Abstract
Hepatocellular carcinoma (HCC) is an aggressively malignant type of cancer with a complex pathogenesis. Multiple studies have identified that lncRNA HOXA11-AS is involved in the development of HCC. Nevertheless, the pathological mechanisms of HOXA11-AS in the development of HCC require further investigation. In the present study, the role and underlying mechanisms of HOXA11-AS in HCC were examined. RT-qPCR revealed that HOXA11-AS expression was increased, while that of miR-506-3p was decreased in HCC tissues and cells compared with that in adjacent non-tumor tissues and normal hepatic cells. Dual-luciferase reporter assay and RNA pull-down assay indicated that HOXA11-AS directly interacted with miR-506-3p. miR-506-3p downregulation reversed the inhibitory effects of HOXA11-AS deletion on cell proliferation, invasion and epithelial-mesenchymal transition (EMT), as shown by CCK-8 and Transwell assays, as well as western blot analysis. Bioinformatics analysis and dual-luciferase reporter assay indicated that Slug was a target gene of miR-506-3p. The overexpression of Slug reversed the effects of HOXA11-AS deletion on the viability, invasion and the EMT of HCC cells. Taken together, the present study demonstrates that HOXA11-AS functions as an oncogene to promote the progression of HCC via the miR-506-3p/Slug axis, providing a therapeutic target for patients with HCC.
Keywords: hepatocellular carcinoma, proliferation, epithelial-mesenchymal transition, HOXA11-AS, miR-506-3p, Slug
Introduction
Hepatocellular carcinoma (HCC) is one of the most aggressive types of cancer worldwide and accounts for >80% of primary hepatic cancers. The majority causes of HCC are liver cirrhosis, genetic alterations and living habits (1). It is characterized by a delayed diagnosis, complex pathogenesis, vascular invasion, metastasis and multidrug resistance (2,3). Although interventional prevention and therapeutic strategies, including vaccination, surgical resection, transplantation and immunotherapy have been adopted clinically, a high incidence and low survival rate are still insurmountable challenges for HCC (4–6). Therefore, the investigation of the biological mechanisms responsible for the development of HCC and the identification of novel biomarkers for HCC therapy are urgently required.
Long non-coding RNAs (lncRNAs) refer to a class of RNAs with >200 endogenous nucleotides in length. Despite not having a protein coding capacity, they participate in a series of physiological and pathological processes, including epithelial-mesenchymal transition (EMT), stem cell pluripotency reprogramming, RNA splicing, transcriptional silencing/activation and chromatin remodeling (7–9). Homeobox A11 antisense RNA (HOXA11-AS) is a highly conserved gene that has been shown to be involved in the regulation of post-transcription during tumorigenesis and embryogenesis (10). The aberrant expression of HOXA11-AS is associated with cell staging, and the metastasis and invasion of various types of cancer, such as glioma, laryngeal squamous cell carcinoma (LSCC) and HCC (11,12). For instance, HOXA11-AS expression has been shown to be upregulated in glioma cells and the knockdown of HOXA11-AS markedly suppresses glioma cell proliferation by regulating the miR-214-3p/EZH2 axis (9). Similarly, it has been demonstrated that the overexpression of HOXA11-AS significantly promotes cisplatin resistance by modulating miR-454-3p/Stat3 in human lung adenocarcinoma cells (13).
lncRNAs exert effects on tumor progression through various mechanisms, including their microRNA (miRNA/miR) binding function. miRNAs are non-coding RNAs comprised of 19–25 endogenous nucleotides. Typically, they function as crucial regulators of various types of cancer, including nasopharyngeal carcinoma (NPC), pancreatic ductal adenocarcinoma (PDAC) and HCC by complementary interacting with their target genes (14,15). As oncogenes or suppressors, miRNAs play essential roles in cell proliferation, differentiation, metabolism and apoptosis. For instance, miR-BART8-3p has been proven to induce EMT and to promote NPC cell progression by deactivating immune response (16,17). Inversely, the upregulation of miR-200b has been shown to inhibit HCC cell adhesion and migration by targeting high mobility protein HMGB3 (18). Currently, miR-506 is regarded as a novel biomarker for cancer diagnosis. Moreover, starBase has indicated that miR-506-3p is a target gene of HOXA11-AS. Thus, the physiological role of miR-506-3p in HCC was investigated in the present study.
In the present study, the expression of HOXA11-AS and miR-506-3p was detected and the interaction between miR-506-3p and HOXA11-AS or Slug was further investigated. It was found that HOXA11-AS accelerates proliferation, invasion and EMT in HCC by modulating the miR-506-3p/Slug axis, thus providing possible, promising biomarkers for the treatment of HCC.
Materials and methods
Tissue samples
A total of 39 pairs HCC tumor and corresponding non-tumor tissue samples were obtained from patients recruited from the Third Hospital of Hebei Medical University. The characteristics of the patients with HCC are presented in Table I. The patients had not received any pre-operative therapy and their basic pathological characteristics were recorded. The recruited patients signed written informed consent forms. All the protocols were proved by the Ethics Committee of the Third Hospital of Hebei Medical University.
Table I.
Association between the clinical characteristics of the patients with hepatocellular carcinoma (n=78) and HOXA11-AS expression.
| Parameter | No. of patients | HOXA11-AS expression
|
P-value | |
|---|---|---|---|---|
| High (n=40) | Low (n=38) | |||
| Age (years) | ||||
| ≤60 | 44 | 15 | 29 | 0.479 |
| ≥60 | 31 | 25 | 6 | |
| Sex | ||||
| Male | 37 | 27 | 10 | 0.535 |
| Female | 41 | 13 | 28 | |
| Tumor size (cm) | ||||
| ≤7 | 46 | 12 | 34 | 0.032a |
| ≥7 | 32 | 28 | 4 | |
| Tumor staging (HCC) | ||||
| I+II | 36 | 10 | 26 | 0.019a |
| III | 42 | 30 | 12 | |
P<0.05, using median expression level of HOXA11-AS as the cut-off value.
Cells and cell transfection
The HCC cell lines, Huh7, Hep3B and PLC/PRF/5, and the human hepatic epithelial cell line, THLE-3, were purchased from ATCC. In addition, the HCC cell line, MHCC97-H, was obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The Huh7, Hep3B and THLE-3 cells were maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and 0.05% penicillin/streptomycin and incubated in a 5% CO2 incubator at 37°C. Small interfering RNA (siRNA) targeting HOXA11-AS (si-HOXA11-AS, including si-HOXA11-AS#1: 5′-TCGTCACTCGGTGTTCTCACCGAAA-3′, si-HOXA11-AS#2: 5′-GCACGGTGACTTGATTACACTCTCT-3′, and si-HOXA11-AS#3: 5′-CGGAAACGGCTAACAAGGAGATTTG-3′) and siRNA negative control (si-NC), pcDNA-HOXA11-AS overexpression vector (HOXA11-AS) and negative control (Vector), pcDNA-Slug overexpression vector (Slug) and its negative control (control) were synthesized by Genepharma. The miRNA mimic (miR-506-3p) and negative control (miR-NC), miR-506-3p inhibitor (anti-miR-506-3p) and negative control inhibitor (anti-miR-NC) were purchased from Guangzhou RiboBio Co., Ltd. 0.2 μg of HOXA11-AS overexpression vector, Slug overexpression vector or pcDNA vector was transfected in Huh7 and Hep3B cells (4×104 cells) with 0.5 μl of Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). 0.5 μg of those oligonucleotides including si-HOXA11-AS, si-NC, miR-506-3p, miR-NC, anti-miR-506-3p or anti-miR-NC was transfected into cells using 0.6 μl of Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After of 48 h transfection, the transfected cells were used in subsequent experimentats.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The Huh7 and Hep3B cells were incubated with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to obtain the RNA. The All-in-One™ miRNA First-Strand cDNA Synthesis kit (GeneCopoeia, Inc.) was applied to synthesize cDNA and the qPCR was performed using TaqMan Gene Expression assay (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 were used as internal controls. The amplification parameters were as follows: Denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 1 min. The primers used were as follows: HOXA11-AS forward, 5′-GCCAAGTTGTACTTACTACGTC-3′ and reverse, 5′-GTTGGAGGAGTAGGAGTATGTA-3′; miR-506-3p forward, 5′-CGGGCTAAGGCACCCTTCTG-3′ and reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′; E-cadherin forward, 5′-GAGCCTGAGTCCTGCAGTCC-3′ and reverse, 5′-TGTATTGCTGCTTGGCCTCA-3′; N-cadherin forward, 5′-GTGCCATTAGCCAAGGGAATTCAGC-3′ and reverse, 5′-GCGTTCCTGTTCCACTCATAGGAGG-3′; Vimentin forward, 5′-TGCCCTTGAAGCTGCTAACTAC-3′ and reverse, 5′-CAACCAGAGGAAGTGCATCCAG-3′; GAPDH forward, 5′-CCCACTCCTCCACCTTTGAC-3′ and reverse, 5′-GGATCTCGCTCCTGGAAGATG-3′; and U6 forward, 5′-GCUUCGGCAGCACAUAUACUAAAAU-3′ and reverse, 5′-CGCUUCACGAAUUUGCGUGUCAU-3′. Relative expression was calculated using the 2−ΔΔCq method (19).
Western blot analysis
Total protein was isolated from tissues and cell lines using RIPA lysis buffer (EMD Millipore). The concentration of total protein was measured using a BCA Protein assay kit (Nanjing KeyGen Biotech Co., Ltd.). Protein (30 μg) was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (EMD Millipore). The membranes were then blocked with 5% skim milk at room temperature for 2 h. Subsequently, the membranes were incubated with the following primary antibodies: E-cadherin (1:1,000; cat. no. 14472), N-cadherin (1:1,000, cat. no. 13116), Vimentin (1:1,000, cat. no. 5741), Slug (1:1,000, cat. no. 9585), GAPDH (1:1,000, cat. no. 5174) (all from Cell Signaling Technology, Inc.) overnight at 4°C. Following incubation with the primary antibodies, the membranes were incubated with a the goat anti-rabbit IgG H&L (HRP) (1:2,000, cat. no. ab205718, Abcam) and goat anti-mouse IgG H&L (HRP) (1:2,000, cat. no. ab205719, Abcam) secondary antibodies for 40 min at room temperature. Finally, protein bands were visualized using the Enhanced Chemiluminescence Plus kit (EMD Millipore) and quantified by densitometric analysis of protein signals using ImageJ version 1.49 (National Institutes of Health).
Nuclear and cytoplasmic localization analysis
RNA was isolated from the cell cytoplasm and nucleus using the Cytoplasmic and Nuclear RNA Purification kit (Norgen Biotek). Subsequently, total RNA in each fraction was measured by RT-qRCR as described above. U6 and GAPDH functioned as internal references for the nucleus and cytoplasm, respectively.
Cell proliferation and invasion
CCK-8 and Transwell assays were utilized for the evaluation of cell proliferation and cell invasive ability. For CCK-8 assay, the transfected Huh7 and Hep3B cells were seeded in 96-well plates (5,000 cells/well) and continuously incubated for a further 24, 48, 72 and 96 h in a 5% CO2 incubator at 37°C. After rinsing with PBS 3 times, 10 μl CCK-8 (5 mg/ml; Beyotime Institute of Biotechnology, Inc.) were added to each well for 2 h. The absorption at 450 nm was measured using a microplate reader (Bio-Rad Laboratories, Inc). For Transwell assay, transfected Huh7 and Hep3B cells (2×105 cells/well) in 200 μl serum-free medium were seeded into the upper chamber pre-treated with Matrigel (BD Biosciences). Complete medium with 10% FBS was seeded into the lower chamber. After incubation for 24 h at 37°C, the invasive cells in the lower chamber were stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature for 10 min and counted using an inverted microscope (Nikon Eclipse TS100; Nikon Corporation).
Luciferase reporter assay
StarBase (http://starbase.sysu.edu.cn/starbase2/) and TargetScan (http://www.targetscan.org/vert_71/) were used to screen the putative target. Wild-type and mutant type sequences (HOXA11-AS-Wt, HOXA11-AS-Mut, Slug-Wt, Slug-Mut) were constructed and cloned into the pGL3 basic vector (Invitrogen; Thermo Fisher Scientific, Inc.). Huh7 and Hep3B cells were co-transfected with the luciferase vectors and miR-506-3p mimics or miR-NC for 24 h using Lipofectamine 2000 transfection reagent. Luciferase activities were measured by a dual-luciferase assay system (Promega). Firefly luciferase activities were normalized to Renilla luciferase activities.
RNA pull-down assay
Biotinylated HOXA11-AS (Bio-HOXA11-AS), miR-506-3p (Bio-miR-506-3p), HOXA11-AS Mut (Bio-HOXA11-AS-Mut), miR-506-3p Mut (Bio-miR-506-3p-Mut), and negative control (Bio-miR-NC) (Sangon Biotech Co., Ltd.) were transfected into the Huh7 and Hep3B cells. Following incubation for 24 h, the transfected cells were lysed, collected and incubated with Dynabeads M-280 Streptavidin (Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min. The bound RNAs were then subjected to RT-qPCR for quantification and analysis as described above.
Statistical analysis
All the data were collected and analyzed using SPSS software (SPSS, Inc.) and GraphPad Prism 7 (GraphPad Inc.). Data are presented as the means ± SD. Comparisons between 2 groups were evaluated using a Student's t-test. One-way ANOVA followed by Tukey's test was used for differences among multiple groups. The correlation between miR-506-3p and HOXA11-AS or Slug was analyzed using Pearson's correlation coefficient. The association between HOXA11-AS and the patient clinicopathological characteristics were analyzed using the χ2 test or Fisher's exact test. A P-value <0.05 (P<0.05) was considered to indicate a statistically significant difference.
Results
HOXA11-AS is upregulated, whereas miR-506-3p is downregulated in HCC tissues and cell lines
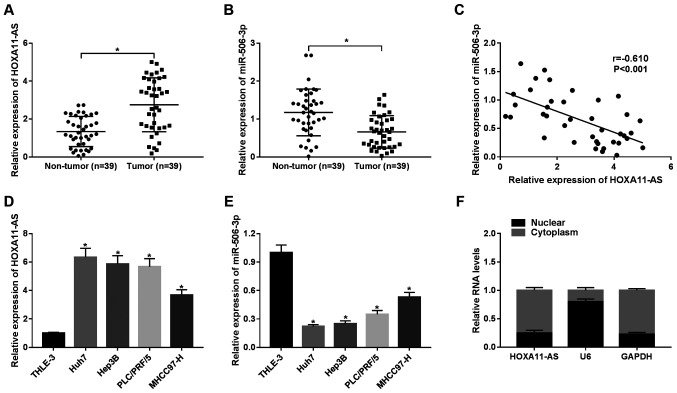
To explore the roles of HOXA11-AS and miR-506-3p in HCC, the expression of HOXA11-AS and miR-506-3p in 39 pairs of HCC tissues and the corresponding non-tumor tissues was detected by RT-qPCR. HOXA11-AS was upregulated, whereas miR-506-3p was downregulated in the HCC tumor samples in comparison to the non-tumor samples (Fig. 1A and B). Moreover, it was found that HOXA11-AS negatively correlated with miR-506-3p (r=−0.610, P<0.001) (Fig. 1C). Furthermore, the expression of HOXA11-AS was significantly enhanced in the HCC cell lines (Huh7, Hep3B, PLC/PRF/5 and MHCC97-H) compared with the human hepatic epithelial cells, THLE-3 (Fig. 1D). On the contrary, the expression of miR-506-3p was lower in the HCC cells than in the THLE-3 cells (Fig. 1E). The data also suggested that HOXA11-AS was mainly located in the cytoplasm (Fig. 1F). These findings demonstrate that HOXA11-AS and miR-506-3p play vital roles in HCC.
Figure 1.
Expression of HOXA11-AS and miR-506-3p in HCC tissues and cell lines. (A and B) The expression of (A) HOXA11-AS and (B) miR-506-3p was measured by RT-qPCR in 39 pairs of HCC tissues and adjacent non-tumor tissues. (C) Correlation between HOXA11-AS and miR-506-3p (r=−0.610, P<0.001). (D and E) Expression of (D) HOXA11-AS and (E) miR-506-3p in HCC cell lines (Huh7, Hep3B, PLC/PRF/5 and MHCC97-H) and THLE-3 cells. (F) RT-qPCR was used to assess the level of the cytoplasmic control transcript (GAPDH), nuclear control transcript (U6) and HOXA11-AS in the nuclear and cytoplasmic fractions. *P<0.05 vs. respective control. HCC, hepatocellular carcinoma.
HOXA11-AS directly interacts with miR-506-3p
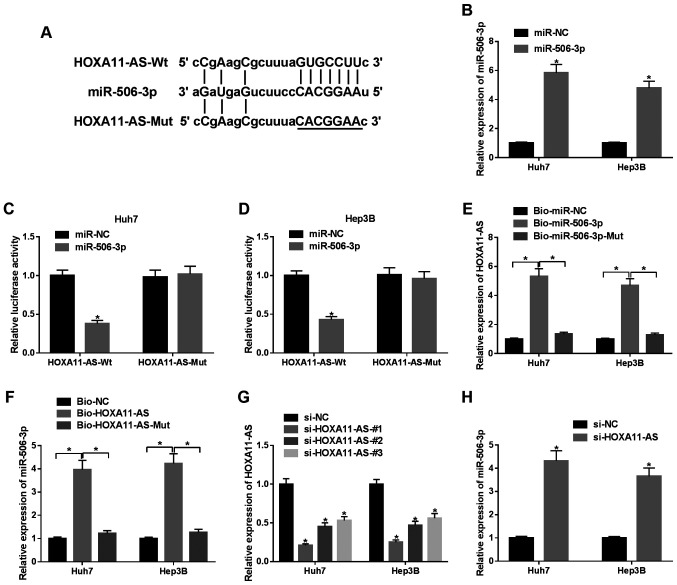
Bioinformatics prediction program StarBase was employed to search the potential target miRNA for HOXA11-AS. It was observed that miR-506-3p may bind to HOXA11-AS (Fig. 2A). As shown in Fig. 2B, miR-506-3p exhibited successful efficiency in both the Huh7 and Hep3B cells. The luciferase activity in the HCC cells co-transfected with miR-506-3p and HOXA11-AS-Wt was markedly suppressed in comparison to the control group (Fig. 2C and D). RNA pull-down assay exhibited that the enrichment of HOXA11-AS was observed in the HCC cells transfected with Bio-miR-506-3p compared with Bio-miR-506-3p-Mut or Bio-miR-NC transfection (Fig. 2E). Similarly, the enrichment of miR-506-3p was observed in the HCC cells transfected with Bio-HOXA11-AS (Fig. 2F). Furthermore, it was observed that the expression of HOXA11-AS was decreased in the HCC cells transfected with si-HOXA11-AS#1, si-HOXA11-AS#2, si-HOXA11-AS#3 compared with the si-NC group (Fig. 2G). Due to the highest knockdown efficiency, si-HOXA11-AS#1 was selected for use in subsequent experiments. It was found that HOXA11-AS knockdown increased the expression of miR-506-3p in HCC cells (Fig. 2H). Taken together, it was thus concluded that miR-506-3p was negatively regulated by HOXA11-AS.
Figure 2.
Identification of the association between HOXA11-AS and miR-506-3p. (A) The putative binding site of HOXA11-AS and miR-506-3p was predicted by StarBase. (B) Expression of miR-506-3p in Huh7 and Hep3B cells transfected with miR-506-3p or miR-NC. (C and D) Luciferase activity of (C) Huh7 and (D) Hep3B cells co-transfected with miR-506-3p or miR-NC and HOXA11-AS-Wt or HOXA11-AS-Mut. (E) Expression of HOXA11-AS in Huh7 and Hep3B cells transfected with Bio-miR-506-3p, Bio-miR-506-3p-Mut or Bio-miR-NC. (F) Expression of miR-506-3p in Huh7 and Hep3B cells transfected with Bio-HOXA11-AS, Bio-HOXA11-AS-Mut or Bio-NC. (G) Expression of HOXA11-AS in Huh7 and Hep3B cells transfected with si-HOXA11-AS#1, si-HOXA11-AS#2, si-HOXA11-AS#3 and si-NC. (H) Expression of miR-506-3p in Huh7 and Hep3B cells transfected with si-HOXA11-AS or si-NC. *P<0.05 vs. respective control.
HOXA11-AS promotes HCC progression by targeting miR-506-3p
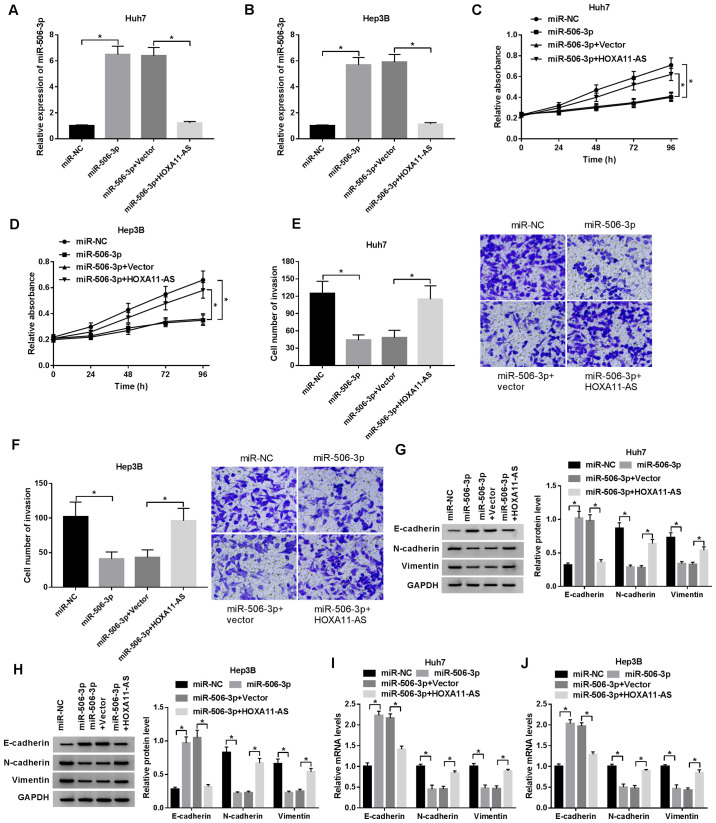
Subsequently, the regulatory mechanisms of the HOXA11-AS/miR-506-3p axis were investigated in the HCC cells. The successful transfection efficiency of anti-miR-506-3p and HOXA11-AS in the HCC cells was observed (Fig. S1A and B). The expression of miR-506-3p, elevated by HOXA11-AS silencing, was reversed by transfection with miR-506-3p inhibitor (Fig. 3A and B), while the increase in miR-506-3p expression induced by transfection with miR-506-3p mimic was suppressed by the overexpression of HOXA11-AS in both the Huh7 and Hep3B cells (Fig. 4A and B). The results of CCK-8 assay revealed that the inhibitory effects of HOXA11-AS silencing on cell proliferation were blocked by transfection with miR-506-3p inhibitor (Fig. 3C and D), and the overexpression of HOXA11-AS reversed the inhibitory effect of miR-506-3p upregulation on cell proliferation (Fig. 4C and D). Moreover, cell invasion was suppressed by HOXA11-AS silencing; however, this effect was reversed by transfection with miR-506-3p inhibitor (Fig. 3E and F). In addition, the inhibition effect of miR-506-3p overexpression on cell invasion was abolished by the upregulation of HOXA11-AS (Fig. 4E and F). Furthermore, the protein expression of EMT markers was examined by western blot analysis in order to determine the effect of HOXA11-AS and miR-506-3p on EMT in HCC cells. The mRNA and protein expression of E-cadherin enhanced by HOXA11-AS silencing was suppressed by transfection with miR-506-3p inhibitor, and the decreased mRNA and protein expression of N-cadherin and vimentin induced by HOXA11-AS silencing was promoted by miR-506-3p downregulation (Fig. 3G-J). Moreover, the promotion of E-cadherin mRNA and protein expression, and the inhibition of the mRNA and protein expression of N-cadherin and vimentin induced by miR-506-3p overexpression were reversed by HOXA11-AS upregulation in both the Huh7 and Hep3B cells (Fig. 4G-J). Collectively, these data demonstrate that HOXA11-AS regulates cell proliferation, invasion and EMT by targeting miR-506-3p.
Figure 3.
miR-506-3p inhibitor reverses the inhibitory effects of HOXA11-AS silencing on HCC progression. Huh7 and Hep3B cells were transfected with si-HOXA11-AS + anti-miR-506-3p, si-HOXA11-AS + anti-miR-NC, si-HOXA11-AS, or si-NC. (A and B) Expression of miR-506-3p in transfected (A) Huh7 and (B) Hep3B cells. (C and D) Viability of transfected (C) Huh7 and (D) Hep3B cells was evaluated by CCK-8 assay at different periods of time (24, 48, 72 and 96 h). (E and F) Transwell assay was used to detect the invasive ability of transfected (E) Huh7 and (F) Hep3B cells. (G-J) mRNA and protein expression of the EMT markers, E-cadherin, N-cadherin and vimentin, in transfected (G and I) Huh7 and (H and J) Hep3B cells determined by RT-qPCR and western blot analysis. GAPDH was used as an internal reference. *P<0.05. HCC, hepatocellular carcinoma.
Figure 4.
HOXA11-AS abolishes the function of miR-506-3p in HCC cells. Huh7 and Hep3B cells were transfected with miR-506-3p, miR-506-3p + HOXA11-AS, miR-506-3p + Vector or miR-NC. (A and B) Expression of miR-506-3p in (A) Huh7 and (B) Hep3B cells 24 h post-transfection. (C and D) Viability of (C) Huh7 and (D) Hep3B cells evaluated by CCK-8 assay 24, 48, 72 and 96 h post-transfection. (E and F) Invasive ability of transfected (E) Huh7 and (F) Hep3B cells detected by Transwell assay. (G-J) mRNA and protein expression of the EMT markers, E-cadherin, N-cadherin and vimentin, determined by RT-qPCR and western blot analysis. GAPDH was used as an internal reference. *P<0.05. HCC, hepatocellular carcinoma.
Slug is a target gene of miR-506-3p
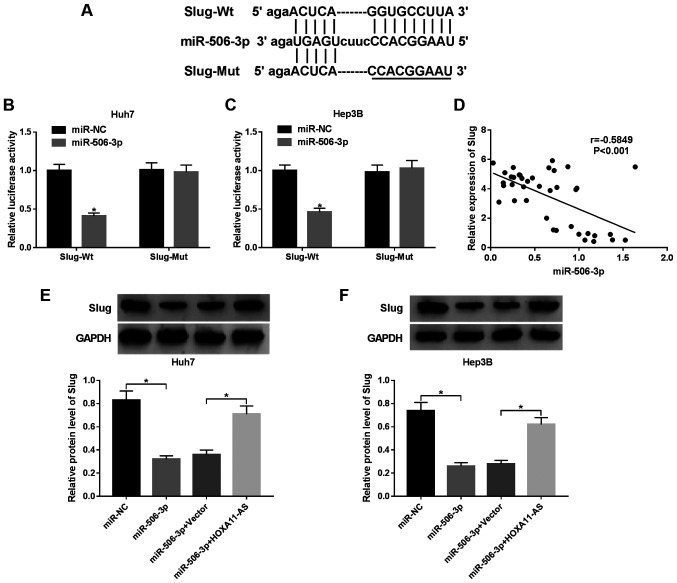
TargetScan predicted that miR-506-3p contained a complementary sequence of Slug (Fig. 5A). To confirm this prediction, wild-type Slug (Slug-Wt) or mutant type Slug (Slug-Mut) was cloned into the luciferase gene and co-transfected with miR-506-3p or miR-NC. The luciferase activity was markeldy reduced in the Huh7 and Hep3B cells co-transfected with Slug-Wt and miR-506-3p compared with miR-NC group, while the luciferase activity was not altered in the HCC cell co-transfected with Slug-Mut and miR-506-3p (Fig. 5B and C). Moreover, we found a negative relationship between miR-506-3p and Slug (r=−5849, P<0.001) (Fig. 5D). In addition, western blot analysis revealed that the protein expression of Slug was decreased by miR-506-3p overexpression, which was reversed by HOXA11-AS overexpression in the Huh7 and Hep3B cells (Fig. 5E and F). These data indicate that HOXA11-AS targets miT-506-3p to regulate Slug expression.
Figure 5.
Slug is a target of miR-506-3p. (A) Bioinformatics analysis prediction using the online website TargetScan determined the putative binding site of Slug and miR-506-3p. (B and C) Luciferase activity of (B) Huh7 and (C) Hep3B cells co-transfected with miR-506-3p or miR-NC and Slug-Wt or Slug-Mut. (D) Correlation between Slug and miR-506-3p (r=−0.5849, P<0.001). (E and F) Slug protein expression in (E) Huh7 and (F) Hep3B cells transfected with miR-506-3p, miR-506-3p + HOXA11-AS, miR-506-3p + Vecter or miR-NC determined by western blot analysis. GAPDH was used as an internal reference. *P<0.05 vs. respective control.
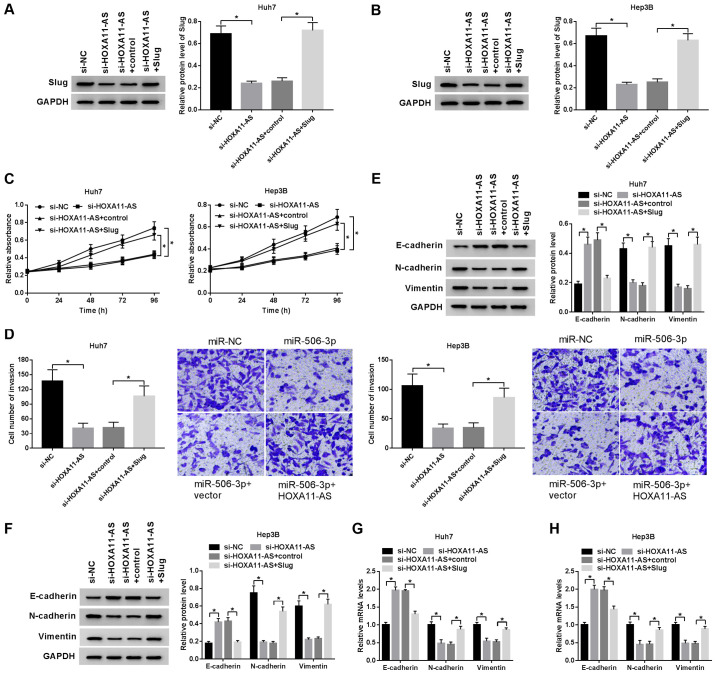
Slug attenuates the inhibitory effect on HCC progression induced by HOXA11-AS silencing
To examine the regulatory effect of the HOXA11-AS/Slug axis on HCC cell proliferation, invasion and EMT, the Huh7 and Hep3B cells were transfected with si-HOXA11-AS+Slug, si-HOXA11-AS, si-HOXA11-AS + control and si-NC. Western blot analysis revealed that overexpression of Slug significantly promoted Slug expression (Fig. S1C and D). Slug protein expression inhibited by HOXA11-AS silencing was recovered by Slug overexpression in the Huh7 and Hep3B cells, demonstrating that Slug overexpression reversed the inhibitory effects mediated by HOXA11-AS silencing (Fig. 6A and B). Moreover, the upregulation of Slug reversed the inhibitory effects of HOXA11-AS downregulation on the proliferation of HCC cells (Fig. 6C). Similarly, Slug overexpression reversed the suppressive effects on cell invasion induced by HOXA11-AS silencing (Fig. 6D). Furthermore, the downregulation of HOXA11-AS promoted E-cadherin mRNA and protein expression, but suppressed N-cadherin and vimentin mRNA and protein expression in the HCC cells; these effects were reversed by Slug overexpression (Fig. 6E-H). Overall, HOXA11-AS promoted HCC cell proliferation, invasion and EMT by upregulating Slug expression.
Figure 6.
Slug abrogates the inhibitory effect on HCC progression induced by HOXA11-AS silencing. Huh7 and Hep3B cells were transfected with si-HOXA11-AS + Slug, si-HOXA11-AS, si-HOXA11-AS + control, or si-NC. (A and B) Slug protein expression in (A) Huh7 and (B) Hep3B cells at 24 h post-transfection. GAPDH was used as an internal reference. The (C) proliferative and (D) invasive ability of transfected Huh7 and Hep3B cells were measured by CCK-8 and Transwell assays, respectively. (E-H) mRNA and protein expression of the EMT markers E-cadherin, N-cadherin and vimentin in transfected Huh7 (E and G) and Hep3B (F and H) cells was examined by RT-qPCR and western blot analysis. GAPDH was used as an internal reference. *P<0.05.
Discussion
Previous studies have identified that lncRNAs play fundamental roles as promoters or suppressors of the cell cycle, infiltration, differentiation and metabolism in various types of cancer, including HCC (20-22). For example, UCA1 has been shown to function as a promoter to enhance HCC cell migration and G1/S transition by improving CDK6 expression (23). Huang et al found that SNHG1 was highly expressed in HCC tissues and cells, which served as an oncogene in HCC progression (24). miRNAs have also been confirmed to be involved in various cellular biological behaviors, such as cell proliferation, differentiation and death in HCC (25,26). For instance, miR-212 has been shown to be expressed at low levels in HCC and to exert inhibitory effects on tumor angiogenesis, migration and invasion by inactivating Wnt/b-Catenin signaling (27). Wang et al suggested that miR-383 functions as a suppressor of HCC cell growth (28).
In the present study, HOXA11-AS was found to be significantly increased HCC in tissues and cells. Moreover, HOXA11-AS has been confirmed to function as a ceRNA to participate in tumor progression by interacting with specific miRNAs (29,30). For instance, HOXA11-AS upregulation has been shown to regulate cell growth via targeting miR-761 in papillary thyroid cancer (31). HOXA11-AS has been demonstrated to play a promoting role in the progression of oral squamous cell carcinoma by sponging miR-98-5p (32). Furthermore, HOXA11-AS has been proven to function as a sponge for miR-125a-5p to upregulate PADI2 expression and further promote colorectal cancer cell metastasis (33). In addition, Zhan et al demonstrated that sufficiency of HOXA11-AS accelerated cell growth and EMT by suppressing miR-214-3p in HCC (34).
StarBase predicted that miR-506-3p contained the potential binding sites of HOXA11-AS. The results indicated that miR-506-3p was a target of HOXA11-AS and negatively regulated by HOXA11-AS. miR-506-3p has been identified to play tumor suppressive role in several human cancers, such as ovarian cancer and retinoblastoma (35,36). For example, miR-506-3p has been demonstrated to suppress tumor progression in prostate cancer and pancreatic cancer (37,38). Furthermore, miR-506-3p has been shown to be downregulated in non-small lung cancer tissues and cells, and the overexpression of miR-506-3p suppresses cell growth, migration and invasion (39). Consistent with these previous studies, in the present study, miR-506-3p was found to be significantly decreased in HCC tissues and cells. Moreover, HOXA11-AS exerted promoting effects on cell growth, invasion and EMT via sponging miR-506-3p in HCC.
Slug (Snai2), a member of the Snail family (transcription factor of C2H2-type zinc finger), is a transcription factor involved in the process of EMT. Accumulating evidence has indicated that Slug is related to cell invasion and metastasis in various types of tumor (40). For example, SNHG15 regulates Slug expression to enhance colon cancer progression (41). Moreover, a high expression of Slug has been observed in HCC, which plays a promoting role in HCC progression (42). In the present research, the results revealed that Slug was a target gene of miR-506-3p. HOXA11-AS could sponge miR-506-3p to regulate the expression of Slug in HCC cells. Furthermore, Slug overexpression blocked the inhibitory effects of HOXA11-AS downregulation on cell growth, invasion and EMT.
In conclusion, the present study demonstrated that HOXA11-AS functioned as an oncogene to promote HCC progression and EMT by elevating Slug expression via targeting miR-506-3p. Furthermore, the regulatory mechanisms of the HOXA11-AS/miR-506-3p/Slug axis in HCC development were elucidated, providing promising biomarkers for the diagnosis and therapy of HCC.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Task Force of Hebei Education Department (grant no. 2008505) and Artificial liver in patients with liver failure clinical study on the individualized treatment (G201734).
Availability of data and materials
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
YL was involved in the conceptualization of the study and in data curation, and in the writing of the original draft. YL and WY were involved in formal analysis and in the investigative aspects of the study. WY was involved in the study methodology. DZ was involved in project administration. GJ provided resources. XC provided software. GJ and XC were also involved in the writing, reviewing and editing of the manuscript. DZ, GJ and XC were also involved in the conception and design of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The recruited patients signed written informed consent forms. All the protocols were proved by the Ethics Committee of the Third Hospital of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Soliman B, Salem A, Ghazy M, Abu-Shahba N, El Hefnawi M. Bioinformatics functional analysis of let-7a, miR-34a, and miR-199a/b reveals novel insights into immune system pathways and cancer hallmarks for hepatocellular carcinoma. Tumour Biol. 2018;40:1010428318773675. doi: 10.1177/1010428318773675. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Wang S, Xu J, Kou B, Chen D, Wang Y, Zhu X. Extract of Stellerachamaejasme L(ESC) inhibits growth and metastasis of human hepatocellular carcinoma via regulating microRNA expression. BMC Complement Altern Med. 2018;18:99. doi: 10.1186/s12906-018-2123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes A, Dhoot GK. Dysregulated cancer cell transdifferentiation into erythrocytes is an additional metabolic stress in hepatocellular carcinoma. Tumour Biol. 2018;40:1010428318811467. doi: 10.1177/1010428318811467. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Sun J, Zhang N, Yang R, Li H, Zhang Y, Chen K, Kong D. PES1 enhances proliferation and tumorigenesis in hepatocellular carcinoma via the PI3K/AKT pathway. Life Sci. 2019;219:182–189. doi: 10.1016/j.lfs.2018.12.054. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Zhu P, Sun B, Guo J, Zhou H, Shu Y, Li Q. Modulation of YrdC promotes hepatocellular carcinoma progression via MEK/ERK signaling pathway. Biomed Pharmacother. 2019;114:108859. doi: 10.1016/j.biopha.2019.108859. [DOI] [PubMed] [Google Scholar]
- 6.Sheng N, Li Y, Qian R, Li Y. The clinical significance and biological function of lncRNA RGMB-AS1 in hepatocellular carcinoma. Biomed Pharmacother. 2018;98:577–584. doi: 10.1016/j.biopha.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 7.XU CH, Xiao LM, Liu Y, Chen LK, Zheng SY, Zeng EM, Li DH. The lncRNA HOXA11-AS promotes glioma cell growth and metastasis by targeting miR-130a-5p/HMGB2. Eur Rev Med Pharmacol Sci. 2019;2:241–252. doi: 10.26355/eurrev_201901_16770. [DOI] [PubMed] [Google Scholar]
- 8.Jin QS, Huang LJ, Zhao TT, Yao XY, Lin LY, Teng YQ, Kim SH, Nam MS, Zhang LY, Jin YJ. HOXA11-AS regulates diabetic arteriosclerosis-related inflammation via PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:6912–6921. doi: 10.26355/eurrev_201810_16161. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, He T, Li Z, Liu H, Ding B. Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth, migration and invasion of glioma cells. Biomed Pharmacother. 2017;95:1504–1513. doi: 10.1016/j.biopha.2017.08.097. [DOI] [PubMed] [Google Scholar]
- 10.Lu CW, Zhou DD, Xie T, Hao JL, Pant OP, Lu CB, Liu XF. HOXA11 antisense long noncoding RNA (HOXA11-AS): A promising lncRNA in human cancers. Cancer Med. 2018;7:3792–3799. doi: 10.1002/cam4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu L, Jin M, Yang L, Sun C, Wang P, Li Y, Tian L, Liu M, Sun Y. Expression of long non-coding RNA HOXA11-AS is correlated with progression of laryngeal squamous cell carcinoma. Am J Transl Res. 2018;10:573–580. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang FQ, Zhang JQ, Jin JJ, Yang CY, Zhang WJ, Zhang HM, Zheng JH, Weng ZM. HOXA11-AS promotes the growth and invasion of renal cancer by sponging miR-146b-5p to upregulate MMP16 expression. J Cell Physiol. 2018;233:9611–9619. doi: 10.1002/jcp.26864. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Li X, Zhou L, Ni J, Yan W, Ma R, Wu J, Feng J, Chen P. LncRNA HOXA11-AS drives cisplatin resistance of human LUAD cells via modulating miR-454-3p/Stat3. Cancer Sci. 2018;109:3068–3079. doi: 10.1111/cas.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang TS, Zheng YJ, Wang J, Zhao JY, Yang DK, Liu ZS. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38:97. doi: 10.1186/s13046-019-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.He H, Liao X, Yang Q, Liu Y, Peng Y, Zhong H, Yang J, Zhang H, Yu Z, Zuo Y, et al. MicroRNA-494-3p promotes cell growth, migration, and invasion of nasopharyngeal carcinoma by targeting sox7. Technol Cancer Res Treat. 2018;17:1533033818809993. doi: 10.1177/1533033818809993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C, Zong J, Lin W, Wang M, Xu Y, Zhou R, Lin S, Guo Q, Chen H, Ye Y, et al. EBV-miR-BART8-3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways. J Exp Clin Cancer Res. 2018;37:283. doi: 10.1186/s13046-018-0953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi F, Hao Y, Chong X, Zhong W. Overexpression of microRNA-506-3p aggravates the injury of vascular endothelial cells in patients with hypertension by downregulating Beclin1 expression. Exp Ther Med. 2018;15:2844–2850. doi: 10.3892/etm.2018.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LK, Xie XN, Song XH, Su T, Chang XL, Xu M, Liang B, Huang DY. Upregulation of miR-200b inhibits hepatocellular carcinoma cell proliferation and migration by targeting HMGB3 protein. Technol Cancer Res Treat. 2018;17:1533033818806475. doi: 10.1177/1533033818806475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, Yang L, Li L, Zeng C. Long non-coding RNA KCNQ1OT1 modulates oxaliplatin resistance in hepatocellular carcinoma through miR-7-5p/ABCC1 axis. Biochem Biophys Res Commun. 2018;503:2400–2406. doi: 10.1016/j.bbrc.2018.06.168. [DOI] [PubMed] [Google Scholar]
- 21.Lu S, Jiang X, Su Z, Cui Z, Fu W, Tai S. The role of the long non-coding RNA HOXA11-AS in promoting proliferation and metastasis of malignant tumors. Cell Biol Int. 2018;42:1596–1601. doi: 10.1002/cbin.11045. [DOI] [PubMed] [Google Scholar]
- 22.Misawa A, Takayama K, Urano T, Inoue S. Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes cell growth and inhibits apoptosis in prostate cancer cells. J Biol Chem. 2016;291:17861–17880. doi: 10.1074/jbc.M116.718536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YL, Liu JY, Yang JE, Yu XM, Chen ZL, Chen YJ, Kuang M, Zhu Y, Zhuang SM. Lnc-UCID promotes G1/S transition and hepatoma growth by preventing DHX9-mediated CDK6 down-regulation. Hepatology. 2019;70:259–275. doi: 10.1002/hep.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang D, Wei Y, Zhu J, Wang F. Long non-coding RNA SNHG1 functions as a competitive endogenous RNA to regulate PDCD4 expression by sponging miR-195-5p in hepatocellular carcinoma. Gene. 2019;714:143994. doi: 10.1016/j.gene.2019.143994. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Chen Y, Liu K. Erratum: miR-185 inhibits cell migration and invasion of hepatocellular carcinoma through CDC42. Oncol Lett. 2020;19:1089. doi: 10.3892/ol.2019.11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu QL, Xu ZP, Lan YF, Li B. miR-636 represses cell survival by targeting CDK6/Bcl-2 in cervical cancer. Kaohsiung J Med Sci. 2020;36:328–335. doi: 10.1002/kjm2.12181. [DOI] [PubMed] [Google Scholar]
- 27.Jia P, Wei G, Zhou C, Gao Q, Wu Y, Sun X, Li X. Upregulation of MiR-212 inhibits migration and tumorigenicity and inactivates Wnt/β-catenin signaling in human hepatocellular carcinoma. Technol Cancer Res Treat. 2018;17:1533034618765221. doi: 10.1177/1533034618765221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Lu L, Luo Z, Li W, Lu Y, Tang Q, Pu J. miR-383 inhibits cell growth and promotes cell apoptosis in hepatocellular carcinoma by targeting IL-17 via STAT3 signaling pathway. Biomed Pharmacother. 2019;120:109551. doi: 10.1016/j.biopha.2019.109551. [DOI] [PubMed] [Google Scholar]
- 29.Cui Y, Yi L, Zhao JZ, Jiang YG. Long noncoding RNA HOXA11-AS functions as miRNA sponge to promote the glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol. 2017;36:822–828. doi: 10.1089/dna.2017.3805. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Peng W, Jiang H, Sha H, Li J. LncRNA HOXA11-AS promotes proliferation and invasion by targeting miR-124 in human non-small cell lung cancer cells. . Tumour Biol. 2017;39:1010428317721440. doi: 10.1177/1010428317721440. [DOI] [PubMed] [Google Scholar]
- 31.Yin X, Zhang J, Li C, Zhang Z, Jin T, Song L, Zhang R, Wang W, Tao Y, Wang X. LncRNA HOXA11-AS accumulation-induced microRNA-761 downregulation regulates cell growth by targeting TRIM29 in papillary thyroid cancer. Am J Transl Res. 2019;11:6826–6837. [PMC free article] [PubMed] [Google Scholar]
- 32.Niu X, Yang B, Liu F, Fang Q. LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression. Biomed Pharmacother. 2020;121:109623. doi: 10.1016/j.biopha.2019.109623. [DOI] [PubMed] [Google Scholar]
- 33.Chen D, Sun Q, Zhang L, Zhou X, Cheng X, Zhou D, Ye F, Lin J, Wang W. The lncRNA HOXA11-AS functions as a competing endogenous RNA to regulate PADI2 expression by sponging miR-125a-5p in liver metastasis of colorectal cancer. Oncotarget. 2017;8:70642–70652. doi: 10.18632/oncotarget.19956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan M, He K, Xiao J, Liu F, Wang H, Xia Z, Duan X, Huang R, Li Y, He X, et al. LncRNA HOXA11-AS promotes hepatocellular carcinoma progression by repressing miR-214-3p. J Cell Mol Med. 2018;22:3758–3767. doi: 10.1111/jcmm.13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Lei X, Gao C, Xue Y, Li X, Wang H, Feng Y. MiR-506-3p suppresses the proliferation of ovarian cancer cells by negatively regulating the expression of MTMR6. J Biosci. 2019;44:126. doi: 10.1007/s12038-019-9952-9. [DOI] [PubMed] [Google Scholar]
- 36.Wu L, Chen Z, Xing Y. MiR-506-3p inhibits cell proliferation, induces cell cycle arrest and apoptosis in retinoblastoma by directly targeting NEK6. Cell Biol Int. 2018 Aug 6; doi: 10.1002/cbin.11041. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Hu CY, You P, Zhang J, Zhang H, Jiang N. MiR-506-3p acts as a novel tumor suppressor in prostate cancer through targeting GALNT4. Eur Rev Med Pharmacol Sci. 2019;23:5133–5138. doi: 10.26355/eurrev_201906_18177. [DOI] [PubMed] [Google Scholar]
- 38.Huang B, Liu C, Wu Q, Zhang J, Min Q, Sheng T, Wang X, Zou Y. Long non-coding RNA NEAT1 facilitates pancreatic cancer progression through negative modulation of miR-506-3p. Biochem Biophys Res Commun. 2017;482:828–834. doi: 10.1016/j.bbrc.2016.11.120. [DOI] [PubMed] [Google Scholar]
- 39.Guo S, Yang P, Jiang X, Li X, Wang Y, Zhang X, Sun B, Zhang Y, Jia Y. Genetic and epigenetic silencing of mircoRNA-506-3p enhances COTL1 oncogene expression to foster non-small lung cancer progression. Oncotarget. 2017;8:644–657. doi: 10.18632/oncotarget.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang H, Li T, Qu Y, Wang X, Li B, Song J, Sun X, Tang Y, Wan J, Yu Y, et al. Long non-coding RNA SNHG15 interacts with and stabilizes transcription factor Slug and promotes colon cancer progression. Cancer Lett. 2018;425:78–87. doi: 10.1016/j.canlet.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Cui RJ, Fan JL, Lin YC, Pan YJ, Liu C, Wan JH, Wang W, Jiang ZY, Zheng XL, Tang JB, Yu XG. miR-124-3p availability is antagonized by LncRNA-MALAT1 for Slug-induced tumor metastasis in hepatocellular carcinoma. Cancer Med. 2019;8:6358–6369. doi: 10.1002/cam4.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.