Abstract
Background.
Endometrial cancer arising in adenomyosis (EC-AIA) is a rare entity of endometrial cancer, and its clinical significance has not been well studied. This study aimed to examine the tumor characteristics and survival outcomes of EC-AIA.
Methods.
An exploratory analysis was performed to compare EC-AIA and historical control cases. For this study, EC-AIA cases were identified via a systematic literature search using PubMed/MEDLINE with entry keywords “endometrial cancer OR uterine cancer” AND “adenomyosis” (n = 46). The control group comprised consecutive non-EC-AIA cases from four institutions that had hysterectomy-based surgical staging (n = 1294). Patient demographics, pathology results, and survival outcomes were evaluated between the two groups.
Results.
The EC-AIA group was significantly older than the control group (58.9 vs. 55.3 years; P = 0.032). In terms of tumor characteristics, 56.5 % of the EC-AIA cases showed tumor within the myometrium without endometrial extension, and the EC-AIA group was significantly more likely to have tumors with more than 50 % myometrial invasion (51.6 vs. 26.6 %; P = 0.002) and serous/clear cell histology (22.2 vs. 8.2 %, P = 0.002) while less likely to express estrogen receptor (14.3 vs. 84.6 %; P < 0.001). Grade and stage distributions were similar (P > 0.05). In the univariate analysis, the EC-AIA group had a significantly poorer disease-free survival than the control group (5-year rate: 71.4 vs. 80.6 %; P = 0.014). In the multivariate analysis, with control for age, ethnicity, histology, grade, and stage, EA-CIC remained an independent prognostic factor for decreased disease-free survival (adjusted hazard ratio, 3.07; 95 % confidence interval 1.55–6.08; P = 0.001).
Conclusions.
The study suggested that endometrial cancer arising in adenomyosis may be an aggressive variant of endometrial cancer.
Uterine cancer is the most common type of gynecologic cancer affecting women, with an annual incidence of approximately 54,000 cases in the United States, which is steadily increasing.1 Surgery remains the mainstay of the management for endometrial cancer, and the surgical specimen obtained from hysterectomy is valuable for identifying histologic markers for prognosis.2 Adenomyosis is a common benign histopathologic finding in hysterectomy specimens of endometrial cancer patients, and the coexistence of these two conditions is variable, ranging from1 0 to 70 %.3,4 Adenomyosis is histologically defined as the presence of ectopic endometrial glands and stroma located deep within the surrounding myometrium.5 An association of adenomyosis with uterine lesions such as leiomyoma, endometrial hyperplasia, and endometrial cancer has been described previously.
Although many studies have reported carcinoma arising in endometriosis at extra-uterine sites,6,7 malignant transformation of adenomyosis is a rare occurrence, and it remains unknown whether this unique presentation has an impact on outcome. This uncommon form is histologically characterized by the transition from adenomyotic glandular epithelium to adenocarcinoma within the myometrium, and clearly apart from the endometrial cavity, a feature that often poses one of many challenges to proper diagnosis.8,9
Malignant transformation to ovarian cancer arising from endometriosis was first described in 1910,25 and the diagnostic criteria were later modified to apply to endometrial carcinoma arising in adenomyosis (EC-AIA) as follows: (1) the carcinoma must not be situated in the endometrium or elsewhere in the pelvis, (2) the carcinoma must be seen to arise from the epithelium of adenomyosis and not to have invaded from another source, and (3) endometrial stromal cells should be surrounding the aberrant glands to support the diagnosis of adenomyosis.11
As an extremely rare clinical entity, EC-AIA is estimated to occur in <1 % of endometrial cancer cases.12 In addition, existing evidence for EC-AIA has been limited to case reports. For these reasons, tumor characteristics and survival outcome of EC-AIA still are poorly delineated. This study aimed to conduct a systematic review of the literature to examine oncologic patterns and outcomes of EC-AIA versus those of historical control subjects with endometrial cancer.
MATERIALS AND METHODS
Study Design and Eligibility
An exploratory analysis compared the case group with EC-AIA and the control group consisting of historical endometrial cancer cases. For the case group, a systematic literature search was performed using the public search engines PubMed and MEDLINE with entry keywords “endometrial cancer OR uterine cancer” AND “adenomyosis” in the English literature (29 April 2015). Eligible cases were EC-AIA cases with adequate description of clinical information. Cases with cancer from other sites, sarcomas, and endometrial hyperplasia were excluded.
For the control group, consecutive cases with endometrial cancer that had hysterectomy-based surgical staging from four institutions were examined. No EC-AIA case was reported in the control group. Institutional review board or local ethical committee approval was obtained in each participating institution. The STROBE guidelines were consulted for this case–control study. Some of the study population were within the context of our previous studies.4,13-17
Clinical Information
For the eligible cases, the following information was abstracted: patient demographics, tumor characteristics, and survival outcomes. The patient demographics included patient age and ethnicity. In addition, year and country of publication, menopausal status, presenting symptom, and use of endometrial biopsy and its results were collected for the case group. The tumor characteristics included histologic subtype, grade, stage, depth of myometrial tumor invasion, lymph node metastasis, and estrogen receptor (ER) expression. The presence or absence of tumor extension to the endometrial layer also was abstracted. For survival outcome, disease-free survival (DFS) and overall survival (OS) were collected.
Definition
Histologic subtypes were grouped into endometrioid, serous, clear cell, and other. Grade was grouped into low-versus high grade. Grades 1 and 2 endometrioid tumors were considered low-grade. Grade 3 endometrioid, serous, and clear-cell tumors were grouped as high grade.
All EC-AIA cases in which the tumor grade described was high grade without detail were allocated to the high-grade arm of the study. Stage was reclassified based on the most recent International Federation of Gynecology and Obstetrics (FIGO) system.18 Deep myometrial tumor invasion was defined as more than 50 % of tumor invasion into the uterine myometrium layer. All EC-AIA cases in which depth of myometrial tumor invasion was described as deep invasion without detail were allocated to the deep invasion arm. For EC-AIA cases in which the tumor was incidentally found in a hysterectomy specimen without preoperative diagnosis of endometrial cancer, the tumor was considered an incidental tumor. DFS was defined as the interval between the date of hysterectomy and the date of the first recurrence or the last follow-up visit. OS was defined as the interval between the date of hysterectomy and the date of death due to endometrial cancer or the last follow-up visit.
Statistical Analysis
The primary aim of our analysis was to examine DFS and OS between the case and control groups. The secondary aim of the analysis was to examine DFS and OS in subgroups of women with stage I disease and endometrioid histology. Continuous variables were assessed for normality expressed as mean ± standard deviation or median (range). The statistical significance of continuous variables was assessed using Student’s t test or the Mann–Whitney U test as appropriate. Categorical or ordinal variables were assessed for statistical significance by the Chi square test or Fisher’s exact test and expressed as odds ratio (OR) and 95 % confidence interval (CI).
For survival analysis, a log-rank test for univariate analysis and a Cox proportional hazard regression model for multivariate analysis were performed. The covariates entered into the final model were EC-AIA (no vs. yes), age (<50, 50–59, and ≥60 years), ethnicity (Caucasian, African, Hispanic, or Asian), histology (endometrioid, serous, clear-cell, and other), grade (low vs. high grade), and stage (I, II, III, and IV). The statistical significance of survival analysis was expressed in terms of hazard ratio (HR) and 95 % CI. The Kaplan–Meier method was used to construct survival curves. All analyses were two-tailed, and P values lower than 0.05 were considered statistically significant. The Statistical Package for Social Sciences (version 22.0; SPSS, Chicago, IL, USA) was used for the analysis.
RESULTS
The selection criteria for the EC-AIA group are shown in Fig. S1. With the searching keywords, 588 articles were initially screened for title and abstract. Of these articles, 554 were excluded, with the remaining 34 articles assessed for eligibility. Six articles were further excluded due to separate foci of cancer and adenomyosis, and the remaining 28 articles, including 46 cases of EC-AIA, met the inclusion criteria and formed the case group (meta-data in Table S1).8,9,12,19-43
For the control group, 1294 cases of endometrial cancer that had hysterectomy-based surgical staging were examined for statistical analysis (Los Angeles County Medical Center: n = 771; Osaka University Hospital: n = 287; Keck Medical Center of University of Southern California: n = 138, and Niigata University Hospital: n = 98). None of the control group had EC-AIA.
The patient demographics are shown in Tables 1 and 2. The EC-AIA group was significantly older than the control group (58.9 vs. 55.3 years; P = 0.032). More than three-fourths of the EC-AIA group were postmenopausal (81.4 %). The majority of the EC-AIA patients were Asian (56.8 %), a statistically higher proportion than in the control group (39.7 %; P < 0.001).
TABLE 1.
Patient demographics
| EC-AIA n (%) | Control n (%) | P value | |
|---|---|---|---|
| Number | 46 | 1294 | |
| Mean age (years) | 58.9 ± 9.9 | 55.3 ± 11.4 | 0.032 |
| <50 | 7 (15.6) | 384 (29.7) | |
| 50–59 | 16 (35.5) | 466 (36.0) | |
| ≥60 | 22 (48.9) | 444 (34.3) | |
| Missing | 1 | 0 | |
| Ethnicity | <0.001 | ||
| Caucasian | 14 (31.8) | 176 (13.6) | |
| African American | 3 (6.8) | 38 (2.9) | |
| Hispanic | 2 (4.6) | 566 (43.8) | |
| Asian | 25 (56.8) | 514 (39.7) | |
| Missing | 2 | 0 | |
| Histology | 0.002 | ||
| Endometrioid | 35 (77.8) | 1063 (82.1) | |
| Serous | 7 (15.5) | 77 (6.0) | |
| Clear cell | 3 (6.7) | 28 (2.2) | |
| Other | 0 | 126 (9.7) | |
| Missing | 1 | 0 | |
| Grade | 0.16 | ||
| Low grade | 28 (68.3) | 1005 (77.7) | |
| High grade | 13 (31.7) | 289 (22.3) | |
| Missing | 5 | 0 | |
| Deep myometrial invasion | 0.002 | ||
| No | 15 (48.4) | 923 (73.4) | |
| Yes | 16 (51.6) | 335 (26.6) | |
| Missing | 15 | 36 | |
| Stage | 0.63 | ||
| I | 30 (73.2) | 921 (71.2) | |
| II | 1 (2.4) | 100 (7.8) | |
| III | 6 (14.6) | 185 (14.3) | |
| IV | 4 (9.8) | 87 (6.7) | |
| Missing | 5 | 1 | |
| Nodal metastasisa | 0.075 | ||
| Negative | 24 (72.7) | 701 (84.4) | |
| Positive | 9 (27.3) | 130 (15.6) | |
| Not performed | 13 | 463 | |
| ER expression | <0.001 | ||
| Negative | 12 (85.7) | 57 (15.4) | |
| Positive | 2 (14.3) | 313 (84.6) | |
| Not evaluated | 32 | 924 |
Student t test or Chi square was performed for P values
EC-AIA Endometrial cancer arising in adenomyosis, ER estrogen receptor
Nodal metastasis for pelvic and/or paraaortic lymph nodes
TABLE 2.
Symptom and diagnostic test for endometrial cancer arising in adenomyosis
| Number | n (%) = 46 |
|---|---|
| Menopause | |
| Yes | 35 (81.4) |
| No | 8 (18.6) |
| Missing | 3 |
| Symptom | |
| Abnormal uterine bleeding | 23 (50.0) |
| Abdominal or pelvic pain | 12 (26.1) |
| Other | 4 (8.7) |
| No symptoms | 7 (15.2) |
| Endometrial biopsy | |
| Not performed | 22 (47.8) |
| Performed | 24 (52.2) |
| Normal or atrophic endometrium | 9 (37.5) |
| Atypical hyperplasia | 6 (25.0) |
| Carcinoma | 9 (37.5) |
| Preoperative diagnosis | |
| Endometrial cancer | 16 (34.8) |
| Other | 30 (65.2) |
The most common symptom of EC-AIA was abnormal uterine bleeding (50 %) followed by abdominopelvic pain (26.1 %). Seven patients (15.2 %) in the EC-AIA group were asymptomatic. Endometrial biopsy was performed preoperatively in 24 cases (52.2 %), of which 9 cases (37.5 %) had a normal/atrophic endometrium and 6 cases (25 %) had endometrial hyperplasia. In 16 EC-AIA cases (34.8 %), hysterectomy was performed for a preoperative diagnosis of endometrial cancer, and in the remaining 30 (65.2 %) cases, the tumor was incidentally discovered in the hysterectomy specimens.
The majority of the EC-AIA patients had the endometrioid histologic subtype (77.8 %), low-grade tumors (68.3 %), or stage I disease (73.2 %). These proportions of grade and stage were similar between the EC-AIA and control groups (P > 0.05, respectively). More than half (56.5 %) of the tumors in the EC-AIA group were confined within the myometrial layer of the uterus without expansion to the endometrium. The EC-AIA group had a significantly greater risk of serous/clear-cell histology (22.2 vs. 8.2 %; P = 0.002) and deep myometrial invasion (51.6 vs. 26.6 %; P = 0.002) than the control group. The proportion of lymphadenectomy was similar between the two groups (P = 0.30). The EC-AIA group had a higher risk of lymph nodal metastasis than the control group, but the difference did not quite reach statistical significance (27.3 vs. 15.6 %; P = 0.075). The tumors in the EC-AIA group also were less likely to express ER than the tumors in the control group (14.3 vs. 84.6 %; P < 0.001). No case underwent genetic mutation testing in the EC-AIA group.
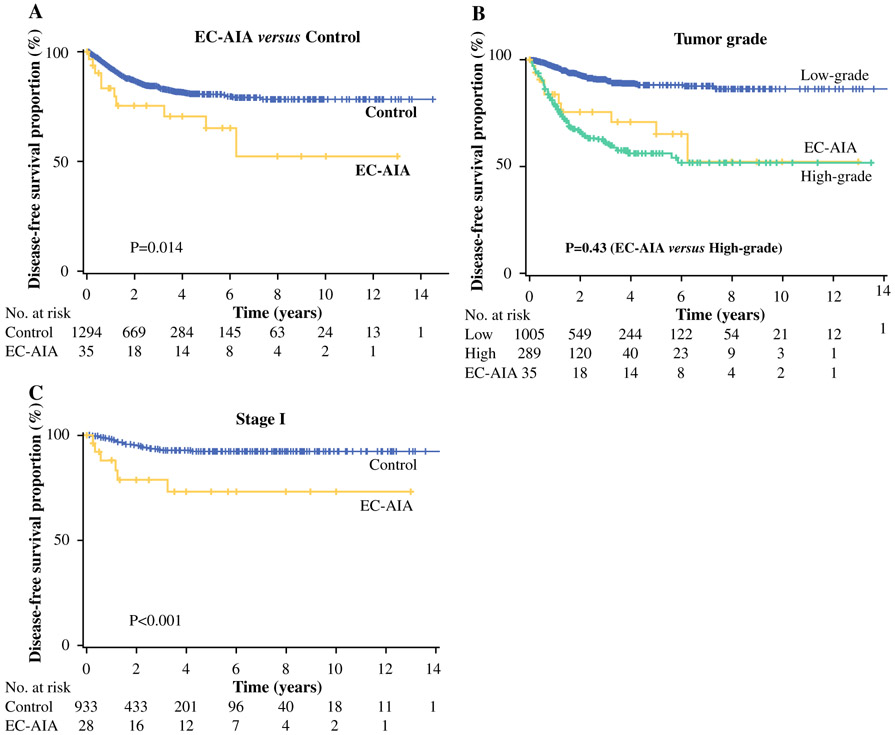
Survival analysis was performed for all the cases (Tables 3, S2). The median follow-up period was 28.6 months. The EC-AIA group had 10 events of disease recurrence (21.7 %) and 6 events of death due to cancer progression (13 %). In the univariate analysis, EC-AIA was significantly associated with decreased DFS compared with the control group (5-year rates: 71.4 vs. 80.6 %; P = 0.014, Fig. 1a). In addition, older age, African ethnicity, non-endometrioid histology, high-grade tumor, and advanced stage were associated with decreased DFS (P < 0.05 for all).
TABLE 3.
Risk factors for disease-free survival in all cases
| n | 5-Year (%) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |||
| Age (years) | <0.001 | |||||
| <50 | 391 | 89.7 | 1 | 1 | ||
| 50–59 | 482 | 79.5 | 2.09 (1.36–3.22) | 1.96 (1.26–3.04) | 0.003 | |
| ≥60 | 466 | 72.3 | 3.19 (2.09–4.86) | 2.67 (1.70–4.21) | <0.001 | |
| Ethnicity | 0.003 | |||||
| Caucasian | 190 | 70.0 | 1 | 1 | ||
| African American | 41 | 60.7 | 1.69 (0.85–3.38) | 0.53 (0.26–1.10) | 0.09 | |
| Hispanic | 568 | 83.5 | 0.61 (0.40–0.93) | 0.76 (0.49–1.18) | 0.22 | |
| Asian | 539 | 81.2 | 0.76 (0.50–1.17) | 0.80 (0.52–1.23) | 0.31 | |
| Histology | <0.001 | |||||
| Endometrioid | 1098 | 86.3 | 1 | 1 | ||
| Serous | 84 | 41.6 | 5.79 (3.95–8.48) | 1.02 (0.63–1.67) | 0.92 | |
| Clear cell | 31 | 47.1 | 6.34 (3.55–11.3) | 1.34 (0.71–2.55) | 0.37 | |
| Other | 126 | 62.5 | 3.14 (2.14–4.60) | 1.28 (0.94–1.97) | 0.25 | |
| Grade | <0.001 | |||||
| Low grade | 1033 | 88.0 | 1 | 1 | ||
| High grade | 302 | 54.8 | 5.33 (3.99–7.12) | 1.89 (1.31–2.73) | 0.001 | |
| Stage | <0.001 | |||||
| I | 951 | 91.3 | 1 | 1 | ||
| II | 101 | 79.8 | 2.67 (1.52–4.68) | 2.87 (1.62–5.08) | <0.001 | |
| III | 192 | 60.1 | 5.59 (3.84–8.14) | 4.73 (3.18–7.02) | <0.001 | |
| IV | 90 | 22.2 | 22.1 (15.2–32.0) | 15.8 (10.2–24.5) | <0.001 | |
| EC-AIA | 0.014 | |||||
| No | 1294 | 80.6 | 1 | 1 | ||
| Yes | 46 | 71.4 | 2.18 (1.15–4.13) | 3.07 (1.55–6.08) | 0.001 | |
The log-rank test was performed for univariate analysis and Cox the proportional hazard regression test for multivariate analysis. Significant P values are in bold type. Chi square value: 551.6 (P < 0.001)
HR Hazard ratio, CI confidence interval, EC-AIA endometrial cancer arising in adenomyosis
FIG. 1.
Survival curves for disease-free survival. The Kaplan–Meier method was used to construct survival curves for a all cases based on endometrial cancer arising in adenomyosis (EC-AIA), b all cases based on grade and EC-AIA, and c stage I disease based on EC-AIA. The log-rank test was performed for P values
In the multivariate analysis, with control for age, ethnicity, histology, grade, and stage, EC-AIA remained an independent prognostic factor associated with decreased DFS (HR, 3.07; P = 0.001; Table 3). Other independent prognostic factors associated with decreased DFS included age of 50–59 years (HR 1.96; P = 0.003), age of 60 years or older (HR 2.67; P < 0.001), high-grade tumor (HR 1.89; P = 0.001), stage II disease (HR 2.87; P < 0.001), stage III disease (HR 4.73; P < 0.001), and stage IV disease (HR 15.8; P < 0.001).
When survival outcomes were compared between the EC-AIA group and high-grade tumors in the control group, the 5-year DFS rates were similar between the two groups (71.4 vs. 55.7 %; P = 0.43; Fig. 1b). The 5-year OS rates were similar in the EC-AIA and control groups (86 vs. 88.4 %; P = 0.15).
A subgroup of cases with stage I disease was examined (Tables 4, S3). In the univariate analysis, EC-AIA was significantly associated with decreased DFS (5-year rates: 68.3 vs. 92.0 %; P < 0.001; Fig. 1c). In the multivariate analysis, with control for age, ethnicity, histology, and grade, EC-AIA remained an independent prognostic factor associated with decreased DFS (HR 3.90; 95 % CI 1.46–10.4; P = 0.007). In this subgroup, EC-AIA had the second largest magnitude of statistical significance for decreased DFS after high-grade tumor (HR 4.19; P < 0.001). For OS, EC-AIA was significantly associated with a lower 5-year OS rate than the control group in the univariate analysis (88.4 vs. 95.9 %; P = 0.009). In the multivariate analysis, EC-AIA did not remain an independent prognostic factor for OS in stage I disease (P = 0.56; Table S3). Similarly, a subgroup of cases with endometrioid histology was examined (Tables S4, S5). In the multivariate analysis, EC-AIA remained an independent prognostic factor for decreased both DFS (HR 3.82; 95 %CI 1.36–10.8; P = 0.011) and OS (HR 4.58; 95 %CI 1.04–20.3; P = 0.045).
TABLE 4.
Risk factors for disease-free survival in stage I
| n | 5-Year (%) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |||
| Age (years) | 0.001 | |||||
| <50 | 280 | 96.2 | 1 | 1 | ||
| 50–59 | 337 | 92.1 | 2.05 (0.85–4.94) | 1.98 (0.81–4.82) | 0.13 | |
| ≥60 | 334 | 85.6 | 3.97 (1.73–9.09) | 2.34 (0.97–5.63) | 0.057 | |
| Ethnicity | 0.019 | |||||
| Caucasian | 135 | 83.6 | 1 | 1 | ||
| African American | 24 | 100 | NA | NA | 0.97 | |
| Hispanic | 423 | 93.8 | 0.35 (0.16–0.75) | 0.49 (0.22–1.14) | 0.098 | |
| Asian | 369 | 90.6 | 0.72 (0.35–1.46) | 0.71 (0.33–1.54) | 0.39 | |
| Histology | <0.001 | |||||
| Endometrioid | 837 | 93.6 | 1 | 1 | ||
| Serous | 36 | 64.2 | 4.39 (1.84–10.5) | 0.73 (0.24–2.19) | 0.58 | |
| Clear cell | 14 | 62.2 | 9.14 (3.23–25.8) | 1.99 (0.60–6.59) | 0.26 | |
| Other | 64 | 80.0 | 3.70 (1.77–7.73) | 1.82 (0.79–4.16) | 0.16 | |
| Grade | <0.001 | |||||
| Low grade | 815 | 94.4 | 1 | 1 | ||
| High grade | 134 | 72.3 | 6.00 (3.48–10.3) | 4.19 (2.08–8.46) | <0.001 | |
| EC-AIA | <0.001 | |||||
| No | 921 | 92.0 | 1 | 1 | ||
| Yes | 30 | 68.9 | 5.21 (2.22–12.2) | 3.90 (1.46–10.4) | 0.007 | |
The log-rank test for univariate analysis and the Cox proportional hazard regression test for multivariate analysis. Significant P values are in bold type. Chi square value: 87.5 (P < 0.001)
HR Hazard ratio, CI confidence interval, NA not available, EC-AIA endometrial cancer arising in adenomyosis
DISCUSSION
Adenomyosis is ectopic endometrium within the myometrium, which is essentially an endometriosis, and lesions of both adenomyosis and endometriosis share an identical origin with respect to structure and function. Malignant transformation of endometriosis occurs in <1 % of cases and has been reported mainly in ovarian endometriosis.6 It also is known that endometriosis is a risk factor for epithelial ovarian cancer.6 Clear-cell carcinoma is reported to be the most common histologic subtype of endometriosis-associated ovarian cancer followed by low-grade serous and endometrioid carcinomas.7 Among malignancy types related to extra-ovarian endometriosis, however, endometrioid type was found to be the most common histologic subtype.6 These findings were found to be consistent with our study findings in that endometrioid type was the most common histologic pattern of EC-AIA.
Few studies have described the molecular mechanism of adenomyosis formation. It has been suggested that loss of heterozygosity in the DNA mismatch repair family (hMSH2, hMLH1, p16, and GALT) is associated with adenomyosis and its pathogenesis.44 However, the etiology of malignant transformation of adenomyosis remains unknown and seems to be dependent on genetic and epigenetic alterations in a multistep process.
In contrast, the mechanism for malignant transformation of endometriosis has been well described. A transition from endometrial epithelium of adenomyosis to the premalignant single-layered tumor cells and finally to carcinoma has been described previously.45 However, the focus of the current study was on examination of the de novo carcinogenesis pathways involved in the malignant transformation of the ectopic endometrium.
Previous studies have described a mutation of ARID1A, a classic tumor suppressor and negative regulator of the cell cycle, as a possible key mechanism in the transformation of ectopic endometrium to malignant neoplasm.46 An ARID1A mutation is found in approximately 41–57 % of the clear-cell type, in 30–48 % of the endometrioid type of endometriosis-associated ovarian cancer, and in approximately 40 % of contiguous endometriosis cases, next to cancerous tissue.47,48 Findings show that ARID1A encodes BAF250a, a protein involved in chromatin remodeling via the multi-protein SWI-SNF complex. The ARID1A gene mutation has been described as an early event in precursors of endometriosis-associated cancers.46,49 Unlike BRCA and TP53 mutations, in which genetic alteration occurs in germ-line DNA, all ARID1A mutations are somatic. However, the mechanism by which somatic mutations in ARID1A enable the progression of benign endometriosis to carcinoma remains unclear.46
Findings show that the concurrent loss of BAF250a and ER expression is rare in benign endometriosis and more frequent in atypical endometriosis (up to 23 %) and in ovarian clear cell carcinoma (up to 42 %).50 A number of previous studies have suggested that loss of ARID1A is associated with TP53 wild-type tumors,51 and it has been noted that direct protein–protein interaction may occur between ARID1A and TP53.52 Another possible mechanism is a mutation in the PI3K/AKT pathway that has a crucial role in cell cycle regulation and is suggested to be an important key to be the pathogenesis of endometriosis-associated ovarian cancer, particularly in association with loss of ARID1A.53,54
Interestingly, findings have demonstrated that ARID1A mutations lead to activation of the PI3K/AKT pathway in endometrial cancer, and it has been shown that PI3K/AKT can activate ERα in the absence of estrogen.55,56 Whether these genetic alterations occur in EC-AIA or not requires further investigation.
The negative prognostic impact of EC-AIA may be affected by several possibilities. The first reason is a possible delay in diagnosis due to the absence of a lesion in the endometrium. Theoretically, EC-AIA occurs within the myometrial layer of the uterus without endometrial involvement initially. Then, as the tumor progresses, it may expand into the endometrium, which then triggers symptoms and a diagnostic workup such as an endometrial biopsy. However, the tumor characteristics and survival outcomes of EC-AIA with or without endometrial expansion were found to be similar.
A second potential reason is the unique location in which EC-AIA occurs. That is, in contrast to endometrial cancer, which needs a mechanical process to invade through the anatomic barriers of the basal layer of the endometrium to reach the stroma, EC-AIA is already in the stromal layer with close proximity to lymphatic and vascular channels. Indeed, this may be causality for the risk of nodal metastasis in the EC-AIA group. A highly vascularized proximity to angiolymphatic structures in histologic sections of EC-AIA has been reported, which may be a reason for easy invasion to the lymphatic system and nodal metastasis.41
A limitation of the study was the relatively small sample size for EC-AIA, reflecting its rarity. Only 46 cases were identified during the 50 years of the study period that we searched. During such a long period, treatment paradigms have changed, introducing treatment bias. Another limitation was that this study, as a retrospective investigation, may have missed variables confounded for the analysis. For instance, there may be a publication bias for EC-AIA. A central pathology review for the historical control group to rule out EC-AIA was not performed, although all the pathology reports were examined for the presence of EC-AIA.
A potential weakness of this study was that its case-control design was not a typical approach in that we did not match the cases with the control subjects for known prognostic factors as well as area and time period. Also, we were unable to ascertain what treatment the patients received, so we could not correct for treatment regimen in our analyses. Therefore, further validation is warranted to support our results. Finally, whether survival outcome of endometrial cancer coexisting with adenomyosis differs from that of EC-AIA remains unanswered in our study and merits further investigation.57
Supplementary Material
ACKNOWLEDGMENT
The study was supported by the Ensign Endowment for Gynecologic Cancer Research (K.M. and L.D.R.).
Footnotes
CONFLICT OF INTEREST The authors declare that there is no conflict of interest in all authors.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4952-y) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. [DOI] [PubMed] [Google Scholar]
- 3.Musa F, Frey MK, Im HB, Chekmareva M, Ellenson LH, Holcomb K. Does the presence of adenomyosis and lymphovascular space invasion affect lymph node status in patients with endometrioid adenocarcinoma of the endometrium? Am J Obstet Gynecol. 2012;207:417e1–6. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo K, Cahoon SS, Gualtieri M, Scannell CA, Jung CE, Jung T, et al. Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer. Ann Surg Oncol. 2014;21:4246–55. [DOI] [PubMed] [Google Scholar]
- 5.Ferenczy A Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312–22. [DOI] [PubMed] [Google Scholar]
- 6.Bats AS, Zafrani Y, Pautier P, Duvillard P, Morice P. Malignant transformation of abdominal wall endometriosis to clear cell carcinoma: case report and review of the literature. Fertil Steril. 2008;90:1197e13–6. [DOI] [PubMed] [Google Scholar]
- 7.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb P. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol. 2012;13:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motohara K, Tashiro H, Ohtake H, Saito F, Ohba T, Katabuchi H. Endometrioid adenocarcinoma arising in adenomyosis: elucidation by periodic magnetic resonance imaging evaluations. Int J Clin Oncol. 2008;13:266–70. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Sugiyama T, Nanjo H, Hoshi N, Murakami M, Sugita A, et al. Endometrioid adenocarcinoma arising from adenomyosis: report and immunohistochemical analysis of an unusual case. Pathol Int. 2001;51:308–13. [DOI] [PubMed] [Google Scholar]
- 10.Sampson JA. Endometrial carcinoma of the ovary arising in endometrial tissue of that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 11.Colman HI, Rosenthal AH. Carcinoma developing in areas of adenomyosis. Obstet Gynecol. 1959;14:342–8. [PubMed] [Google Scholar]
- 12.Koshiyama M, Suzuki A, Ozawa M, Fujita K, Sakakibara A, Kawamura M, et al. Adenocarcinomas arising from uterine adenomyosis: a report of four cases. Int J Gynecol Pathol. 2002;21:239–45. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo K, Gray MJ, Yang DY, Srivastava SA, Tripathi PB, Sonoda LA, et al. The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecol Oncol. 2013;128:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo K, Gualtieri MR, Cahoon SS, Jung CE, Paulson RJ, Shoupe D, et al. Surgical menopause and increased risk of non-alcoholic fatty liver disease in endometrial cancer. Menopause. 2015. doi: 10.1097/GME.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo K, Hom MS, Moeini A, Machida H, Takeshima N, Roman LD, et al. Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol. 2015;138:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol. 2015;125:424–33. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo K, Yessaian AA, Lin YG, Roman LD, Pham HQ, Muderspach LI, et al. Predictive model of venous thromboembolism in endometrial cancer. Gynecol Oncol. 2013;128:544–51. [DOI] [PubMed] [Google Scholar]
- 18.Pecorelli S Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- 19.Taga S, Sawada M, Nagai A, Yamamoto D, Hayase R. A case of endometrioid adenocarcinoma arising from adenomyosis. Case Rep Obstet Gynecol. 2014;2014:5692–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff JD, Erozan YS, Genadry R. Adenocarcinoma arising in adenomyosis detected by atypical cytology. Obstet Gynecol. 1986;67:145–8. [PubMed] [Google Scholar]
- 21.Kuwashima Y, Uehara T, Kishi K, Tajima H, Shiromizu K, Matsuzawa M, et al. Intramural adenocarcinoma of the uterus, arisen from adenomyosis uteri, showing unique histologic appearances: report of two cases. Eur J Gynaecol Oncol. 1994;15:418–23. [PubMed] [Google Scholar]
- 22.Hayata T, Tanaka Y, Miyakawa I. Endometrial cancer associated with adenomyosis. Int J Gynaecol Obstet. 1994;44:76–7. [DOI] [PubMed] [Google Scholar]
- 23.Takai N, Akizuki S, Nasu K, Etoh Y, Miyakawa I. Endometrioid adenocarcinoma arising from adenomyosis. Gynecol Obstet Investig. 1999;48:141–4. [DOI] [PubMed] [Google Scholar]
- 24.Abushahin N, Zhang T, Chiang S, Zhang X, Hatch K, Zheng W. Serous endometrial intraepithelial carcinoma arising in adenomyosis: a report of 5 cases. Int J Gynecol Pathol. 2011;30:271–81. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez E, Woodruff JD. Endometrial adenocarcinoma arising in adenomyosis. Am J Obstet Gynecol. 1980;138:827–32. [DOI] [PubMed] [Google Scholar]
- 26.Winkelman J, Robinson R. Adenocarcinoma of endometrium involving adenomyosis: report of an unusual case and review of the literature. Cancer. 1966;19:901–8. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa Y, Takano K, Higa S, Tanabe M, Wada A, Sugita M, et al. Endometrial carcinoma coexisting with pregnancy, presumed to derive from adenomyosis: a case report. Int J Gynecol Cancer. 2001;11:488–90. [DOI] [PubMed] [Google Scholar]
- 28.Couto D, Mota F, Silva T, de Oliveira C. Adenocarcinoma arising in adenomyosis: report of an unusual case. Acta Obstet Gynecol Scand. 2004;83:406–8. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi K, Yamanaka Y, Hamana S, Ohara N, Maruo T. Invasive adenocarcinoma arising from uterine adenomyosis involving the rectosigmoid colon. Int J Gynecol Cancer. 2004;14:1004–6. [DOI] [PubMed] [Google Scholar]
- 30.Hsu MI, Chou SY, Lin SE, Liang SJ, Chiu HC, Hsu CS. Very-early-stage adenocarcinoma arising from adenomyosis in the uterus. Taiwan J Obstet Gynecol. 2006;45:346–9. [DOI] [PubMed] [Google Scholar]
- 31.Izadi-Mood N, Samadi N, Sarmadi S, Eftekhar Z. Papillary serous carcinoma arising from adenomyosis presenting as intramural leiomyoma. Arch Iran Med. 2007;10:258–60. [PubMed] [Google Scholar]
- 32.Puppa G, Shozu M, Perin T, et al. Small primary adenocarcinoma in adenomyosis with nodal metastasis: a case report. BMC Cancer. 2007;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohta Y, Hamatani S, Suzuki T, Ikeda K, Kiyokawa K, Shiokawa A, et al. Clear-cell adenocarcinoma arising from a giant cystic adenomyosis: a case report with immunohistochemical analysis of laminin-5 gamma 2 chain and p53 overexpression. Pathol Res Pract. 2008;204:677–82. [DOI] [PubMed] [Google Scholar]
- 34.Hirabayashi K, Yasuda M, Kajiwara H, Nakamura N, Sato S, Nishijima Y, et al. Clear-cell adenocarcinoma arising from adenomyosis. Int J Gynecol Pathol. 2009;28:262–6. [DOI] [PubMed] [Google Scholar]
- 35.Kazandi M, Zeybek B, Terek MC, Zekioglu O, Ozdemir N, Oztekin K. Grade 2 endometrioid adenocarcinoma arising from adenomyosis of the uterus: report of a case. Eur J Gynaecol Oncol. 2010;31:719–21. [PubMed] [Google Scholar]
- 36.Boes AS, Tousseyn T, Vandenput I, Timmerman D, Vergote I, Moerman P, et al. Pitfall in the diagnosis of endometrial cancer: case report of an endometrioid adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol Oncol. 2011;32:431–4. [PubMed] [Google Scholar]
- 37.Heo SH, Lee KH, Kim JW, Jeong YY. Unusual manifestation of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis of the uterus: emphasis on MRI and positron emission tomography CT findings. Br J Radiol. 2011;84:e210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori M, Furusawa A, Kino N, Uno M, Ozaki Y, Yasugi T. Rare case of endometrioid adenocarcinoma arising from cystic adenomyosis. J Obstet Gynaecol Res. 2015;41:324–8. [DOI] [PubMed] [Google Scholar]
- 39.Bae HS, Kim IS, Kang JS, Song JY. Endometrioid adenocarcinoma arising from adenomyosis after black cohosh with St John’s wort. J Obstet Gynaecol. 2014;34:213–4. [DOI] [PubMed] [Google Scholar]
- 40.Zhang SQ. Adenoacanthoma developing in adenomyosis uteri. Chin Med J Engl. 1992;105:343–6. [PubMed] [Google Scholar]
- 41.Olsen K An unusual case of adenocarcinoma-adenomyosis of the uterus with diffuse embolic lung metastases. Acta Obstet Gynecol Scand. 1955;34:269–72. [DOI] [PubMed] [Google Scholar]
- 42.Kawana K, Shirai T, Jimbo H, Yoshida M, Takahashi M, Shiromizu K, et al. Endometrial cytology in early diagnosis of adenocarcinoma arising from adenomyosis uteri. Acta Cytol. 2002;46:612–4. [PubMed] [Google Scholar]
- 43.Takeuchi K, Tateiwa H, Hamana S, Yoshida S, Kitazawa S, Maruo T. Invasive adenocarcinoma arising from adenomyosis in a septate uterus. Acta Obstet Gynecol Scand. 2006;85:1146–7. [DOI] [PubMed] [Google Scholar]
- 44.Goumenou AG, Arvanitis DA, Matalliotakis IM, Koumantakis EE, Spandidos DA. Loss of heterozygosity in adenomyosis on hMSH2, hMLH1, p16Ink4, and GALT loci. Int J Mol Med. 2000;6:667–71. [DOI] [PubMed] [Google Scholar]
- 45.Koike N, Tsunemi T, Uekuri C, Akasaka J, Ito F, Shigemitsu A, et al. Pathogenesis and malignant transformation of adenomyosis (review). Oncol Rep. 2013;29:861–7. [DOI] [PubMed] [Google Scholar]
- 46.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowery WJ, Schildkraut JM, Akushevich L, Bentley R, Marks JR, Huntsman D, et al. Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer. 2012;22:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chene G, Ouellet V, Rahimi K, Barres V, Provencher D, Mes-Masson AM. The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynaecol Obstet. 2015;130:27–30. [DOI] [PubMed] [Google Scholar]
- 49.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear-cell carcinoma. Science. 2010;330:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao W, Awadallah A, Xin W. Loss of ARID1A/BAF250a expression in ovarian endometriosis and clear cell carcinoma. Int J Clin Exp Pathol. 2012;5:642–50. [PMC free article] [PubMed] [Google Scholar]
- 51.Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci. 2013;14:18824–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozmik Z, Machon O, Kralova J, Kreslova J, Paces J, Vlcek C. Characterization of mammalian orthologues of the Drosophila osa gene: cDNA cloning, expression, chromosomal localization, and direct physical interaction with Brahma chromatin-remodeling complex. Genomics. 2001;73:140–8. [DOI] [PubMed] [Google Scholar]
- 53.Wiegand KC, Hennessy BT, Leung S, Wang Y, Ju Z, McGahren M, et al. A functional proteogenomic analysis of endometrioid and clear-cell carcinomas using reverse-phase protein array and mutation analysis: protein expression is histotype-specific and loss of ARID1A/BAF250a is associated with AKT phosphorylation. BMC Cancer. 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. PIK3CA mutations and loss of ARID1A protein expression are early events in the development of cystic ovarian clear-cell adenocarcinoma. Virchows Arch. 2012;460:77–87. [DOI] [PubMed] [Google Scholar]
- 55.Nagl NG Jr., Wang X, Patsialou A, Van Scoy M, Moran E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J. 2007;26:752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for antiestrogen resistance. J Biol Chem. 2001;276:9817–24. [DOI] [PubMed] [Google Scholar]
- 57.Taneichi A, Fujiwara H, Takahashi Y, Takei Y, Machida S, Saga Y, et al. Influences of uterine adenomyosis on muscle invasion and prognosis of endometrioid adenocarcinoma. Int J Gynecol Cancer. 2014;24:1429–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.