the fourth ventricle, bronchial epithelial cells, the tips of lung buds, the sacs of the lungs and kidney (3,6,7). However, CAPN6 expression is significantly downregulated after birth (6).
Structure
Based on the differences in the C-terminal domains of members within the calpain protein family, calpain is divided into 2 groups, classic (typical) and non-classic (atypical) (8).
Classic calpains, such as CAPN1, 2, 3, 8, 9, 11, 12, 13 and 14 comprise a large subunit (80 kDa) with catalytic activity and a small subunit (28 kDa) with regulatory activity (2,4,8). The large subunit contains 4 domains, namely I, II, III and IV. Located on the N-terminus, domain I is the region where autolysis occurs when the large subunit is activated by Ca2+, a prerequisite for the hydrolysis of calpain. Domain II is the key site for hydro-lytic activity with a conserved calpain-like cysteine protease sequence motif defined in the conserved domain database at the National Center for Biotechnology Information (cd00044; CysPc) catalytic region, which consists of 2 protease core (PC) domains (PC1 and PC2). Related to the conserved domain 2 originally defined for protein kinase C (C2), domain III is the calpain-type β-sandwich (CBSW) domain that plays a regulatory role by undergoing conformational changes during calcium-binding. Domain IV at the C-terminus with a penta-EF-hand (PEF) is the calcium-binding site (2,9,10). Moreover, the small subunit contains 2 domains, namely V and VI. The glycine-rich domain V is a hydrophobic region where calpain binds to cell membranes or membrane proteins. Similar to domain IV, the PEF-containing domain VI is the calmodulin binding region (11).
Non-classic calpains, such as CAPN5, 6, 7, 10, 13, 15 and 16 have a different motif that replaces the IV domain (4). Although most residues of CAPN6 are highly conserved among members of the calpain family, it lacks a C-terminal calmodulin-like domain, and it contains a CBSW domain instead of a PEF domain at the catalytic subunit (7,12). Due to the lack of a key active site that contains catalytic cysteine residues for proteolytic activity, it is believed that the CAPN6 protein may employ different sites for proteolysis. Since the entire coding region exhibits a significant homology with TRA-3 that is involved in the sex determination of nematodes, the C-terminal structure of CAPN6 is defined as a forked C2 domain (also known as domain T) (3,5,13-15), rather than domain IV (summarized in Fig. 1).
Figure 1.
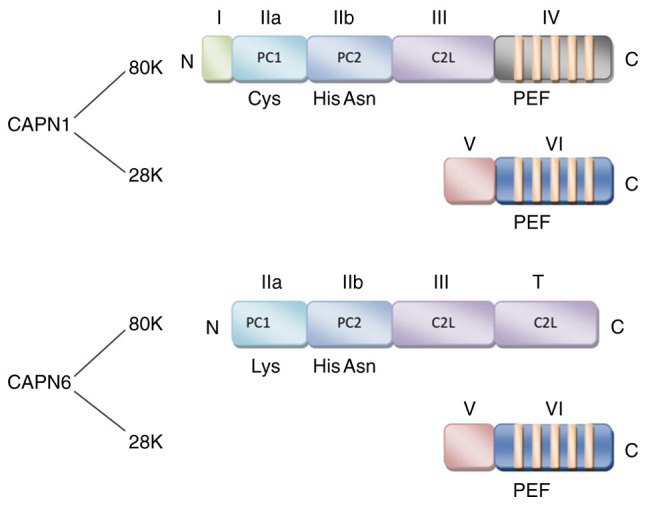
Domain structure of CAPN1 and CAPN6. CAPN1 comprise a large subunit (80 kDa) and a small subunit (28 kDa). The large subunit contains four domains, namely I, II, III and IV, domain IV at the C-terminus with a PEF. The small subunit contains two domains, namely V and VI. The main difference in the structure of CAPN6 is that it has a domain T at the C-terminus, which contains a CBSW domain instead of a PEF domain at the catalytic subunit. CAPN calpain; PEF, penta-EF-hand (E: E-helix; F: F-helix); CBSW, calpain-type β-sandwich.
Physiological function
Classic calpain is a highly conserved protease widely expressed in a number of tissues that participates in various physiological and pathological processes of the cell, including cell proliferation, apoptosis, cell migration and invasion, cytoskeleton remodeling, signal transduction, etc. (4,16-18).
To date, there are only a few studies available that address the physiological functions of CAPN6 protein, even though CAPN6 has a unique structure which may confer a different functional and regulatory mechanism from that of members of the classic calpain (7,12,19-21). During cytokinesis, CAPN6 is co-localized to the microtubule structure, including the central spindle and intermediates. As a microtubule-stabilizing protein, the domain III of CAPN6 binds to microtubules to induce stabilization through a non-proteolytic activity (7). Conversely, the inhibition of CAPN6 destroys the cytoskeletal tissues and the absorption capacity of cells, which then lowers the microtubule stability, the levels of acetylation and b3-integrin subunit proteins in osteoclasts, further damages the factors that regulate osteoclast cytoskeleton function, and ultimately causes cytoskeleton remodeling (19).
Rac Family Small GTPase1 (Rac1GTPase) and vimentin are the key factors for cell movement (22). CAPN6 inhibits the activity of Rac1GTPase through the interaction with Rho guanine nucleotide exchange factor GEF-H1 (officially known as ARHGEF2) in order to control lamellipodial formation and cell movement (20). The crosstalk between microtubules and actin as the main cytoskeletal components is essential for cell migration, diffusion and cytokinesis. Changes in microtubule stability have an impact on microtubule-actin interactions and cell movement (23). Therefore, the inactivation of CAPN6 not only causes the instability of microtubules, the loss of acetylated β-tubulin, and the reorganization of actin, it also leads to the formation of lamellipodium, laminar pseudofoot and laminar folds, which affect the actin tissues, and ultimately promotes cell movement and diffusion (7,20).
The activity of microtubule cytoskeleton and small GTPases are the key regulators of muscle cell differentiation and skeletal muscle development (24,25). CAPN6 regulates microtubule dynamics and actin reorganization by affecting the activity of Rac1GTPase. CAPN6 is highly expressed in embryonic muscle. The knockout of CAPN6 gene in mice can promote the differentiation of the muscle cells into myotubes. CAPN6 mRNA and CAPN6 protein can be detected in the regenerated skeletal muscle of adult mice. Compared with that of CAPN6lacZ/+ mice, the regenerated skeletal muscle of CAPN6lacZ/lacZ mice has more nuclei per muscle fiber and a larger cross-sectional area (21). Therefore, CAPN6 deficiency can promote skeletal muscle differentiation during skeletal muscle development and regeneration.
In addition, studies have demonstrated that in uterine leiomyoma, cervical cancer, osteosarcoma and liver cancer cells, an increased level of CAPN6 expression can promote cancer cell proliferation and inhibit cell apoptosis (12,26-28). In white matter damage, a decreased level of CAPN6 expression can promote oligodendrocyte precursor cell apoptosis (29).
Overall, CAPN6 plays a role in maintaining cell stability, in controlling cell movement and diffusion, and in inhibiting skeletal muscle differentiation and growth. CAPN6 also plays an anti-apoptotic role.
2. CAPN6 in disease: An introduction
Due to the abnormal protein expression levels, members of the calpain protein family have been found to be associated with the development of a number of diseases, such as Alzheimer's disease, gastric cancer, muscular dystrophy, diabetes, atherosclerosis, psoriasis, etc. (30-35). These are common diseases with a high incidence rate and a significant impact on society. Based on the different mechanisms involved in the pathogenesis of diseases, calpain-related diseases can be classified into 3 categories as follows: Diseases caused or aggravated by calpain activity, diseases caused by pathogenic microorganisms which use the host and/or their own calpain, and infection and survival caused by calpain gene defects (36). Calpain has been demonstrated as an effective therapeutic target for a number of diseases (37). Treatment strategies include the inhibition of calpain, such as α-ketoamide inhibitor A-705253 (also known as BSF 419961 and CAL 9961) in treating Alzheimer's disease; and the activation of calpain (direct or replacement), such as the restoration or compensation of the loss of CAPN3 function in improving limb-girdle muscular dystrophy type 2A (LGMD2A) (36,38,39). As a target of disease treatment, the use of calpain is very challenging as classic calpains are expressed in almost all cells. However, non-classic calpains are mainly expressed in specific tissues and organs, exhibiting their unique potential. In recent years, with an improved knowledge of physiological functions of CAPN6, a number of diseases have been identified to be specifically linked to CAPN6 (Table I), including tumors, neurological diseases, vascular diseases, muscle diseases and skin diseases (21,28,40-47). Among all the CAPN6-associated diseases, tumors have gained the most research attention.
Table I.
CAPN6 expression and active pathways in tissues in different diseases.
| Disease | Effect | Active pathwaya | Refs. |
|---|---|---|---|
| Tumors | |||
| Uterine tumors | |||
| Uterine leiomyomas | Overexpression | Rac1/PAK1/CAPN6 | (26,49) |
| Uterine sarcoma | Overexpression | - | (40,50,52) |
| Cervical cancer | Overexpression | PI3K/AKT/CAPN6 | (12,51) |
| Osteosarcoma | Overexpression | EDN-1/ERK1/2, PI3K/AKT, NF-κB/CAPN6 | (27,41,60,61) |
| Liver cancer | Overexpression | PI3K/AKT/CAPN6 | (12,28,70) |
| Head and neck squamous cell carcinoma | Low expression | - | (42) |
| Neurological diseases | |||
| White matter injury | Low expression | miR-142-3p/miR-466b-5p/CAPN6 | (29) |
| Prion diseases | Overexpression | - | (43) |
| Vascular diseases | |||
| Atherosclerosis | Overexpression | CWC22/EJC/Rac1 | (46,78-80) |
| Target organ damage in hypertension | Low expression | - | (44) |
| Type 2 diabetic nephropathy | Overexpression | - | (45) |
| Muscular diseases | |||
| Muscular dystrophy | Overexpression | - | (21) |
| Muscular atrophy | Overexpression | - | (21) |
| Skin diseases | |||
| Atopic dermatitis | - | Key factors related to YWHAE | (47) |
Not all diseases involved have clear active pathways. CAPN6, calpain6; EDN-1, endothelin-1; NF-κB, nuclear factor-κ-gene binding; ERK1/2, extracellular regulated protein kinase 1/2; AKT, protein kinase B.
3. CAPN6 in tumors
Uterine tumors
Uterine leiomyomas (UtLMs) are the most common benign tumor of the female reproductive system. Overexpressed CAPN6 in UtLMs interacts with other abnormally expressed factors, such as neuron navigator 2 (NAV2), kinesin family member 5C (KIF5C), doublecortin (DCX), etc., which may lead to the pathological transformation of the normal myometrium through related biochemical path-ways (26,48). It has recently been found that, whilst CAPN6 may regulate proliferation and apoptosis in UtLM through the Rac1/p21-activated kinase 1 (PAK1) signaling pathway, the downregulation of CAPN6 inhibits cell proliferation and promotes cell apoptosis in UtLM (49). Therefore, the overexpression of CAPN6 promotes the occurrence and development of UtLM.
CAPN6 has been found to be overexpressed in the tissues of other malignant tumors of the uterus, such as leiomyosarcoma, endometrial stromal sarcoma and cervical cancer (12,40,50-52). As previously demonstrated, there is a modest association between the intensity of CAPN6 expression and tumor subtype, but not tumor stage or survival rate (50).
The expression of CAPN6 in cervical cancer is regulated by the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) pathway (12), which plays an important role in a number of cellular processes, and promotes tumor growth and survival (53). The PI3K/Akt pathway inhibits the proteasome degradation of CAPN6 protein through glycogen synthase kinase (GSK)-3β, increases the stability of CAPN6 protein, upregulates CAPN6mRNA expression, and uses transcription factors, such as activator protein 1 (AP1), forkhead box D3 (FoxD3) and organic cation transporter 1 (Oct-1) to stimulate the activity of the CAPN6 promoter (12). A main prerequisite for tumor formation and progression is to prevent apoptosis, and promote cell proliferation and angiogenesis. Cisplatin is used to induce cervical cancer cell apoptosis by activating the caspase-3 pathway (54). A previous study using cervical cancer cells (HeLa cells) transfected with CAPN6 cDNA (Calpain 6) or CAPN6 siRNA (siCalpain 6) demonstrated that CAPN6 antagonized cisplatin-mediated apoptosis by inhibiting caspase-3 activity (51). Consistently, another CAPN6 knockdown study using HeLa cells demonstrated an upregulation of caspase-3 and a downregulation of Bcl-2 (12), indicating that CAPN6 induced resistance to apoptosis. HeLa cells with a downregulated CAPN6 expression grow at a more rapid rate than their parental cells, with a reduced S phase and cell-specific cyclin (cyclin D1) expression, suggesting that CAPN6 can promote the proliferation of cancer cells (12). Vascular endothelial growth factor (VEGF) and basic fibro-blast growth factor (bFGF) promote endothelial cell migration, proliferation and angiogenesis, and activate calcium signal regulation in endothelial cells (55-57). Since the secretion of VEGF and bFGF is related to cervical cancer (58), a study using human umbilical vein endothelial cells (HUVECs) demonstrated that CAPN6 overexpression enhanced the VEGF-induced invasiveness of endothelial cells, and the tissue formation of endothelial cell network, indicating that calpain 6 promotes HUVEC invasion, migration and angiogenesis. Since calpain 6 is a Ca2+-dependent cysteine protease, it is considered that the intracellular calcium signals induced by VEGF or bFGF can possibly activate CAPN6 (51). A recent study found that the binding between the domain III of CAPN6 and the C-terminus of VEGFA, produced a unique interaction that leads to the secretion of VEGF, which in turn increases and promotes angiogenesis (59). Although it is generally accepted that CAPN6 plays a role in promoting the occurrence and development of cervical cancer, CAPN6 is not associated with cancer cell migration and epithelial-mesenchymal transition (12).
Osteosarcoma
Osteosarcoma is one of the most common primary malignant bone tumors. Metastasis and recurrence are important factors leading to a poor prognosis. Chemoresistance is the main cause of the poor treatment efficacy and disease recurrence in patients with osteosarcoma.
It has been demonstrated that CAPN6 is expressed in human osteosarcoma tissue, metastatic bone and lung, and the majority of recurrent tumors at high levels (41). The CAPN6 level in the primary tumor is found to be negatively associated with the chemotherapeutic response. Compared with original U2OS sensitive cells, cells with a resistant U2OS osteosarcoma origin have been shown to express higher CAPN6 levels (27).
The inhibition of CAPN6 expression in osteosarcoma cells can reduce the incorporation of 5-bromodeoxyuridine (BrdU) into DNA, reduce cell proliferation, increase spontaneous apoptosis, rescue the apoptotic response of resistant cells, and increase the sensitivity to cytotoxic drugs. Therefore, CAPN6 promotes osteosarcoma cell proliferation, survival and growth, and provides protection by increasing the chemical resistance of cells (27). CAPN6 is a downstream molecule of endo-thelin-1 (EDN-1) signaling during embryonic development (7). EDN-1 can induce the continuous activation of the nuclear factor-κ-gene binding (NF-κB), extracellular regulated protein kinase ½ (ERK1/2) and AKT pathways, and can promote the expression of CAPN6 in osteosarcoma (60,61). Tumor cyto-chemical resistance is associated with the activation of the NF-κB, Ras/MAPK and PI3K/AKT pathways (62,63), further demonstrating the association between CAPN6 expression and osteosarcoma cell chemical resistance. In addition, in liposarcoma, it has been found that the higher the tumor grade, the higher the CAPN6 expression (64), indicating that the expression of CAPN6 is associated with the malignancy of sarcoma.
Syndecan-2 is a key regulator of cell death, and has the function of promoting apoptosis. Since there is a decreased level of expressed syndecan-2 in osteosarcoma tissue, it is inferred that the abnormal expression or induction of syndecan-2 in osteosarcoma is associated with the imbalanced apoptosis of tumor cells (65). The overexpression of syndecan-2 in osteosarcoma cells can enhance the apoptotic response to cytotoxic drugs, restore the sensitivity of drug-resistant osteosarcoma cells to chemotherapeutic drugs, decrease the levels of CAPN6 in the tumor cells and ultimately inhibit tumor growth; thus, a decrease in CAPN6 expression contributes to syndecan-2-induced apoptosis (41,66). EDN-1 is overexpressed in osteosarcoma to promote the invasion, metastasis and survival of bone cancer cells, and to resist cisplatin-induced apoptosis through EDNA receptor (67). Syndecan-2 can alter EDN-1 signaling. The PI3K/AKT and NF-κB pathways are controlled during the pro-apoptotic process. In cells with syndecan-2 overexpression, the steady-state activation of ERK1/2 and AKT is low (60,61). Therefore, syndecan-2 can inhibit EDN-1 signal transduction, and can downregulate CAPN6 expression through the ERK1/2, PI3K/AKT and NF-κB pathways to exert its function to promote apoptosis (summarized in Fig. 2).
Figure 2.
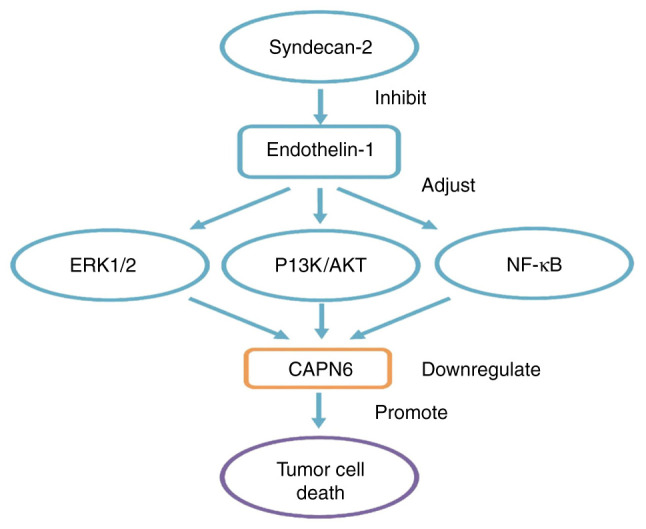
In osteosarcoma, CAPN6 involves related signal pathways. CAPN6 is a downstream molecule of EDN-1 signaling. EDN-1 can induce the continuous activation of NF-κB, ERK1/2 and AKT pathways, and promote the expression of CAPN6 in osteosarcoma. Syndecan-2 is a key regulator of cell death. Overexpression of syndecan-2 in osteosarcoma cells can decrease the levels of CAPN6 in the tumor cells, and can alter EDN-1 signaling. PI3K/AKT and NF-κB are new pathways controlled during their pro-apoptotic process. In cells with syndecan-2 overexpression, steady-state activation of ERK1/2 and AKT is low. Therefore, syndecan-2 can inhibit EDN-1 signal transduction, and down-regulate CAPN6 expression through ERK1/2, PI3K/AKT and NFκB pathways to exert its function to promote apoptosis. CAPN6, calpain6; EDN-1, endothelin-1; NF-κB, nuclear factor-κ-gene binding; ERK1/2, extracellular regulated protein kinase 1/2; AKT, protein kinase B.
Cancer stem cells (CSC) have been shown to promote the development of malignancies. Oct4, Nanog and Sox2 are stem cell transcription factors that can be upregulated by hypoxia in osteosarcoma. Hypoxia can also activate NF-κB and increase the expression of EDN-1 receptors, thereby promoting the EDN-1/NF-κB pathway to induce increased mRNA and protein levels of CAPN6, resulting in a marked decrease in the silencing of Oct4, Nanog or Sox2 (68). In a previous study, in an osteosarcoma mouse model, it was found that cells expressing CAPN6 had unique tumor initiation and metastasis capabilities; CAPN6 knockdown inhibited hypoxia, promoted autophagy, prevented aging, induced cancer stem cell population depletion and blocked mouse tumor development (64). CAPN6 expression also recognizes CSCs. The metastatic potential of bone tumors is dependent on CSCs that express CAPN6, and CSCs communicate with other tumor cells through exosomes to regulate the cell migratory capacity (69).
Liver cancer
Consistent with that in cervical cancer, the enhanced expression of CAPN6 in liver cancer (70), which is regulated by the PI3K/AKT signaling pathway, plays a role in promoting cancer cell proliferation and inhibiting apoptosis, but not necessarily tumor metastasis (12). MicroRNAs (miRNAs or miRs) play a key role in post-transcriptional gene regulation (71), and an miRNA imbalance may lead to the inactivation of liver cancer suppressor genes and the activation of oncogenes, mainly by inhibiting the expression of its target genes (72). miR-449a expression is decreased in liver cancer (27). In a previous study, luciferase assay confirmed that CAPN6 and POU2F1 are the target genes of miR-449a (29). The downregulation of CAPN6 or POU2F1 increases the levels of Bax, decreases the levels of Bcl-2, promotes G1 phase arrest, and inhibits cancer cell proliferation and induces apoptosis, thereby preventing the occurrence and development of liver cancer (28).
Head and neck squamous cell carcinoma (HNSCC)
Xiang et al examined CAPN6 expression in HNSCC, including tongue cancer, laryngeal cancer and nasal cancer (42). The results of their study demonstrated that the expression of CAPN6 in HNSCC tissues was significantly decreased, which was in complete contrast to the observations of other tumor studies, as mentioned above. CAPN6 expression also varies between different tumor stages of HNSCC, and it differs significantly between different T stages, although there is no significant difference between different N stages and different tumor grades. In addition, the expression of CAPN6 and the survival rate of patients with HNSCC exhibit a positive association (42). However, it is not clear whether the expression of CAPN6 is directly linked to the occurrence of HNSCC, or whether HNSCC tumor growth or metastasis leads to CAPN6 downregulation.
These studies have provided new knowledge for the further understanding of the pathogenesis of these tumors. CAPN6 may be used as a biomarker, and in the diagnosis, prevention and management of these tumors. Most importantly, CAPN6 may be an ideal specific therapeutic target. For uterine tumors, osteosarcoma and liver cancer that can be caused or aggravated by CAPN6 activity, the inhibition of CAPN6 may be an effective treatment strategy, particularly for chemoresistant and recurring tumors. Therefore, CAPN6 can be inhibited to produce targeted drugs (including targeted cell drugs and targeted vascular drugs) or tumor stem cells to block the initiation, proliferation or metastasis of tumor cells, to promote the occurrence of apoptosis or autophagy, and to achieve the purpose of treatment. The combination of CAPN6 targeting with anticancer drug cisplatin may also produce a synergistic effect. However, it is worth noting that studies have found the pros and cons of calpain inhibition, depending on the stage of tumor progression (73), and that CAPN6 inhibition has an opposite effect in HNSCC. Therefore, further systematic studies are warranted to determine whether CAPN6 exerts differential effects in different stages of the same tumor, and different cell types of tumors, as well as to determine whether CAPN6 targeting is beneficial for the treatment of tumors.
4. CAPN6 in neurological diseases
White matter injury (WMI)
Studies have found that hypoxia-ischemia (HI) can lead to reduced myelin sheath, the death of oligodendrocyte precursor cells and the dysplasia of oligodendrocytes, and can alter the expression of specific mature miRNAs in demyelinating and oligodendrocyte precursor cells, ultimately resulting in WMI (29,71,74). The co-expression network of miRNAs/mRNAs indicates that miR-142-3p, miR-466b-5p and miR-146a-5p have differentially expressed targets. CAPN6 is the target gene of miR-142-3p and miR-466b-5p. The abundance of CAPN6 mRNAs in HI is significantly decreased. Therefore, it is considered that miR-142-3p and miR-466b-5p may promote the apoptosis of oligodendrocyte precursor cells by inhibiting CAPN6, and that miR-142-3p/miR-466b-5p/CAPN6 pathway may be involved in the pathogenesis of WMI (29).
Prion diseases
Prion diseases are a group of lethal neurodegenerative disorders; however, the molecular mechanisms responsible for these diseases are poorly understood. Other members of the calpain family are associated with prion diseases and several other neurodegenerative diseases (75). Calpain-mediated proteolytic cleavage by prion protein (PrPSc) may be an important event in prion reproduction (76). CAPN6 is overexpressed in the medulla oblongata, spinal cord, cerebellar cortex and several other areas of prion disease-affected brain tissues. CAPN6 is involved in the pathogenesis of prion diseases (43).
CAPN6 participates in different pathogenesis of WMI and prion diseases; thus, the corresponding targeted treatment strategies vary. The decreased activity of CAPN6 in WMI the promotes the development of disease. Hence, treatment strategies should consider activating or replacing CAPN6, as no calpain activator has been developed to date, at least to the best of our knowledge. The only treatment option available is calpain replacement or gene therapy (36). However, to the best of our knowledge, to date, there is no research available on the overexpression of CAPN6 in the treatment of diseases in live animals; thus, it is difficult to assess whether gene therapy will bring other effects. In prion diseases, it is speculated that prion proteins can reproduce and survive to promote disease by mediating CAPN6. Therefore, inhibiting CAPN6 to ameliorate the invasion and growth of the infecting virus may be a novel treatment strategy. For the inhibition of calpain in the treatment of neurodegenerative diseases, mature drugs are already available, such as the aforementioned α-ketoamide inhibitor, A-705253. There are also drugs that have been tested on animals, such as Gabadul for the treatment of Parkinson's disease and Lewy body dementia (36,77). Hence, therapeutic strategies with the inhibition of CAPN6 may be worthy of investigation.
5. CAPN6 in vascular diseases
Degenerative vascular diseases
In degenerative vascular diseases, the dysfunction of the calpain system has a prominent effect, since the increased activity of calpain can induce diseases (78). As a non-proteolytic calpain, CAPN6 disrupts the CWC22/exon junction complex (EJC) system in macro-phages to affect CWC22/EJC/Rac1 signal transduction, and enhances the cell phagocytosis of natural low-density lipoprotein (LDL), causing the cytosolic DL cholesterol deposition in cells, thereby attenuating the clearance of dead cell corpses, and eventually promoting the progression of degenerative vascular diseases, such as atherosclerosis (46,78-80).
Hypertensive heart disease
Damage to target organs is the most harmful effect caused by hypertension. Experiments have established a model of target organ damage in hypertension. It has been found that in the heart tissue of Dahl salt-sensitive rats with high salt (4% NaCl), CAPN6 and CAPN9 are down-regulated by >50%, and that endogenous calpain inhibitor calpastatin is upregulated by 225%. However, the specific role of CAPN6 in hypertension target organ damage is unknown, as CAPN6 lacks key amino acids in the catalytic triad and may not have proteolytic activity (44).
Diabetic nephropathy
Diabetic nephropathy is one of the comorbidities of diabetic systemic microangiopathies. Due to the higher expression levels, CAPN6 is one of the 50 functional genes that may be responsible for the occurrence of type 2 diabetes (45).
In vascular diseases, the expression and site of action of CAPN6 differ due to the nature of the disease and the different organs involved. In degenerative vascular diseases, CAPN6 mainly affects lipid metabolism by participating in the inter-ference of mRNA splicing in macrophages. It has been found that the transgenic overexpression of calpastatin and calpain inhibitors (such as MDL28170 and BDA 410), are effective in the treatment of degenerative disorders (36). Although there is no drug that inhibits calpain-6, the development of atypical calpain inhibitors is promising. In acute cardiovascular diseases, calpain-mediated myocardial proteolysis is involved in ischemia-reperfusion and pressure overload mechanisms that lead to the pathogenesis. Studies performing animal experiments have indicated that calpain inhibitors can improve the symptoms of these diseases (81,82). However, it has also been reported that the inhibition of calpain may lead to a decrease in endogenous calpain activity, which is unfavorable (83). This is consistent with the decrease in CAPN6 activity and the upregulation of endogenous calpain inhibitors in the hypertensive heart disease model. The inhibition of calpain can also cause cardiac dysfunction under pressure overload (82). Therefore, calpain may play multiple roles in cardiovascular diseases, either in a therapeutically inhibited or activated manner. As regards diabetic nephropathy, only the specific biological pathways and sites of action of CAPN6 involved in the pathogenesis are clarified, the treatment measures need to be further addressed.
6. CAPN6 in muscle disorders
Muscular dystrophy and muscle atrophy
With CAPN6 being overexpressed, the developing skeletal muscle is in a continuous cycle of degeneration and regeneration. CAPN1 is also related to muscle differentiation. CAPN1 overexpression inhibits muscle cell differentiation (84). During muscle degeneration, the expression of CAPN1 decreases, which then recovers during muscle regeneration. However, CAPN6 deficiency delays the expression of CAPN1 in regenerating skeletal muscles (21). The exact association between CAPN1 and CAPN6 needs to be clarified.
Muscular dystrophy is a muscle degeneration disease caused by genetic mutations in slow progressive symmetrical muscle weakness and atrophy; while muscle atrophy is muscle reduction and rhabdomyolysis caused by the thinning or even disappearance of muscle fibers. Studies have found that CAPN6 is one of the genes associated with LGMD2A, and its expression is up-regulated in the skeletal muscles of patients (85). As an inhibitor of skeletal muscle differentiation, CAPN6 downregulation assists the growth of skeletal muscles and the induction of pluripotent stem cells to produce skeletal muscles in vitro. Therefore, the modification of specific antibodies or siRNA to offset CAPN6 may regulate the quality of skeletal muscles, and may improve the conditions of muscular dystrophy or muscle atrophy. However, the inhibition of calpain does not necessarily improve muscle function. The inhibition of calpain through inhibitors or calpastatin overexpression can lead to the compensatory upregulation of calpain (86). Therefore, the results of such treatments need to be analyzed dialectically. Limb-girdle muscular dystrophy type 2 (LGMD2A) is a recessive genetic disease caused by CAPN3 mutation and loss of function (85). The inhibition of CAPN6 alone may not be able to treat LGMD2A completely. A combination of CAPN6 inhibition with other treatment methods may be more beneficial.
7. CAPN6 in skin diseases
Atopic dermatitis
Atopic dermatitis is a familial hereditary skin disease. The tyrosine 3-monooxygenase/tryptophan 5-mono-oxygenase activation protein, epsilon polypeptide (YWHAE) isoform located in the human keratinocytes is involved in the pathogenesis of atopic dermatitis (87). CAPN6 is one of the key factors associated with YWHAE (47). Addressing the causal association and signal transduction pathways between CAPN6 and YWHAE may facilitate the discovery of novel clinical treatments for YWHAE-related atopic dermatitis.
8. Conclusions and future perspectives
Calpain-related diseases are a threat to human health. The development of therapeutic drugs is the focus of current research. Although some studies have facilitated the under-standing of the disease pathogenesis and the application of calpain in disease treatment, there are several aspects that require further investigations. For instance, knowledge about calpains, particularly non-classical calpains with natural advantages warrant further attention. CAPN6 has great potential as an emerging therapeutic target, although there are a number of research areas that require further clarifications, including the characteristics of CAPN6 itself, the molecular pathways involved in the associated diseases, the identification of targets, the development of CAPN6 inhibitors or activators, and the effective testing of these therapies. However, the future research direction of CAPN6 holds promise.
Acknowledgements
Not applicable.
Abbreviations
- CAPN6
calpain6
- PI3K
phosphatidylinositol 3 kinase
- AKT
protein kinase B
- CysPc
calpain-like cysteine protease sequence motif defined in the conserved domain database at the National Center for Biotechnology Information (cd00044)
- PC
protease core
- PC1
protease core domain 1
- PC2
protease core domain 2
- C2
conserved domain 2 originally defined for protein kinase C
- CBSW
calpain-type β-sandwich
- PEF
penta-EF-hand (E, E-helix, F, F-helix)
- GEF-H1
officially known as ARHGEF2
- LGMD2A
limb girdle muscular dystrophy type 2A
- UtLMs
uterine leiomyomas
- Rac1
Rac Family Small GTPase1
- PAK1
p21-activated kinase1
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
- HUVECs
human umbilical vein endothelial cells
- BrdU
5-bromodeoxyuridine
- EDN-1
endothelin-1
- NF-κB
nuclear factor-κ-gene binding
- ERK1/2
extracellular regulated protein kinase 1/2
- CSC
cancer stem cells
- miRNA/miRs
microRNAs
- HNSCC
head and neck squamous cell carcinoma
- HI
hypoxia-ischemia
- WMI
white matter injury
- EJC
exon junction complex
- LDL
low-density lipoprotein
- YWHAE
tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon polypeptide
Funding
The present study was supported by the National Key R&D Programme of China (grant nos. 2017YFA 0104201 and 2017YFA 0104200), the National Science Foundation of China (grant nos. 81330016, 81630038 and 81771634), and the Science and Technology Bureau of Chengdu City (grant no. 2015-HM01-00424-SF).
Availability of data and materials
Not applicable.
Authors' contributions
LC and DX were involved in the conceptualization of the study. LC, DX, FT and XL were involved in software applications. LC, DX, HG and XL provided the study resources. FT, HG and XL also played a role in the conceptualization of the study. LC and DX were involved in the writing and preparation of the original draft, and in the writing, reviewing and editing of the study. HG and XL were involved in the processing of the figures. XL supervised the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sorimachi H, Hata S, Ono Y. Calpain chronicle-an enzyme family under multidisciplinary characterization. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:287–327. doi: 10.2183/pjab.87.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretti D, Del Bello B, Allavena G, Maellaro E. Calpains and cancer: Friends or enemies? Arch Biochem Biophys. 2014;564:26–36. doi: 10.1016/j.abb.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Dear N, Matena K, Vingron M, Boehm T. A new subfamily of vertebrate calpains lacking a calmodulin-like domain: Implications for calpain regulation and evolution. Genomics. 1997;45:175–184. doi: 10.1006/geno.1997.4870. [DOI] [PubMed] [Google Scholar]
- 4.Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. J Biochem. 2011;150:23–37. doi: 10.1093/jb/mvr070. [DOI] [PubMed] [Google Scholar]
- 5.Matena K, Boehm T, Dear N. Genomic organization of mouse Capn5 and Capn6 genes confirms that they are a distinct calpain subfamily. Genomics. 1998;48:117–120. doi: 10.1006/geno.1997.5133. [DOI] [PubMed] [Google Scholar]
- 6.Dear TN, Boehm T. Diverse mRNA expression patterns of the mouse calpain genes Capn5, Capn6 and Capn11 during development. Mech Dev. 1999;89:201–209. doi: 10.1016/S0925-4773(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 7.Tonami K, Kurihara Y, Aburatani H, Uchijima Y, Asano T, Kurihara H. Calpain 6 is involved in microtubule stabilization and cytoskeletal organization. Mol Cell Biol. 2007;27:2548–2561. doi: 10.1128/MCB.00992-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature. 2008;456:409–412. doi: 10.1038/nature07451. [DOI] [PubMed] [Google Scholar]
- 9.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: Calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/S0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Sorimachi H. A novel aspect of calpain activation. FEBS Lett. 1998;433:1–4. doi: 10.1016/S0014-5793(98)00856-4. [DOI] [PubMed] [Google Scholar]
- 11.Campbell RL, Davies PL. Structure-function relationships in calpains. Biochem J. 2012;447:335–351. doi: 10.1042/BJ20120921. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Mei C, Sun L, Li X, Liu M, Wang L, Li Z, Yin P, Zhao C, Shi Y, et al. The PI3K-Akt pathway regulates calpain 6 expression, proliferation, and apoptosis. Cell Signal. 2011;23:827–836. doi: 10.1016/j.cellsig.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Barnes TM, Hodgkin J. The tra-3 sex determination gene of Caenorhabditis elegans encodes a member of the calpain regulatory protease family. EMBO J. 1996;15:4477–4484. doi: 10.1002/j.1460-2075.1996.tb00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 15.Mugita N, Kimura Y, Ogawa M, Saya H, Nakao M. Identification of a novel, tissue-specific calpain htra-3; a human homologue of the Caenorhabditis elegans sex determination gene. Biochem Biophys Res Commun. 1997;239:845–850. doi: 10.1006/bbrc.1997.7571. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini M, Najmabadi H, Kahrizi K. Calpains: Diverse functions but enigmatic. Arch Iran Med. 2018;21:170–179. [PubMed] [Google Scholar]
- 17.Lebart MC, Benyamin Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 2006;273:3415–3426. doi: 10.1111/j.1742-4658.2006.05350.x. [DOI] [PubMed] [Google Scholar]
- 18.Sato K, Kawashima S. Calpain function in the modulation of signal transduction molecules. Biol Chem. 2001;382:743–751. doi: 10.1515/bchm.2001.382.5.743. [DOI] [PubMed] [Google Scholar]
- 19.Hong JM, Teitelbaum SL, Kim TH, Ross FP, Kim SY, Kim HJ. Calpain-6, a target molecule of glucocorticoids, regulates osteoclastic bone resorption via cytoskeletal organization and microtubule acetylation. J Bone Miner Res. 2011;26:657–665. doi: 10.1002/jbmr.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonami K, Kurihara Y, Arima S, Nishiyama K, Uchijima Y, Asano T, Sorimachi H, Kurihara H. Calpain-6, a microtubule-stabilizing protein, regulates Rac1 activity and cell motility through interaction with GEF-H1. J Cell Sci. 2011;124:1214–1223. doi: 10.1242/jcs.072561. [DOI] [PubMed] [Google Scholar]
- 21.Tonami K, Hata S, Ojima K, Ono Y, Kurihara Y, Amano T, Sato T, Kawamura Y, Kurihara H, Sorimachi H. Calpain-6 deficiency promotes skeletal muscle development and regeneration. PLoS Genet. 2013;9:e1003668. doi: 10.1371/journal.pgen.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margiotta A, Progida C, Bakke O, Bucci C. Rab7a regulates cell migration through Rac1 and vimentin. Biochim Biophys Acta Mol Cell Res. 2017;1864:367–381. doi: 10.1016/j.bbamcr.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 24.Mogessie B, Roth D, Rahil Z, Straube A. A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation. Elife. 2015;4:e05697. doi: 10.7554/eLife.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryan BA, Li D, Wu X, Liu M. The Rho family of small GTPases: Crucial regulators of skeletal myogenesis. Cell Mol Life Sci. 2005;62:1547–1555. doi: 10.1007/s00018-005-5029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skubitz KM, Skubitz AP. Differential gene expression in uterine leiomyoma. J Lab Clin Med. 2003;141:297–308. doi: 10.1016/S0022-2143(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 27.Marion A, Dieudonné FX, Patiño-Garcia A, Lecanda F, Marie PJ, Modrowski D. Calpain-6 is an endothelin-1 signaling dependent protective factor in chemoresistant osteosarcoma. Int J Cancer. 2012;130:2514–2525. doi: 10.1002/ijc.26246. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Wang Y, Sun X, Mei C, Wang L, Li Z, Zha X. miR-449a promotes liver cancer cell apoptosis by downregulation of Calpain 6 and POU2F1. Oncotarget. 2016;7:13491–13501. doi: 10.18632/oncotarget.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su X, Xiao D, Huang L, Li S, Ying J, Tong Y, Ye Q, Mu D, Qu Y. MicroRNA alteration in developing rat oligodendrocyte precursor cells induced by hypoxia-ischemia. J Neuropathol Exp Neurol. 2019;78:900–909. doi: 10.1093/jnen/nlz071. [DOI] [PubMed] [Google Scholar]
- 30.Mahaman YAR, Huang F, Kessete Afewerky H, Maibouge TMS, Ghose B, Wang X. Involvement of calpain in the neuropatho-genesis of Alzheimer's disease. Med Res Rev. 2019;39:608–630. doi: 10.1002/med.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng P, Wu W, Zhao J, Song S, Wang X, Jia D, Shao M, Zhang M, Li L, Wang L, et al. Decreased expression of Calpain-9 predicts unfavorable prognosis in patients with gastric cancer. Sci Rep. 2016;6:29604. doi: 10.1038/srep29604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fichna JP, Macias A, Piechota M, Korostyński M, Potulska- Chromik A, Redowicz MJ, Zekanowski C. Whole-exome sequencing identifies novel pathogenic mutations and putative phenotype-influencing variants in Polish limb-girdle muscular dystrophy patients. Hum Genomics. 2018;12:34. doi: 10.1186/s40246-018-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, Arata S, Ohata H, Ota H, Murakami M, Miyazaki A. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–2532. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita Y, Shimada Y, Kawara S, Takehara K, Sato S. Autoantibodies directed against the protease inhibitor calpastatin in psoriasis. Clin Exp Immunol. 2005;139:355–362. doi: 10.1111/j.1365-2249.2005.02701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono Y, Saido TC, Sorimachi H. Calpain research for drug discovery: Challenges and potential. Nat Rev Drug Discov. 2016;15:854–876. doi: 10.1038/nrd.2016.212. [DOI] [PubMed] [Google Scholar]
- 37.Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat Rev Drug Discov. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikkel AL, Martino B, Markosyan S, Brederson JD, Medeiros R, Moeller A, Bitner RS. The novel calpain inhibitor A-705253 prevents stress-induced tau hyperphosphorylation in vitro and in vivo. Neuropharmacology. 2012;63:606–612. doi: 10.1016/j.neuropharm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Ono Y, Ojima K, Shinkai-Ouchi F, Hata S, Sorimachi H. An eccentric calpain, CAPN3/p94/calpain-3. Biochimie. 2016;122:169–187. doi: 10.1016/j.biochi.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Skubitz KM, Skubitz AP. Differential gene expression in leiomyosarcoma. Cancer. 2003;98:1029–1038. doi: 10.1002/cncr.11586. [DOI] [PubMed] [Google Scholar]
- 41.Marion A, Dieudonné F, Patiño-García A, Lecanda F, Marie P, Modrowski D. Identification of calpain-6 as a new target involved in cell death of bone cancer cells. Bone. 2009;44:S247. doi: 10.1016/j.bone.2009.03.117. [DOI] [Google Scholar]
- 42.Xiang Y, Li F, Wang L, Zheng A, Zuo J, Li M, Wang Y, Xu Y, Chen C, Chen S, et al. Decreased calpain 6 expression is associated with tumorigenesis and poor prognosis in HNSCC. Oncol Lett. 2017;13:2237–2243. doi: 10.3892/ol.2017.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filali H, Vidal E, Bolea R, Márquez M, Marco P, Vargas A, Pumarola M, Martin-Burriel I, Badiola JJ. Gene and protein patterns of potential prion-related markers in the central nervous system of clinical and preclinical infected sheep. Vet Res. 2013;44:14. doi: 10.1186/1297-9716-44-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markmann A, Schäfer S, Linz W, Löhn M, Busch AE, Wohlfart P. Down-regulation of calpain 9 is linked to hypertensive heart and kidney disease. Cell Physiol Biochem. 2005;15:109–116. doi: 10.1159/000083643. [DOI] [PubMed] [Google Scholar]
- 45.Guttula SV, Rao AA, Sridhar GR, Chakravarthy MS, Nageshwararo K, Rao PV. Cluster analysis and phylogenetic relationship in biomarker identification of type 2 diabetes and nephropathy. Int J Diabetes Dev Ctries. 2010;30:52–56. doi: 10.4103/0973-3930.60003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyazaki T, Tonami K, Hata S, Aiuchi T, Ohnishi K, Lei XF, Kim-Kaneyama JR, Takeya M, Itabe H, Sorimachi H, et al. Calpain-6 confers atherogenicity to macrophages by dysregulating pre-mRNA splicing. J Clin Invest. 2016;126:3417–3432. doi: 10.1172/JCI85880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin SJ, Lee JR, Kwak H, Lee BN, Han JW, Hahn MJ, Park YD, Yang JM. Functional study of 143-3 protein epsilon (YWHAE) in keratinocytes: Microarray integrating bioinformatics approaches. J Biomol Struct Dyn. 2020;38:2633–2649. doi: 10.1080/07391102.2019.1637282. [DOI] [PubMed] [Google Scholar]
- 48.Xia L, Liu Y, Fu Y, Dongye S, Wang D. Integrated analysis reveals candidate mRNA and their potential roles in uterine leiomyomas. J Obstet Gynaecol Res. 2017;43:149–156. doi: 10.1111/jog.13172. [DOI] [PubMed] [Google Scholar]
- 49.Zhu L, Sun Y, Wu Q, Zhang C, Ling J. CAPN6 regulates uterine leiomyoma cell proliferation and apoptosis through the Rac1-dependent signaling pathway. Ann Clin Lab Sci. 2020;50:24–30. [PubMed] [Google Scholar]
- 50.Lee SJ, Choi YL, Lee EJ, Kim BG, Bae DS, Ahn GH, Lee JH. Increased expression of calpain 6 in uterine sarcomas and carci-nosarcomas: An immunohistochemical analysis. Int J Gynecol Cancer. 2007;17:248–253. doi: 10.1111/j.1525-1438.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 51.Rho SB, Byun HJ, Park SY, Chun T. Calpain 6 supports tumorigenesis by inhibiting apoptosis and facilitating angiogenesis. Cancer Lett. 2008;271:306–313. doi: 10.1016/j.canlet.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Lee SJ, Kim BG, Choi YL, Lee JW. Increased expression of calpain 6 during the progression of uterine cervical neoplasia: Immunohistochemical analysis. Oncol Rep. 2008;19:859–863. [PubMed] [Google Scholar]
- 53.LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34:3803–3815. doi: 10.1200/JCO.2014.59.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu XX, Kakehi Y. Enhancement of lexatumumab-induced apoptosis in human solid cancer cells by Cisplatin in caspase-dependent manner. Clin Cancer Res. 2009;15:2039–2047. doi: 10.1158/1078-0432.CCR-08-2667. [DOI] [PubMed] [Google Scholar]
- 55.Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Köhler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of micro-vascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- 56.Tomatis C, Fiorio Pla A, Munaron L. Cytosolic calcium microdomains by arachidonic acid and nitric oxide in endothelial cells. Cell Calcium. 2007;41:261–269. doi: 10.1016/j.ceca.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: Beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Meng X, Hu J, Zhang Y, Dang Y, Wei L, Shi M. Heparanase promotes radiation resistance of cervical cancer by upregulating hypoxia inducible factor 1. Am J Cancer Res. 2017;7:234–244. [PMC free article] [PubMed] [Google Scholar]
- 59.Oh M, Rho SB, Son C, Park K, Song SY. Non-proteolytic calpain-6 interacts with VEGFA and promotes angiogenesis by increasing VEGF secretion. Sci Rep. 2019;9:15771. doi: 10.1038/s41598-019-52364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marion A, Dieudonné FX, Marie PJ, Modrowski D. Endothelin-1 up regulates the survival factor calpain-6 in osteo-sarcoma cells through Mapk and PI3K pathways. Bone. 2010;47:S112. doi: 10.1016/j.bone.2010.04.238. [DOI] [Google Scholar]
- 61.Marion A, Dieudonné FX, Patiño-Garcia A, Lecanda F, Marie PJ, Modrowski D. Abnormal expression of calpain-6 due to endothelin-1/NFκB signalling contributes to cell survival and chemoresistance in osteosarcoma cells. Bone. 2011;48:S38. doi: 10.1016/j.bone.2010.10.105. [DOI] [Google Scholar]
- 62.Jin W, Wu L, Liang K, Liu B, Lu Y, Fan Z. Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br J Cancer. 2003;89:185–191. doi: 10.1038/sj.bjc.6601048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geismann C, Schäfer H, Gundlach JP, Hauser C, Egberts JH, Schneider G, Arlt A. NF-κB dependent chemokine signaling in pancreatic cancer. Cancers (Basel) 2019;11:1445. doi: 10.3390/cancers11101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrique C, Morardet L, Linares LK, Cissé MY, Merle C, Chibon F, Provot S, Haÿ E, Ea HK, Cohen-Solal M, Modrowski D. Calpain-6 controls the fate of sarcoma stem cells by promoting autophagy and preventing senescence. JCI insight. 2018;3:e121225. doi: 10.1172/jci.insight.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orosco A, Fromigué O, Bazille C, Entz-Werle N, Levillain P, Marie PJ, Modrowski D. Syndecan-2 affects the basal and chemotherapy-induced apoptosis in osteosarcoma. Cancer Res. 2007;67:3708–3715. doi: 10.1158/0008-5472.CAN-06-4164. [DOI] [PubMed] [Google Scholar]
- 66.Dieudonné FX, Marion A, Marie PJ, Modrowski D. Targeted inhibition of T-cell factor activity promotes syndecan-2 expression and sensitization to doxorubicin in osteosarcoma cells and bone tumors in mice. J Bone Miner Res. 2012;27:2118–2129. doi: 10.1002/jbmr.1650. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Liao Q, Zhu Y, Long H. Endothelin-1 promotes osteosarcoma cell invasion and survival against cisplatin-induced apoptosis. Clin Orthop Relat Res. 2011;469:3190–3199. doi: 10.1007/s11999-011-1939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaci I, Mansouri R, Marie P, Modrowski D. Hypoxia upregulates calpain-6expression in osteosarcoma cells: Implication in cancer stem cells. J Bone Min Res. 2013;28 [Google Scholar]
- 69.Bouanga JT, Yoon J, Modrowski D. Contribution of cancer stem cells to the metastatic capacities of osteosarcoma. Calcif Tissue Int. 2019;104:S85. [Google Scholar]
- 70.Zha X. The PI3K-Akt pathway regulates calpain 6 expression, proliferation, and apoptosis. FASEB J. 2011;25 doi: 10.1016/j.cellsig.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Singh NK. microRNAs databases: Developmental methodologies, structural and functional annotations. Interdiscip Sci. 2017;9:357–377. doi: 10.1007/s12539-016-0166-7. [DOI] [PubMed] [Google Scholar]
- 72.Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: Molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018;15:137–151. doi: 10.1038/nrgastro.2017.169. [DOI] [PubMed] [Google Scholar]
- 73.Raimbourg Q, Perez J, Vandermeersch S, Prignon A, Hanouna G, Haymann JP, Baud L, Letavernier E. The calpain/calpastatin system has opposing roles in growth and metastatic dissemination of melanoma. PLoS One. 2013;8:e60469. doi: 10.1371/journal.pone.0060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birch D, Britt BC, Dukes SC, Kessler JA, Dizon ML. MicroRNAs participate in the murine oligodendroglial response to perinatal hypoxia-ischemia. Pediatr Res. 2014;76:334–340. doi: 10.1038/pr.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, Gong HS, Zhang J, Xie WL, Tian C, Chen C, Shi Q, Wang SB, Xu Y, Zhang BY, Dong XP. Remarkable reduction of MAP2 in the brains of scrapie-infected rodents and human prion disease possibly correlated with the increase of calpain. PLoS One. 2012;7:e30163. doi: 10.1371/journal.pone.0030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yadavalli R, Guttmann RP, Seward T, Centers AP, Williamson RA, Telling GC. Calpain-dependent endoproteolytic cleavage of PrPSc modulates scrapie prion propagation. J Biol Chem. 2004;279:21948–21956. doi: 10.1074/jbc.M400793200. [DOI] [PubMed] [Google Scholar]
- 77.Donkor I. An update on the therapeutic potential of calpain inhibitors: A patent review. Expert Opin Ther Pat. 2020:1–17. doi: 10.1080/13543776.2020.1797678. [DOI] [PubMed] [Google Scholar]
- 78.Miyazaki T, Miyazaki A. Dysregulation of calpain proteolytic systems underlies degenerative vascular disorders. J Atheroscler Thromb. 2018;25:1–15. doi: 10.5551/jat.RV17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyazaki T, Miyazaki A. Emerging roles of calpain proteolytic systems in macrophage cholesterol handling. Cell Mol Life Sci. 2017;74:3011–3021. doi: 10.1007/s00018-017-2528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyazaki T, Miyazaki A. Impact of dysfunctional protein catabolism on macrophage cholesterol handling. Curr Med Chem. 2019;26:1631–1643. doi: 10.2174/0929867325666180326165234. [DOI] [PubMed] [Google Scholar]
- 81.Kang MY, Zhang Y, Matkovich SJ, Diwan A, Chishti AH, Dorn GW., II Receptor-independent cardiac protein kinase Calpha activation by calpain-mediated truncation of regulatory domains. Circ Res. 2010;107:903–912. doi: 10.1161/CIRCRESAHA.110.220772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taneike M, Mizote I, Morita T, Watanabe T, Hikoso S, Yamaguchi O, Takeda T, Oka T, Tamai T, Oyabu J, et al. Calpain protects the heart from hemodynamic stress. J Biol Chem. 2011;286:32170–32177. doi: 10.1074/jbc.M111.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y, Dorn GW., II Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 84.Moyen C, Goudenege S, Poussard S, Sassi A, Brustis J, Cottin P. Involvement of micro-calpain (CAPN 1) in muscle cell differentiation. Int J Biochem Cell Biol. 2004;36:728–743. doi: 10.1016/S1357-2725(03)00265-6. [DOI] [PubMed] [Google Scholar]
- 85.Sáenz A, Azpitarte M, Armañanzas R, Leturcq F, Alzualde A, Inza I, García-Bragado F, De la Herran G, Corcuera J, Cabello A, et al. Gene expression profiling in limb-girdle muscular dystrophy 2A. PLoS One. 2008;3:e3750. doi: 10.1371/journal.pone.0003750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hollinger K, Selsby J. The physiological response of protease inhibition in dystrophic muscle. Acta Physiol (Oxf) 2013;208:234–244. doi: 10.1111/apha.12114. [DOI] [PubMed] [Google Scholar]
- 87.Raaby L, Otkjær K, Salvskov-Iversen ML, Johansen C, Iversen L. Characterization of the expression of 143-3 isoforms in psoriasis, basal cell carcinoma, atopic dermatitis and contact dermatitis. Dermatol Rep. 2010;2:e14. doi: 10.4081/dr.2010.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


