Abstract
Objective.
To examine changes in performance and outcomes of pelvic exenteration for gynecologic malignancies.
Methods.
This is a population-based retrospective study examining the Nationwide Inpatient Sample between 2001 and 2015. Women with cervical, uterine, vaginal, and vulvar malignancies who underwent pelvic exenteration were examined. Comorbidity, perioperative complications, total charges, length of stay, and mortality were assessed.
Results.
There were 2647 cases included. Cervical cancer was the most common malignancy (45.1%), followed by vaginal cancer (27.6%). 26.9% of women had a Charlson Comorbidity Index ≥3, which significantly increased from 23.3% in 2001–2005 to 33.3% in 2011–2015 (42.9% relative increase, P < 0.001). Obese women undergoing exenteration increased significantly from 4.5% in 2001–2005 to 19.4% in 2011–2015 (3.3-fold relative increase, P < 0.001). The perioperative complication rate was 68.1%, including 38.7% with multiple complications. The mortality rate was 1.9%. The number of women with multiple perioperative complications increased from 29.4% in 2001–2005 to 52.8% in 2011–2015 (78.6% relative increase, P < 0.001). More recent year of surgery, obesity, higher comorbidity, higher household income, surgery at large bedsize hospital, urinary diversion, vaginal reconstruction, and vulvar cancer were associated with an increased risk of multiple complications on multivariable analysis (all, P < 0.05). Median length of stay was 14 (IQR 9–21) days, and the number of women hospitalized ≥28 days significantly increased from 12.6% in 2001–2005 to 19.1% in 2011–2015 (51.6% relative increase, P < 0.001). The median corrected total charges increased from $121,854 to $185,100 between 2001 and 2015 (net difference +$63,246, 51.9% relative increase, P < 0.001).
Conclusion.
Women undergoing pelvic exenteration for gynecologic malignancies became more obese and comorbid during the study period. Pelvic exenteration for women with gynecologic malignancies is associated with high morbidity and mortality as well as substantial treatment-related costs.
Keywords: Gynecologic cancer, Pelvic exenteration, Trend, Morbidity, Mortality, Outcome
1. Introduction
Pelvic exenteration is a rare radical surgical procedure to remove the visceral pelvic organs with or without the perineum in an en-bloc fashion [1,2]. In the area of gynecologic malignancies, pelvic exenteration is typically performed for recurrent cervical, uterine, vaginal, and vulvar cancers located in the central pelvis. Survival rates after pelvic exenteration have been reported as 32–47% for overall survival and 40–52% for recurrence-free survival, highlighting the importance of this surgical procedure as an option for salvage therapy with a therapeutic intent [3-9]. Pelvic exenteration, however, is associated with high perioperative morbidity due to the nature and extent of surgery, with complication rates previously quoted as 51–88% [5,6,10]. Therefore, careful patient selection balancing risks and benefits is a crucial step when discussing this high-risk, high-return surgery.
The clinical characteristics of women with gynecologic malignancies who undergo pelvic exenteration have been changing over past decades in the United States. First, demographics of US adults have significantly shifted to a more obese and older population [11,12]. Second, the indications for and utilization of pelvic irradiation have expanded in the treatment of gynecologic malignancies [13,14]. Third, pelvic exenteration as a treatment option has expanded to include the non-central pelvic lesion or for palliative use as chemotherapy options for control of non-symptomatic distant metastases have improved [6]. Lastly, the evolution of surgical techniques such as reconstructive surgery, the availability of newer surgical instruments that hemostatically incise, and improving perioperative care in recent years may affect patient selection by surgeons [1,5].
All these factors may directly or indirectly impact the performance and outcome of pelvic exenteration. Thus, we hypothesized that there may be changes in patient characteristics and perioperative outcomes of women with gynecologic malignancies who undergo pelvic exenteration over time. To date, population-based statistics have been lacking to examine trends and outcomes of pelvic exenteration in gynecologic malignancies: here, we examined recent trends and performance of this surgical procedure performed for cervical, uterine, vaginal, and vulvar cancers.
2. Materials and methods
2.1. Data source
The Nationwide Inpatient Sample is a publically available and deidentified population-based database that is distributed as part of the Healthcare Cost and Utilization Project by the Agency for Healthcare Research and Quality [15]. This database includes hospital discharge data for >36 million hospitalizations per year when weighted, and it provides patient demographic, clinical, and resource-use information, such as patient length of stay and hospital charges, as well as hospital-specific data, including location, bed-size, and teaching status. On average, over 90% of the United States population is represented in this database between 2001 and 2015. The University of Southern California Institutional Review Board deemed the study exempt due to the use of publicly available deidentified data.
2.2. Design and eligibility
This is a retrospective study examining the Nationwide Inpatient Sample between January 2001 and September 2015. Women with cervical, uterine, vaginal, and vulvar malignancies who underwent pelvic exenteration were eligible for the study. Exclusion criteria included pelvic exenteration performed for non-gynecologic malignancies including bladder, anal, and rectal cancers, and pelvic exenteration for unknown cancer type. Cases of ovarian and fallopian tubal malignancies were also excluded because distinguishing pelvic exenteration from radical cytoreductive surgery is not feasible in this database and this procedure is rarely performed for these diseases in general.
2.3. Clinical information
Among eligible cases, the following information was abstracted from the database: patient baseline demographics, hospital information, operative details, and outcome of the index admission. Patient demographics included age, race/ethnicity, medical comorbidities, obesity, primary expected payer, and median household income. Charlson Comorbidity Index was determined for each patient based on the codes for the specified medical conditions in each category and weighted appropriately to calculate a final score (Table S1) [16]. This index, proposed in 1987, is determined based on the type, number, and extent of medical comorbidities, and it has been widely utilized to predict outcomes of interest such as surgical morbidity and mortality as well as survival prognosis [16]. Hospital data included calendar year of hospitalization, hospital bedsize, teaching status, and hospital region. Hospital bedsize is defined by hospital geographic region, urban-rural designation, and teaching status [17]. Operative details included use of lymphadenectomy, colostomy, urinary diversion, flap, and vaginal reconstruction at pelvic exenteration. The outcomes for analysis were perioperative complications, length of hospital stay, and total charges at time of discharge.
2.4. Study definition
In 2012, the Nationwide Inpatient Sample was redesigned to improve national estimates and patient confidentiality and minimize sampling errors. We accounted for these changes by weighting and merging the corresponding variables properly before and after 2012. The International Classification of Disease 9th revision codes remained the same during the study period between 2001 and 2015.
Study period was grouped every five years (2001–2005, 2006–2010, and 2011 −2015). The International Classification of Disease 9th revision (ICD-9) code for pelvic evisceration (68.8) was used as the surrogate for pelvic exenteration in the study as described previously [2,18]. There is no specific ICD-9 code for total, anterior, and posterior exenteration. Obesity was defined by ICD-9 coding as body mass index of ≥30 kg/m2. The crude total charge in each case was corrected for the 2015 value based on the medical inflation rate from the year of pelvic exenteration, ranging from 2.63% for 2014–2015 to 63.79% for 2001–2015 (Table S2) [19].
Perioperative complications, including both intraoperative and postoperative complications before hospital discharge, were defined as the presence of any of the following: hemorrhage, shock, wound complications, thromboembolism, cerebrovascular disease or stroke, cardiac failure, myocardial infarction, pneumonia, respiratory failure, systemic inflammatory response syndrome or sepsis, ileus or small bowel obstruction, vascular injury, acute kidney injury, pyelonephritis, abscess, fistula, intestinal perforation, and death during the index admission (Table S1). Designation of multiple complications in this study refers to any two or more of these complications during the index admission. The database does not caption the information for complication after the initial discharge.
2.5. Study objective
The primary objective of the analysis was to estimate the trends and outcomes during hospital admission for pelvic exenteration performed for gynecologic malignancies between 2001 and 2015. Specifically, perioperative complications, length of stay, and total charge for the index admission for pelvic exenteration were examined. The secondary objective was to identify clinico-demographics associated with multiple complications or death during the index admission.
2.6. Statistical methods
All the analyses were based on weighted values. Normality of continuous variables was assessed with the Kolmogorov-Smirnov test. The Kruskal Wallis H test and chi-square test were used to assess the difference in multiple groups of more than two groups. The Spearman's coefficient was used for assessing the correlation between continuous variables.
A binary logistic regression model was used to identify contributing factors for perioperative multiple complications at pelvic exenteration on multivariable analysis. All the covariates with P < 0.05 on univariable analysis were entered in the initial model. Conditional backward method was used to retain covariates with P < 0.05 in the final model. This methodology was used based on relatively small sample size in our study. The magnitude of statistical significance was expressed with adjusted odds ratio (OR) and 95% confidence interval (CI).
The Joinpoint Regression Program (version 4.4.0.0), which is provided by the National Cancer Institute, was utilized to evaluate temporal trends. Time point data was examined annually to identify temporal changes as previously described [20]. Temporal trend was examined with a linear segmented regression test, and log-transformation was performed to determine the annual percent change and 95%CI.
In an attempt to predict a subgroup of women with increased risk of death during the index admission, a recursive partitioning analysis was performed to construct a classification-tree model for mortality pattern [21]. All perioperative complications were entered in the analysis, and the chi-square automatic interaction detector method was used for the model. In a sensitivity analysis, only cervical cancer cases were examined. This is based on the rationale that cervical cancer is the most common gynecologic malignancy for which pelvic exenteration is performed [3-9].
All statistical analyses were based on two-tailed hypotheses, and a P < 0.05 was considered statistical significant. Statistical Package for Social Sciences (IBM SPSS, version 24.0, Armonk, NY) was used for the analysis. The STROBE guidelines were consulted for the performance of the observational cohort study [22].
3. Results
There were 23,740 cases of pelvic exenteration initially identified in the database during the study period. Of those, 20,229 cases with non-gynecologic malignancy and unknown disease types were excluded. Among 3511 cases of pelvic exenteration for gynecologic malignancies, 864 cases of ovarian and fallopian tubal cancers were excluded, and 2647 women who underwent pelvic exenteration for cervical, uterine, vaginal, and vulvar cancers represented the study population.
The patient demographics are shown in Table 1. Cervical cancer was the most common malignancy (n = 1194, 45.1%) followed by vaginal cancer (n = 729, 27.6%). During the study period, the number of women with vulvar cancer who underwent exenteration increased from 9.8% to 15.6% (59.2% relative increase; P < 0.001); similarly, the number of women with uterine cancer increased from 12.2% to 15.4% (26.2% relative increase; P < 0.001). Median age at surgery was 56 (interquartile range [IQR] 47–66) years, which was similar over the study period (P = 0.45).
Table 1.
Patient demographics.
| Characteristic |
All |
2001–2005 |
2006–2010 |
2011–2015 |
P-value |
|---|---|---|---|---|---|
| Number | N = 2647 | n = 716 | n = 994 | n = 937 | |
| Cancer type | 0.001 | ||||
| Cervical | 1194 (45.1%) | 347 (48.5%) | 437 (44.0%) | 410 (43.8%) | |
| Uterine | 394 (14.9%) | 87 (12.2%) | 163 (16.4%) | 144 (15.4%) | |
| Vaginal | 729 (27.6%) | 211 (29.5%) | 282 (28.4%) | 236 (25.2%) | |
| Vulvar | 328 (12.4%) | 70 (9.8%) | 112 (11.3%) | 146 (15.6%) | |
| Age (yr) | 56 (IQR 47–66) | 56 (IQR 46–65) | 56(48–65) | 56 (47–66) | 0.45 |
| <50 | 792 (29.9%) | 226 (31.6%) | 284 (28.6%) | 282 (30.1%) | |
| 50–69 | 1411 (53.3%) | 354 (49.4%) | 543 (54.6%) | 514 (54.9%) | |
| ≥70 | 444 (16.8%) | 136 (19.0%) | 167 (16.8%) | 141 (15.0%) | |
| Race/ethnicity | <0.001 | ||||
| White | 1661 (62.8%) | 433 (60.5%) | 614(61.8%) | 614 (65.6%) | |
| Black | 233 (8.8%) | 38 (5.3%) | 67 (6.7%) | 128 (13.7%) | |
| Hispanic | 214 (8.1%) | 22 (3.1%) | 107 (10.8%) | 85 (9.1%) | |
| Others | 142 (5.4%) | 45 (6.3%) | 47 (4.7%) | 50 (5.3%) | |
| Missing | 396 (15.0%) | 178 (24.9%) | 159 (16.0%) | 59 (6.3%) | |
| Obesity | <0.001 | ||||
| No | 2343 (88.5%) | 684 (95.5%) | 904 (90.9%) | 755 (80.6%) | |
| Yes | 304 (11.5%) | 32 (4.5%) | 90 (9.1%) | 182 (19.4%) | |
| Charlson Index | <0.001 | ||||
| 0 | 1076 (40.7%) | 315 (44.0%) | 426 (42.9%) | 336 (35.8%) | |
| 1 | 562 (21.2%) | 181 (25.3%) | 184(18.5%) | 197 (21.0%) | |
| 2 | 296 (11.2%) | 53 (7.4%) | 151 (15.2%) | 93 (9.9%) | |
| 3–5 | 262 (9.9%) | 38 (5.3%) | 60 (6.0%) | 164(17.5%) | |
| ≥6 | 450 (17.0%) | 129 (18.0%) | 173 (17.4%) | 148 (15.8%) | |
| Median household income | <0.001 | ||||
| ≤$38,999 | 565 (21.4%) | 112 (15.6%) | 269 (27.1%) | 184 (19.7%) | |
| $39,000–$47,999 | 677 (25.6%) | 184 (25.7%) | 232 (23.3%) | 261 (27.9%) | |
| $48,000–$62,999 | 711 (26.9%) | 192 (26.8%) | 258 (26.0%) | 261 (27.9%) | |
| ≥$63,000 | 573 (21.7%) | 201 (28.1%) | 192 (19.3%) | 180 (19.2%) | |
| Missing | 120 (4.5%) | 27 (3.8%) | 43 (4.3%) | 50 (5.3%) | |
| Primary expected payer | <0.001 | ||||
| Medicare | 777 (29.4%) | 205 (28.6%) | 262 (26.4%) | 310 (33.1%) | |
| Medicaid | 578 (21.8%) | 130 (18.2%) | 236 (23.8%) | 212 (22.6%) | |
| Private including HMO | 1096 (41.4%) | 359 (50.1%) | 381 (38.4%) | 356 (38.0%) | |
| Others/missing | 195 (7.4%) | 22 (3.1%) | 114(11.5%) | 59 (6.3%) | |
| Hospital bedsize | ** | ||||
| Small | 207 (7.8%) | 62 (8.7%) | 70 (7.0%) | 75 (8.0%) | |
| Medium | 447 (16.9%) | 138 (19.3%) | 136 (13.7%) | 173 (18.5%) | |
| Large | 1977 (74.7%) | 516 (72.1%) | 783 (78.8%) | 678 (72.4%) | |
| Missing | 16 (0.6%) | * | * | 11 (1.2%) | |
| Hospital teaching status | ** | ||||
| Rural | 14 (0.5%) | * | * | * | |
| Urban non-teaching | 331 (12.5%) | 116 (16.2%) | 112 (11.3%) | 103 (11.0%) | |
| Urban teaching | 2286 (86.4%) | 590 (82.4%) | 873 (87.8%) | 823 (87.8%) | |
| Missing | 16 (0.6%) | * | * | 11 (1.2%) | |
| Hospital region | <0.001 | ||||
| Northeast | 385 (14.6%) | 81 (11.3%) | 152 (15.3%) | 152 (16.2%) | |
| Midwest | 535 (20.2%) | 183 (25.6%) | 170 (17.1%) | 182 (19.4%) | |
| South | 969 (36.6%) | 283 (39.6%) | 381 (38.3%) | 305 (32.6%) | |
| West | 757 (28.6%) | 168 (23.5%) | 291 (29.3%) | 298 (31.8%) |
Median (IQR) or number (percent per column) is shown. Kruskal-Wallis H test or chi-square test for P-values. Significant P-values are emboldened.
indicated number of ≤10, required to suppress per the Healthcare Cost and Utilization Project.
not assessed due to suppressed number.
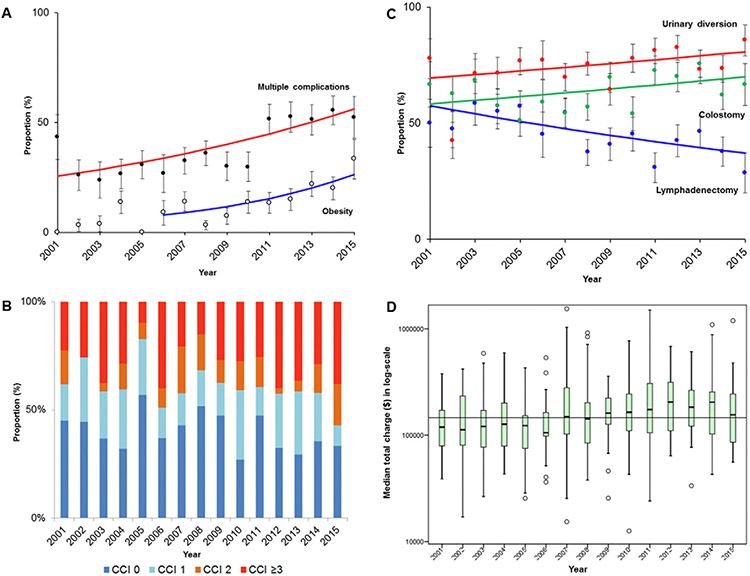
Obese women undergoing exenteration increased significantly from 4.5% in 2001–2005 to 19.4% in 2011–2015 (3.3-fold relative increase; P < 0.001). Annual percent change was 14.5 (95%CI 4.7–25.2) between 2006 and 2015 (P = 0.008; Fig. 1A). There were 1571 (59.4%) women who had a Charlson Comorbidity Index of ≥1, with a median of 2 (IQR 2–6; Fig. 1B). The number of women who had a Charlson Comorbidity Index ≥3 was 712 (26.9%), which significantly increased from 23.3% in 2001–2005 to 33.3% in 2011–2015 (42.9% relative increase; P < 0.001). Higher Charlson Comorbidity Index was significantly correlated with older age (r = 0.18), high number of perioperative complications (r = 0.31), longer length of stay (r = 0.12), and high total charges (r = 0.16) (all, P < 0.001).
Fig. 1.
Trends of comorbidity and outcome of pelvic exenteration between 2001 and 2015. Year-specific trends of A) obesity and multiple complications during the index admission, B) Charlson Comorbidity Index (P < 0.001), C) surgical performance, and D) median total charge of the index admission converted to 2015 value are shown (median total charge of $146,366 for horizontal line; P < 0.001). For panel A and C, dots represent actual observed value, bars represent confidence interval, and lines represent modeled value. For panel D, box plots are shown with log-transformed values. Abbreviation: CCI, Charlson Comorbidity Index.
The vast majority of surgeries were performed at large bedsize hospitals (>70%) and at urban teaching hospitals (>80%). Charlson Comorbidity Index was similar across the three bedsize groups (proportion of women with Charlson Comorbidity Index of ≥2: 37.0% for small, 35.6% for medium, and 38.9% for large, P = 0.406). The number of women with private insurance decreased from 50.1% in 2001–2005 to 38.0% in 2011–2015 (24.2% relative decrease), and those who had median household income of ≥$63,000 decreased from 28.1% to 19.2% during the same period (31.7% relative decrease) (both, P < 0.001).
Trends in treatment interventions were assessed. The number of women who underwent lymphadenectomy at the time of pelvic exenteration decreased from 53.8% in 2001–2005 to 38.1% in 2011–2015 (29.2% relative decrease, P< 0.001; Table 2 and Fig. 1C). Both colostomy formation (59.5% to 69.6%; 17.0% relative increase) and urinary diversion (67.5% to 78.3%; 16.0% relative increase) have modestly increased during the study period (both, P < 0.001; Fig. 1C).
Table 2.
Surgical performance and perioperative outcome of pelvic exenteration.
| Characteristic |
All |
2001–2005 |
2006–2010 |
2011–2015 |
P-value |
|---|---|---|---|---|---|
| Number | N = 2647 | n = 716 | n = 994 | n = 937 | |
| Lymphadenectomy | <0.001 | ||||
| No | 1463 (55.3%) | 331 (46.2%) | 552 (55.5%) | 579 (61.9%) | |
| Yes | 1184 (44.7%) | 385 (53.8%) | 442 (44.5%) | 357 (38.1%) | |
| Colostomy | <0.001 | ||||
| No | 987 (37.3%) | 290 (40.5%) | 413 (41.5%) | 285 (30.4%) | |
| Yes | 1659 (62.7%) | 426 (59.5%) | 581 (58.5%) | 652 (69.6%) | |
| Urinary diversion | <0.001 | ||||
| No | 707 (26.7%) | 233 (32.5%) | 271 (27.3%) | 203 (21.7%) | |
| Yes | 1940 (73.3%) | 483 (67.5%) | 723 (72.7%) | 734 (78.3%) | |
| Vaginal reconstruction | 0.350 | ||||
| No | 2055 (77.7%) | 561 (78.4%) | 781 (78.7%) | 713 (76.1%) | |
| Yes | 591 (22.3%) | 155 (21.6%) | 212 (21.3%) | 224 (23.9%) | |
| Complication (any) | <0.001 | ||||
| 0 | 845 (31.9%) | 299 (41.8%) | 351 (35.3%) | 195 (20.8%) | |
| 1 | 779 (29.4%) | 206 (28.8%) | 325 (32.7%) | 248 (26.5%) | |
| 2 | 426 (16.1%) | 127 (17.7%) | 111 (11.2%) | 188 (20.1%) | |
| ≥3 | 597 (22.6%) | 84 (11.7%) | 207 (20.7%) | 306 (32.7%) | |
| Death | ** | ||||
| No | 2598 (98.1%) | 709 (99.0%) | 967 (97.3%) | 922 (98.4%) | |
| Yes | 49 (1.9%) | * | 27 (2.7%) | 15 (1.6%) | |
| Length of stay (day) | |||||
| <28 | 2205 (83.3%) | 626 (87.4%) | 821 (82.6%) | 758 (80.9%) | |
| ≥28 | 442 (16.7%) | 90 (12.6%) | 173 (17.4%) | 179 (19.1%) | 0.001 |
| Total charge (crude) | $119,274 (IQR 75,484–203,744) | $81,732 (IQR 51,368–190,189) | $120,581 (IQR78,118–290,591) | $177,095 (IQR101,341–264,317) | <0.001 |
| Total charge (corrected)† | $146,366 (IQR 94,655–236,040) | $121,854 (IQR 78,632–172,350) | $146,332 (IQR 98,539–232,101) | $185,100 (IQR 106,665–282,394) | <0.001 |
Median (IQR) or number (percent per column) is shown. Kruskal-Wallis H test or chi-square test for P-values. Significant P-values are emboldened.
indicated number of ≤10, required to suppress per the Healthcare Cost and Utilization Project.
not assessed due to suppressed number.
converted to 2015 value based on medical inflation rate (Table S2).
Outcomes related to pelvic exenteration were assessed (Tables 2-3). Perioperative complications were seen in 1802 (68.1%, 95%CI 66.3–69.9) women. There were 1023 (38.6%, 95%CI 36.8–40.5) women who had multiple complications (Table 2). The most common complication was hemorrhage (31.8%) followed by ileus/small bowel obstruction (25.8%), wound complication (21.3%), respiratory failure (16.1%) and acute kidney injury (13.8%). Sepsis, thromboembolism, and pneumonia were seen in 8.4%, 7.1%, and 6.0% of the study population, respectively (Table 3). Death during the index admission was seen in 49 (1.9%, 95% CI 1.3–2.4) women.
Table 3.
Frequency of complications.
| Characteristic | No. | (%) |
|---|---|---|
| Hemorrhage | 841 | 31.8% |
| Ileus/SBO | 684 | 25.8% |
| Wound complications | 564 | 21.3% |
| Respiratory failure | 427 | 16.1% |
| AKI | 366 | 13.8% |
| Sepsis/SIRS | 223 | 8.4% |
| Thromboembolism | 188 | 7.1% |
| Pneumonia | 158 | 6.0% |
| Cardiac failure | 96 | 3.6% |
| Shock | 58 | 2.2% |
| Fistula | 58 | 2.2% |
| Abscess | 53 | 2.0% |
| Death | 49 | 1.8% |
| Pyelonephritis | 34 | 1.3% |
| MI | 29 | 1.1% |
| Intestinal perforation | 19 | 0.7% |
Abbreviations: SBO, small bowel obstruction; AKI, acute kidney injury; SIRS, systemic inflammatory response syndrome; MI, myocardial infarction.
The number of women with multiple perioperative complications increased from 25.7% in 2001–2005 to 52.7% in 2011–2015 (78.6% relative increase; P < 0.001). Annual percent change was 5.8 (95%CI 3.2–8.5) between 2001 and 2015 (P < 0.001; Fig. 1A). Contributing factors for multiple complications were examined (Table 4). On multivariable analysis, Charlson Comorbidity Index (OR per unit 1.197), obesity (OR 1.519), higher household income, recent year (OR per year 1.100), surgery at large or small bedsize hospital (OR 1.588 and 1.484 compared to medium size hospital), vulvar cancer (OR 1.697 compared to cervical cancer), urinary diversion (OR 2.251), and vaginal reconstruction (OR 1.390) were associated with increased risk of multiple complications (all, P < 0.05). Black women had lower risk of multiple complications (OR 0.559 compared to white women, P < 0.001).
Table 4.
Multivariable analysis for multiple complications/death at pelvic exenteration.
| Number | Multiple complications§ |
Perioperative death |
||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Year† | 1.100 (1.075–1.126) | <0.001 | ||
| Race/ethnicity | <0.001* | |||
| White | 1 | |||
| Black | 0.559 (0.402–0.778) | 0.001 | ||
| Hispanic | 1.011 (0.722–1.415) | 0.949 | ||
| Others | 1.343 (0.910–1.984) | 0.138 | ||
| Missing | 1.347 (1.052–1.724) | 0.018 | ||
| Obesity | ||||
| No | 1 | |||
| Yes | 1.519 (1.162–1.987) | 0.002 | ||
| Charlson Comorbidity Index† | 1.197 (1.156–1.240) | <0.001 | 1.617 (1.434–1.823) | <0.001 |
| Median household income | <0.001* | |||
| ≤$38,999 | 1.171 (0.901–1.522) | 0.238 | ||
| $39,000–$47,999 | 1 | |||
| $48,000–$62,999 | 1.572 (1.241–1.992) | <0.001 | ||
| ≥$63,000 | 1.418 (1.097–1.833) | 0.008 | ||
| Missing | 0.598 (0.375–0.955) | 0.031 | ||
| Primary expected payer | <0.001* | <0.001* | ||
| Medicare | 1 | 1 | ||
| Medicaid | 0.639 (0.488–0.838) | 0.001 | na | 0.990 |
| Private including HMO | 0.769 (0.614–0.962) | 0.022 | 0.155 (0.069–0.351) | <0.001 |
| Others/missing | 1.253 (0.885–1.774) | 0.204 | 0.603 (0.215–1.691) | 0.336 |
| Hospital bedsize | 0.001* | <0.001* | ||
| Small | 1.484(1.016–2.169) | 0.041 | 1.071 (0.412–2.785) | 0.887 |
| Medium | 1 | 1 | ||
| Large | 1.588 (1.245–2.025) | <0.001 | 0.231 (0.111–0.481) | <0.001 |
| Urinary diversion | ||||
| No | 1 | |||
| Yes | 2.251 (1.799–2.816) | <0.001 | ||
| Vaginal reconstruction | ||||
| No | 1 | |||
| Yes | 1.390 (1.127–1.713) | 0.002 | ||
| Cancer type | 0.005* | 0.034* | ||
| Cervical | 1 | 1 | ||
| Uterine | 1.123 (0.844–1.496) | 0.426 | 0.949 (0.378–2.284) | 0.911 |
| Vaginal | 1.092 (0.877–1.359) | 0.432 | 2.642 (1.094–6.379) | 0.031 |
| Vulvar | 1.697 (1.267–2.273) | <0.001 | 0.824 (0.246–2.758) | 0.753 |
Binary logistic regression model for analysis. All the covariates with P < 0.05 on univariable analysis were entered in the initial model. Conditional backward method was used to retain covariates with P < 0.05 in the final model. All the covariates in the list were entered in the final model.
any two or more complications listed in Table 3.
continuous variable.
P-value for interaction. Abbreviations: OR, odds ratio; and CI, confidence interval.
Contributing clinico-demographics for death were assessed (Table 4). On multivariable analysis, Charlson Comorbidity Index (OR per unit 1.617) and vaginal cancer (OR 2.642 compared to cervical cancer) were associated with increased mortality (both, P < 0.05). Private insurance (OR 0.155 compared to Medicare) and surgery at a largesized hospital (OR 0.231 compared to medium size) were associated with decreased mortality (both, P < 0.01). When perioperative complication types were assessed for death during the index admission (Fig. S1), those who developed shock, seen in 2.2% of the study population, had the highest mortality rate of 23.3%, followed by respiratory failure with sepsis/SIRS seen in 3.0% of study population but had 18.8% of mortality rate (all, P < 0.001).
Median length of stay was 14 (IQR 9–21) days, and the number of women hospitalized ≥28 days significantly increased from 12.6% in 2001–2005 to 19.1% in 2011–2015 (51.6% relative increase, P < 0.001; Table 2). The median uncorrected total charges were $119,274 (IQR 75,535-203,744), which consistently exceeded $100,000 as of 2007. Median total charges increased from $81,732 to $177,095 between 2001 and 2015 (net difference +$95,363, 2.2-fold relative increase, P < 0.001; Fig. S2). After correcting for the 2015 medical inflation rate, median total charges were $146,366, and charges significantly increased from $121,854 to $185,100 between 2001 and 2015 (net difference +$63,246, 51.9% relative increase, P < 0.001; Fig. 1D and Table 2).
A subgroup of only cervical cancer cases was examined (n = 1194). Similar to the whole cohort, a trend of increased obesity and comorbidity was observed during the study period, and in more recent years women were less likely to undergo lymphadenectomy but more likely to undergo urinary diversion (Tables S3-4). Unlike the whole cohort, the number of women who had colostomy remained unchanged during the study period. The number of women with multiple complications also increased significantly (68.3% relative increase, P < 0.001).
4. Discussion
Key findings of this study are that women undergoing pelvic exenteration for gynecologic malignancies became more obese and had greater comorbidity during the study period and that pelvic exenteration for women with gynecologic malignancies is associated with high morbidity and mortality as well as substantial treatment-related costs.
Our study showed that women who undergo pelvic exenteration were characterized by high medical comorbidity. While this may be partly due to treatment-related factors such as prior radiotherapy, chemotherapy, and surgery, it may also be that high medical comorbidity is secondary to patient factors such as obesity. There was a significant increase in obese women in this study population during the study period. This trend parallels to what is observed in the general U.S. population [12]. Increasing obesity is most likely a contributing factor to increasing medical comorbidity, resulting in more complications, as has also been demonstrated in other studies [23,24]. For instance, a prior study showed that obese patients had a significantly higher rate of early postoperative complications within 2 months of surgery compared to those of normal weight (82.8% versus 59.3%) [23].
In addition to obesity, the extent of medical comorbidity was also a factor associated with multiple complications related to pelvic exenteration in this study cohort. Similar to our findings, a recent study found that presence of ≥3 comorbidities was independently associated with severe postoperative complications after pelvic exenteration [24]. Our study even found that the extent of medical comorbidity is one of a few factors associated with surgical mortality. This information is new in the literature, and it is paramount to emphasize the impact of medical comorbidities when counseling patients for pelvic exenteration.
The number of women who underwent concurrent lymphadenectomy significantly decreased during the study period. The exact causality for this association is unknown, but it may be secondary to increasing utilization of radiotherapy for gynecologic cancer. That is, surgeons are not in favor of performing lymphadenectomy within the radiated field, as it is associated with increased risks of multiple complications. It is also speculated that in-field recurrence after radiation within the lymphatic chains is less common and that lymphadenectomy within an irradiated field is not indicated at the time of pelvic exenteration.
Vulvar cancer was associated with higher risk of multiple complications, whereas vaginal cancer was associated with high mortality compared to cervical cancer. We observed that women with vulvar cancer were more likely to be older and have comorbidities compared to those with cervical cancer (Table S5). This is likely the reason for higher rates of multiple complications in vulvar cancer. Causality of increased mortality in vaginal cancer is unexplained, but vaginal cancer seems to have poorer outcomes compared to cervical cancer after pelvic exenteration [5].
We not only observed that the vast majority of pelvic exenterations are performed in large hospitals but also that surgery in large hospitals was associated with a higher rate of multiple complications but a lower rate of perioperative mortality. It is likely that unmeasured factors such as resources, trained surgical specialists, and management of complications differ in such large hospitals.
Median total charges for pelvic exenteration more than doubled during the study period. This increase was also observed even after correcting for medical inflation rates. Thus, the increase observed in this study is beyond what is seen for historical medical inflation in the United States, with >$60,000 being accounted for by the interval increase between 2001 and 2015. It is most likely that increasing numbers of women with medical comorbidities, multiple complications, and prolonged hospital stays are contributing to this increase in treatment-related cost.
Another factor to explain the association between increasing complications and total charges may be the increasing number of procedures. For example, although interval increases of ostomy use were modest, this may lead to extra procedure-related complications/charge, particularly urinary diversion, which had a clinically meaningful impact on multiple complications (OR 2.257). Vaginal reconstruction was also independently associated with increased multiple complications. As the database has no information for surgeon's surgical volume or subspecialist (gynecologic oncologists versus other subspecialists), further study is warranted to examine if these factors possibly affect performance and outcome [25,26].
With regards to perioperative mortality, our result of 1.9% is similar to recent studies reporting 2.2–2.3% mortality rates (2011–2018 reports) [9,24]. These recent perioperative mortality rates seem improved in comparison to older studies, ranging from 4.0–4.2% (2005 reports) to 6.3–7.2% (1989 reports) [8,27-29]. It is speculated that while patients have more comorbidities in recent years, improving perioperative care and surgical techniques may have resulted in this decrease in perioperative mortality.
The vaginal reconstruction rate was relatively low in our study population (22.3%). This statistic is similar to what has reported previously (24.0%) [30]. The low rate of vaginal reconstruction in our study may be related to the fact that our patient population was older (median age 56). In a post-hoc analysis, women who had vaginal reconstruction were younger compared to those who did not in our cohort (median, 53 versus 65, P < 0.001). Our study also demonstrated that vaginal reconstruction is an independent factor for multiple complications. This finding seems consistent with a recent study that showed high morbidity after vaginal reconstruction at the time of pelvic exenteration [31].
There are a number of limitations in this study. First, this is a retrospective study and there may be missing confounders for analysis. For example, we do not know the indication for pelvic exenteration, and it is unknown if recurrent sites were single or multiple or central pelvic or sidewall. We also do not know if the surgical intent was therapeutic or palliative due to the lack of codes to specify and distinguish these. Second, type of pelvic exenteration (anterior, posterior, or total), use of rectal reanastomosis after exenteration, details of pelvic floor reconstruction, history of pelvic radiotherapy, and use of prosthetic grafts were also not available in this study due to lack of specific codes for these factors, but these are all salient factors impacting exenteration outcomes.
Third, patient performance status, frailty, albumin level, surgeon's surgical volume, hospital's care quality, and subspecialty for reconstruction were not available in this database but most likely affect the outcome. Fourth, information for surgical instrumentation and costs were not available in this study, and it remains unknown if these factors contributed to treatment cost inflation. Last, this database does not have a long-term endpoint of oncologic outcome, and a composite outcome analysis combining survival outcome and perioperative complications was not assessable in this study. A recent retrospective study suggested that survival after pelvic exenteration has not changed despite improving surgical technique and patient selection [3]. Thus, whether or not increasing complication rates are offset by improving overall survival after pelvic exenteration, or whether patients with worse prognostic factors undergoing this operation without a decrease in survival rates, are not answered in this study.
In summary, our study showed that women undergoing pelvic exenteration for gynecologic malignancies became more obese and comorbid during the study period, resulting in more complications, longer length of stay, and higher treatment-related costs. This data helps to define changes and trends in the use and outcomes of pelvic exenteration for gynecologic cancer. Integrating Charlson Comorbidity Index into preoperative assessment routinely may be useful because every single point increase in the index is associated with a 20% increase in multiple complications and a 60% increase in mortality. Similarly, awareness of obesity as the risk factor for multiple complications is necessary (50% increased risk). Standardizing and centralizing surgical care would also be beneficial for patients given the rarity and complexity of the procedure [2].
Supplementary Material
HIGHLIGHTS.
Pelvic exenteration for gynecologic malignancies was examined between 2001 and 2015.
Patients became more obese and comorbid during the study period.
Multiple complications, prolonged hospitalization, and total charge have increased.
Patient factors for multiple complications: obesity, comorbidity, and recent year surgery
Tumor/surgeon factors: vulvar cancer, vaginal reconstruction, and urinary diversion
Acknowledgments
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.)
Footnotes
Disclosure statement
Consultant, Tesaro and Clovis Oncology (J.D.W.); consultant, Tempus Labs (L.D.R.); honorarium, Chugai (K.M.); none for others.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.02.002.
Abstract of the study was presented at 50th Annual Meeting on Women’s Cancer, Honolulu, HI, March 16–19, 2019.
References
- [1].Sideri M, Surgery for cervical neoplasia, in: Morrow CP (Ed.), Morrow's Gynecologic Cancer Surgery, 2nd editionSouth Coast Medical Publishing, Encinitas, CA: 2013, pp. 513–698. [Google Scholar]
- [2].Chatterjee S, Chen L, Jones N, Tergas AI, Burke WM, Hou JY, Wright JD, National trends in total pelvic exenteration for gynecologic malignancies, Am. J. Obstet. Gynecol 215 (2016) 395–396. [DOI] [PubMed] [Google Scholar]
- [3].Westin SN, Rallapalli V, Fellman B, Urbauer DL, Pal N, Frumovitz MM, Ramondetta LM, Bodurka DC, Ramirez PT, Soliman PT, Overall survival after pelvic exenteration for gynecologic malignancy, Gynecol. Oncol 134 (2014) 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goldberg GL, Sukumvanich P, Einstein MH, Smith HO, Anderson PS, Fields AL, Total pelvic exenteration: the Albert Einstein College of Medicine/Montefiore Medical Center Experience (1987 to 2003), Gynecol. Oncol 101 (2006) 261–268. [DOI] [PubMed] [Google Scholar]
- [5].Maggioni A, Roviglione G, Landoni F, Zanagnolo V, Peiretti M, Colombo N, Bocciolone L, Biffi R, Minig L, Morrow CP, Pelvic exenteration: ten-year experience at the European Institute of Oncology in Milan, Gynecol. Oncol 114 (2009) 64–68. [DOI] [PubMed] [Google Scholar]
- [6].Schmidt AM, Imesch P, Fink D, Egger H, Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer, Gynecol. Oncol 125 (2012) 604–609. [DOI] [PubMed] [Google Scholar]
- [7].Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, Zighelboim I, Pelvic exenteration in gynecologic oncology: a single institution study over 20 years, Gynecol. Oncol 122 (2011) 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharma S, Odunsi K, Driscoll D, Lele S, Pelvic exenterations for gynecological malignancies: twenty-year experience at Roswell Park Cancer Institute, Int. J. Gynecol. Cancer 15 (2005) 475–482. [DOI] [PubMed] [Google Scholar]
- [9].McLean KA, Zhang W, Dunsmoor-Su RF, Shah CA, Gray HJ, Swensen RE, Goff BA, Pelvic exenteration in the age of modern chemoradiation, Gynecol. Oncol 121 (2011) 131–134. [DOI] [PubMed] [Google Scholar]
- [10].Huang M, Iglesias DA, Westin SN, Fellman B, Urbauer D, Schmeler KM, Frumovitz M, Ramirez PT, Soliman PT, Pelvic exenteration: impact of age on surgical and oncologic outcomes, Gynecol. Oncol 132 (2014) 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].An Aging World, International Population Reports, United States Census Bureau, 2015https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf, Accessed date: 20 November 2018. [Google Scholar]
- [12].Health, United States, Centers for Disease Control and Prevention, https://www.cdc.gov/nchs/data/hus/hus16.pdf#053 2016, Accessed date: 20 November 2018.
- [13].National Comprehensive Cancer Network Clinical Practice Guideline in Oncology, Cervical Cancer, http://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf, Accessed date: 24 November 2018. [Google Scholar]
- [14].National Comprehensive Cancer Network Clinical Practice Guideline in Oncology, Uterine Neoplasms, http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf, Accessed date: 24 November 2018. [Google Scholar]
- [15].HCUP National Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, Rockville, MD, 2001–2015 https://www.hcup-us.ahrq.gov/nisoverview.jsp (November/18/2018). [Google Scholar]
- [16].Charlson ME, Pompei P, Ales KL, MacKenzie CR, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J. Chronic Dis 40 (1987) 373–383. [DOI] [PubMed] [Google Scholar]
- [17].NIS description of data element, https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp, Accessed date: 23 January 2019.
- [18].International classification of diseases, 9th revision, https://simba.isr.umich.edu/restricted/docs/Mortality/icd_09_codes.pdf, Accessed date: 26 November 2018.
- [19].Historical Price Inflation for Medical Care, http://www.in2013dollars.com/Medical-care/price-inflation/, Accessed date: 26 November 2018.
- [20].Matsuo K, Ross MS, Machida H, Blake EA, Roman LD, Trends of uterine carcinosarcoma in the United States, J. Gynecol. Oncol 29 (2018), e22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R, Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials, Int. J. Radiat. Oncol. Biol. Phys 37 (1997) 745–751. [DOI] [PubMed] [Google Scholar]
- [22].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Iglesias DA, Westin SN, Rallapalli V, Huang M, Fellman B, Urbauer D, Frumovitz M, Ramirez PT, Soliman PT, The effect of body mass index on surgical outcomes and survival following pelvic exenteration, Gynecol. Oncol 125 (2012) 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tortorella L, Casarin J, Mara KC, Weaver AL, Multinu F, Glaser GE, Cliby WA, Scambia G, Mariani A, Kumar A, Prediction of short-term surgical complications in women undergoing pelvic exenteration for gynecological malignancies, Gynecol. Oncol 152 (2019) 151–156. [DOI] [PubMed] [Google Scholar]
- [25].Jalloul RJ, Nick AM, Munsell MF, Westin SN, Ramirez PT, Frumovitz M, Soliman PT, The influence of surgeon volume on outcomes after pelvic exenteration for a gynecologic cancer, J. Gynecol. Oncol 29 (2018), e68. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aviki EM, Esselen KM, Barcia SM, Nucci MR, Horowitz NS, Feltmate CM, Berkowitz RS, Orgill DG, Viswanathan AN, Muto MG, Does plastic surgical consultation improve the outcome of patients undergoing radical vulvectomy for squamous cell carcinoma of the vulva? Gynecol. Oncol 137 (2015) 60–65. [DOI] [PubMed] [Google Scholar]
- [27].Berek JS, Howe C, Lagasse LD, Hacker NF, Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA, Gynecol. Oncol 99 (2005) 153–159. [DOI] [PubMed] [Google Scholar]
- [28].Shingleton HM, Soong SJ, Gelder MS, Hatch KD, Baker VV, Austin JM Jr., Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix, Obstet. Gynecol 73 (1989) 1027–1034. [DOI] [PubMed] [Google Scholar]
- [29].Soper JT, Berchuck A, Creasman WT, Clarke-Pearson DL, Pelvic exenteration: factors associated with major surgical morbidity, Gynecol. Oncol 35 (1989) 93–98. [DOI] [PubMed] [Google Scholar]
- [30].Mirhashemi R, Averette HE, Lambrou N, Penalver MA, Mendez L, Ghurani G, Salom E, Vaginal reconstruction at the time of pelvic exenteration: a surgical and psychosexual analysis of techniques, Gynecol. Oncol 87 (2002) 39–45. [DOI] [PubMed] [Google Scholar]
- [31].Cortinovis U, Sala L, Bonomi S, Gallino G, Belli F, Ditto A, Martinelli F, Bogani G, Leone Roberti Maggiore U, Raspagliesi F, Rectus abdominis myofascial flap for vaginal reconstruction after pelvic exenteration, Ann. Plast. Surg 81 (2018) 576–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.