Abstract
Checkpoint blockade immunotherapy has revolutionized cancer treatment, however, the cellular and molecular factors that govern responsiveness to immunotherapy are still poorly understood. One emerging area of clinical importance is differential responsiveness to checkpoint blockade immunotherapy across different tissues sites of tumor growth. Each tissue site in the body can contain unique tissue-resident immune cells from both the lymphoid and myeloid compartments, and differences in tissue-specific immune cell composition might predispose tumors in certain tissue sites to be more or less responsive to immunotherapy. Understanding the interplay between tissue-resident and systemic immune responses against tumors will help to determine how to better therapeutically target the immune system to fight cancer. This review summarizes clinical and preclinical investigations of tissue specific anti-tumor immune responses, and how they influence the tumor immune microenvironment and the efficacy of immunotherapy.
Keywords: Checkpoint Blockade Immunotherapy, T cell, metastasis, dendritic cells, myeloid cells
Immunotherapy and the Cancer Immunity Cycle
The ability of immunotherapy to induce long-term clinical benefit against metastatic disease is one major advantage over conventional cancer therapies. The most prominent immunotherapy, so-called checkpoint blockade immunotherapy (CBT), targets immune inhibitory receptors/ligand interactions on T cells. Engagement of these inhibitory receptors, CTLA-4 or PD-1, on activated T cells contributes to T cell dysfunction in the tumor microenvironment, and blockade of these receptor/ligand interactions is sufficient to re-invigorate anti-tumor T cell responses [1, 2]. The presence of a T cell infiltrate in a tumor has long been a positive prognostic indicator [3], and both pre-clinical and clinical evidence suggests that responsiveness to CBT is strongly associated with the presence of tumor-reactive effector T cells within the tumor microenvironment [4, 5]. Studies in pre-clinical mouse models have further emphasized that the mechanism of action of CBT is to reinvigorate existing anti-tumor immune responses within the tumor-microenvironment itself [5, 6]. Our current understanding of how T cells infiltrate a tumor is represented by the cancer immune cycle, which is primarily derived from syngeneic, subcutaneous tumor models and correlative data from patients [7]. The cancer immune cycle can be summarized as follows: dendritic cells (DC) infiltrate the tumor microenvironment where they take up tumor-derived materials including dsDNA (Fig. 1A). The activation of the cGAS/STING pathway by dsDNA leads to the production of type-I interferons and activation of cross-presenting, migratory, Batf3-dependent dendritic cells [8]. These migratory DCs traffic processed tumor-associated antigens to draining lymph nodes, where they present these antigens to activate antigen-specific cytotoxic T cells. The activated T cells then traffic back through the circulation to the tumor in a CXCL9/CXCL10-dependent manner, where they carry out their effector functions including tumor cell killing [7, 8]. Despite this infiltration of activated, antigen-specific CD8+ T cells, tumors still progress. Prolonged activation in the tumor microenvironment promotes T cell dysfunction, mediated in part through the engagement of inhibitory receptors like CTLA-4 and PD-1, and this dysfunction allows for immune escape and tumor progression [9–11]. While several clinical studies provide strong evidence for the cancer immune cycle to function in a similar fashion in humans, not all cancers have apparent infiltration of immune cells, and not all inflamed cancers respond to CBT [12, 13]. The cancer immune cycle currently ignores contributions from the tumor microenvironment, including tissue-resident immune cell populations present in organs before and during tumor growth. These tissue resident immune cells have the potential to impact anti-tumor immune responses at any given point in the cancer immune cycle. Myeloid cells collaborate closely with the anti-tumor specific T cells, and varying subsets and activation states can skew the cancer immune cycle in a tissue-specific fashion. This review highlights both pre-clinical and clinical evidence that the anatomic site of primary or metastatic tumor growth, and its tissue-resident or tissue-specific cell populations, can have drastic impacts on anti-tumor immunity.
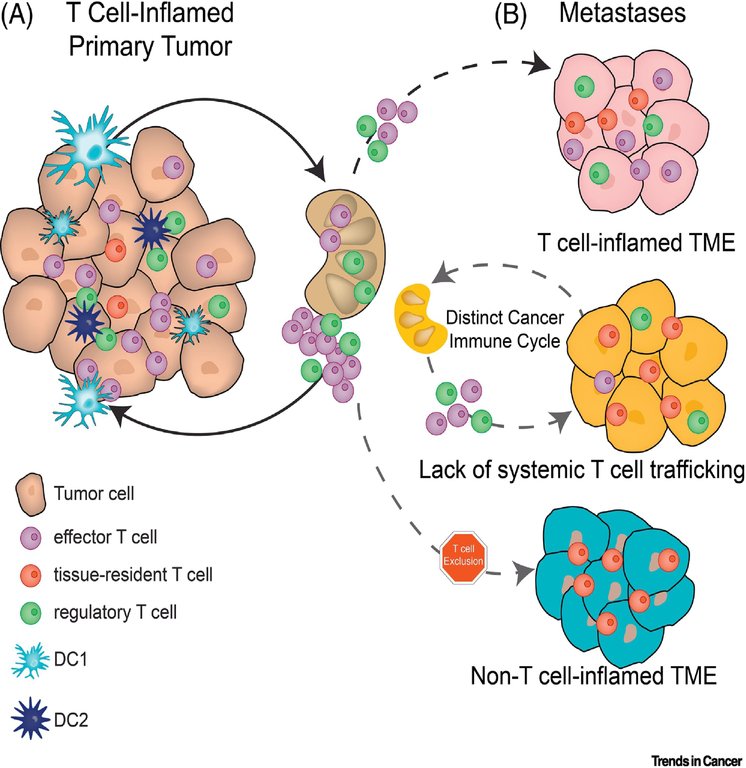
Figure 1 –
The influence of tissue site on T cell inflammation of tumors
A) T cell-inflamed tumors are the result of dendritic cell activation that leads to tumor-specific T cell priming in the tumor-draining lymph node (TdLN) followed by T cell trafficking to the tumor microenvironment. Tissue-resident T cells may also expand in response to tumors, likely through pathways independent of T cell priming. B) T cell responses against metastatic cancer might infiltrate the lesion from an existing systemic immune response (top) or generate their own cancer immune cycle independent of the immune response against the primary tumor (middle). Further metastasis may exclude T cells from infiltrating even if a systemic immune response is generated.
Tissue site impacts anti-tumor T cell response and response to checkpoint blockade
Recent clinical data has demonstrated the importance of the tissue microenvironment in the response to immunotherapy: studies in both melanoma and lung cancer have shown that response rates to CBT within individual metastatic patients varies depending on the anatomic location of the metastasis [14–16]. These results imply the importance of the local tumor microenvironment in anti-tumor immunity, and suggest that similar tumors growing in different tissues may lead to very different immune responses and have different susceptibilities to immunotherapy. Interestingly, tissue-specific response to CBT are dependent on cancer types, meaning responsive and non-responsive sites are different between patients with lung cancer or melanoma [14–16]. For instance, metastatic melanomas growing in the lung respond particularly well to CBT, while primary and metastatic lung lesions growing in the lung respond similarly to lung metastases in other sites [14–16]. This indicates the existence of a complex set of interactions between the immune system, tissue sites of tumor growth, and tumor organ of origin, and raises the possibility that tissue-specific T cells may play different roles in tumor progression and response to CBT depending on the site of tumor growth.
Autochthonous mouse models of cancer have also found that the tissue site of tumor growth plays an important role in the anti-tumor T cell response. A series of studies using KRasG12DLSL+/− p53fl/fl mice found that induced sarcomas and lung adenocarcinomas harbored intrinsically different degrees of immunogenicity, with sarcomas being highly immunogenic and prone to immunoediting, while lung adenocarcinomas were found to be poorly immunogenic and rarely edited [17, 18]. However, the immunological mechanisms behind these differences remains mostly elusive. These results reinforce the notion that the tissue in which the tumor grows can drastically affect the anti-tumor immune response. The lung has many resident immune populations, and further studies with the KRasG12DLSL+/− p53fl/fl mouse model found that T cells present in the lung, especially regulatory T cells (Tregs) and γδ T cells, suppressed anti-tumor immune responses and promoted tumor development [19, 20]. Therefore, lung resident T cells may establish an immune-suppressive environment that enables lung tumor growth. Evidence from human patients suggests, however, that not all lung tissue-resident T cells promote tumor growth. In contrast to Treg, tissue-resident memory CD8+ T cells are thought to be beneficial to anti-tumor immunity, and their presence correlates with a better prognosis in lung cancer patients [21, 22]. A similar study found that the presence of CD8+ T cells with a resident-memory phenotype was a positive prognostic indicator in melanoma, and that these T cells expanded upon immunotherapy [23]. In mouse viral models, tissue-resident memory CD8+ T cells can act as sentinels to induce rapid immune responses and can help enhance responses against antigens other than their own specificities [24]. To date however, tumor studies have used systems with tissue-resident memory T cells specific for model antigens expressed by tumor cells [25]. In melanoma, this may be a relevant model, as both human and mouse studies have found that tissue-resident CD8+ T cells can be reactive towards melanocyte differentiation antigens [23, 25], however this notion is less well-defined for other cancer types. Tissue-resident T cells could therefore play a major role in promoting or suppressing anti-tumor immune responses, potentially explaining differences in immune responses and tumor growth between tissue sites. Whether local immune-suppressive or tumor-promoting T cells, such as lung Treg and γδ T cells, can influence the growth of metastatic tumors or the generation of systemic immune responses remains unknown, and will be important to understand in the context of metastasis and immunotherapy.
T cell responses can be locally restricted to one anatomic site
While analyses of anti-tumor immune responses against individual tumor lesions provided, and will continue to generate critical insights into the impact of tissue resident immune cells on anti-tumor immunity, they fail to assess the crosstalk between different lesions. Clinical studies utilizing longitudinal human biopsies have begun to investigate immune responses against tumors growing in different tissues within individual patients. After activation in the tumor-draining lymph node, tumor-specific T cells traffic through the circulation back to the tumor site [7, 8]. Two distinct hypotheses exist about the distribution of effector T cells during an ongoing anti-tumor immune response: the first postulates that any induced anti-tumor T cell response will be systemic and equally disseminated between all metastatic lesions, while the second proposes that each metastatic lesion harbors a distinct set on tumor-reactive effector T cells (Fig. 1a and b). While most mouse models suggest the first hypothesis to be true, several recent studies using patient biopsies have revealed a large heterogeneity of immune infiltrates between different metastases within a patient, and between primary and metastatic tumors [26–28]. A study of primary colon cancers found that protective, systemic immune responses might take place in some patients but not others. The authors found that a stronger immune response in the primary tumor correlated with a lack of metastases [27]. Examining patients with only early stage metastatic tumors, the authors ruled out that the presence of metastases decreased the immune response at the primary tumor site. This indicated that generating a sufficient immune response in the primary lesion can lead to control of metastasis in a systemic fashion, and that a protective systemic immune response is possible only if the primary tumor is sufficiently immunogenic. The strength of the immune response in the primary tumors was also correlated with increased lymphatic vessel density of the tumor, suggesting that lymphatic drainage was critical for allowing a strong immune response. These data suggest that systemic, protective immune responses can be generated, but do not always take place, and that the physical characteristics of the primary tumors such as lymphatic drainage can impact the strength of anti-tumor immunity. A different study by Galon and colleagues followed 2 patients longitudinally, assessing T cell infiltration in 36 lesions (primaries and metastases) over 11 years [26]. They found that the lesion with the least amount of T cell infiltration was predictive for overall survival, with no or very low T cell infiltration being correlated with shorter survival. These data indicate that metastases can be differentially controlled by the immune system within a single patient, and that immune control of metastases plays a critical role in patient survival [28]. Interestingly, the authors not only found that different metastases have drastically different amounts of infiltrating T cells, but that the T cell receptor repertoires between metastases can differ greatly. Further, the authors identified non-overlapping TCR repertoires between metastatic lesions within patients, indicating that each metastasis harbors its own unique T cell environment [26]. These distinct T cell environments were observed in simultaneously growing metastases with overlapping profiles of non-synonymous mutations. These results suggest that within the same patient, each metastasis is its own unique immunological event, and that systemic immune responses are not always generated, or cannot always reach every metastasis. It could be possible that even if a systemic immune response is generated individual lesions could blunt T cell infiltration. In a mouse model of melanoma, it was found that an activating β-catenin mutation could inhibit the recruitment of circulating, tumor antigen-specific T cells [29]. Additionally, it is possible that mutations leading to neoantigens could take place after metastatic dissemination (often referred to as branch mutations), leading to T cell clones reactive against only individual metastases. However, a recent study of 39 patients with primary lung tumors and matched brain metastases found that despite a high rate of shared mutations and infiltrating T cell clonotypes, T cells were significantly less abundant in brain metastases than in primary lung tumors [30]. These results highlight that even when tumors are genetically similar and a systemic T cell response has been generated, the anatomic site of tumor growth can have a strong influence on the anti-tumor T cell response.
Locally restricted T cell responses due to distinct T cell repertoires between metastatic sites
Studies in mice and humans have shown that different anatomic sites harbor different antigen-specific T cells within the tissues and tissue-draining lymph nodes [31]. These locally restricted T cell pools can potentially give different tissues access to distinct TCR repertoires that can respond and expand during tumor growth [32]. While many T cells recirculate throughout the blood and lymph, subsets of T cells have been found to be preferentially localized to certain metastatic sites [33]. Sequencing of TCRs from different lymph nodes in mice found that the repertoire of TCRs varied between lymph nodes that drained different tissue sites [31]. This was true for both Treg and activated CD4+ effector T cells. Thus, different sets of tumor-reactive T cells would be activated depending on the pre-existing repertoire found within the tissue-draining lymph node. Work in an autochthonous mouse prostate cancer model found that prostate-antigen specific Treg preferentially reside in prostate-draining lymph nodes, and are expanded upon tumor growth by tumor-expressed self-antigen [32, 34]. These Treg then infiltrate tumors as they grow and suppress effector T cell responses [35]. Whether these tissue-specific Treg are able to traffic to metastases in other tissue sites is largely unknown. Interestingly, biopsies from human tumors have provided evidence of increased Treg in primary tumors compared to metastatic lesions [28]. Different metastases within a patient could therefore harbor different Treg populations, depending on which tissue site they grow in (Fig 1c). This could potentially affect the level of immune suppression in different lesions, contributing to different immune responses and different responses to CBT between metastases.
Different T cell responses mediated by local factors in the tumor microenvironment
After T cell activation occurs, local factors within the tumor microenvironment can further modulate a T cell response. Recently described examples include expression of Fas-L and TGF-β. While reports of Fas-FasL mediated T cell apoptosis as a form of immune suppression date back to the 1990’s, recent studies have again highlighted the importance of TIL apoptosis in dampening anti-tumor immune responses [36, 37]. Several groups have shown that apoptosis of both endogenous and transferred T cells in the tumor environment is a key obstacle limiting anti-tumor immunity. These studies found that decreasing T cells’ ability to undergo cell death, whether by inhibiting FAS signaling or through the overexpression of anti-apoptotic molecules, increases anti-tumor immune responses and synergizes with immunotherapy. Both a neutralizing antibody and the introduction of a dominant-negative FAS receptor into CAR T cells was effective at decreasing CD8+ T cell apoptosis and increasing tumor control in mouse models [37, 38]. This suggests that inhibition of FAS-FASL interactions could synergize with current modes of immunotherapy. Interestingly, FASL is differentially expressed across both normal tissues and tumor sites, suggesting the extent of FAS-mediated T cell apoptosis could be tumor and tissue specific [38]. FAS-FASL interactions may also play a role in shaping tissue-resident T cell populations outside of the cancer setting [38, 39]. It was recently shown that healthy lung tissue expresses much higher levels of FASL than healthy skin, and that memory T cells express increased levels of FAS [38]. Another group found that pathogen-specific TRM T cells in lung are more prone to undergo apoptosis than those in skin [39]. Thus FAS-FASL interactions could be a tissue-specific mediator of T cell apoptosis in both normal immune responses and in tumors.
TGFβ is generally considered immune suppressive [40], but recent data suggests it plays a specific role in the tumor microenvironment by restraining T cell infiltration into tumors [41]. Powles and colleagues found that in metastatic urothelial cancer patients receiving anti-PD-L1 treatment could be grouped into one of three pre-treatment groups based on immune phenotype: immune inflamed, immune excluded, or immune desert. In the immune excluded group, tumors had immune cells restricted to the periphery of the tumor and T cells were often interacting with fibroblasts or stroma. In this group, the authors found that high fibroblast TGFβ expression correlated with stable or progressive disease, while lower TGFβ correlated with partial or complete responses [41]. In a mouse model, blocking TGFβ synergized with anti-PD-L1 to induce greater tumor regression and allowed for greater infiltration of T cells into tumors. It was not discussed if patients could exhibit multiple immune phenotypes, but it is imaginable that a patient with multiple lesions might have both immune-inflamed, excluded, and desert tumors, leading to localized immune responses that differ across tumor sites. What drives stroma to produce TGFβ and what determines whether this will restrict T cells’ ability to infiltrate tumors remains unknown, but should be explored further.
T cell exclusion via tumor cell-intrinsic factors
An obvious explanation for differential T cell infiltration into the TME is a substantially different expression of immunogenic antigens. This notion has been predominantly tested for mutationally derived neo-antigens. While human studies have found significant correlations between overall response to immunotherapy and the presence of highly immunogenic neo-antigens [42, 43], no correlation has been found between the absence of T cells and neo-antigen quantity or quality [44–46]. In contrast, multiple tumor cell-intrinsic pathways have now been associated with differences in T cell infiltration and resistance to CBT. The first pathway to be associated with differences in T cell infiltration of tumors was the Wnt/β-catenin pathway in metastatic melanoma [47]. Using TCGA, we found that increased Wnt/β-catenin signaling was correlated with decreased T cell infiltration into the tumor. This analysis has subsequently been expanded to at least 19 cancer types including bladder and ovarian cancer [48, 49]. The mechanism blocking T cell infiltration was further associated with diminished infiltration by dendritic cells, which will be covered in more detail below [29, 47]. Other tumor cell-intrinsic signaling alterations associated with T cell exclusion include PTEN, Myc, Cox1/2, PPARγ, and FGF3 [48, 50–52]. It is therefore plausible that as metastases in a patient evolve, some may acquire mutations in pathways that exclude an ongoing, systemic T cell response.
Impact of tissue-specific myeloid cells on anti-tumor T cell responses
Myeloid cell types exert a broad array of functions in the TME ranging from activation of tumor-specific T cell responses to local and systemic immune suppression [53]. Numerous studies have highlighted the tissue-specific distribution of myeloid cells [54] and therefore it is plausible that myeloid cell populations may also directly impact tumor-reactive T cells in individual metastatic lesions [53, 55–57]. Similarly, the developmental origin of myeloid cells from circulating vs. tissue-resident pools may impinge on their functions within the TME. Here we summarize our current knowledge on the impact of tissue-specific myeloid cell populations on anti-tumor T cell responses.
Conventional dendritic cells impact T cell function beyond T cell priming
Upon their development and initial seeding into peripheral tissues, DC rapidly ingest cell debris and particles from their surroundings through phagocytosis or pinocytosis [58, 59]. Pattern or Danger Associated Molecular Patterns (PAMPs or DAMPs, respectively) triggers DC maturation, resulting in a decrease in cell debris uptake, increases in surface expression of peptide bound Major Histocompatibility Complex receptors as well as costimulatory ligands. Migratory DC further upregulate the chemokine receptor CCR7 to traffic into draining lymph nodes [60]. Together, these highly specialized behaviors endow DC with unrivaled ability to activate T cells [61]. While all DC respond to danger signals by maturation, different subtypes orchestrate different T cell responses, and inadequate activation might result in immune suppressive DC phenotypes [62].
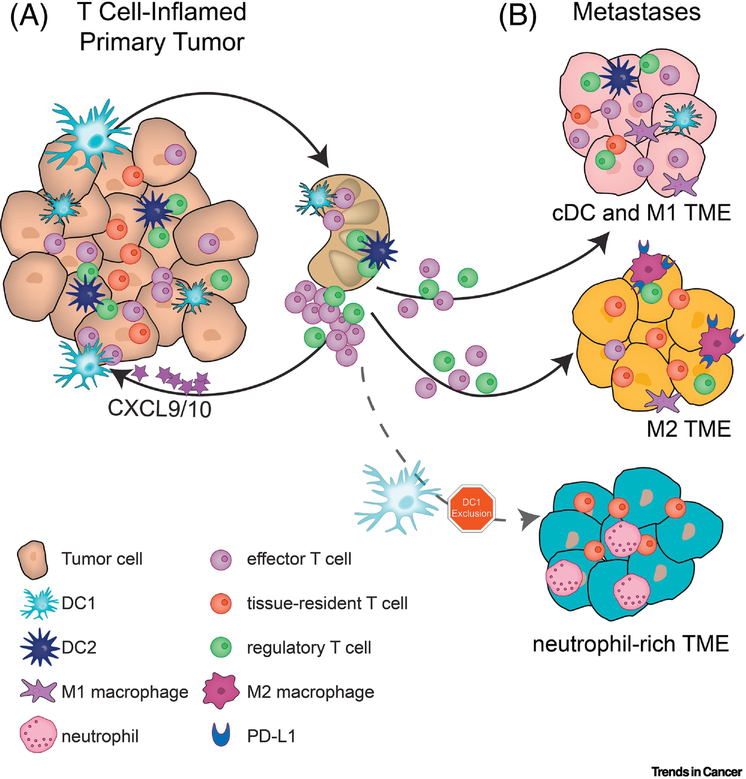
The most prominent DC in anti-tumor immunity are conventional DC type 1 (cDC1), which depend on the transcription factor Batf3 and express the integrin CD103 in peripheral tissues or CD8a homodimers in lymphoid organs [63, 64]. This subset of DC is especially adept at ingesting and cross-presenting tumor cell-derived antigens to CD8+ T cells, thereby inducing cytotoxic effector T cells [29, 60, 65]. Preclinical models of melanoma have identified unique roles for cDC1 beyond cross-priming T cells in draining lymph nodes. cDC1 residing within the TME secrete CXCL9 and CXCL10 to recruit effector T cells (Fig. 2a) [29, 65, 66]. This unique role of cDC1 ensures continuous infiltration and activation of CD8+ T cells into the tumor. cDC1 however are rare cells and can be excluded by tumor-intrinsic signaling through beta-catenin or the lipid signaling molecule prostaglandin-E2 (PGE2) in melanoma and ovarian cancer among others [47, 48, 50, 67, 68]. This experimental evidence aligns with sequencing data from primary tumors showing correlations between the presence of cDC1 in tumors and the presence of CD8+ T cells and also with improved patient outcomes, in breast, lung, and head and neck cancer [29, 69]. Interventions that stimulate cDC1 accumulation in melanomas can potently augment anti-PD1 or anti-CTLA4 immunotherapies, consistent with their role in regulating T cell responses to melanoma [70, 71]. These results primarily obtained in melanoma models indicate that cDC1s are required for a potent anti-tumor T cell response. Data beyond melanoma however strongly suggest that the role of cDC1 in the cancer immune cycle appears to be broadly applicable.
Figure 2 –
Myeloid cells act both systemically and locally to direct T cell responses in the tumor environment
A) DC1 mediate T cell activation in lymph nodes and recruit effector T cells to the local tumor environment through the secretion of CXCL9/10. While DC1 activate CD8+ T cells, DC2 can interact with Treg, and these interactions lead to enhanced Treg functions and blunted DC2 maturation. B) Tumors with high T cell infiltration are associated with DC1 and M1 macrophage infiltration. The presence of M2 macrophages is correlated with a suppressive microenvironment and high PD-L1 expression, however the state of T cell infiltration may vary. Tumors lacking T cell infiltration are characterized by a lack of DC1 and the recruitment of neutrophils.
Besides cross-presenting cDC1, other subsets of DC haJournal Pre-proofve been shown to impact anti-tumor immunity [72, 73]. Conventional DC type 2 (cDC2) present tumor-derived antigens to activate CD4+ T cells but can also interact directly with Tregs, driving a suppressive T cell response. In fact, a cross-talk between Tregs and cDC2 has been described in pancreas and melanoma cancer models, resulting both in enhanced Treg function but also inadequate cDC2 maturation [74–76]. However, patient data suggests a positive correlation between a cDC2 signature and overall survival for melanoma, HNSCC, and lung cancer cohorts, indicating that cDC2 can be both immune potentiating as well as immune dampening [75, 77]. Additional studies are required to fully elucidate the role of cDC2 on anti-tumor immunity and whether this role might differ depending on the organ site.
Macrophages impact anti-tumor T cell responses in two distinct fashions
Macrophages constitute a diverse set of myeloid cells whose functions are intimately linked with the properties of their surrounding tissue [78, 79]. While they can impact the adaptive immune response through antigen presentation and cytokine production, macrophages specialize in uptake and digestion of cellular debris and pathogens [80]. Their plasticity is evident during the response to infection, and is mirrored in the tumor microenvironment: macrophages can adopt an inflammatory phenotype that participates in tumor destruction, but more frequently mature to subtypes responsible for tissue repair and neoangiogenesis which support tumor growth [81]. These opposing roles have been designated M1 and M2 respectively, which in vivo are thought to correspond to a continuum of activation states [82, 83]. In colon cancer, macrophages are placed at the nexus of an inflammatory state that has been well-documented as a driver of this malignancy, for instance through secretion of PGE2 [84, 85]. Chemokines such as colony-stimulating factor recruit macrophages to the colonic epithelium, where they may exclude T cells or become major sources of PD-L1 (Fig. 2b) [52, 86, 87]. While tumor-promoting macrophages may dominate the tumor microenvironment, it is critical to note that tumors do not induce de novo macrophage functions but coopt existing cell behaviors. Indeed, macrophages deprived of M2-polarizing signals participate with DC and T cells in the anti-tumor immune response in breast cancer [88, 89]. Given that macrophage populations differ drastically form one anatomic site to the other it is plausible that the immune dampening effects might differ between sites in the metastatic setting.
Monocytes and Neutrophils – friend or foe?
A common feature of neutrophil and monocyte biology is that immature states, defined differently for different subsets [79], are by default immune suppressive. As tumors often lack required maturation signals for infiltrating neutrophils and monocytes, these cell types accumulate within the TME in their immature state and are often referred to as myeloid-derived suppressor cells (MDSC). Many studies have documented the tumor-promoting effects of immature granulocyte lineage cells in a variety of tumor types [90]. This cell population has been designated on a functional basis with little insights into their ontogeny, but a recent study linked ER stress with differentiation of granulocyte-lineage cells into MDSC marked by Lectin-type Oxidized LDL Receptor-1 [91]. Although the drivers of this differentiation remain unclear, this marker identified substantial increases in circulating MDSC in patients bearing colon, head and neck, and lung cancers, but not melanoma. Mature neutrophils are short-lived members of the myeloid lineage and are the most abundant nucleated cells in circulation. Neutrophils have been reported to both collaborate with and protect against metastatic tumor cells during seeding to the lung and are frequently associated with circulating tumor cells [92–94]. Further, accumulation of neutrophils in lung cancer lesions has been associated with T cell exclusion in LKB1-positive lung cancer patients [95] (Fig. 2b). Similar to DC and macrophages, neutrophils can come in many flavors and we are only beginning to appreciate the impact of different neutrophil subsets on tumor progression and anti-tumor immunity. For example, a recent study identified a novel neutrophil subset defined by expression of type I interferon sensitive genes. Presence of this subset, but not other neutrophil subsets, negatively correlated with survival in lung cancer patients, offering clues to the potential mechanisms underlying their tumor promoting effects [77].
Monocytes are circulating precursor cells which upon stimulation can differentiate into highly plastic macrophage-like or DC-like states. The most prominent example impacting anti-tumor immune responses are monocyte-derived DC (moDC) producing high levels of TNF-alpha and iNOS, so called TiP-DC [96]. This subset has been correlated with increased anti-tumor immune responses in colon cancer via CD40:CD40 ligand interaction. The exact cues required to mediate TiP-DC differentiation versus induction of MDSC remain somewhat elusive. Notably, monocyte-derived DC are the basis of most DC vaccination therapies, which can mediate potent tumor control of melanoma [97].
Concluding Remarks
Recent findings in mice and humans have provided evidence that the anatomic site of tumor growth can greatly impact response to immunotherapy. Specifically, metastases in some organs respond to CBT at much higher rates than metastases in other organs, indicating an underappreciated role of tissue-specific immune responses against cancer [14, 15]. These heterogeneous responses pose a clinical problem, as patients with responses to CBT in all lesions survive longer than patients with responses in only some lesions [98]. Determining how the tissue microenvironment impacts anti-tumor immunity and the response to CBT could facilitate the development of new strategies to improve patient survival. Possible mechanisms by which tissue and organ environments impact anti-tumor immunity range from evasion of the immune system through myeloid cell exclusion [47], skewing which T cell repertoires become activated in response to tumor growth [32], to local factors such as inducing T cell apoptosis in the tumor microenvironment [36]. However, the interplay among these is ill-defined, and the role of tissue-resident and tissue-specific T cells in anti-tumor immunity is not well understood (see Outstanding Questions). Tissue resident T cells have been shown to both promote and suppress tumor progression, potentially dependent on the context [20, 25]. Similarly, few studies delineate tissue-resident vs infiltrating myeloid cell populations [77], leaving unclear how cell origin and exposure to tumor cells dictates the ensuing myeloid response. Further, local physical features such as lymphangiogenesis impact how tumors access the lymphatics, affecting how they trigger immune system activation [27]. At the same time other features of the host environment, especially commensal bacteria, have also been shown to have significant impacts on both tissue resident immune cells and anti-tumor immune responses [19, 99]. An immense amount of work will be needed to fully elucidate and integrate the importance of these complex interactions affecting anti-tumor immune responses at metastatic sites.
Outstanding Questions.
What factors define the tumor-reactive T cell pool at a given tissue site?
What features of the immune response to a primary tumor ensure the generation of a systemic immune response?
Can immune suppressive T cell populations in one tissue site impact systemic immunity?
Are myeloid responses restricted to one metastatic lesion or can they be systemic as well?
Can certain myeloid cell subsets suppress or potentiate existing systemic T cell immunity?
Clinical correlates will be critical to understand the impact of organ-site specific immune responses on the responsiveness of tumors to immunotherapy. Reporting the responses of every lesion in an individual patient, instead of overall changes in tumor burden, will help to determine patterns of response between tumors and patients. Pairing changes in tumor size with information from biopsies of individual tumors, such as genetic and transcriptomic information, will allow for more meaningful biological conclusions from immunotherapy clinical trials. These approaches will help to increase our understanding of the complex web of interactions that determines immunotherapy response, and help guide us towards improving immunotherapy efficacy.
Highlights.
Generating CD8+ T cell responses to primary or metastatic lesions depends on both lymphoid and myeloid cell populations that impact CD8+ effector T cell activation and infiltration into the tumor microenvironment.
T cell responses may be limited to specific tissue sites and are not detectable in all metastatic tumors.
Type 1 conventional dendritic cells are indispensable for initiating an anti-tumor T cell response,
M2 type macrophages and neutrophils can dampen the anti-tumor response or even mediate T cell exclusion.
Acknowledgments
Funding: This work was supported by the NCI K99/R00 award, R00CA204595. SS is the Howard S (1953) & Linda B Stern CD Assistant Professor. TBF is an Irvington Postdoctoral Fellow of the Cancer Research Institute and BLH was supported by the Ludwig Center for Metastasis.
Footnotes
Conflict of Interest: BLH and TBF have nothing to declare. SS is member of the SAB of Venn Therapeutics, Tango Therapeutics, Replimune and serves as an advisor for Dragonfly Therapeutics, Merck, Ribon, Torque and TAKEDA. There is no conflict between those activities and this review article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ribas A, et al. , Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol, 2005. 23(35): p. 8968–77. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR, et al. , Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol, 2010. 28(19): p. 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galon J, et al. , Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science, 2006. 313(5795): p. 1960–4. [DOI] [PubMed] [Google Scholar]
- 4.Spranger S, et al. , Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med, 2013. 5(200): p. 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumeh PC, et al. , PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 2014. 515(7528): p. 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger S, et al. , Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer, 2014. 2: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DS and Mellman I, Oncology meets immunology: the cancer-immunity cycle. Immunity, 2013. 39(1): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 8.Spranger S, Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol, 2016. 28(8): p. 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JB, et al. , The EGR2 targets LAG-3 and 4–1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med, 2017. 214(2): p. 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leach DR, Krummel MF, and Allison JP, Enhancement of antitumor immunity by CTLA-4 blockade. Science, 1996. 271(5256): p. 1734–6. [DOI] [PubMed] [Google Scholar]
- 11.Iwai Y, et al. , Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A, 2002. 99(19): p. 12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayers M, et al. , IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest, 2017. 127(8): p. 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji RR, et al. , An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother, 2012. 61(7): p. 1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid S, et al. , Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother, 2018. 67(12): p. 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoja L, et al. , Patterns of response to anti-PD-1 treatment: an exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients. Br J Cancer, 2016. 115(10): p. 1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JHJ, et al. , Metastasis-specific patterns of response and progression with anti-PD-1 treatment in metastatic melanoma. Pigment Cell Melanoma Res, 2018. 31(3): p. 404–410. [DOI] [PubMed] [Google Scholar]
- 17.DuPage M, et al. , Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell, 2011. 19(1): p. 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPage M, et al. , Expression of tumour-specific antigens underlies cancer immunoediting. Nature, 2012. 482(7385): p. 405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin C, et al. , Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell, 2019. 176(5): p. 998–1013 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi NS, et al. , Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity, 2015. 43(3): p. 579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djenidi F, et al. , CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol, 2015. 194(7): p. 3475–86. [DOI] [PubMed] [Google Scholar]
- 22.Ganesan AP, et al. , Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol, 2017. 18(8): p. 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards J, et al. , CD103(+) Tumor-Resident CD8(+) T Cells Are Associated with Improved Survival in Immunotherapy-Naive Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin Cancer Res, 2018. 24(13): p. 3036–3045. [DOI] [PubMed] [Google Scholar]
- 24.Schenkel JM, et al. , T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science, 2014. 346(6205): p. 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik BT, et al. , Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol, 2017. 2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelova M, et al. , Evolution of Metastases in Space and Time under Immune Selection. Cell, 2018. 175(3): p. 751–765 e16. [DOI] [PubMed] [Google Scholar]
- 27.Mlecnik B, et al. , The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med, 2016. 8(327): p. 327ra26. [DOI] [PubMed] [Google Scholar]
- 28.Van den Eynde M, et al. , The Link between the Multiverse of Immune Microenvironments in Metastases and the Survival of Colorectal Cancer Patients. Cancer Cell, 2018. 34(6): p. 1012–1026 e3. [DOI] [PubMed] [Google Scholar]
- 29.Spranger S, et al. , Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell, 2017. 31(5): p. 711–723 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo Y, et al. , Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small cell lung cancer. Ann Oncol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lathrop SK, et al. , Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med, 2008. 205(13): p. 3105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malchow S, et al. , Aire-dependent thymic development of tumor-associated regulatory T cells. Science, 2013. 339(6124): p. 1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong MT, et al. , A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity, 2016. 45(2): p. 442–56. [DOI] [PubMed] [Google Scholar]
- 34.Leonard JD, et al. , Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity, 2017. 47(1): p. 107–117 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klages K, et al. , Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res, 2010. 70(20): p. 7788–99. [DOI] [PubMed] [Google Scholar]
- 36.Horton BL, et al. , Intratumoral CD8(+) T-cell Apoptosis Is a Major Component of T-cell Dysfunction and Impedes Antitumor Immunity. Cancer Immunol Res, 2018. 6(1): p. 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, et al. , Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun, 2017. 8(1): p. 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto TN, et al. , T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J Clin Invest, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slutter B, et al. , Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol, 2017. 2(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batlle E and Massague J, Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity, 2019. 50(4): p. 924–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariathasan S, et al. , TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature, 2018. 554(7693): p. 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGranahan N, et al. , Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science, 2016. 351(6280): p. 1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder A, et al. , Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med, 2014. 371(23): p. 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danilova L, et al. , Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci U S A, 2016. 113(48): p. E7769–E7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spranger S, et al. , Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A, 2016. 113(48): p. E7759–E7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Allen EM, et al. , Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science, 2015. 350(6257): p. 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spranger S, Bao R, and Gajewski TF, Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature, 2015. 523(7559): p. 231–5. [DOI] [PubMed] [Google Scholar]
- 48.Sweis RF, et al. , Molecular Drivers of the Non-T-cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol Res, 2016. 4(7): p. 563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luke JJ, et al. , WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin Cancer Res, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casey SC, et al. , MYC regulates the antitumor immune response through CD47 and PD-L1. Science, 2016. 352(6282): p. 227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng W, et al. , Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov, 2016. 6(2): p. 202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prima V, et al. , COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A, 2017. 114(5): p. 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engblom C, Pfirschke C, and Pittet MJ, The role of myeloid cells in cancer therapies. Nat Rev Cancer, 2016. 16(7): p. 447–62. [DOI] [PubMed] [Google Scholar]
- 54.Guilliams M, et al. , Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity, 2016. 45(3): p. 669–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binnewies M, et al. , Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med, 2018. 24(5): p. 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitamura T, Qian BZ, and Pollard JW, Immune cell promotion of metastasis. Nat Rev Immunol, 2015. 15(2): p. 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Headley MB, et al. , Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature, 2016. 531(7595): p. 513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarez D, Vollmann EH, and von Andrian UH, Mechanisms and consequences of dendritic cell migration. Immunity, 2008. 29(3): p. 325–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez-Martinez E, et al. , Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets. Front Immunol, 2015. 6: p. 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts EW, et al. , Critical Role for CD103 + /CD141 + Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell, 2016. 30(2): p. 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guermonprez P, et al. , Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol, 2002. 20: p. 621–67. [DOI] [PubMed] [Google Scholar]
- 62.Dudziak D, et al. , Differential Antigen Processing by Dendritic Cell Subsets in Vivo. Science, 2007. 315(5808): p. 107–111. [DOI] [PubMed] [Google Scholar]
- 63.Hildner K, et al. , Batf3 Deficiency Reveals a Critical Role for CD8 + Dendritic Cells in Cytotoxic T Cell Immunity. Science, 2008. 322(5904): p. 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edelson BT, et al. , Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α + conventional dendritic cells. The Journal of Experimental Medicine, 2010. 207(4): p. 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broz Miranda L., et al. , Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell, 2014. 26(5): p. 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engelhardt JJ, et al. , Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell, 2012. 21(3): p. 402–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zelenay S, et al. , Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell, 2015. 162(6): p. 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Motz GT, et al. , Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med, 2014. 20(6): p. 607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barry KC, et al. , A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nature Medicine, 2018. 24(8): p. 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salmon H, et al. , Expansion and Activation of CD103 + Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity, 2016. 44(4): p. 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Böttcher JP, et al. , NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell, 2018. 172(5): p. 1022–1037.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radford KJ, Tullett KM, and Lahoud MH, Dendritic cells and cancer immunotherapy. Curr Opin Immunol, 2014. 27: p. 26–32. [DOI] [PubMed] [Google Scholar]
- 73.Ma Y, et al. , Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity, 2013. 38(4): p. 729–41. [DOI] [PubMed] [Google Scholar]
- 74.McLachlan JB, et al. , Dendritic Cell Antigen Presentation Drives Simultaneous Cytokine Production by Effector and Regulatory T Cells in Inflamed Skin. Immunity, 2009. 30(2): p. 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binnewies M, et al. , Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity. Cell, 2019. 177(3): p. 556–571.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang J-E, et al. , Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Reports, 2017. 20(3): p. 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zilionis R, et al. , Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Consortium t.I.G., et al. , Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology, 2012. 13(11): p. 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geissmann F, et al. , Development of Monocytes, Macrophages, and Dendritic Cells. Science, 2010. 327(5966): p. 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wynn TA, Chawla A, and Pollard JW, Macrophage biology in development, homeostasis and disease. Nature, 2013. 496(7446): p. 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantovani A, et al. , Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology, 2002. 23(11): p. 549–555. [DOI] [PubMed] [Google Scholar]
- 82.Mosser DM and Edwards JP, Exploring the full spectrum of macrophage activation. Nature Reviews Immunology, 2008. 8(12): p. 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruffell B and Coussens LM, Macrophages and therapeutic resistance in cancer. Cancer Cell, 2015. 27(4): p. 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erreni M, Mantovani A, and Allavena P, Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenvironment, 2011. 4(2): p. 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko SC, et al. , Paracrine cyclooxygenase-2-mediated signalling by macrophages promotes tumorigenic progression of intestinal epithelial cells. Oncogene, 2002. 21(47): p. 7175–86. [DOI] [PubMed] [Google Scholar]
- 86.Mroczko B, et al. , Serum macrophage-colony stimulating factor levels in colorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin Chim Acta, 2007. 380(1–2): p. 208–12. [DOI] [PubMed] [Google Scholar]
- 87.Llosa NJ, et al. , The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov, 2015. 5(1): p. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolny C, et al. , HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell, 2011. 19(1): p. 31–44. [DOI] [PubMed] [Google Scholar]
- 89.Ruffell B, et al. , Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell, 2014. 26(5): p. 623–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bronte V, et al. , Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun, 2016. 7: p. 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Condamine T, et al. , Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol, 2016. 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spiegel A, et al. , Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discovery, 2016. 6(6): p. 630–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Granot Z, et al. , Tumor Entrained Neutrophils Inhibit Seeding in the Premetastatic Lung. Cancer Cell, 2011. 20(3): p. 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huh SJ, et al. , Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res, 2010. 70(14): p. 6071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koyama S, et al. , STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Research, 2016. 76(5): p. 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marigo I, et al. , T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell, 2016. 30(4): p. 651. [DOI] [PubMed] [Google Scholar]
- 97.Gross S, et al. , Twelve-year survival and immune correlates in dendritic cell-vaccinated melanoma patients. JCI Insight, 2017. 2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gauci ML, et al. , Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome upon Treatment Discontinuation. Clin Cancer Res, 2019. 25(3): p. 946–956. [DOI] [PubMed] [Google Scholar]
- 99.Matson V, et al. , The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science, 2018. 359(6371): p. 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]