Abstract
Cell senescence is caused by the activation of cell cycle inhibition pathways induced by an accumulation of cellular damage, where cells permanently leave the cell cycle. Senescent cells undergo changes in cell morphology, transcription, protein homeostasis, metabolism and other characteristic alterations. At the same time, senescent cells are able to resist apoptosis and accumulate in multiple organs and tissues in vivo. Senescent cells are capable of activating inflammatory factor secretion pathways, generating local, non-infectious inflammatory microenvironments within tissues, leading to organ degeneration and the development of aging-associated diseases. A large number of recently published studies have demonstrated that removing senescent cells from the body delays the occurrence of various aging-associated diseases. Therefore, the targeted killing of senescent cells potentially has important clinical applications in the treatment of various aging-associated diseases, aiming to improve the life span of patients. The present review summarizes recent progress that has been made in the field of senescent cell clearance and various anti-aging strategies. The history of cell senescence research is briefly reviewed, along with the association between cell senescence and tumor therapy. Furthermore, the potential of senescent cells to be used as therapeutic targets in various senescence-associated diseases is primarily discussed, and the limitations, as well as the future prospects of this line of research, are reviewed.
Keywords: senescence, tumor therapy, senescence-associated secretory phenotype
1. Introduction
Cell senescence refers to the stable cell-cycle arrest caused by telomere erosion, DNA damage, the abnormal activation of oncogenes and other conditions, which is accompanied by morphological, biochemical and epigenetic changes to the cells. Cell senescence plays important roles in non-disease-related physiological processes, including embryo development, wound healing, tissue repair and aging (1). A large number of previously published studies have demonstrated that cell senescence plays an important role in the inhibition of potential cancer cell proliferation pathways (2-4). However, as the detailed scientific knowledge in this field continues to expand, it has been demonstrated that senescent cells also secrete a variety of cytokines, chemokines, matrix-remodeling proteases and growth factors to form the senescence-associated secretory phenotype (SASP), which is a 'double-edged sword' in that it may either inhibit or promote tumor formation (5). Therefore, establishing an in-depth understanding of the association between cell senescence and tumors that focuses on the inhibitory function of cell senescence in tumors, rather than on its promoting function, may provide more options in terms of tumor therapies.
2. Characteristics of cell senescence related with tumors
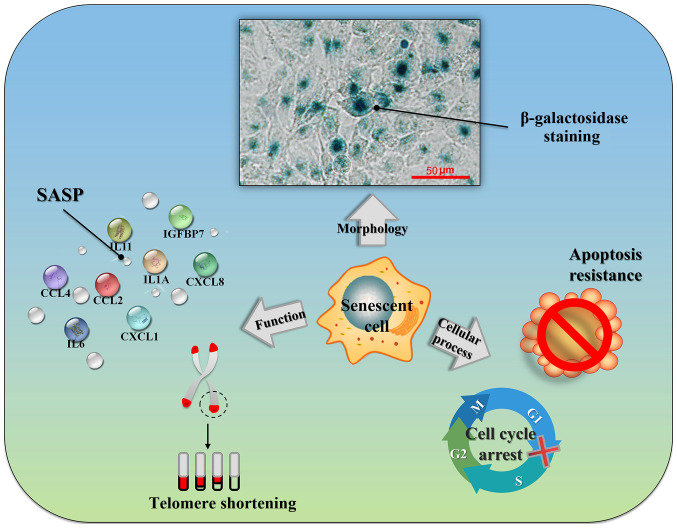
When cells become senescent, they undergo changes in cellular morphology, function and cellular process (Fig. 1).
Figure 1.
Overview of characteristics of cell senescence related to tumors. Senescent cells will undergo changes in cellular morphological, senescence-associated β-galactosidase (SA-β-gal) activity staining, senescence-related secretory phenotype (SASP), telomere dysfunction, cell cycle arrest, and apoptosis resistance.
Cellular morphological changes
After entering the aging phase, the senescent cells may be observed as polygonal-shaped flat cells occupying a large volume, with increased nuclear and nucleolar volumes, as observed under an optical microscope. Moreover, the numbers of Golgi apparatus and lysosomes in senescent cells are increased, along with a marked increase in intracytoplasmic particles (6). In 1995, Dimri et al (7) discovered that when the pH value was 6 in diploid fibroblasts cultured in vitro, the positive rate of β-galactosidase staining gradually increased with a rise in generation age. They defined this neutral galactosidase as SA-β-gal. When cells become senescent, lysosomes expand and increase, and galactosidase clearly accumulates within lysosomes (8). Galactosidase is produced by senescent cells and tissues, and is able to catalyze the hydrolysis of its substrate, X-Gal, to produce an insoluble, intensely dark blue product, which can be easily observed under an optical microscope. The SA-β-gal is a type of biological marker that can be simple and easy to use, it has been widely applied in the detection of senescent cells.
Functional alterations
After the cells have entered the aging process, their protein expression profiles are markedly altered. Most importantly, senescent cells will secrete a large number of cytokines and chemokines in this regard. The concept of SASP was first proposed by Coppé et al (9) in 2008. These researchers discovered that senescent cells are able to promote malignancy in adjacent precancerous cells by secreting inflammatory and oncogene-associated factors, and the SASP includes pro-inflammatory cytokines [interleukin (IL)-6 and IL-8], chemokines [monocyte chemoattractant proteins (MCP) and macrophage inflammatory proteins (MIPs)], growth factors [transforming growth factors (TGFs) and granulocyte-macrophage colony-stimulating factor (GM-CSF)] and proteases (10). Different biological activities induced by SASP components indicate that SASP functions with other cells and regulates the local microenvironment of tissues, which may either lead to or promote tumor formation.
When telomeres decay to a critical extent, cells begin a spontaneous aging process (11). Telomeres were first identified by Muller in 1938 (12). He found that the damaged and broken chromosome ends in Drosophila were easily connected, forming various types of chromosome aberrations. Telomeres, a special structure located at the chromosome ends, maintain the stability of chromosome ends by forming protective 'caps', thereby preventing them from being exposed and fusing with adjacent chromosome ends. However, since DNA polymerase may not completely replicate the lagging strands of chromosomes, telomere DNA repeat sequences are continuously shortened according to the increase in replication times. This irreversible telomere DNA damage leads to a damage response that may accelerate cell aging by activating tumor suppressor genes, such as p53, and triggering the expression of age-associated genes.
Cellular process alterations
Unlike resting cells, the growth stagnation of senescent cells is relatively permanent. Even when subjected to physiological stimulation, or after eliminating factors that induce aging, senescent cells cannot regain the ability to divide (13). Flow cytometric analysis has been employed to demonstrated that senescent cells are mainly held in a state of G1 phase arrest, although they may also be found in G2/M phase arrest, or can be induced into a state of G2/M phase arrest in certain cases (14). In addition, in certain types of cell, low-level DNA damage activates DNA damage repair, triggers cell senescence, causes the expression of certain anti-apoptosis proteins, and leads to the inhibition of apoptosis (15). A number of studies have confirmed that the occurrence of aging is closely related to several oncogenes and tumor suppressor genes (16-18).
3. Cell senescence has important functions in tumors
Senescence initiation in cancer is able to rely upon genetic alterations, such as oncogene-induced senescence (OIS) and tumor-suppressor gene (TSG) loss-induced senescence, or upon therapeutic interventions [therapy-induced senescence (TIS)]. OIS generally occurs early in tumorigenesis, and is able to limit cell proliferation due to oncogene mutations, maintaining tumors in a non-invasive precancerous state. By examining lesions associated with OIS, new patterns of tumorigenesis have been proposed (19,20). When cells have cancer gene mutations, they are generally prevented from being able to proliferate through the processes of apoptosis and senescence. When the apoptotic or senescence program cannot be initiated, and other genetic mutations occur at the same time, the cells experience continuous growth and eventually enter into the state of malignancy due to the lack of effective defense mechanisms. Senescent cells remain senescent for decades, and are presented as benign lesions. It is certainly possible that other genetic mutations may also be acquired, and the cells are subsequently transformed into malignant tumors. Other studies have found that certain aging markers are only present in precancerous lesions, but are rarely expressed in corresponding malignancies. Using a mouse prostate model in which PTEN tumor suppressor was inactivated, Chen et al (21) observed similar results, i.e., precancerous lesions or non-lethal tumors were able to express senescence markers, whereas malignant tumors were not. In cultured cells, as well as in vivo, p53 restricts the growth and malignant evolution of PTEN-deficient cells by inducing cellular senescence. This is similar to the situation with human prostate cancer, in that PTEN loss-induced cellular senescence (PICS) appears during the early stage, but is not expressed in the advanced malignant stages (22,23). Patients with cancer often receive radiotherapy and chemotherapy as a means of treating the tumor. A previous study demonstrated that the chemoradiotherapy of tumors induced cellular senescence (24). Common chemoradiotherapeutic treatments include cisplatin treatment, docetaxel treatment, cyclophosphamide treatment, vincristine treatment, doxorubicin treatment or gamma-ray irradiation. Notably, when lower drug concentrations are used for chemotherapy or lower radiation intensities are used for radiotherapy, such chemoradiotherapeutic treatments can induce cell senescence. Conversely, if higher chemotherapeutic drug concentrations or radiation intensities are used, apoptosis can be induced (25). The main cause of cellular senescence induced by chemoradiotherapy is the induction of intracellular DNA damage; for example, in cultured cells and mouse models of cancer, tumor cells expressing wild-type p53 gene are more sensitive to chemotherapy than tumor cells with a p53 gene mutation are, findings that are consistent with p53 playing a key role in DNA damage-induced cellular senescence (26). Other explanations underlying cellular senescence induced by chemoradiotherapy are that chemotherapeutic drugs cause the production of intracellular reactive oxygen species (ROS), or that the chemoradiotherapy leads to the inhibition of telomerase activity in the cells; these processes are also involved in the induction of cellular senescence, and may accelerate the cellular senescence process (17). After the body receives chemoradiotherapy, even though the tumor cells undergo senescence, exit the cell cycle, and lose the ability to proliferate, these cells are potentially tumorigenic, and after the tumor cells enter the state of senescence, the SASP phenomenon may occur.
The role of SASP in vivo is closely associated with the microenvironment where it is located. The secretion of SASP is a dynamic process, and SASP factors can inhibit tumor development by altering the tissue microenvironment, promoting embryonic development and tissue repair, and recruiting and activating immune cells. On the other hand, as senescent cells accumulate in organisms, SASP drives tissue senescence in a cell-involuntary manner, thereby inducing body inflammation through autocrine and paracrine pathways, and transmitting senescence signals to adjacent cells, resulting in increased levels of inflammatory factors. The inflammatory environment can promote the development of tumors by inhibiting immune surveillance and/or stimulating malignant phenotypes (27). SASP is secreted into the surrounding environment, where it blocks the differentiation of cells, prevents the renewal of damaged cells, interferes with the rejuvenation of tissues, further accelerates aging and promotes tumor migration, proliferation, invasion and angiogenesis, ultimately leading to tumor metastasis. Tumor extracellular matrix proteins are involved in the occurrence, development and immune adaptation of tumors, and senescent fibroblasts can regulate the secretion of integrins mediated by surrounding cells, thereby affecting extracellular matrix proteins. Integrins can enhance both contractility and the focal adhesions produced by ERK and Rho kinase (ROCK) activation, which interfere with epithelial morphology and promote the malignant transformation of tumors (28). MMP members often exhibit an increasing trend in SASP factors secreted by senescent fibroblasts, whereas during tumor development, MMP mainly degrades the extracellular matrix and destroys the basement membrane, which, in turn, promotes the infiltration and metastasis of tumor cells (29). Acosta and Gil (30) demonstrated that IL-8, as one of the SASP factors secreted by senescent tumor cells, is an effective agonist of chemokine (C-X-C motif) ligand 1 (CXCL1)/CXCL-2 receptors in granulocytes and monocytes, which can mediate the migration of target cells to the tumor microenvironment and promote tumor angiogenesis and tumor cell proliferation. Ortiz-Montero et al (31) demonstrated that IL-6 was a major regulator of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, which promoted tumor cell proliferation, invasion and immunosuppression and induce fibroblast morphological changes in MCF-7 breast cancer cells.
Recently, it has been reported that senescence-inducing factors can promote tumor formation. Carrière et al (32) demonstrated that the oncogene Kras was able to induce an aging response, upregulating proinflammatory cytokines in the pancreas and increasing the incidence of cystic tumors, while accelerating the process of pancreatic ductal adenocarcinoma. Malaquin et al (33) suggested that skin carcinoma may be activated through a paracrine axis in which matrix metalloproteinases (MMPs) are secured via the oversynthesis of the MMP-active receptor, protease-activated receptor-1. Chen et al (34) demonstrated that stromal cell populations, after having suffered DNA damage via radiotherapy and chemotherapy, entered the aging stage and presented SASP, leading to the formation of an inflammatory microenvironment. Their clinical investigations have indicated that the high expression of serine proteinase inhibitor Kazal Type 1 (SPINK1) in the tumor microenvironment is associated with a worse clinical prognosis and a shorter the survival time of patients. Zhang et al (35) observed that certain drugs, such as vinblastine, taxanes, nitrogen mustard, nucleoside analogues and platinum compounds, which directly or indirectly cause DNA damage, may stimulate the SASP in cells at a high frequency, while triggering cell senescence. They also found that Zscan4 which is highly expressed in plasma cells that suffer from DNA damage, plays a key role in the cascade amplification of SASP over time. Nacarelli et al (36) found that an increased metabolism of NAD+ promoted the proliferation and progression of tumor cells in pancreatic cancer and ovarian cancer in a mouse model by manipulating the expression levels of high-mobility group AT-hook 1 (HMGA1) protein and nicotinamide phosphoribosyltransferase (NAMPT). Correspondingly, this demonstrated that the increase in NAD+ metabolism caused by the increase in HMGA1 and NAMPT expression promoted high levels of SASP. Simultaneously, it strengthened the inflammatory environment around the tumor, and stimulated tumor growth. Xu et al (37) demonstrated that human stromal cells passively entered the aging state during chemotherapy, where they developed the typical SASP. Stromal cells generated and released an exocrine factor termed AREG (amphiregulin, bidirectional modulin), which continuously enters damaged microenvironments. AREG promotes the malignant phenotype of residual cancer cells in lesions, thereby conferring drug resistance and causing resistance in clinical care. However, it also induces cancer cells to upregulate the expression of programmed death-ligand 1, forming an immunosuppressive microenvironment, which enables cancer cells to escape from immune monitoring. A recent study stated that SASP counteracted atorvastatin-induced senescence in hepatocellular carcinoma cells, and also found that the poor overall survival of patients with hepatocellular carcinoma was associated with an increased hTERT expression (38). Miyazoe et al (39) reported that senescent human hepatic stellate cells released increased quantities of extracellular vesicles particles compared with normal human hepatic stellate cells. Extracellular vesicles resulted in increased EGF expression levels and may create a more conducive tumor microenvironment for proliferation of hepatoma cells (Table I).
Table I.
Senescence-inducing factors as targets for the promotion of tumor formation.
| Category | Inducement | Target gene | Tumor histology | Year, (Refs.) |
|---|---|---|---|---|
| Oncogenes activated | Kras | p53 | Pancreatic cancer | 2011, (32) |
| Telomere shortening | 50-60 population doublings | PAR-1 | Skin cancer | 2013, (33) |
| DNA damage | Radiotherapy, chemotherapy | SPINK1 | Prostate cancer | 2018, (34) |
| DNA damage | Chemotherapy | Zscan4 | Breast cancer, prostate cancer | 2018, (35) |
| Oncogenes activated | Ras | NAD+ | Pancreatic cancer, ovarian cancer | 2019, (36) |
| DNA damage | Chemotherapy | AREG | Prostate cancer | 2019, (37) |
| DNA damage | Chemotherapy | hTERT | Hepatocellular carcinoma | 2020, (38) |
| DNA damage | Extracellular vesicles | EGF | Hepatocellular carcinoma | 2020, (39) |
Kras, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; PAR-1, protease-activated receptor 1; SPINK1, serine protease inhibitor kazal type 1; Zscan4, zinc finger and SCAN domain containing 4; NAD+, nicotinamide adenine dinucleotide; AREG, amphiregulin; hTERT, human telomerase reverse transcriptase; EGF, epidermal growth factor.
4. Targeting senescent cells to inhibit tumor formation
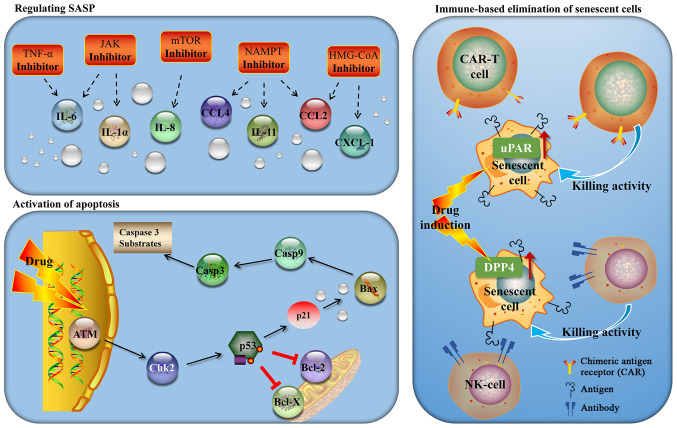
Aging is a double-edged sword. When senescent cells are retained, they promote chronic inflammation, which in turn leads to aging-associated diseases, such as atherosclerosis, cancer and fibrosis. Therefore, the targeted elimination of senescent cells has become a promising new avenue for therapeutic interventions, and researchers have confirmed the feasibility of this approach. Currently, regulating SASP, activating apoptosis and immune-based elimination of senescent cells are the three most important targeting strategies (Fig. 2).
Figure 2.
Various strategies of cancer therapy based on the characteristics of senescent cells. Regulating senescence-related secretory phenotype (SASP), activating apoptosis and immune-based elimination of senescent cells are three mainstream targeting strategies.
Regulating SASP
Tumor necrosis factor (TNF) is the main component of SASP, and adalimumab is a monoclonal antibody that directly inhibits TNF. It has previously been demonstrated that adalimumab can inhibit the secretion of SASP and markedly reduce the release of IL-6, attenuating the cancer-promoting effect of SASP (40). Xu et al (41) demonstrated that JAK inhibitors reduce the secretion of SASP in adipocyte precursor cells and human umbilical vein endothelial cells (HUVECs), suggesting that the JAK pathway is a potential target for anti-senescence-associated treatments. Mammalian target of rapamycin (mTOR) controls SASP by regulating the translation of IL-1 and MAPK-activated protein kinase 2 (MAPKAPK2) (42,43). Rapamycin, an inhibitor of mTOR, reduces the mRNA levels of IL-6 and other cytokines, and selectively inhibits the translation of membrane-bound cytokine IL-1, regulating SASP. A previous study confirmed that rapamycin improved aging-associated diseases and tumors by inhibiting aging-associated inflammation (44). Simvastatin is an inhibitor of β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase, and Liu et al (45) demonstrated that simvastatin reduced the SASP of aging human fibroblasts by inhibiting protein isopentenylation without affecting the stagnation of age growth. The study by Nacarelli et al (36) used a Ras-induced IMR90 cell senescence model to demonstrate that the down-regulation of HMGA1 or NAMPT, or using FK886 in senescent cells, may inhibit the expression of SASP. They confirmed that NAD+ promotes the activity of NF-κB to mediate SASP by inhibiting the AMP-activated protein kinase (AMPK) signaling pathway, thereby inhibiting the proliferation of tumor cells. They postulated that targeting NAMPT may be an effective strategy to inhibit proinflammatory and tumor microenvironment promotion in tumors with high expression levels of HMGA1 for patients who are undergoing lifespan-extending cancer treatment (such as chemotherapy or radiotherapy).
Activation of apoptosis
Wang et al(46) demonstrated that a cell division cycle kinase 7 (CDC7) inhibitor (XL413) specifically induced aging in TP53 mutant liver cancer cells, whereas sertraline, a drug used to treat depression, specifically promoted the apoptosis of aging liver cancer cells by down-regulating the mTOR signaling pathway. Guerrero et al (47) discovered that cardiac glycosides are anti-aging drugs that can selectively kill senescent cells in the body, thereby avoiding the toxic side-effects of conventional drug therapies. It has been demonstrated that senescent cells exert a sensitizing effect with regard to ouabain-induced apoptosis, which is partly mediated by inducing the apoptosis-promoting Bcl-2 family protein, NOXA. Hickson et al (48) demonstrated for the first time that anti-aging drugs may successfully remove senescent cells in human trials, where networks of senescent cell-resisting apoptosis pathways (SCAPs) were discovered. The anti-aging drugs, dasatinib and quercetin (a flavonoid), have been used to target these SCAP nodes: Dasatinib targets tyrosine kinase, whereas the flavonoid quercetin targets Bcl-2, hypoxia-inducible factor-1, phosphoinositide 3-kinase and p21, promoting apoptosis in senescent cells and allowing cancer cells to start the process of apoptosis for self-destructive purposes (49).
Immune-based elimination of senescent cells
Researchers have indicated that the body removes senescent cells by recruiting immune cells. NRAS-H12V can cause oncogene-induced cell senescence, and these senescent cells can be removed via both the innate and adaptive immune systems, such as natural killer (NK) cells and macrophages. Ta et al (50) demonstrated that dipeptidyl-peptidase 4 (DPP4; also known as CD26) was highly expressed in aging human fibroblasts, and senescent cells were preferentially cleared by NK cells that recognize anti-DPP4 antibodies. Removing aging and damaged cells provides a favorable microenvironment for tissue regeneration, which stimulates the proliferation and differentiation of neighboring cells, including tissue stem cells, enabled damaged tissues to be replenished with healthy cells, aiding in tissue repair. The results revealed that, in different models, the immune system possesses a variety of mechanisms concerning the clearance of senescent cells. Amor et al (51) analyzed the expression of transmembrane proteins found in human and mouse senescent cells, and demonstrated that urokinase-type proenzyme-activating receptor (uPAR) was highly expressed in senescent cells both in vivo and in vitro. Of note, a soluble uPAR (suPAR) that lacked the transmembrane region was shown to be a component secreted during the SASP response. The presence of suPAR is a hallmark of several chronic diseases, including diabetes and nephropathy. Subsequently, the investigators designed CAR-T cells targeting uPAR, and tested these in mouse models of several aging-associated diseases, including cancer and liver fibrosis. It was found that CAR-T cell therapy targeting uPAR led to the elimination of senescent cells in mouse models of liver and lung cancer. Similarly, CAR-T cell therapy improved the survival rate in a mouse model of lung cancer when used in association with a drug previously shown to induce senescence in this cancer type (Navitoclax).
As research has progressed in this area, Childs et al (52) subsequently demonstrated that a series of drugs can be targeted to induce the death of senescent cells, although they exert minimal effects on non-senescent cells; these drugs have been termed 'senolytics', and they have been used to remove senescent cells from the human body. A number of senolytic drugs have been successively developed, such as dasatinib, mistletoe extracts, navitoclax (ABT263), piperine, phenanthrone, A1331852 and A1155463, and numerous other drugs are undergoing clinical studies and are expected to be successively applied in the future (53,54) (https://clini-caltrials.gov/ct2/results?pg=1&load=cart&id=NCT04063124). Jeon et al (55) collected chondrocyte samples from patients with severe osteoarthritis and cultured 3D-stereoscopic cartilage tissue models in vitro, and their results revealed that the drug under investigation, UBX0101, was able to both selectively remove senescent cells from the joints and effectively prevent the generation and development of osteoarthritis. The results of an interim report of a clinical trial for the treatment of dysfunction in diabetic patients with chronic kidney disease indicated that the use of dasatinib and quercetin in combination chemotherapy removed senescent cells from tissues, delayed physical dysfunction and reduced inflammatory cytokine secretion associated with human aging (48). Similarly, another study (56) featuring a clinical trial study conducted on a small group of patients with pulmonary fibrosis revealed that dasatinib and quercetin selectively removed senescent cells to treat idiopathic pulmonary fibrosis. Clinical trials of another senolytic drug, UBX1967, for the treatment of ocular diseases in the elderly are also advancing (57).
5. Conclusion
As the understanding of cell aging gradually develops, and the mechanisms underlying the etiology of aging-associated diseases are better understood, researchers will also better understand the pathogenesis of aging-associated diseases, and this knowledge should provide novel therapeutic targets, giving rise to novel therapeutic approaches in the treatment of aging-associated diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from National Key R&D Program of China (grant no. 2016YFC1303100), and the National Natural Science Foundation of China (grant nos. 31570803, 81773090, 81272879 and 81402151).
Availability of data and materials
Not applicable.
Authors' contributions
ZW, JG, YO and CX were involved in the conception of the study. JG and HL were involved in the literature search and critical reviewing of the manuscript. ZW and JG were involved in the preparation of the draft of the manuscript. ZW, JG, YO and CX were involved in the revising and editing of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kuwano K, Araya J, Hara H, Minagawa S, Takasaka N, Ito S, Kobayashi K, Nakayama K. Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) Respir Investig. 2016;54:397–406. doi: 10.1016/j.resinv.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu H, Bolati D, Adijiang A, Muteliefu G, Enomoto A, Nishijima F, Dateki M, Niwa T. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physio. 2011;301:C1201–C1212. doi: 10.1152/ajpcell.00471.2010. [DOI] [PubMed] [Google Scholar]
- 3.Lansu K, Gentile S. Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. 2013;4:e652. doi: 10.1038/cddis.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, Hu X, Liao C, Xiao H, Zhu Q, Li Y, Liu Z, Tao A, He Z, Xu C, Zheng K. Gypenoside L inhibits proliferation of liver and esophageal cancer cells by inducing senescence. Molecules. 2019;24:1054. doi: 10.3390/molecules24061054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schosserer M, Grillari J, Breitenbach M. The dual role of cellular senescence in developing tumors and their response to cancer therapy. Front Oncol. 2017;7:278. doi: 10.3389/fonc.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113(Pt 20):3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 7.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosińska P, Mikuła-Pietrasik J, Ryżek M, Naumowicz E, Książek K. Specificity of cytochemical and fluorescence methods of senescence-associated β-galactosidase detection for ageing driven by replication and time. Biogerontology. 2014;15:407–413. doi: 10.1007/s10522-014-9505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovadya Y, Krizhanovsk V. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology. 2014;15:627–642. doi: 10.1007/s10522-014-9529-9. [DOI] [PubMed] [Google Scholar]
- 11.Donate LE, Blasco MA. Telomeres in cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:76–84. doi: 10.1098/rstb.2010.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller HJ. The Remaking of Chromosomes. The Collecting Net, Woods Hole. 1938;13:181–198. [Google Scholar]
- 13.Leontieva OV, Blagosklonny MV. Tumor promoter-induced cellular senescence: Cell cycle arrest followed by geroconversion. Oncotarget. 2014;5:12715–12727. doi: 10.18632/oncotarget.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien M, Rinker-Schaeffer C, Stadler WM. A G2/M growth arrest response to low-dose intermittent H2O2 in normal uroepithelial cells. Int J Oncol. 2000;17:425–432. doi: 10.3892/ijo.17.3.425. [DOI] [PubMed] [Google Scholar]
- 15.Campisi J, Fabrizio FD. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 16.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010;2:471–474. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochetkova EY, Blinova GI, Bystrova OA, Martynova MG, Pospelov VA, Pospelova TV. Targeted elimination of senescent Ras-transformed cells by suppression of MEK/ERK pathway. Aging (Albany NY) 2017;9:2352–2375. doi: 10.18632/aging.101325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooi WJ, Peeper DS. Oncogene-induced cell senescence-halting on the road to cancer. N Engl J Med. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- 20.Braig M, Schmitt CA. Oncogene-induced senescence: Putting the brakes on tumor development. Cancer Res. 2006;66:2881–2884. doi: 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeper DS. PICS-ure This: Prosenescence Therapy? Cancer Cell. 2010;17:219–210. doi: 10.1016/j.ccr.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Tato-Costa J, Casimiro S, Pacheco T, Pires R, Fernandes A, Alho I, Pereira P, Costa P, Castelo HB, Ferreira J, Costa L. Therapy-induced cellular senescence induces epithelial-to-mesenchymal transition and increases invasiveness in rectal cancer. Clin Colorectal Cancer. 2016;15:170–178. doi: 10.1016/j.clcc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: An emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Tonnessen-Murray CA, Lozano G, Jackson JG. The regulation of cellular functions by the p53 protein: Cellular senescence. Cold Spring Harb Perspect Med. 2017;7:a026112. doi: 10.1101/cshperspect.a026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meiyanto E, Putri H, Larasati YA, Utomo RY, Jenie RI, Ikawati M, Lestari B, Yoneda-Kato N, Nakamae I, Kawaichi M, Kato JY. Anti-proliferative and anti-metastatic potential of curcumin analogue, pentagamavunon-1 (PGV-1), toward highly metastatic breast cancer cells in correlation with ROS generation. Adv Pharm Bull. 2019;9:445–452. doi: 10.15171/apb.2019.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasgupta J, Kar S, Liu R, Joseph J, Kalyanaraman B, Remington SJ, Chen C, Melendez JA. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J Cell Physiol. 2010;225:52–62. doi: 10.1002/jcp.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta JC, Gil J. A role for CXCR2 in senescence, but what about in cancer? Cancer Res. 2009;69:2167–2170. doi: 10.1158/0008-5472.CAN-08-3772. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz-Montero P, Londoño-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal. 2017;15:17. doi: 10.1186/s12964-017-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrière C, Gore AJ, Norris AM, Gunn JR, Young AL, Longnecker DS, Korc M. Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic kras and impairs senescence in premalignant lesions. Gastroenterology. 2011;141:1091–1101. doi: 10.1053/j.gastro.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malaquin N, Vercamer C, Bouali F, Martien S, Deruy E, Wernert N, Chwastyniak M, Pinet F, Abbadie C, Pourtier A. Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PLoS One. 2013;8:e63607. doi: 10.1371/journal.pone.0063607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen F, Long Q, Fu D, Zhu D, Ji Y, Han L, Zhang B, Xu Q, Liu B, Li Y, et al. Targeting SPINK1 in the damaged tumour micro-environment alleviates therapeutic resistance. Nat Commun. 2018;9:4315. doi: 10.1038/s41467-018-06860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Fu D, Xu Q, Cong X, Wu C, Zhong X, Ma Y, Lv Z, Chen F, Han L, et al. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat Commun. 2018;9:1723. doi: 10.1038/s41467-018-04010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol. 2019;21:397–407. doi: 10.1038/s41556-019-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q, Long Q, Zhu D, Fu D, Zhang B, Han L, Qian M, Guo J, Xu J, Cao L, et al. Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD-L1)-mediated immunosuppression. Aging Cell. 2019;18:e13027. doi: 10.1111/acel.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang ST, Huang SW, Liu KT, Lee TY, Shieh JJ, Wu CY. Atorvastatin-induced senescence of hepatocellular carcinoma is mediated by downregulation of hTERT through the suppression of the IL-6/STAT3 pathway. Cell Death Discov. 2020;6:17. doi: 10.1038/s41420-020-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyazoe Y, Miuma S, Miyaaki H, Kanda Y, Nakashiki S, Sasaki R, Haraguchi M, Shibata H, Honda T, Taura N, Nakao K. Extracellular vesicles from senescent hepatic stellate cells promote cell viability of hepatoma cells through increasing EGF secretion from differentiated THP-1 cells. Biomed Rep. 2020;12:163–170. doi: 10.3892/br.2020.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prattichizzol F, Giuliani A, Recchioni R, Bonafè M, Marcheselli F, De Carolis S, Campanati A, Giuliodori K, Rippo MR, Brugè F, et al. Anti-TNF-α treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells. Oncotarget. 2016;7:11945–11958. doi: 10.18632/oncotarget.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, et al. JAK inhibition alleviates the cellular senescence associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA. 2015;112:E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alimbetov D, Davis T, Brook AJ, Cox LS, Faragher RG, Nurgozhin T, Zhumadilov Z, Kipling D. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology. 2016;17:305–315. doi: 10.1007/s10522-015-9610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsubouchi K, Araya J, Kuwano K. PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses. Inflamm Regen. 2018;38:18. doi: 10.1186/s41232-018-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Uppal H, Demaria M, Desprez PY, Campisi J, Kapahi P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep. 2015;5:17895. doi: 10.1038/srep17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Vegna S, Jin H, Benedict B, Lieftink C, Ramirez C, de Oliveira RL, Morris B, Gadiot J, Wang W, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574:268–272. doi: 10.1038/s41586-019-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, Wolter K, Pombo J, Irvine EE, Innes AJ, et al. Cardiac glycosides are broad-spectrum senolytics. Nat Metab. 2019;1:1074–1088. doi: 10.1038/s42255-019-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovacovicova K, Skolnaja M, Heinmaa M, Mistrik M, Pata P, Pata I, Bartek J, Vinciguerr M. Senolytic cocktail dasatinib+quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front Oncol. 2018;8:459. doi: 10.3389/fonc.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ta NN, Li Y, Schuyler CA, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits TLR4-mediated ERK activation and ERK-dependent MMP-1 expression by U937 histiocytes. Atherosclerosis. 2010;213:429–435. doi: 10.1016/j.atherosclerosis.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 51.Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM. Senescent cells: An emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkland JL, Tamara T, Yi Z, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Deursen JM. Senolytic therapies for healthy longevity. Science. 2019;364:636–637. doi: 10.1126/science.aaw1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.