Appreciation of the widespread prevalence of non-histone protein lysine-residue acetylation (AcK) arose from several major proteomic analyses in the mid-2000’s.1 Initial studies uncovered that AcK was enriched in mitochondria, and modified multiple metabolic and redox stress-sensing enzymes, in addition to regulating the overall rate of oxidative phosphorylation.2, 3 Early enthusiasm that mitochondrial protein acetylation may play an important regulatory role was somewhat tempered by evidence suggesting that this post-translational modification was present at low stoichiometric occupancy on the putative functional lysine residues of candidate regulatory mitochondrial enzymes.4
In parallel, the regulation of mitochondrial protein lysine-residue acetylation/deacetylation has also proven to be complex. On the one hand, the major deacetylase enzyme, Sirt3, appears to predominantly operate under extreme stress conditions e.g. prolonged fasting, caloric restriction and/or with major redox stress. On the other hand, the debate whether high acetyl-CoA concentrations and the intrinsic mitochondrial pH is sufficient to permit non-enzymatic lysine acetylation,5 or whether a more regulated enzymatic mechanism involving the putative acetyl-CoA interacting molecule and facilitator of acetylation, GCN5L1, remains unresolved in vivo.6
Genome-wide depletion of Sirt3 disrupts cardiac mitochondrial oxidative metabolism and exacerbates pressure overload-induced heart failure,7 while aged Sirt3 KO mice exhibit accelerated cardiac hypertrophy and fibrosis.7, 8 Outstanding questions included whether these effects are heart-intrinsic, or whether systemic Sirt3 effects contributed to these phenotypes. Furthermore, what is the functional contribution of mitochondrial protein acetylation, given the proposed low stoichiometric occupancy of acetylation on lysine residues?4 In this issue of Circulation Research, Davidson et al.9 employ a very elegant cardiac- and skeletal muscle-restricted genetic model to markedly accentuate cardiac mitochondrial protein hyperacetylation. The model was created by crossing striated muscle creatine kinase cre-recombinase (MCK-cre) transgenic mice with floxed-Sirt3 (mitochondrial deacetylase) or floxed-carnitine acetyltransferase (CrAT; which sequesters free acetyl groups to carnitine) animals, followed by subsequent interbreeding to generate double knockout (DKO) or double floxed control (DFC) mice. The Muoio and Kelly laboratories then asked whether tissue-restricted mitochondrial protein hyperacetylation in the DKO mice modified cardiac mitochondrial function, and whether this, in turn, exacerbated cardiac dysfunction in response to pressure overload induced by thoracic transverse aortic constriction (TAC).
Acetyl-proteomic analysis showed an additive effect of knocking out both Sirt3 and CrAT on mitochondrial protein acetylation, and showed that the DKO mice had a more robust (albeit overlapping) increase in mitochondrial protein acetylation when compared to DFC mice subjected to an acute pathophysiological stress (transverse aortic constriction plus apical myocardial infarction, or TAC-MI).10 Interestingly, despite this robust increase in acetylation, DKO mice exhibited remarkably similar mitochondrial bioenergetic and redox function compared to the DFC mice. In parallel, DKO and DFC mice subjected to TAC for 16 weeks showed a similar level of left ventricular decompensation and fibrosis, supporting the finding that mitochondrial function was not qualitatively different between DKO and DFC mice. A repeat of the proteomic analyses after TAC alone again showed robustly increased mitochondrial protein acetylation in the ventricles of DKO-TAC versus the DFC-TAC mice. However, it was noted that mitochondrial protein acetylation was not augmented in TAC versus sham surgery controls in either genetic model, which differed from the robust induction of AcK in the TAC-MI model discussed above. Finally, bioinformatic pathway analyses were performed to compare proteome remodeling in wildtype mice subjected to 4 weeks of TAC-MI and DFC mice subjected to 16 weeks of TAC alone. The most robust concordantly altered pathways showed reduced fatty acid catabolism in both interventions compared to their sham controls. Interestingly, comparing each model to its sham control found no distinct pathway differences in the acute TAC-MI:sham model, but did find a reduction in complex I proteins of the electron transfer chain in the chronic TAC:sham model. These data reinforce the role of changes to mitochondrial oxidative metabolism and mitochondrial content in progressive cardiac decompensation in response to pressure overload, and buttress the dissociation of these metabolic signatures from the magnitude of mitochondrial protein acetylation.9
This study profoundly disrupts our understanding of the functional role of mitochondrial protein acetylation in the heart in response to pressure overload. It also raises the question why both the bioenergetic and ventricular phenotype in response to pressure overload differs in the whole body Sirt3 knockout mouse compared to the cardiac and skeletal muscle DKO? One obvious difference is the additional depletion of CrAT in the DKO mice. CrAT buffers the mitochondrial acetyl-CoA pool by converting short chain acyl-CoAs to membrane permeant acyl-carnitine, and enables the efflux of acetyl-moieties out of mitochondria. The role of CrAT has been most extensively explored in skeletal muscle, using the same MCK-cre directed CrAT ablation as employed here. These studies demonstrated that CrAT alleviates excessive acetylation of mitochondrial proteins, and prevents insulin resistance and glucose intolerance in response to high fat feeding.11 Interestingly, these dual skeletal muscle and cardiac CrAT KO mice showed reduced exercise tolerance and increased skeletal muscle fat oxidation.12 The previous evaluation of skeletal muscle function in this DKO mouse model mirrors the current cardiac assessment, showing a robust increase in mitochondrial protein acetylation with minimal changes in bioenergetic or redox biology.13 In contrast to the cardiac TAC stress, high fat feeding resulted in robust systemic insulin resistance in the DKO mice.13 Given the contributions of skeletal muscle and insulin resistance to the pathophysiology of heart failure, a question arises whether systemic effects of skeletal muscle perturbations alters the cardiac response to pressure overload in this DKO model? Additionally, whether disruptions in the efflux of acetyl-moieties from mitochondria via CrAT depletion results in changes to nuclear histone acetylation, with subsequent gene regulatory effects, should be explored as a potential variable that distinguishes the whole body Sirt3 KO from the DKO response to TAC. A further question that arises is whether there are systemic effects from the loss of whole body Sirt3 expression that modulate cardiac bioenergetic and stress adaptation programs. One such system may be the immune system, as heart failure progression is attenuated by blocking IL-1β and Sirt3 KO mice are known to display excessive IL-1β activation.14 Whether additional Sirt3 effects in the liver, brain, endothelium or adipose tissue could be operational in conferring these differences are also worthy of interrogation. At the same time, different phenotypes have been observed in response to the same genetic manipulation in different mouse strains, and whether this is a variable that distinguishes whole body Sirt3 KO mice used in previous studies from the DKO mouse reported here could also be considered. Finally, the impact of AcK stoichiometry (reviewed elsewhere4) remains an enigma, and further studies are required to understand how potentially low levels of non-histone protein acetylation modifies cellular and mitochondrial function.
In summary, the current study by Davidson et al. has challenged the paradigm that excessive mitochondrial protein acetylation plays a primary role in the disruption of cardiac mitochondrial function, and predisposes hearts to accelerated adverse remodeling in response to pressure overload. The data are truly compelling however, the story remains incomplete until we understand the mitochondrial and cellular effects of acetyl-moiety flux disruption by CrAT depletion, and explore how Sirt3 depletion in other organs may modulate the cardiac response to biomechanical stressors. These questions could be resolved by performing TAC in CrAT KO mice, and by comparing the TAC response in cardiac restricted versus whole body Sirt3 KO mice. Finally, crossing the Sirt3 KO or DKO mice with a cardiac GCN5L1 KO15 mouse may also help resolve the question whether cardiac mitochondrial protein AcK is exclusively non-enzymatic, or requires functional GCN5L1 activity.
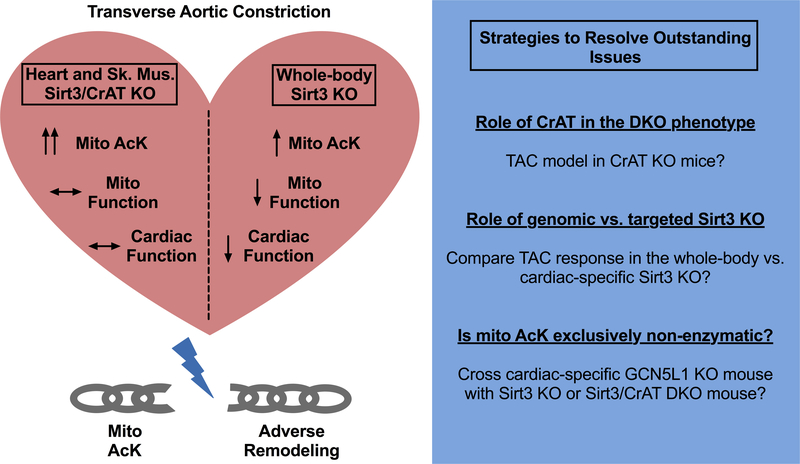
Figure 1. Breaking the link between mitochondrial protein acetylation and pressure-overload induced adverse remodeling.
The differences in the effect of the DKO to the whole body Sirt3 KO mice are shown within the heart subjected to pressure overload. The dissociation of the extent to mitochondrial protein acetylation in the DKO mice with both adverse effects on mitochondrial function and cardiac adaptation to TAC are depicted with the broken chain link. The text box on the right suggests approaches to be pursued to answer outstanding questions. Abbreviations: CrAT - carnitine acetyl transferase; DKO - double Sirt3 and CrAT KO mice; GCN5L1 - General control of amino acid synthesis 5 like 1 and TAC – transverse aortic constriction.
Acknowledgments
Funding: Funding for this project was supported by NHLBI extramural grants (IS R01-HL132917 and R01-HL147861), and by the NHLBI Division of Intramural Research (MNS ZIA-HL005102).
Footnotes
Disclosures: None
References
- 1.Lu Z, Scott I, Webster BR, Sack MN. The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circulation research. 2009;105:830–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harbor perspectives in biology. 2012;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster BR, Lu Z, Sack MN, Scott I. The role of sirtuins in modulating redox stressors. Free radical biology & medicine. 2012;52:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeza J, Smallegan MJ, Denu JM. Mechanisms and dynamics of protein acetylation in mitochondria. Trends Biochem Sci. 2016;41:231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Molecular cell. 2014;54:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu K, Scott I, Wang L, Thapa D, Sack MN. The emerging roles of gcn5l1 in mitochondrial and vacuolar organelle biology. Biochim Biophys Acta Gene Regul Mech. 2020:194598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC, Meyer-Steenbuck M, Cenkerova K, Hoffmann MM, Jaeger C, et al. Sirt3 deficiency impairs mitochondrial and contractile function in the heart. Basic research in cardiology. 2015;110:36. [DOI] [PubMed] [Google Scholar]
- 8.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging 2010;2:914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson MT, Grimsrud P, Lai L, Draper J, Fisher-Wellman KH, Narowski TM, Koves TR, Kelly DP, Muoio DM. Extreme acetylation of the cardiac mitochondrial proteome does not promote heart failure. Circ Res. 2020; 127: xx–xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinheimer CJ, Lai L, Kelly DP, Kovacs A. Novel mouse model of left ventricular pressure overload and infarction causing predictable ventricular remodelling and progression to heart failure. Clin Exp Pharmacol Physiol. 2015;42:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MN, Kjalarsdottir L, Thompson JW, Dubois LG, Stevens RD, Ilkayeva OR, Brosnan MJ, Rolph TP, Grimsrud PA, Muoio DM, et al. The acetyl group buffering action of carnitine acetyltransferase offsets macronutrient-induced lysine acetylation of mitochondrial proteins. Cell reports. 2016;14:243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seiler SE, Koves TR, Gooding JR, Wong KE, Stevens RD, Ilkayeva OR, Wittmann AH, DeBalsi KL, Davies MN, Lindeboom L, et al. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell metabolism. 2015;22:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams AS, Koves TR, Davidson MT, Crown SB, Fisher-Wellman KH, Torres MJ, Draper JA, Narowski TM, Slentz DH, Lantier L, Wasserman DH, Grimsrud PA, Muoio D, et al. Disruption of acetyl-lysine turnover in muscle mitochondria promotes insulin resistance and redox stress without overt respiratory dysfunction. Cell metabolism. 2020;31:131–147 e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traba J, Geiger SS, Kwarteng Siaw M, Han K, Ra OH, Siegel RM, Gius D, Sack MN. Prolonged fasting suppresses mitochondrial nlrp3 inflammasome assembly and activation via sirt3 mediated activation of superoxide dismutase 2. The Journal of biological chemistry. 2017;292:12153–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning JR, Thapa D, Zhang M, Stoner MW, Traba J, McTiernan CF, Corey C, Shiva S, Sack MN, Scott I. Cardiac-specific deletion of gcn5l1 restricts recovery from ischemia-reperfusion injury. Journal of molecular and cellular cardiology. 2019;129:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]