Abstract
Belonging to a social group is one of the most important factors contributing to well-being. The Belonging Regulation model proposes that humans possess a social monitoring system (SMS) that evaluates social inclusion and monitors belonging needs. Here, we utilized a prospective longitudinal design to examine links between peer victimization experienced across 7 years and social monitoring at the behavioral and neural level in adolescent girls (N= 38, Mage=15.43 years, SD=.33). Participants completed a social evaluation task during a functional magnetic resonance imaging (fMRI) scan. In whole-brain regression analyses, more severe peer victimization was associated with increased activation to in-group versus out-group peers in the amygdala, ventral striatum, fusiform gyrus, and temporoparietal junction. Moreover, participants who displayed increased activation in these regions reported higher levels of internalizing and externalizing symptoms. These results suggest that exposure to peer victimization across the school years is associated with heightened social monitoring at the neural level during adolescence, which has potential adverse implications for girls’ adjustment and well-being.
Keywords: fMRI, adolescence, peer victimization, social monitoring
Across all age groups, cultures, and societies, belonging to a social group is one of the most important factors contributing to physical and psychological health and well-being (Aboud, 2003; Brown, 1991; Dunbar, 2018; Dunham, Baron, & Carey, 2011; Dunham & Emory, 2014) and is proposed to be essential for survival (Baumeister & Leary, 1995). Indeed, social isolation increases risk for premature death as much as smoking, diabetes, or obesity (Holt-Lunstad, Smith, Baker, Harris, & Stephenson, 2015; House, Landis, & Umberson, 1988). It has therefore been proposed that social belonging is one of the most fundamental human needs (Baumeister & Leary, 1995; Cruwys, Haslam, Dingle, Haslam, & Jetten, 2014; Pickett & Gardner, 2005). The importance of belonging to a group becomes increasingly salient as youth enter adolescence, a time when individuals strive to achieve a sense of connection within a valued social group (Crockett, Losoff, & Petersen, 1984; Furman & Buhrmester, 1992; Hart & Fegley, 1995; Newman & Newman, 2001).
The Belonging Regulation Model
One promising theory that seeks to explain the processes supporting the human need to belong is the Belonging Regulation model (Gardner, Pickett, Jefferis, & Knowles, 2005). According to this model, all humans possess an innate Social Monitoring System (SMS) to regulate belonging needs (Gardner et al., 2005). The SMS assesses current levels of belonging need. When belonging levels are too low, the SMS produces a type of social hunger, such that increased need to belong leads to increased social monitoring, which involves increased attention, processing, and memory for social information. This leads to an intensified focus on opportunities for social interaction and belonging (Gardner et al., 2005). Similar to physical hunger directing attention towards food cues, social exclusion produces a social hunger that heightens attention, processing, and memory of social cues (Gardner, Pickett, & Brewer, 2000). Indeed, findings from behavioral research show that individuals who have been socially rejected demonstrate improved perspective taking skills (Knowles, 2014), greater ability to detect subtle social cues in vocal tone and facial expressions (Pickett, Gardner, & Knowles, 2004), show heightened nonverbal affiliative behaviors (Lakin, Chartrand, & Arkin., 2008), improved memory for social events about others (Hess & Pickett, 2010; Knowles, 2014), and are more cooperative and generous with group members (Maner, DeWall, Baumeister, & Schaller, 2007; Williams & Sommer, 1997), all of which are thought to reflect reinclusion strategies. Indeed, the final stage of the belonging regulation model is to use the information acquired through increased social monitoring to re-establish social inclusion (Gardner et al., 2005).
While the need to belong is important across the lifespan, it is particularly salient during adolescence, a developmental period marked by a strong need to affiliate with peers (Crockett, Losoff, & Petersen, 1984; Furman & Buhrmester, 1992; Hart & Fegley, 1995; Kroger, 2000; Newman & Newman, 2001; Tajfel & Turner, 1979). Because the need to belong is so strong in adolescence, adolescents are acutely aware of their peers’ behaviors and perceptions, even more so than adults and children. Compared to children and adults, adolescents display heightened neural, behavioral, and autonomic arousal when being observed by a peer (Somerville et al., 2013), are more likely to alter their behavior in the presence of peers (Gardner & Steinberg, 2005), and, following both acute and chronic peer rejection, are more likely to engage in risky behaviors (Peake, Dishion, Stormshak, Moore, & Pfeifer, 2013; Telzer, Miernicki, & Rudolph, 2018). Thus, due to their increased peer focus and elevated need to belong, adolescents may have a particularly strong SMS.
Importantly, salient group membership influences social monitoring, whereby individuals seek to fulfill their belonging needs by affiliating more with members of their own group (i.e., in-group) relative to disliked or even unknown others (i.e., out-group; Van Bavel, Swencionis, O’Connor, & Cunningham, 2012). According to social identity theory, individuals orient more towards in-group members, which contributes to their sense of self (Tajfel, Billig, Bundy, & Flament, 1971; Turner, 1982). Because in-groups are more likely to strengthen one’s sense of belonging, these types of group memberships are most likely to be activated after rejection (Knowles & Gardner, 2008). Consistent with social identity theory, individuals generally display increased social monitoring for in-group members. For instance, following an exclusion experience from an in-group, individuals show increased nonverbal affiliative behaviors with a subsequent in-group but not out-group partner, suggesting that people are selective in their reinclusion strategies (Lakin et al., 2008). Even in minimal group paradigms, where in-group and out-group distinctions are artificial, individuals display an in-group bias, including improved memory for in-group members (Bernstein, Young, & Hugenberg, 2007; Van Bavel et al., 2012); moreover, following social rejection from a minimally constructed in-group, individuals increase their social monitoring by demonstrating preferential recall for social information about peers (Gardner et al., 2000) and by working harder at a collective task (Williams & Sommer, 1997). Even just remembering a real past experience of social exclusion from one’s in-group can increase the entitativity (i.e., perceived meaningfulness of a group) and importance of in-groups but not out-groups (Knowles & Gardner, 2008). In each of these studies, rejection from an in-group increased social monitoring, presumably in an attempt to demonstrate commitment to the group in the hopes of improving their belonging. Ultimately, socially rejected individuals may use this greater awareness of social cues to satiate belonging needs with salient in-group members.
The Belonging Regulation System in the Brain
Within any regulatory system, there are biological mechanisms in place to monitor and regulate needs in order to maintain homeostasis. When an individual’s state of belonging is satisfactory, the system is in a state of equilibrium and can remain at rest. However, when an individual’s belonging level is low, the regulatory system becomes engaged in an attempt to restore adequate levels (Gardner et al., 2005). Although prior studies have not directly identified a neural SMS, research with adults and adolescents has identified several neural networks that may be involved in social monitoring given their role in processing group belonging, including regions involved in affective salience, social perception, and mentalizing.
Affective regions of the brain, including the amygdala, ventral striatum, and orbitofrontal cortex (OFC) are involved in detecting the salience of group membership in adults (Van Bavel, Packer, & Cunningham, 2008) and adolescence (Guassi Moreira, Van Bavel, & Telzer, 2017). The amygdala tracks developmental changes in the salience of social identities, including race (Telzer et al., 2013), gender (Telzer et al., 2015), and novel in-groups (Guassi Moreira, Van Bavel, & Telzer, 2017). The ventral striatum and OFC track the subjective value of important social groups and tend to be activated when favoring in-group over out-group members (Telzer, Ichien, & Qu, 2015), which increases from childhood to adolescence (Guassi Moreira, Van Bavel, & Telzer, 2017). These developmental increases in activation to in-groups coincide with adolescents’ strong need to affiliate with peers (Crockett, Losoff, & Petersen, 1984; Furman & Buhrmester, 1992; Hart & Fegley, 1995; Kroger, 2000; Newman & Newman, 2001; Tajfel & Turner, 1979), underscoring the salience of group belonging in adolescence.
In addition, social perception regions, such as the fusiform gyrus, facilitate deeper perceptual encoding of in-group faces in adults (Van Bavel et al., 2008). Moreover, adolescents show heightened fusiform activation when receiving positive feedback from peers (Guyer et al., 2012), and there are linear increases in fusiform activation to in-group relative to out-group faces from childhood to adolescence (Guassi Moreira et al., 2017). These studies suggest that group belonging facilitates deeper perceptual processing of in-group faces across development, a process involving the fusiform.
Finally, regions supporting mentalizing (e.g., temporoparietal junction [TPJ], posterior superior temporal sulcus [pSTS], and dorsal medial prefrontal cortex [dmPFC]) may promote attention to in-group peers. When monitoring a social environment, individuals anticipate and infer the intentions of others. Especially in a context in which group belonging and a shared group identity are emphasized, adolescents may focus on inferring the mental states of in-group peers. Neural regions involved in mentalizing show developmental changes in activation across development (Blakemore, 2010; Burnett, Bird, Moll, Frith, & Blakemore, 2009; Gweon, Dodell- Feder, Bedny, & Saxe, 2012; van den Bos, van Dijk, Westenberg, Rombouts, & Crone, 2011). For example, adolescents display greater TPJ activation during social perspective taking than children, with increased activation correlating with increased sensitivity to another’s perspective (van den Bos et al., 2011). Moreover, adolescents (Guassi Moreira et al., 2017) and adults (Van Bavel et al., 2008; Van Bavel et al., 2011) who have stronger in-group biases show heightened activation to in-group relative to out-group members in regions involved in mentalizing (e.g., TPJ, pSTS), with such activation increasing from childhood to adolescence (Guassi Moreira et al., 2017). Together, this collection of research underscores adolescence as a key developmental period during which neural regions involved in affective salience, social perception, and mentalizing may be particularly sensitive, highlighting several candidate neural regions involved in the SMS.
Peer Victimization and Social Hunger
The SMS is an adaptive mechanism for satisfying the need to belong. When belonging levels are too low, the system will activate to guide information processing in an effort to regain social connection (Pickett et al., 2004). In the short-term, this system may motivate individuals to maintain healthy social bonds. However, frequent activation of this system will result in unsatiated social hunger, or a socially starved individual (Pickett et al., 2004). As such, the SMS may become maladaptive when belonging needs remain unfulfilled (Gardner et al., 2000). Belonging needs are likely unmet among youth who are exposed to frequent or severe victimization by their peers (i.e., being the recipient of peers’ physical, verbal, or psychological threats and aggression). Victimized youth show heightened sensitivity to social threat (Taylor, Sullivan, & Kliewer, 2013) and are more concerned about being negatively socially evaluated (Storch, Nock, Masia-Warner, & Barlas, 2003) and becoming socially isolated (Hunter & Boyle, 2004), which may intensify their motivation toward group belonging. Indeed, chronically victimized adolescents report a greater threat to their need to belong after an acute exclusion experience then do nonvictimized youth (Rudolph, Miernicki, Troop-Gordon, Davis, & Telzer, 2016). A history of exposure to peer victimization may therefore lower the threshold for activation of the SMS.
While victimized youth may display increased attention to social information and cues about their in-group, they may lack the social skills and social resources to correctly interpret these social cues or effectively use this information to establish social inclusion. Indeed, victimized youth struggle with many processes that enable individuals to turn social information into effective social action. For instance, while victimized youth generally correctly perceive their own victim status (Bellmore & Cillessen, 2006; Prinstein, Cheah, & Guyer, 2005), they tend to perform worse than non-victimized children on perspective-taking tasks (Gasser & Keller, 2009; Gini, 2006), often do not interpret social cues correctly (Ziv, Leibovich, & Shechtman, 2013), and have lower quality friendships, potentially because of less sophisticated social reasoning (Parker & Asher, 1993), poorer conflict resolution skills (Champion, Vernberg, & Shipman, 2003), or over-disclosure that might put them at risk for future victimization (Holt & Espelage, 2007). In addition, youth who are victimized may misperceive group membership, orienting to their peers who do not reciprocate in-group belonging, which may elicit more peer victimization. Indeed, victimized youth tend to have fewer reciprocal friends (Scholte, Overbeek, Ten Brink, Rommes, De Kemp, et al., 2009).
Although being tuned to social information is an important social skill, hyper-attunement to social cues may be maladaptive. Indeed, adolescents who show very low or very high attunement to social cues (measured at the behavioral and neural level) show poor decision-making skills, whereas moderate levels of social sensitivity are adaptive (van Hoorn et al., 2018). Thus, moderate social sensitivity is crucial for competently interacting with others and engaging in flexible social behavior, whereas too much social sensitivity may be maladaptive, as it hinders effectively navigating the social world. For instance, socially anxious individuals are hyper- attentive to social cues and misinterpret affiliative social signals, hampering their social reintegration and resulting in distress and impaired social functioning in daily life (see Gilboa-Schechtman & Shachar-Lavie, 2013). Therefore, victimized youth may have difficulty translating their increased social monitoring into effective re-inclusion strategies, which may place them at risk for maladjustment. Although some research suggests victimized youth show altered processing of socially threatening information (e.g., Rosen, Milich, & Harris, 2007), to our knowledge, no prior research has expressly examined victimized youth’s attention to, or memory of, social information in non-threatening situations. We would expect that peer victimized youth’s pattern of belonging regulation would mirror that of lonely individuals, who struggle to translate heightened social monitoring into actual opportunities for positive social interaction (Gardner et al., 2005).
Current Study
In the present study, we used a minimal group paradigm to examine the association between a history of exposure to peer victimization and heightened social monitoring at the behavioral and neural levels in adolescent girls. In minimal group designs, individuals are assigned to an arbitrary group (Tajfel, Billig, Bundy, & Flament, 1971). Even when such groups are based on random assignment and group members do not meet each other, both children (Dunham et al., 2011) and adults (Ashburn-Nardo, Voils, & Monteith, 2001; Tajfel et al., 1971) develop strong in-group favoritism, highlighting how readily people identify with social in-groups. Minimal group designs are optimal for well-controlled neuroimaging studies, because familiarity with the in- and out-group members is matched, and group membership is not based on a history of learned associations. In the current study, we randomly assigned participants to the red or blue team. After being exposed to pictures of 20 in-group and 20 out-group peers during training, participants completed an fMRI scan during which they rated how much they liked/disliked each peer, and then completed a memory test following the scan (see Figure 1). We utilized a longitudinal design, in which exposure to peer victimization was prospectively assessed across 2nd through 8th grade, providing a robust measure of peer victimization across mid childhood through adolescence that is not affected by recall biases. We examined whether experiencing more severe peer victimization across seven years is associated with heightened social monitoring in adolescent girls.
Figure 1.
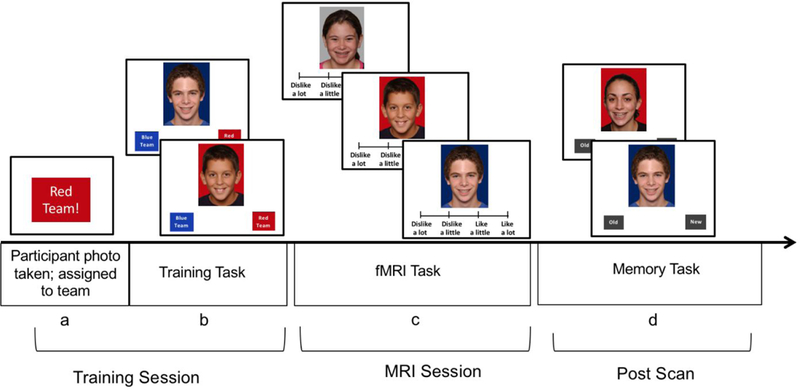
Task Design. (a) A photo of the participant was taken and a computer randomly assigned them to a team (Red Team or Blue Team). (b) Participants completed a training task, in which they saw all 40 peers and indicate whether each peer was on the red team or blue team. (c) During an fMRI scan, participants rated their peers, 20 in-group, 20 out-group, and 20 unaffiliated peers. (d) After the scan, participants completed a memory task, in which they indicated whether each face is a new or old peer.
Motivational accounts of social identity suggest that individuals will experience differential motivation to encode relevant targets that belong to a social group as a means of social affiliation (Van Bavel et al., 2012). Thus, we predicted that higher levels of victimization would be associated with stronger engagement in social monitoring of in-group relative to out-group peers. Reflecting this stronger in-group orientation, and consistent with prior work in adults (Van Bavel et al., 2012; Van Bavel & Cunningham, 2012), we hypothesized that more severe peer victimization would be associated with behavioral biases favoring the in-group, including implicit biases (i.e., slower reaction time to rating in-group relative to out-group peers, representing longer processing time and selective allocation of attention to in-group peers; improved memory for in-group relative to out-group peers, reflecting greater encoding of the in-group) and explicit biases (i.e., preferential liking of in-group relative to out-group peers; greater self-reported collective belonging to in-group). Indeed, prior research with adolescents and adults has shown that individuals report greater preferential liking of minimal in-group peers (Brewer, 1979; Van Bavel et al., 2008; Guassi Moreira et al., 2017), adults show longer response time biases when encoding in-group relative to out-group members, which relates to greater memory bias for in-group peers (Van Bavel & Cunningham, 2012), and adults exposed to social rejection show greater memory for in-group relative to out-group peers (Van Bavel et al., 2012). At the neural level, based on prior studies using a similar task in youth (Guassi Moreira et al., 2017) and adults (Van Bavel et al., 2008; Van Bavel et al., 2011), we predicted that more severe peer victimization would be associated with neural biases, including greater activation to in-group relative to out-group peers in regions that code for affective salience (e.g., amygdala, ventral striatum), social perception (e.g., fusiform gyrus), and mentalizing (e.g., TPJ, pSTS, dmPFC).
We also conducted follow-up analyses as a way to validate our neural results. The Belonging Regulation model proposes that feelings of low self-esteem alert the SMS that in-group belonging levels have dropped too low (Leary, 1999). Further, excessive social monitoring suggests a need to belong that is not being met (Gardner et al., 2005; Slavich, Donovan, Epel, & Kemeny, 2010), which is linked to internalizing and externalizing symptoms (Donnellan, Trzesniewski, Robins, Moffitt, & Caspi, 2005; Haney & Durlak, 1998; Orth, Robins, Trzesniewski, Maes, & Schmitt, 2009). If indeed, patterns of neural activation linked to peer victimization are indicative of an over-active neural SMS, low self-esteem should be associated with heightened social monitoring at the neural level, and heightened neural processing should be linked with higher levels of internalizing and externalizing symptoms.
We focused on adolescent girls given their heightened need to belong relative to males, which is a stronger predictor of self-esteem, internalizing symptoms, and externalizing symptoms in girls than in boys (see Leibovich, Schmid, & Calero, 2018; Newman, Lohman, & Newman, 2007). Moreover, adolescent girls’ show greater endorsement of connection-oriented goals in relationships, increased sensitivity to peer evaluation, and heightened reactivity to interpersonal stress relative to adolescent boys (La Greca & Lopez, 1998; Rose & Rudolph, 2006; Telzer & Fuligni, 2013). Girls also show heightened sensitivity to social threats that peak in adolescence, as evidenced by greater activation in affective regions of the brain during peer evaluation (Guyer et al., 2009, 2012) and heightened cortisol peaks following social rejection challenges (Stroud, Salovey, & Epel, 2002). Thus, we expected that the processes of interest would be particularly relevant for adolescent girls.
Method
Participants and Procedures
Thirty-eight 9th grade adolescent girls (Mage=15.43 years, SD=.33 range=14.90–16.34 years; see Table 1 for demographics) were recruited from a longitudinal study that tracked 636 youth from 2nd-8th grade. Of the 636 participants, 115 were identified as eligible to participate based on being female and meeting criteria for low or high peer victimization across the seven years. Of these, 8 had contraindications for MRI; 6 were not interested in participating; 51 were not recruited because they had moved out of town, were not reachable, or we reached our target sample size of 50 prior to their recruitment; an additional 12 girls participated but were not included in the current manuscript due to technical problems with the task during the scan (n=6) or non-compliance on the task (i.e., not responding, pressing the incorrect buttons; n=61). Based on participants’ annual reports of peer victimization scores across the seven years, we recruited adolescent girls who had been chronically victimized (N=21) or non-victimized (N=17). Selection criteria for victimization was based on scoring ≥ .75 SD above or below the mean on victimization for at least three years, at least one of which was in middle school. We selected .75 standard deviations to distinguish girls who showed fairly extreme deviations from the mean but would still provide a large enough sample to select from as well as variability in victimization experiences. Chronically victimized girls scored ≥ .75 SD above the mean on victimization for at least three years (range = 3 to 7 years), with an average of 1.22 standard deviations above the mean across the 7 years (SD=.46). Non-victimized girls scored ≤ .75 below the mean on victimization for at least three years (range = 3 to 7 years) with an average of .78 standard deviations below the mean across the 7 years (SD=.15). Parents provided written consent and adolescents provided written assent in accordance with the University’s Institutional Review Board. Participants were given a minimal-group assignment and then completed a social-evaluation task during an fMRI scan. Following the scan, they completed a memory task. Participants completed questionnaires assessing internalizing and externalizing symptoms at the time of the scan as well as three, six, and nine months following the scan. Thirty-five participants (92.1%) completed all four assessments, two participants completed three of the assessments, and one participant only completed the assessment at the time of the scan. We used a composite score of their internalizing and externalizing symptoms, averaging across the four waves, and thus did not have any missing data. The number of waves participants completed the assessments was included as a control in analyses examining associations with symptoms.
Table 1.
Family demographics.
| Variable | N (%) |
|---|---|
| Race | |
| White/Caucasian | 26 (68.4) |
| Black/African American | 10 (26.3) |
| Asian | 1 (2.6) |
| Hispanic/Latina | 1 (2.6) |
| Female Caregiver’s Highest Education | |
| Completed some high school | 1 (2.6) |
| High School Diploma | 4 (10.5) |
| Completed some college | 15 (39.5) |
| Associate’s degree | 2 (5.3) |
| Bachelor’s degree | 8 (21.1) |
| Master’s Degree | 8 (21.1) |
| Male Caregiver’s Highest Education | |
| Completed some high school | 1 (2.6) |
| High School Diploma | 6 (15.8) |
| Completed some college | 7 (18.4) |
| Associate’s degree | 2 (5.3) |
| Bachelor’s degree | 7 (18.4) |
| Master’s Degree | 4 (10.5) |
| Family Income | |
| $0–14,999 | 6 (15.8) |
| $15,000–29,999 | 8 (21.1) |
| $30,000–44,999 | 1 (2.6) |
| $45,000–59,999 | 2 (5.3) |
| $60,000–74,999 | 2 (5.3) |
| $75,000–89,999 | 6 (15.8) |
| $90,000+ | 13 (34.2) |
| Parents’ Marital Status | |
| Married | 20 (52.6) |
| Separated or divorced | 5 (13.2) |
| Never married | 10 (26.3) |
| Other | 2 (5.2) |
Note. Percentages are based on the full sample (N=38). Columns that do not sum to 100% represent missing data
Self-Report Measures
Peer victimization.
Each year from the 2nd through 8th grades, youth reported on their victimization experiences using the Social Experiences Questionnaire-Revised (Rudolph et al., 2014; Rudolph, Troop-Gordon, Hessel, & Schmidt, 2011). The measure taps overt victimization (i.e., being the target of behaviors intended to harm others through physical damage, threat of such damage, or verbal aggression; 11 items; e.g., “How often do you get hit by another kid?” “How often does another kid insult you or put you down?”) and relational victimization (i.e., being the target of behaviors intended to harm others through manipulation of relationships; 10 items; e.g., “How often does another kid say they won’t like you unless you do what they want you to do?”). Using a 5-point scale, participants indicated how often they experienced each type of victimization. Scores were computed as the mean of the 21 items. The scale had high internal reliability across all seven waves (αs=.95-.98). The correlations between consecutive waves ranged from rs = .35-.77. For instance, the correlation between grade 5 and grade 6 (i.e., pre and post middle school transition) was r=.77, p<.0001. The correlation between grade 4 and grade 5 was r=.69, p<.0001. These correlations suggest stability in victimization across grades, with stability across the middle school transition similar to stability prior to the transition. To capture individual variability in exposure to victimization, we computed a continuous index of victimization severity, reflecting the mean level across the seven waves.
Social Self-Esteem.
Each year from the 2nd through 7th grades, youth completed the negative self-perceptions subscale of the Perceptions of Peers and Self Questionnaire (Caldwell, Rudolph, Troop-Gordon, & Kim, 2004), which assesses low self-esteem in the context of relationships (7 items; e.g., “It’s a waste of other kids’ time to be friends with me.”). For each item, youth checked a box indicating how true each statement was on a 4-point scale. This measure shows strong internal consistency, test-retest reliability, and convergent and predictive validity (Caldwell et al., 2004). In the current study, the scale had good internal reliability across waves (αs=.70-.81). We formed a composite variable of low social self-esteem by averaging this measure across the six waves, where higher scores indicate lower social self-esteem.
Internalizing symptoms.
At the time of the scan and three, six, and nine months following the scan, participants completed two measures of internalizing symptoms. First, youth completed the Short Mood and Feelings Questionnaire (Angold, Costello, Messer, & Pickles, 1995) to assess depressive symptoms (e.g., “I felt unhappy or miserable.”). Youth responded to 12 items to indicate how much they experienced each symptom over the past two weeks on a four-point scale. Across the four waves, the scale had high internal reliability (αs=.93-.96). Second, youth completed the Revised Child Manifest Anxiety Scale (Reynolds & Richmond, 1978), a 28-item measure that taps general anxiety over the past two weeks. Participants responded “yes” or “no” to each item (e.g., “I worry about what is going to happen.” “I am nervous.”). Across the four waves, the scale had high internal reliability (αs=.93-.95). The correlations between consecutive waves ranged from rs = .65-.80 for depression and rs = .84-.86 for anxiety . Moreover, depression and anxiety were highly correlated within (rs =.77-.85) and between waves (rs = .69-.86). Given these strong correlations and our similar predictions regarding the association between heightened social monitoring and both types of internalizing symptoms, we formed a composite variable of internalizing symptoms by standardizing and averaging depression and anxiety at each time point and then averaging this index across the four waves. This index provides a more robust measure of internalizing symptoms that captures the stability of internalizing symptoms across the 9 months following the fMRI scan.
Externalizing symptoms.
At the time of the scan and three, six, and nine months following the scan, participants completed a measure of their externalizing symptoms using an antisocial behavior questionnaire adapted from Nolen-Hoeksema and colleagues (Nolen-Hoeksema, Stice, Wade, & Bohon, 2007). Participants completed 13 items using a 5-point scale to indicate how much each item described them (e.g., “I stole things.” “I cut classes or skipped school.” “I hung around with kids who get in trouble.”). The scale had high internal reliability across the 4 waves (αs=.90-.93). The correlations between consecutive waves ranged from rs = .75-.91. Again, we formed a composite variable of follow-up externalizing symptoms by averaging this index across the four waves.
Minimal Group Task
Establishment of minimal group.
Participants arrived at the imaging center and posed for a digital photograph. Participants were then assigned to the red team or blue team, which was randomly selected by a computer (Figure 1a) and were instructed that they would be a part of this team for the duration of the study (Bernstein et al., 2007; Van Bavel & Cunningham, 2009; Van Bavel et al., 2012). Participants were told they could win points for their team to earn a prize as part of another task published previously (Telzer et al., 2018). Next, participants were shown pictures of in-group and out-group team members (totaling 40 peers), who were described as participants who had already completed the study. Each face was displayed in random order, one at a time. Two labels appeared at the bottom of the screen indicating “red team” and “blue team”, and participants were instructed to press one of two buttons to indicate the correct team of each peer (Figure 1b). Photos were placed on blue or red backgrounds to provide a visual cue to team membership. Participants also saw their own face two times on the colored background and categorized themselves into the appropriate team in order to enhance their in-group identification (Van Bavel et al., 2008). The next trial proceeded after participants pressed a button.
The face stimuli included equal numbers of males and females and were racially and ethnically diverse. All faces were looking into the camera and smiling. Faces were taken from several databases, including the National Institute of Mental Health Child Emotional Faces Picture Set (NIMH-ChEFS (Egger et al., 2011)), as well as internal pictures collected from prior studies. Faces were randomly assigned to the teams ensuring equal representation of race, gender, and age across the teams, and assignment was fully counterbalanced so that participants were equally likely to see each face as an in-group or out-group member. This ensured that any visual differences in the stimuli (e.g., attractiveness, luminance) could not account for observed differences between in-group and out-group members.
Social evaluation task.
After completing the learning task, participants were placed in the scanner and completed a social evaluation task. During the task, participants were presented with 60 faces: 20 in-group faces, 20 out-group faces, and 20 novel faces of individuals who were unaffiliated with the in-group or out-group (Van Bavel et al., 2011). The in-group and out-group faces were identical to those seen during the learning task and the unaffiliated peers had not been seen previously. The in-group and out-group faces were presented on their respective colored backgrounds, and the unaffiliated faces were presented on grey backgrounds. For each trial of the task, participants were instructed to indicate how much they like or dislike each person (Figure 1c). Participants pressed one of four buttons to indicate their response (1=dislike a lot, 2=dislike a little, 3=like a little, 4=like a lot). Each face was presented for 3 seconds with an inter-trial interval that was jittered randomly between 1.5 to 3 seconds.
We calculated a preferential bias score by subtracting the mean ratings for in-group faces minus out-group faces, such that higher scores indicate preferential biases for in-group peers. We also calculated a response time bias score by calculating the mean response time (MRT) for rating in-group faces minus out-group faces.
Memory task.
After completing the scan, participants were tested on their memory of the faces. They were presented with 40 faces, half of which were new faces and half of which were old faces (i.e., seen during the learning task and social evaluation task; balanced between in- and out-group members), all of which were displayed on blue and red backgrounds (Van Bavel et al., 2011). Participants indicated whether the face was old or new (Figure 1d). Faces were presented in random order. Memory was relatively high for all 3 groups (% hit: in-group 86% (range 65%- 100%; out-group 85.25% (range 50%−100%); new faces 87.15% (range 35% −100%)). We calculated a memory bias score, which represented the percent of hits (i.e., correctly remembered faces) for in-group minus percent of hits for out-group, such that higher scores indicate a memory bias towards in-group faces.
Collective group identity.
Finally, participants responded to questions indicating their collective group identity using items commonly used in the social identity literature (Ashmore, Deaux, & McLaughlin-Volpe, 2004). For both the red and blue team, participants indicated (1) whether they value being a member of the team, (2) whether they are proud of being a member of the team, and (3) being a member of the team is important to their identity (1=strongly disagree to 6=strongly agree). As done in other work (Van Bavel et al., 2012; Do et al., 2019; Guassi Moreira et al., 2017), we took the average of these three items to create an index for in-group and out-group identity and calculated a group identity bias score by subtracting the mean ratings for in-group identity minus out-group identity, such that higher scores indicate higher in-group identity.
Summary of behavioral bias scores.
We calculated four bias scores in the current study, which represented different ways of examining biases towards in-group relative to out-group peers. Preferential Liking Bias represents the mean ratings for in-group faces minus out-group faces (calculated from the social evaluation task). Response Time Bias represents the MRT for rating in-group faces minus out-group faces (calculated from the social evaluation task). Memory Bias represents the percent of faces correctly remembered for in-group faces minus out-group faces (calculated in the post-scan memory task). Group Identity Bias represents the mean ratings for in-group minus out-group identity (calculated in the post-scan questionnaire). Two of these measures (Preferential Liking Bias and Group Identity Bias) represent explicit biases, and two of these measures (Response Time Bias and Memory Bias) represent implicit biases. .
fMRI Data Acquisition and Analysis
fMRI data acquisition.
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. The task included T2*-weighted echoplanar images (EPI) [slice thickness=3 mm; 38 slices; TR=2sec; TE=25msec; matrix=92×92; F0V=230 mm; voxel size 2.5×2.5×3mm3]. Structural scans consisted of a T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR=4sec; TE=64msec; F0V=230; matrix=192×192; slice thickness=3mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR=1.9sec; TE=2.3msec; F0V=230; matrix=256×256; sagittal plane; slice thickness=1mm; 192 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
fMRI Data Preprocessing and Analysis.
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant’s images included spatial realignment to correct for head motion (no participant exceeded 2mm of maximum image-to-image motion in any direction). The realigned functional data were coregistered to the high resolution MPRAGE, which was then segmented into cerebrospinal fluid, grey matter, and white matter. The normalization transformation matrix from the segmentation step was then applied to the functional and T2 structural images, thus transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8mm Gaussian kernel, full-width-at-half maximum, to increase the signal-to-noise ratio.
Statistical analyses were performed using the general linear model in SPM8. Each trial was convolved with the canonical hemodynamic response function. High-pass temporal filtering with a cutoff of 128 seconds was applied to remove low-frequency drift in the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1. In each participant’s fixed-effects analysis, a general linear model (GLM) was created with 3 regressors of interest, modeled as events: in-group faces, out-group faces, and unaffiliated faces. Null events, consisting of the jittered inter-trial intervals, were not explicitly modeled and therefore constituted an implicit baseline. The parameter estimates resulting from the GLM were used to create linear contrast images. Our primary contrast of interest in the current study was In-group>Out-group.
Random effects, group-level analyses were performed on all individual subject contrasts using GLMFlex. GLMFlex corrects for variance-covariance inequality, partitions error terms, removes outliers and sudden activation changes in the brain, and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLMFlex). We conducted whole brain regression analyses with continuous scores of victimization entered as the regressor to examine neural regions that showed increased activation as a function of peer victimization when rating in-group relative to out-group peers. In order to examine brain-behavior relationships, we extracted parameter estimates of signal intensity from the clusters of activation that correlated with peer victimization and ran correlation analyses in SPSS with behavioral biases (Preferential Liking Bias, Reaction Time Bias, Memory Bias, and Group Identity Bias) and adjustment (social self-esteem, internalizing and externalizing symptoms).
To correct for multiple comparisons, we conducted a Monte Carlo simulation implemented using 3dClustSim in the software package AFNI (Ward, 2000) and the -acf option in 3dFWHMx to estimate the smoothness. Simulations were run separately for each analysis. Results of the simulation for the whole-brain regression with peer victimization on the contrast in-group>out-group yielded a voxel-wise threshold of p<.001 combined with a minimum cluster size of 32 voxels for the whole brain, corresponding to p<.05, False Wise Error (FWE) corrected. Because the ventral striatum and amygdala are anatomically small structures, and we had a priori hypotheses about their involvement in monitoring social inclusion, we used a small volume correction by conducting a Monte Carlo simulation using anatomically defined regions for the amygdala and ventral striatum, which yield a voxel-wise threshold of p<.001 combined with a minimum cluster size of 5 voxels for the amygdala and ventral striatum, corresponding to p<.05, small volume corrected. All fMRI analyses presented in the results are available on Neurovault (see https://neurovault.org/collections/5959/)
Results
Peer Victimization and Behavioral Correlates of In-group Bias
We first examined the hypothesis that peer victimization would be associated with in-group bias at the behavioral level. To this end, we ran four separate correlations, with peer victimization correlating with each behavioral bias score. Higher levels of peer victimization correlated with the two implicit bias scores (Table 2). In particular, peer victimization was associated with response time bias, such that girls exposed to more victimization took longer to rate in-group relative to out-group peers during the social evaluation task, suggesting that victimization is associated with longer processing time to rate in-group peers. Peer victimization was also associated with a greater memory bias, such that girls exposed to more victimization were more likely to accurately remember in-group relative to out-group faces, suggesting greater encoding of in-group peers during the fMRI task. Peer victimization was not associated with the explicit bias scores. For descriptives and correlations between all study variables, see Table 2.
Table 2.
means, standard deviations, and Correlations between all study variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Peer Victimization | 1 | |||||||
| 2. Preferential Liking Bias | −.13 | 1 | ||||||
| 3. Response Time Bias | .35 | −.23 | 1 | |||||
| 4. Memory Bias | .37* | .23 | .03 | 1 | ||||
| 5. Group Identity Bias | .01 | .23 | .22 | −.09 | 1 | |||
| 6. Internalizing | .66 | −.22 | .36* | .22 | −.02 | 1 | ||
| 7. Externalizing | 64*** | −.22 | .27 | .25 | −.03 | .83*** | 1 | |
| 8. Low Social Self-Esteem | 74*** | −.28 | .35* | .28 | .12 | .68*** | .58*** | 1 |
| Mean | 2.05 | .09 a | −8.49 a | .14 a | 2.36b | −.09 | 1.49 | 1.81 |
| (SD) | (.69) | (.48) | (123.65) | (1.72) | (168) | (.90) | (.63) | (.47) |
Note.
p<.001,
p<.05,
p<.10.
Peer victimization represents average victimization across the 7 years prior to the scan. Preferential Liking Bias represents the mean ratings for in-group faces minus out-group faces. Response Time Bias represents the mean RT for rating in-group faces minus out-group faces. Memory bias represents the percent of faces correctly remembered for in-group faces minus out-group faces during the post-scan memory task. Group Identity Bias represents the mean ratings for in-group minus out-group identity. Internalizing and Externalizing represent the average symptoms at the time of the scan, 3-, 6-, and 9-months following the scan. Social self-esteem represents the average social self-esteem across grades 2 through 7.
One-sample t-tests relative to 0 indicates no significant difference, suggesting that preferential liking bias, response time bias, and memory bias are not different for the in-group relative to the out-group.
One-sample t-tests relative to 0 indicates significant difference, suggesting that group identity is higher for the in-group than out-group.
Peer Victimization and Neural Correlates of In-group Bias
Before examining how victimization correlates with neural activation, we conducted a whole-brain t-test to examine the main effect for the contrast in-group>out-group (and vice versa). No regions were significantly activated. Next, in whole-brain regression analyses, we regressed adolescents’ mean victimization across the 7 years onto neural activation for the contrast in-group>out-group. As hypothesized, a history of greater exposure to peer victimization was associated with heightened activation to in-group relative to out-group peers in the amygdala, ventral striatum, fusiform gyrus, and TPJ (see Table 3, Figure 2). Peer victimization was not associated with greater activation to out-group peers in any region.
Table 3.
Neural regions that correlate with peer victimization when rating In-group>Out-group peers
| Region Label | k | t | x | y | z |
|---|---|---|---|---|---|
| L TPJ | 56 | 5.46 | −51 | −37 | 16 |
| R Fusiform Gyrus | 45 | 5.12 | 45 | −28 | −26 |
| L Ventral Striatum | 20 | 3.82 | −15 | 5 | −2 |
| L Amygdala | 22 | 4.36 | −27 | 2 | −18 |
| R Lingual Gyrus | 71 | 4.69 | 6 | −52 | −14 |
Note: L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation in each cluster; x, y, and z refer to MNI coordinates. No regions correlated negatively with peer victimization for this contrast.
Figure 2.
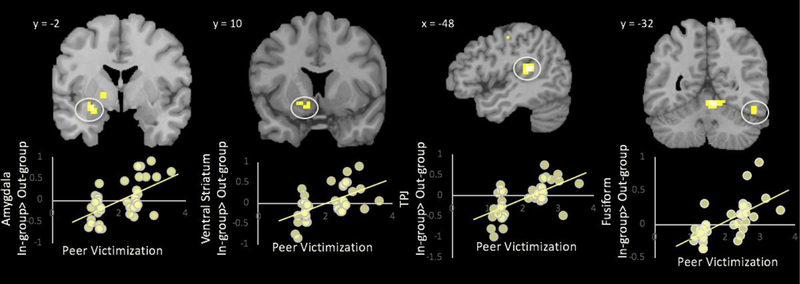
Peer victimization correlated with activation in the amygdala, ventral striatum, TPJ, and fusiform to in-group>out-group peers. For descriptive purposes, parameter estimates of signal intensity were extracted from each region and plotted with peer victimization.
Brain-Behavior Bias Correlations
To elucidate the implications of heightened neural activation to in-group peers, we conducted analyses to examine (1) whether there was a correlation between neural and behavioral biases for in-group versus out-group faces, and (2) whether there was a correlation between neural biases, social self-esteem, and maladjustment. To this end, we created functional ROIs from the brain regions that showed a significant correlation with victimization. From the in-group>out-group contrast, we extracted parameter estimates of signal intensity from the functional ROIs and examined correlations with the behavioral bias measures and with the self-esteem, internalizing, and externalizing self-report measures.
Behavioral correlates.
Activation in the ventral striatum (r=.33, p<.05), TPJ (r=.46, p<.005), and amygdala (r=.35, p<.05) was significantly associated with a greater memory bias for in-group faces. These results indicate that participants with greater in-group bias at the neural level have greater in-group memory bias. Activation in the fusiform was significantly associated with slower RT to in-group relative to out-group peers (r=.33, p<.05). These results suggest that the fusiform may be involved in deeper encoding of in-group faces, resulting in slowing down and processing those faces in more depth. Neural activation was not associated with ratings of liking in-group relative to out-group members or collective group identity.
Self-esteem and adjustment correlates.
Heightened activation in the amygdala, ventral striatum, fusiform, and TPJ to in-group relative to out-group peers was associated with lower social self-esteem across the school years and with elevated internalizing and externalizing symptoms across the 9-month follow-up (see Table 4).
Table 4.
Correlations between Neural Biases to In-group>Out-group and Social Self-Esteem, Internalizing and Externalizing Symptoms
| Variable | Low Social Self-Esteem |
Internalizing Symptoms1 |
Externalizing Symptoms |
|---|---|---|---|
| Amygdala | 42 | .34* | .40* |
| Ventral Striatum | .38 | .44 | .54*** |
| TPJ | .46*** | .49*** | .51*** |
| Fusiform | .42** | .41* | .56*** |
We ran separate analyses with anxiety and depression, each of which were nearly identically correlated with neural activation ***p<. 005, **p < .01, -*p< .05; corrections for multiple comparisons (i.e., correlations with 4 brain regions) required a statistical threshold of p < .0125.
Discussion
Belonging to a social group is one of the most fundamental human needs (Baumeister & Leary, 1995; Dunbar, 2018). When belonging levels are too low, the SMS produces social hunger, leading to increased social monitoring (Gardner et al., 2005). Our study is the first to demonstrate that exposure to peer victimization across the school years is associated with social monitoring at the behavioral and neural level during adolescence. By measuring prospective reports of victimization across 7 years, we were able to capture a rich measure of peer victimization experiences that was not confounded by recall biases. We found that a history of more severe victimization in adolescent girls was associated with longer response times for rating in-group peers, better memory for in-group peers, and increased activation to in-group peers in neural regions associated with affective processing, social perception, and mentalizing. Such heightened social monitoring may have implications for youths’ adjustment.
Behavioral Correlates of Social Monitoring
The primary goal of the SMS is to attune individuals to information that will help them navigate their social environment in order to fulfill their belonging needs (Pickett & Gardner, 2005). Social exclusion is thought to produce a social hunger that heightens attention to and memory of social cues (Gardner, Pickett, & Brewer, 2000). Motivational accounts of social identity suggest that individuals will experience differential motivation to encode relevant targets that belong to a social group as a means of social affiliation (Van Bavel et al., 2012). Such social motives will influence attention and social memory. Consistent with this model, prior behavioral studies in adults have shown that experimentally manipulated rejection increases social monitoring behaviorally (Gardner et al., 2000; Knowles, 2014; Van Bavel et al., 2012), chronic loneliness increases social monitoring (Gardner et al., 2005; Pickett et al., 2004), and high trait level need to belong increases encoding of, and memory biases for, in-group relative to out-group peers (Van Bavel et al., 2012).
The current study builds upon prior work in adults by demonstrating that a history of more severe peer victimization throughout childhood is associated with selective, in-group focused social monitoring in adolescence, including heightened memory biases for and slower reaction time to rating in-group peers. Heightened social memory and slower reaction time to in-group peers may reflect selective allocation of attention to in-group peers, whereby individuals slow down to encode and process in-group peers in more depth. Although we suggest that longer response time to rating in-groups represents differential motivation to encode salient group members (Van Bavel et al., 2012), it is also possible that faster response times to rating out-group peers indicates biased or vigilant attention to the out-group. Indeed, adolescents demonstrate a response time bias, such that they show faster reaction times for predicting that their peers will dislike them versus like them (Rodman et al, 2017). Such faster response times are interpreted to reflect biased expectations of rejection in adolescence. Interestingly, we did not find associations between peer victimization and explicit biases, and analyses focusing on mean level behavioral biases across the whole sample showed that only group identity bias was significantly higher for in-group relative to out-group peers. This is inconsistent with prior behavioral findings in adults and youth, which revealed significantly higher preferential liking biases for minimal in-group peers (Van Bavel et al., 2008; Guassi Moreira et al., 2017).
Neural Correlates of Social Monitoring
At the main effect level, we did not find significant differences in neural activation when processing in-group relative to out-group peers. Prior research in children and adolescents has also shown no meaningful mean level differences in activation to this contrast (Guassi Moreira et al., 2017). This is likely due to strong individual differences in the salience of group membership, Indeed, we found that adolescent girls exposed to more severe peer victimization showed greater amygdala and ventral striatum activation to in-group relative to out-group peers. The amygdala belongs to a neural detection network that draws attention to salient stimuli (Cunningham & Brosch, 2012), and the ventral striatum codes for subjective value of important social groups and is involved in directing focus to the salient elements of group membership in adults (Van Bavel et al., 2008) and adolescents (Guassi Moreira et al., 2017; Telzer et al., 2015). Moreover, amygdala-ventral striatum connectivity increases from childhood to adolescence when detecting in-group peers (Guassi Moreira et al., 2017), suggesting that in-group belonging becomes particularly salient as youth transition into adolescence.
We also found that more severe peer victimization was associated with heightened fusiform activation when encoding in-group relative to out-group peers. The fusiform plays a key role in social perception (Haxby, Hoffman, & Gobbini, 2002) and individuating faces (Gauthier et al., 2000; Kanwisher, McDermott, & Chun, 1997; Rhodes, Byatt, Michie, & Puce, 2004). Indeed, the fusiform is more activated in response to in-group than out-group members (Golby, Gabrieli, Chiao, & Eberhardt, 2001; Van Bavel et al., 2008; Van Bavel et al., 2012), a pattern that increases from childhood to adolescence (Guassi Moreira et al., 2017). Moreover, adolescents show increased fusiform activation when receiving acceptance feedback from peers (Guyer et al., 2012). Because in- and out-group faces were based on a minimal group design, fusiform activation in the current study does not represent perceptual expertise, but instead likely reflects attentional biases and greater individuation and encoding of in-group faces, as found in adults (Van Bavel et al., 2008). Thus, more severe victimization may activate the fusiform to increase the perceptual encoding of motivationally relevant faces.
Finally, we found that more severe peer victimization was associated with heightened activation in the TPJ when evaluating in-group relative to out-group peers. The TPJ is a key region of the mentalizing network (Blakemore, 2008), suggesting that more severe peer victimization is associated with intensified social cognition toward the in-group. Indeed, individuals tend to mentalize about the needs and intentions of in-group peers more often (Harris & Fiske, 2006, 2009) and more accurately (Adams et al., 2010) than out-group peers, and youth show developmental increases from childhood to adolescence in TPJ activation when evaluating members of in-group versus out-group peers (Guassi Moreira et al., 2017). The current study extends this prior work by demonstrating that exposure to peer victimization may intensify this normative developmental process, devoting attentional resources toward evaluating the intentions of in-group peers. Among the social brain regions, we only found this pattern of effects for the TPJ. Prior research has found that the TPJ differentiates social sensitivity in adolescents, where the lowest and highest levels of TPJ activation are indicative of poor social competence (van Hoorn et al., 2018), suggesting that high TPJ activation may signal maladaptive social sensitivity. In contrast, the pSTS is associated with better mental state reasoning toward in-group relative to out-group peers (Adams et al., 2009), suggesting that high pSTS activation might signal adaptive social sensitivity. Thus, we may have only identified TPJ activation in the current study, as it may be uniquely involved in social monitoring under more perilous circumstances.
Implications of Heightened Social Monitoring
Overall, our results suggest that peer victimization is associated with heightened activation to in-group faces in regions associated with affective salience (i.e., amygdala, ventral striatum), social perception (i.e., fusiform), and mentalizing (i.e., TPJ) along with implicit biases (i.e., differential attention and memory) toward in-group relative to out-group peers. Although heightened activation to in-groups in each of these regions is developmentally normative in adolescence (Guassi Moreira et al., 2017), the SMS appears to be even more activated in girls exposed to peer victimization. Consistent with the Belonging Regulation model (Gardner, Pickett, Jefferis, & Knowles, 2005), social exclusion may produce a social hunger that heightens attention to motivationally relevant social cues in the environment in an attempt to seek social inclusion (Gardner, Pickett, & Brewer, 2000). Perhaps this social monitoring at the behavioral and neural levels is aimed at restoring adolescents’ social affiliation with their in-group by focusing on peers who may be more likely to fulfill their belonging needs. These findings suggest that one reason victimized youth may withstand teasing, stigmatization, and mockery within an in-group clique (Adler, & Adler, 1995, 1996) is that their SMS is over-activated, potentially as a way to fulfill their belonging needs and to seek acceptance within their in-group. While moderate levels of social sensitivity tend to be adaptive, high levels of social sensitivity may place youth at risk for psychopathology. For instance, social approach/avoidance motivation, which focuses on sensitivity to cues of acceptance/positive judgments versus rejection/negative judgments, can have adverse effects (Llewellyn & Rudolph, 2014; Rudolph, Troop-Goedon, & Llewewllyn, 2013; Rudolph, Abaied, Flynn, Sugimura, & Agoston, 2011), and children (Chen et al., 2016a, 2018) and adolescents (van Hoorn et al., 2018) with high social sensitivity tend to have poor adjustment, including higher depression and loneliness, lower self-worth, and are more likely to be nominated as the least liked. In the current study, we found that heightened social monitoring at the neural level (i.e., greater neural activation to in-group relative to out-group peers in the amygdala, ventral striatum, fusiform, and TPJ) was associated with lower social self-esteem and higher levels of internalizing and externalizing symptoms. The Belonging Regulation model proposes that feelings of low self-esteem alert the SMS that in-group belonging levels have dropped too low (Leary, 1999). This decline in self-esteem induces social hunger and activates the SMS to find new opportunities for socialization (Pickett et al., 2004). Although increases in the SMS are adaptive following acute instances of social rejection, over-activation of this system becomes maladaptive when belonging needs remain unfulfilled (Gardner et al., 2000). In this study, we were unable to examine whether girls exposed to victimization across childhood show chronic over-activation of the SMS, and our sample size did not allow us to examine emerging trajectories of internalizing and externalizing symptoms over time. However, our results are consistent with theoretical predictions and provide evidence that heightened activation of the SMS linked to a history of victimization is associated with maladjustment in adolescent girls.
Strengths, Limitations, and Future Directions
One important strength of our study involves the use of a sample in which we assessed victimization prospectively across seven years, providing a comprehensive index of girls’ victimization history. However, our analyses are correlational, and we cannot be certain about the casual pathways linking victimization, social monitoring (i.e., neural and behavioral biases to in-group peers), and maladjustment. For example, it is possible that for adolescents in particular, due to more time with peers, a heightened need to belong, and heightened sensitivity to peer evaluation, experiencing depressive and anxiety symptoms activates the social monitoring system at both the behavioral and neural levels, which in turn makes such youth easy targets for victimization. Thus, the direction of effects may start with internalizing and externalizing symptoms, lead to heightened social monitoring, and ultimately induce victimization. Indeed, prior work has shown that depressive symptoms predict increases in victimization (Marsh et al., 2016). It is also possible that the social exclusion that accompanies peer victimization leads to in-group sensitivity, which results in increased social monitoring in an environment without opportunities for improved belonging. This unmet need to belong may then undermine self-esteem and increase internalizing and externalizing symptoms, which may trigger more peer victimization (Hodges & Perry, 1999; Reijntjes et al., 2011), resulting in an even greater sense of social isolation. Risks such as social anxiety may both precede victimization and contribute to heightened social monitoring. Thus, characteristics of victimized youth and their environments may transact over time, creating a self-perpetuating cycle. Thus, it will be important to examine dynamic and reciprocal influences among these processes to unpack the direction of effects.
One way to potentially disrupt this cycle could be to provide opportunities for social inclusion with peers outside of the environment in which peer victimization takes place, helping direct adolescents towards an environment with peers who provide greater opportunities to satiate social hunger. Although victimization tends to be stable across time and context (Paul & Cillessen, 2003), making disruption of the cycle a challenge, promoting dyadic friendships among victimized youth may provide a context for youth to develop self-regulatory skills, enhance self-esteem, and increase security in social relationships (Schwartz et al., 2008), thereby decreasing the need to socially snack. Indeed, peer support buffers the link between victimization and youth adjustment (Hodges, Boivin, Vitaro, & Bukowski, 1999; Schwartz et al., 2008; Waldrip, Malcolm, & Jensen-Campbell, 2008), especially for girls (Cuadros & Berger, 2016). Importantly, it is essential for these friendships to involve well-adjusted peers, as friendships with peers who are aggressive, engage in bullying behaviors, or are not supportive can accelerate trajectories toward negative outcomes (Schwartz et al., 2008).
Another possible locus for intervention is social skills training. Youth suffering from internalizing and externalizing disorders often have reduced social competence (Bornstein, Hahn, & Haynes, 2010; Burt, Obradovic, Long, & Masten, 2008). Interventions that improve social competence could harness the fact that socially hungry individuals actually encode more social information than socially sated individuals due to the ongoing activation of their SMS (Gardner et al., 2005). These interventions could teach specific strategies to use the high volume of social information about in-groups that they have encoded to create positive social interactions.
A strength of our study is the use of a minimal group design, showing that within just minutes of being assigned to an in-group, girls with a history of more severe victimization demonstrate high levels of selective social monitoring specific to their in-group, including response time and memory biases, as well as heightened activation in neural regions supporting the SMS. Operationalizing behavioral markers of social hunger in terms of explicit and implicit biases when processing in-group relative to out-group information is consistent with prior work in adults that focuses on preferential recall for information concerning group membership (Gardner et al., 2000), improved memory for in-group members’ faces (Bernstein, Young, & Hugenberg, 2007; Van Bavel et al., 2012), and increased importance of in-groups but not outgroups (Knowles & Gardner, 2008). Nonetheless, future research utilizing a more explicit task could make a more direct link to social hunger by testing whether unmet belonging needs motivate individuals to work harder at a collective task (e.g., Williams & Sommer, 1997) or sacrifice something valuable in order to connect with others (but see Will, Crone, van Lier, & Güroğlu., 2016, 2018).
Our focus on adolescent girls was based on theory and research suggesting that this group may be most likely to engage in social monitoring due to their greater emphasis on creating and maintaining positive relationships with peers and may be particularly reactive to compromised social ties with peers given their sensitivity to interpersonal stressors (La Greca & Lopez, 1998; Rose & Rudolph, 2006). However, future studies should examine whether these effects are similar in male adolescents, as well as at other developmental stages.
Conclusion
In conclusion, this is the first study to link past exposure to peer victimization with potential neural markers of the SMS. Our research suggests that victimization experiences are associated with differential processing of social categories - even minimal groups - to dynamically shape attention, memory, and neural encoding of group belonging in ways that may be detrimental for their adjustment. Importantly, our findings implicate social sustenance as a fundamental need for adolescent girls’ well-being.
Acknowledgments
This work was supported by a University of Illinois Research Board Award and National Institute of Mental Health Grants MH68444 (to K.D.R.) and MH105655 (to K.D.R. and E.H.T.). We thank the families and schools who participated in this study. We are grateful to Michelle Miernicki, Jamie Abaied, Monica Agoston, Samirah Ali, Suravi Changlani, Megan Flynn, Inge Karosevica, Nicole Llewellyn, Jennifer Monti, Heather Ross, and Niwako Sugimura for their assistance in data collection and management.
Footnotes
Noncompliance was not associated with victimization or any variables of interest in the study
References
- Aboud FE (2003). The formation of in-group favoritism and out-group prejudice in young children: Are they distinct attitudes? Developmental Psychology, 39(1), 48–60. 10.1037//0012-1649.39.1.48 [DOI] [PubMed] [Google Scholar]
- Adams RB, Rule NO, Franklin RG, Wang E., Stevenson MT, Yoshikawa S., ... Ambady N. (2010). Cross-cultural reading the mind in the eyes: An fMRI investigation. Journal of Cognitive Neuroscience, 22(1), 97–108. 10.1162/jocn.2009.21187 [DOI] [PubMed] [Google Scholar]
- Adler PA, & Adler P. (1995). Dynamics of inclusion and exclusion in preadolescent cliques.Social Psychology Quarterly, 55(3), 145–162. [Google Scholar]
- Adler PA, & Adler P. (1996). Preadolescent clique stratification and the hierarchy of identity.Sociological Inquiry, 66(2), 111–142. [Google Scholar]
- Angold A., Costello EJ, Messer SC, & Pickles A. (1995). Development of a shortquestionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research, 54(4), 237–249. [Google Scholar]
- Ashburn-Nardo L., Voils CI, & Monteith MJ (2001). Implicit associations as the seeds of intergroup bias: How easily do they take root? Journal of Personality and Social Psychology, 81(5), 789–799. 10.1037/Z0022-3514.8L5.789 [DOI] [PubMed] [Google Scholar]
- Ashmore RD, Deaux K., & McLaughlin-Volpe T. (2004). An organizing framework for collective identity: Articulation and significance of multidimensionality. Psychological Bulletin, 130(1), 80–114. 10.1037/0033-2909.130.L80 [DOI] [PubMed] [Google Scholar]
- Baumeister RF, & Leary MR (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117(3), 497–529. Retrieved from http://ronaldvoorn.com/wp-content/uploads/2015/09/1995-The-Need-to-Belong-Desire-for-Interpersonal-Attachments-as-a-Fundamental-Human-Motivation.pdf [PubMed] [Google Scholar]
- Bellmore AD, & Cillessen AHN (2006). Reciprocal influences of victimization, perceived social preference, and self-concept in adolescence. Self and Identity, 5(3), 209–229. 10.1080/15298860600636647 [DOI] [Google Scholar]
- Bernstein MJ, Young SG, & Hugenberg K. (2007). The cross-category effect.Psychological Science, 18(8), 706–712. 10.1111/j.1467-9280.2007.01964.x [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2008). Development of the social brain during adolescence. Quarterly Journal of Experimental Psychology, 61(1), 40–49. 10.1080/17470210701508715 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2010). The developing social brain: Implications for education. Neuron, 65(6), 744–747. https://doi.org/10.1016Zj.neuron.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, & Haynes OM (2010). Social competence, externalizing, and internalizing behavioral adjustment from early childhood through early adolescence: Developmental cascades. Development and Psychopathology, 22(4), 717–735. 10.1017/S0954579410000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE (1991). Human Universals. New York: McGraw-Hill. [Google Scholar]
- Burnett S., Bird G., Moll J., Frith CD, & Blakemore S-J (2009). Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience, 21(9), 1736–1750. 10.1162/jocn.2009.21121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt KB, Obradović J., Long JD, & Masten AS (2008). The interplay of social competence and psychopathology over 20 years: Testing transactional and cascade models. Child Development, 79(2), 359–374. 10.1111/j.1467-8624.2007.01130.x [DOI] [PubMed] [Google Scholar]
- Caldwell MS, Rudolph KD, Troop-Gordon W., & Kim DY (2004). Reciprocal influences among relational self-views, social disengagement, and peer stress during early adolescence. Child Development, 75(4), 1140–1154. [DOI] [PubMed] [Google Scholar]
- Card NA, & Little TD (2006). Proactive and reactive aggression in childhood and adolescence: A meta-analysis of differential relations with psychosocial adjustment. International Journal of Behavioral Development, 30(5), 466–480. 10.1177/0165025406071904 [DOI] [Google Scholar]
- Champion K., Vernberg E., & Shipman K. (2003). Nonbullying victims of bullies: Aggression, social skills, and friendship characteristics. Journal of Applied Developmental Psychology, 24(5), 535–551. 10.1016/j.appdev.2003.08.003 [DOI] [Google Scholar]
- Chen X., Fu R., Liu J., Wang L., Zarbatany L., & Ellis W. (2018). Social sensitivity and social, school, and psychological adjustment among children across contexts. Developmental Psychology, 54(6), 1124–1134. 10.1037/dev0000496 [DOI] [PubMed] [Google Scholar]
- Chen X., Liu J., Ellis W., & Zarbatany L. (2016). Social sensitivity and adjustment in Chinese and Canadian children. Child Development, 87, 1115–1129. 10.1111/cdev.12514 [DOI] [PubMed] [Google Scholar]
- Crick N., & Nelson D. (2002). Relational and physical victimization within friendships:Nobody told me there’d be friends like these. Journal of Abnormal Child Psychology, 30(6), 599–607. [DOI] [PubMed] [Google Scholar]
- Crockett L., Losoff M., & Petersen AC (1984). Perceptions of the peer group and friendship in early adolescence. The Journal of Early Adolescence, 4(2), 155–181. 10.1177/0272431684042004 [DOI] [Google Scholar]
- Cruwys T., Haslam SA, Dingle GA, Haslam C., & Jetten J. (2014). Depression and social identity: An integrative review. Personality and Social Psychology Review, 18(3), 215–238. 10.1177/1088868314523839 [DOI] [PubMed] [Google Scholar]
- Cuadros O., & Berger C. (2016). The protective role of friendship quality on the wellbeing of adolescents victimized by peers. Journal of Youth and Adolescence, 45(9), 1877 10.1007/s10964-016-0504-4 [DOI] [PubMed] [Google Scholar]
- Cunningham WA, & Brosch T. (2012). Motivational salience: Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21(1), 54–59. 10.1177/0963721411430832 [DOI] [Google Scholar]
- Do KT & Telzer EH (2019). Corticostriatal connectivity is associated with the reduction of intergroup bias and greater impartial giving in youth. Developmental Cognitive Neuroscience, 37, 1–9. 10.1016/_j.dcn.2019.100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan MB, Trzesniewski KH, Robins RW, Moffitt TE, & Caspi A. (2005). Low self-esteem is related to aggression, antisocial behavior, and delinquency. Psychological Science, 16(4), 328–35. 10.1111/j.0956-7976.2005.01535.x [DOI] [PubMed] [Google Scholar]
- Dunbar RIM (2018). The anatomy of friendship. Trends in Cognitive Sciences, 22(1), 32–51. https://doi.org/10.1016Zj.tics.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Dunham Y., Baron AS, & Carey S. (2011). Consequences of “minimal” group affiliations in children. Child Development, 82(3), 793–811. 10.1111/j.1467-8624.2011.01577.x.Consequences [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham Y., & Emory J. (2014). Of affect and ambiguity: The emergence of preference for arbitrary ingroups. Journal of Social Issues, 70(1), 81–98. 10.1111/josi.12048 [DOI] [Google Scholar]
- Egger H., Pine DS, Nelson E., Leibenluft E., Ernst M., Towbin KE, & Angold A. (2011). The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): A new set of children’s facial emotion stimuli. Int J MethodsPsychiatr Res., 20(3), 145–156. 10.1002/mpr.343.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W., & Buhrmester D. (1992). Age and sex differences in perceptions of networks of personal relationships. Child Development, 63(1), 103–115. [DOI] [PubMed] [Google Scholar]
- Gardner M., & Steinberg L. (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41(4), 625–635. 10.1037/0012-1649.41A625 [DOI] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, & Brewer MB (2000). Social exclusion and selective memory: How the need to belong influences memory for social events. Personality and Social Psychology Bulletin, 26(4), 486–496. [Google Scholar]
- Gardner WL, Pickett CL, Jefferis V., & Knowles M. (2005). On the outside looking in: Loneliness and social monitoring. Personality and Social Psychology Bulletin, 31(11), 1549–1560. 10.1177/0146167205277208 [DOI] [PubMed] [Google Scholar]
- Gasser L., & Keller M. (2009). Are the competent the morally good? Perspective taking and moral motivation of children involved in bullying. Social Development, 18(4), 798–816. 10.1111/j.1467-9507.2008.00516.x [DOI] [Google Scholar]
- Gauthier I., Tarr MJ, Moylan J., Skudlarski P., Gore JC, & Anderson AW (2000). The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience, 12(3), 495–504. 10.1162/089892900562165 [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Shachar-Lavie I. (2013). More than a face: a unified theoretical perspective on nonverbal social cue processing in social anxiety. Frontiers in Human Neuroscience, 7, 904 10.3389/fnhum.2013.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gini G. (2006). Social cognition and moral cognition in bullying: What’s wrong? Aggressive Behavior, 32(6), 528–539. [Google Scholar]
- Giuliani NR, & Pfeifer JH (2015). Age-related changes in reappraisal of appetitive cravings during adolescence. Neuroimage, 108, 173–181. https://doi.org/10.10167j.neuroimage.2014.12.037.Age-related [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, & Eberhardt JL (2001). Differential responses in the fusiform region to same-race and other-race faces. Nature Neuroscience, 4(8), 845–850. 10.1038/90565 [DOI] [PubMed] [Google Scholar]
- Guassi Moreira JF, Van Bavel JJ, & Telzer EH (2017). The neural development of “Us and Them.” Social Cognitive and Affective Neuroscience, 12(2), 184–196. 10.1093/scan/nsw134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A., Benson B., Nelson EE, Perez-Edgar K., ... Ernst M. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gweon H., Dodell-Feder D., Bedny M., & Saxe R. (2012). Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Development, 83(6), 1853–1868. 10.1111/j.1467-8624.2012.01829.x [DOI] [PubMed] [Google Scholar]
- Haney P., & Durlak JA (1998). Changing self-esteem in children and adolescents: A meta-analytical review. Journal of Clinical Child Psychology, 27(4), 423–433. 10.1207/s15374424jccp2704_6 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R., & Roesch L. (2007). Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development, 78(1), 279–295. [DOI] [PubMed] [Google Scholar]
- Harris LT, & Fiske ST (2006). Dehumanizing the lowest of the low: Neuroimaging responses to extreme out-groups. Psychological Science, 17(10), 847–853.https://doi.org/10.11117j.1467-9280.2006.01793_x [DOI] [PubMed] [Google Scholar]
- Harris LT, & Fiske ST (2009). Social neuroscience evidence for dehumanised perception. European Journal of Social Psychology, 20(1), 192–193. [Google Scholar]
- Hart LT, & Fegley ST (1995). Prosocial behavior and caring in adolescence: Relations to self-understanding and social judgment. Child Development, 66(5), 1346–1359. 10.1111/j.1467-8624.1995.tb00939.x [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 5/(1), 59–67. 10.1016/S0006-3223(01)01330-0 [DOI] [PubMed] [Google Scholar]
- Hess YD, & Pickett CL (2010). Social rejection and self- versus other-awareness. Journal of Experimental Social Psychology, 46(2), 453–456. 10.1016/jjesp.2009.12.004 [DOI] [Google Scholar]
- Hodges EVE, & Perry DG (1999). Personal and interpersonal antecedents andconsequences of victimization by peers. Journal of Personality and Social Psychology, 76(4), 677–685. 10.1037/0022-3514.76A677 [DOI] [PubMed] [Google Scholar]
- Holt MK, & Espelage DL (2007). Perceived social support among bullies, victims, and bully-victims. Journal of Youth and Adolescence, 36(8), 984–994. 10.1007/s10964-006-9153-3 [DOI] [Google Scholar]
- Holt-Lunstad J., Smith TB, Baker M., Harris T., & Stephenson D. (2015). Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspectives on Psychological Science, /0(2), 227–237. 10.1177/1745691614568352 [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, & Umberson D. (1988). Social relationships and health. Science, 24/(4865), 540–545. [DOI] [PubMed] [Google Scholar]
- Hunter SC, & Boyle JME (2004). Appraisal and coping strategy use in victims of school bullying. British Journal of Educational Psychology, 74(1), 83–107. 10.1348/000709904322848833 [DOI] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., & Chun MM (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience, 17(11), 4302–11. 10.1098/Rstb.2006.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles ML (2014). Social rejection increases perspective taking. Journal of Experimental Social Psychology, 55, 126–132. 10.1016/jjesp.2014.06.008 [DOI] [Google Scholar]
- Knowles ML, & Gardner WL (2008). Benefits of membership: The activation andamplification of group identities in response to social rejection. Personality and Social Psychology Bulletin, 34(9), 1200–1213. 10.1177/0146167208320062 [DOI] [PubMed] [Google Scholar]
- Kochenderfer BJ, & Ladd GW (1996). Peer victimization: Cause or consequence of school maladjustment? Child Development, 67(4), 1305–1317. [PubMed] [Google Scholar]
- Kroger J. (2000). Ego identity status research in the new millenium. International Journal of Behavioral Development, 24(2), 145–148. 10.1080/016502500383250 [DOI] [Google Scholar]
- La Greca AM, & Lopez N. (1998). Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology, 26(2), 83–94. 10.1023/A:1022684520514 [DOI] [PubMed] [Google Scholar]
- Lakin J., Chartrand TL, and Arkin R. (2008). I am too just like you: nonconscious mimicry as an automatic behavioral response to social exclusion. Psychological Science, 19, 816–822. 10.1111/j.1467-9280.2008.02162.x [DOI] [PubMed] [Google Scholar]
- Leary MR (1999). Making sense of self-esteem. Current Directions in Psychollogical Science, 8(1), 32–35. 10.1111/j.1467-9396.2011.00987.x [DOI] [Google Scholar]
- Leibovich N., Schmid V., Calero A. (2018). The need to belong in adolescence: Adaptation of a scale for its assessment. Psychology and Behavioral Science International Journal, 8, 1–7. [Google Scholar]
- Llewellyn NM, & Rudolph KD (2014). Individual and sex differences in the consequences of peer victimization: Moderation by approach and avoidance motivation. Developmental Psychology, 50, 2210–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner JK, DeWall NC, Baumeister RF, & Schaller M. (2007). Does social exclusion motivate interpersonal reconnection? Resolving the “porcupine problem.” Journal of Personality and Social Psychology, 92(1), 42–55. 10.1037/0022-3514.92.1.42 [DOI] [PubMed] [Google Scholar]
- Marsh HW, Craven RG, Parker PD, Parada RH, Guo J., Dicke T., & Abduljabbar AS (2016). Temporal ordering effects of adolescent depression, relational aggression, and victimization over six waves: Fully latent reciprocal effects models. Developmental Psychology, 52(12), 1994 10.1037/dev0000241 [DOI] [PubMed] [Google Scholar]
- Newman BM, Lohman BJ, & Newman PR (2007). Peer group membership and a sense of belonging: their relationship to adolescent behavior problems. Adolescence, 42(166), 241–263. [PubMed] [Google Scholar]
- Newman BM, & Newman PR (2001). Group identity and alienation: Giving the we its due. Journal of Youth and Adolescence, 30(5), 515–538. 10.1023/A:1010480003929 [DOI] [Google Scholar]
- Nolen-Hoeksema S., Stice E., Wade E., & Bohon C. (2007). Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. Journal of Abnormal Psychology, 116(1), 198–207. 10.1037/0021-843X.116.1.198 [DOI] [PubMed] [Google Scholar]
- Orth U., Robins RW, Trzesniewski KH, Maes J., & Schmitt M. (2009). Low self-esteem is a risk factor for depressive symptoms from young adulthood to old age. Journal of Abnormal Psychology, 118(3), 472–478. 10.1037/a0015922 [DOI] [PubMed] [Google Scholar]
- Parker JG, & Asher SR (1993). Friendship and friendship quality in middle childhood: Links with peer group acceptance and feelings of loneliness and social dissatisfaction. Developmental psychology, 29(4), 611–621. 10.1037/0012-1649.29A611 [DOI] [Google Scholar]
- Paul JJ, & Cillessen AH (2003). Dynamics of peer victimization in early adolescence: Results from a four-year longitudinal study. Journal of Applied School Psychology, 19(2), 25–43. 10.1300/J008v19n0203 [DOI] [Google Scholar]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, & Pfeifer JH (2013). Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. Neuroimage, 82, 23–24. https://doi.org/10.1016Zj.neuroimage.2013.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, & Gardner WL (2005). The Social Monitoring System: Enhanced sensitivity to social cues as an adaptive response to social exclusion In Williams KD, Forgas JP, & Von Hippel W. (Eds.), Sydney Symposium of Social Psychology series. The social outcast: Ostracism, social exclusion, rejection, and bullying (pp. 213–226). New York: Psychology Press. [Google Scholar]
- Pickett CL, Gardner WL, & Knowles M. (2004). Getting a cue: The need to belong and enhanced sensitivity to social cues. Personality and Social Psychology Bulletin, 30(9), 1095–1107. 10.1177/0146167203262085 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Cheah CSL, & Guyer AE (2005). Peer victimization, cue interpretation, and internalizing symptoms: Preliminary concurrent and longitudinal findings for childrenand adolescents. Journal of Clinical Child and Adolescent Psychology, 34(1), 11–24. 10.1207/s15374424jccp3401_2 [DOI] [PubMed] [Google Scholar]
- Reijntjes A., Kamphuis JH, Prinzie P., Boelen PA, Van Der Schoot M., & Telch MJ (2011). Prospective linkages between peer victimization and externalizing problems in children: A meta-analysis. Aggressive Behavior, 37(3), 215–222. 10.1002/ab.20374 [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Richmond BO (1978). What I Think and Feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology, 6(2), 271–280. 10.1207/s15327752jpa4303 [DOI] [PubMed] [Google Scholar]
- Rhodes G., Byatt G., Michie PT, & Puce A. (2004). Is the fusiform face area specialized for faces, individuation, or expert individuation? Journal of Cognitive Neuroscience, 16(2), 189–203. 10.1162/089892904322984508 [DOI] [PubMed] [Google Scholar]
- Rodman AM, Powers KE, & Somerville LH (2017). Development of self-protective biases in response to social evaluative feedback. Proceedings of the National Academy of Sciences, 114(50), 13158–13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, & Rudolph KD (2006). A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin, 132(1), 98–131. 10.1037/0033-2909.132.1.98.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Abaied J., Flynn M., Sugimura N., & Agoston AM (2011). Developing relationships, being cool, and not looking like a loser: Social goal orientation predicts children’s responses to peer aggression. Child Development, 82, 1518–1530. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M., Abaied JL, Groot A., & Thompson R. (2009). Why is past depression the best predictor of future depression? Stress generation as a mechanism ofdepression continuity in girls. Journal of Clinical Child and Adolescent Psychology, 38(4), 473–485. 10.1080/15374410902976296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Lansford JE, Agoston AM, Sugimura N., Schwartz D., Dodge KA, ... Bates JE (2014). Peer victimization and social alienation: Predicting deviant peer affiliation in middle school. Child Development, 85(1), 124–139. 10.1111/cdev.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W., & Llewellyn N. (2013). Interactive contributions of self-regulation deficits and social motivation to psychopathology: Unraveling divergent pathways to aggressive behavior and depressive symptoms. Development and Psychopathology, 25, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Miernicki ME, Troop-Gordon W., Davis MM, & Telzer EH (2016). Adding insult to injury: Neural sensitivity to social exclusion is associated with internalizing symptoms in chronically peer-victimized girls. Social Cognitive and Affective Neuroscience, 11(5), 829–842. 10.1093/scan/nsw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W., Hessel ET, & Schmidt JD (2011). A latent growth curve analysis of early and increasing peer victimization as predictors of mental health across elementary school. Journal of Clinical Child and Adolescent Psychology, 40(1), 111–122. 10.1080/15374416.2011.533413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte RH, Overbeek G., Ten Brink G., Rommes E., De Kemp RA, Goossens L., & Engels RC (2009). The significance of reciprocal and unilateral friendships for peer victimization in adolescence. Journal of Youth and Adolescence, 38(1), 89–100. 10.1007/s10964-008-9287-6 [DOI] [PubMed] [Google Scholar]
- Slavich GM, Donovan AO, Epel ES, & Kemeny ME (2010). Black sheep get the blues:A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews, 35(1), 39–45. https://doi.org/10.1016Zj.neubiorev.2010.01.003.Black [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G., & Casey BJ (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24(8), 1554–1562. 10.1177/0956797613475633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Nock MK, Masia-Warner C., & Barlas ME (2003). Peer victimization and social-psychological adjustment in Hispanic and African-American children. Journal of Child and Family Studies, 12(4), 439–452. 10.1023/A:1026016124091 [DOI] [Google Scholar]
- Tajfel H., Billig MG, Bundy RP, & Flament C. (1971). Social categorization and intergroup behaviour. European Journal of Social Psychology. 10.1002/ejsp.2420010202 [DOI] [Google Scholar]
- Tajfel H., & Turner JC (1979). An integrative theory of intergroup conflict. The Social Psychology of Intergroup Relations, 33(47), 74. [Google Scholar]
- Taylor KA, Sullivan TN, & Kliewer W. (2013). A longitudinal path analysis of peervictimization, threat appraisals to the self, and aggression, anxiety, and depression among urban African American adolescents. Journal of Youth and Adolescence, 42(2), 178–189. 10.1007/s10964-012-9821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Flannery J., Humphreys KL, Goff B., Gabard-Durman L., Gee DG, &Tottenham N. (2015). “The Cooties Effect”: Amygdala reactivity to opposite-versus same-sex faces declines from childhood to adolescence. Journal of Cognitive Neuroscience, 27, 1685–1696. 10.1162/jocn_a_00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, & Fuligni AJ (2013). Positive daily family interactions eliminate gender differences in internalizing symptoms among adolescents. Journal of Youth and Adolescence, 42(10), 1498–1511. 10.1007/s10964-013-9964-y [DOI] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, & Qu Y. (2015). Mothers know best: Redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience, 10(10), 1383–1391. 10.1093/scan/nsv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Miernicki ME, & Rudolph KD (2018). Chronic peer victimization heightens neural sensitivity to risk taking. Development and Psychopathology, 30, 13–26. 10.1017/S0954579417000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JC (1982). Towards a cognitive redefinition of the social group. In Social identity and intergroup relations (pp. 15–40). [Google Scholar]
- Van Bavel JJ, & Cunningham WA (2009). Self-categorization with a novel mixed-race group moderates automatic social and racial biases. Personality and Social Psychology Bulletin, 35(3), 321–335. 10.1177/0146167208327743 [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, & Cunningham WA (2008). The neural substrates of in-group bias: A functional magnetic resonance imaging investigation. Psychological Science, 19(11), 1131–1139. 10.1111/j.1467-9280.2008.02214.x [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, & Cunningham WA (2011). Modulation of the fusiform face area following minimal exposure to motivationally relevant faces: Evidence of in-group enhancement (not out-group disregard). Journal of Cognitive Neuroscience, 23(11), 3343–3354. 10.1162/jocn_a_00016 [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Swencionis JK, O’Connor RC, & Cunningham WA (2012). Motivated social memory: Belonging needs moderate the own-group bias in face recognition. Journal of Experimental Social Psychology, 48(3), 707–713. 10.1016/j_jesp.2012.01.006 [DOI] [Google Scholar]
- Van Bavel JJ, & Cunningham WA (2012). A social identity approach to person memory: Group membership, collective identification, and social role shape attention and memory. Personality and Social Psychology Bulletin, 38, 1566–1578. 10.1177/0146167212455829 [DOI] [PubMed] [Google Scholar]
- van den Bos W., van Dijk E., Westenberg M., Rombouts SARB, & Crone EA (2011). Changing brains, changing perspectives: The neurocognitive development of reciprocity. Psychological Science, 22(1), 60–70. [DOI] [PubMed] [Google Scholar]
- van Hoorn J., McCormick EM, & Telzer EH (2018). Moderate social sensitivity in a risky context supports adaptive decision-making in adolescence: Evidence from brain and behavior. Social Cognitive Affective Neuroscience, 13, 546–556. 10.1093/scan/nsy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip AM, Malcolm KT, & Jensen-Campbell LA (2008). With a little help from your friends: The importance of high-quality friendships on early adolescent adjustment. Social Development, 17(4), 832–852. 10.1111/j.1467-9507.2008.00476.x [DOI] [Google Scholar]
- Ward BD (2000). Simultaneous inference for fMRI data. Retrieved February 23, 2018, from http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf
- Wei HS, & Jonson-Reid M. (2011). Friends can hurt you: Examining the coexistence of friendship and bullying among early adolescents. School Psychology International, 32(3), 244–262. 10.1177/0143034311402310 [DOI] [Google Scholar]
- Will G-J, Crone EA, van Lier PAC, & Güroğlu B. (2018). Longitudinal links between childhood peer acceptance and the neural correlates of sharing. Developmental Science, 21, e12489. 10.1111/desc.12489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will G-J, Crone EA, van Lier PAC, & Güroglu B. (2016). Neural correlates of retaliatory and prosocial reactions to social exclusion: Associations with chronic peer rejection. Developmental Cognitive Neuroscience, 19, 288–297. http://dx.doi.org/10.10167i.dcn.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, & Sommer KL (1997). Social ostracism by one’s coworkers: Does rejection lead to loafing or compensation? Personality and Social Psychology Bulletin, 23(7), 693–706. [Google Scholar]
- Ziv Y., Leibovich I., & Shechtman Z. (2013). Bullying and victimization in early adolescence: Relations to social information processing patterns. Aggressive Behavior, 39(6), 482–492. 10.1002/ab.21494 [DOI] [PubMed] [Google Scholar]


