Summary
By using negatively charged Coomassie brilliant blue G-250 dye to induce a charge shift on proteins, blue native polyacrylamide gel electrophoresis (BN-PAGE) allows resolution of enzymatically active multiprotein complexes extracted from cellular or subcellular lysates while retaining their native conformation. BN-PAGE was first developed to analyze the size, composition, and relative abundance of the complexes and supercomplexes that form the mitochondrial respiratory chain and OXPHOS system. Here, we present a detailed protocol of BN-PAGE to obtain robust and reproducible results.
For complete details on the use and execution of this protocol, please refer to Lobo-Jarne et al. (2018) and Timón-Gómez et al. (2020).
Graphical Abstract
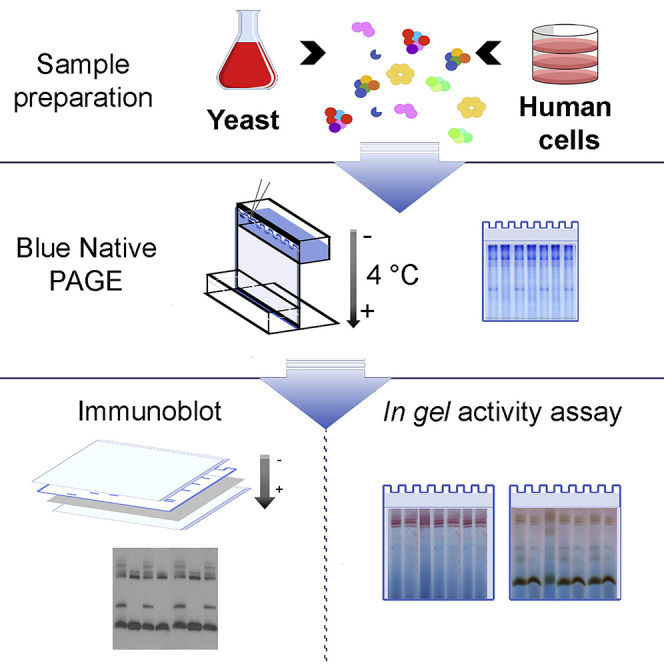
Highlights
-
•
Optimized BN-PAGE protocol to resolve respiratory complexes and supercomplexes
-
•
The protocol can be applied to isolated mitochondria, cultured cells and tissue extracts
-
•
Guidelines to couple BN-PAGE to downstream applications, such as SDS-PAGE, WB, IGA, or MS
By using negatively charged Coomassie brilliant blue G-250 dye to induce a charge shift on proteins, blue native polyacrylamide gel electrophoresis (BN-PAGE) allows resolution of enzymatically active multiprotein complexes extracted from cellular or subcellular lysates while retaining their native conformation. BN-PAGE was first developed to analyze the size, composition, and relative abundance of the complexes and supercomplexes that form the mitochondrial respiratory chain and OXPHOS system. Here, we present a detailed protocol of BN-PAGE to obtain robust and reproducible results.
Before You Begin
Preparation of Buffers and Reagents
Timing: 0.5–2 h
-
1.
Prepare cell culture media and buffers as described in Materials and Equipment.
-
2.
Yeast strains are grown at 30°C using standard procedures (Sherman, 2002).
-
3.
Human cell lines are cultured at 37°C in an incubator supplied with 5% CO2.
Preparation of Whole-Cell Lysates or Isolation of Mitochondria
Timing: 2–6 h
The Blue Native PAGE (BN-PAGE) method can be used to characterize mitochondrial respiratory chain (MRC) subcomplexes, complexes, and supercomplexes ranging between 50 kDa to 10 MDa, extracted from different types of biological materials (e.g., cultured cells or tissues) and organisms. Here, we will explain how to analyze MRC complexes and supercomplexes from yeast strains and human cultured cell lines. MRC complexes and supercomplexes can be isolated from whole-cell lysates and mitochondria, with similar results. However, when detection of low abundance proteins is intended (e.g., subassembly intermediates, assembly factors, or transient interactors), the use of isolated mitochondria is recommended. On the other hand, if the objective is to examine potential modifications in MRC organization under changing nutritional or environmental conditions (e.g., oxygen tension, oxidative stress, carbon source availability), performing the analysis in whole cells will minimize manipulations that could alter the outcome of the experiment. In this section, we describe, in parallel, how to obtain and prepare samples from yeast and human cells, either as whole-cell lysate or isolated mitochondria.
Note: The BN-PAGE method has been applied to multiple fields when required isolating protein complexes, including membrane proteins, under native conditions. For example, it has been used to analyze protein import complexes in mitochondria, chloroplasts, and peroxisomes (Chen and Li, 2017; Okumoto et al., 2017), cytoplasmic complexes such as the proteasome (Couttas et al., 2011), or the photosynthetic complexes present in chloroplasts (Rantala et al., 2018). However, the optimal protein complex extraction conditions and the gradient percentage range of the polyacrylamide gels may differ for each application.
Note: The BN-PAGE method can be applied to virtually any cell type and tissue homogenates. In each case, the optimal protein MRC complex extraction conditions need to be empirically determined. The conditions explained in this article have been optimized for the yeast S. cerevisiae and several human (HeLa, cervical cancer; HEK293T, embryonic kidney; U87, glioblastoma; and 143B, osteosarcoma) and mouse (MEF, embryonic fibroblasts) cell lines grown in culture.
Note: This protocol has been optimized from a procedure previously published by our group (Diaz et al., 2009) based on the original protocol developed by Schägger and von Jagow (Schagger and von Jagow, 1991).
Preparation of Human Whole-Cell Lysates
-
4.
Grow the human cells in complete medium to 70%–80% confluency (preferably in a 10-cm dish or a T-25 flask). The yield is approximately 2–3 × 106 HeLa or HEK293T cells. Cells should not be overgrown, and the culture medium needs to be changed 24 h before harvesting them to prevent nutrient exhaustion or media acidification that could affect the structural and functional integrity of the organelles.
Note: Most common cell lines are grown in DMEM, but use any other medium (e.g., RPMI) if required for your specific cell line.
Note: Specially-supplemented medium is needed when using cells with defects in the mitochondrial respiratory chain and OXPHOS system. See Materials and Equipment for more information. In certain mutant cell lines, the culture medium needs to be changed every 1 or 2 days to prevent excessive acidification that readily compromises cell survival.
Note: This method can be used to analyze cell lines in different environmental, nutritional, or stress conditions. Any stress or nutrient can be added to the media when necessary, or the cell cultures can be placed in any condition relevant to the study.
-
5.
Harvest cells by trypsinization and resuspend them in culture medium.
-
6.
Assess the cell concentration (we use the TC20™ Automated Cell Counter from Bio-Rad) and transfer 2.5 × 106 cells per sample to a 1.5 mL microcentrifuge tube.
-
7.
Wash the cells twice with 1 mL ice-cold PBS and resuspend them in 200 μL of ice-cold PBS.
-
8.
Add 55 μL of digitonin (Sigma) of a concentration of 8 mg/mL in H2O (final concentration of 1.7 mg/mL) and incubate on ice for 10 min, to solubilize the plasma membrane.
-
9.
Add 1 mL of ice-cold PBS to dilute the digitonin and centrifuge at 20,000 × g for 5 min. Washing is critical to detect higher molecular weight supercomplexes (see Troubleshooting).
-
10.
Remove the supernatant and repeat step 9.
-
11.
Discard the supernatant. Cell pellets are ready to use or can be stored at −80°C.
Preparation of Human Mitochondria
-
12.
Grow cells in complete DMEM, as described in the previous section, until they reach 70%–80% confluency in three T-175 flasks or 15-cm dishes (in total, approximately 3–4 × 107 HeLa or HEK293T cells).
Note: Isolate fresh mitochondria for each experiment.
-
13.
Harvest the cells by trypsinization, pellet them at 600 × g for 5 min and wash them twice with an ice-cold isotonic medium as PBS.
-
14.
Isolate intact mitochondria using the following protocol (Fernandez-Vizarra et al., 2010). Place the cell pellets on ice, induce their swelling by adding one volume of hypotonic homogenization buffer (HypoB: 3.5 mM Tris-HCl, pH 7.8, 2.5 mM NaCl, 0.5 mM MgCl2), and homogenize them by ten strokes using a manual glass-Teflon homogenizer with smooth pestle (Thomas). Then, to make the medium isotonic, add 1/10 of the packed cell volume of hypertonic buffer (HyperB: 0.35 M Tris-HCl, pH 7.8, 0.25 M NaCl, 50 mM MgCl2). Centrifuge the homogenate at 1,200 × g for 3 min at 4°C to pellet cell debris and nuclei, and collect the supernatant containing mitochondria. Repeat the centrifugation at the same speed to further pellet any traces of heavy contaminants. Transfer the supernatant to microtubes (approximately 1 mL per tube) and pellet the mitochondria by centrifugation in a microfuge at 15,000 × g for 2 min at 4°C. Wash the pellets in STE buffer (0.32 M sucrose, 1 mM EDTA, and 10 mM Tris-HCl, pH 7.4) 3–4 times while transferring all the pellets to a single microtube. Resuspend the final mitochondrial pellet in STE at a concentration of ∼10–15 mg/mL.
-
15.
Precisely quantify protein concentration in the mitochondrial sample (e.g., by the Lowry procedure (Lowry et al., 1951)) and prepare aliquots of 200–400 μg. Use fresh mitochondria for the next steps. Alternatively, snap-freeze aliquots in liquid nitrogen and store them at −80°C until further use.
Preparation of Yeast Mitochondria
-
16.
For each strain, grow a starter culture in 50 mL YPGal medium for 16 h, inoculate 10 mL of it into 2 × 800 mL YPGal medium, and culture for 16 h to a density of A600 = 0.8–2.
Note: For each strain, the divison time should be calculated to adjust the time of incubation required to reach the optimal density.
-
17.
To isolate yeast mitochondria with intact outer membrane (Horn et al., 2008), harvest the cells by centrifugation at 900 × g for 5 min and wash them once with 250 mL of distilled water. Resuspend the cells in 100 mM Tris-HCl pH 8.8–10 mM DTT solution using 1 mL per 2 g of cells and incubate them at 30°C for 10 min. Pellet the cells by centrifugation at 2,200 × g for 10 min, and wash them once with 250 mL of 1.2 M sorbitol. Resuspend the cells in cell-wall digestion buffer (1.2 M sorbitol, 20 mM K3PO4 pH 7.4, 0.4 mg/mL zymolyase-100T) using 1 mL of solution per 0.15 g of cells and incubate them at 30°C for 30 min precisely. Approximately 80% of the cells will be converted into spheroplasts due to cell-wall digestion. Pellet the spheroplasts by centrifugation at 1,250 × g for 10 min at 4°C and resuspend them in ice-cold homogenization buffer (0.6 M sorbitol, 10 mM Tris-HCl pH 7,5, 1 mM EDTA pH 8, 1 mM PMSF) using 1 mL of solution for every 0.15 g of cells. Homogenize the spheroplasts with ten strokes in a loose glass/Teflon dounce homogenizer with smooth pestle (Thomas), and centrifuge the homogenate at 1,700 × g for 5 min at 4°C to eliminate cell debris and nuclei. Repeat this step on the supernatant and centrifuge the second supernatant at 14,000 × g for 10 min at 4°C to obtain a crude pellet containing mitochondria. Resuspend the mitochondrial pellet in 10 mL of ice-cold SHE buffer (0.6 M sorbitol, 20 mM Hepes pH 7.4, 1 mM EDTA), and centrifuge 5 min at 1,700 × g to pellet and discard remaining cell debris. Centrifuge the supernatant at 14,000 × g for 10 min at 4°C, and resuspend the mitochondrial pellet in SHE buffer at a concentration of ∼10 mg/mL.
-
18.
Quantify protein concentration and prepare aliquots of 200–400 μg of purified mitochondria.
-
19.
Snap-freeze aliquots in liquid nitrogen and store them at −80°C when they are not used fresh.
Pause Point: Cellular and mitochondrial pellets should be freshly used for best results, particularly when analyzing digitonin-extracted MRC supercomplexes, which can easily disintegrate. However, the pellets can be stored at −80°C for several weeks, particularly if they are intended for analysis of MRC complexes extracted with the detergent n-dodecyl-β-δ-maltoside (DDM), also known as lauryl maltoside (LM), which disrupts respiratory supercomplexes into individual complexes.
CRITICAL: Do not freeze and thaw pellets multiple times to avoid sample degradation, protein complex disintegration, and artificial reduction of the respiratory chain activities assessed by in gel assays.
CRITICAL: To assess reproducibility, use at least three independent biological samples to perform all the experiments.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| ATP5A | Abcam | Cat# ab14748 RRID: AB_301447 |
| CORE2 | Abcam | Cat# ab14745 RRID: AB_2213640 |
| COX1 | Abcam | Cat# ab14705 RRID: AB_2084810 |
| COX5B | Santa Cruz | Cat# sc-374417 RRID: AB_10988066 |
| NDUFB11 | Abcam | Cat# ab183716 RRID: AB_2298378 |
| SDHA | Abcam | Cat# ab14715 RRID: AB_301433 |
| TOM20 | SantaCruz | Cat# sc-11415 RRID: AB_2207533 |
| β-ACTIN | Abcam | Cat# ab8227 RRID: AB_2305186 |
| 2˚ Ab-mouse | Rockland Immunochemicals | Cat# 610-103-121 RRID: AB_218457 |
| 2˚ Ab-rabbit | Rockland Immunochemicals | Cat# 611-1302 RRID: AB_219720 |
| Cox1 (yeast) | Abcam | Cat# 110270 |
| Sdh2 (yeast) | (Kim et al., 2012) | N/A |
| Atp2 (yeast) | (Liang and Ackerman, 1996) | N/A |
| Rip1 (yeast) | (Strogolova et al., 2012) | N/A |
| Porin (yeast) | Abcam | Cat# 110326 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco’s Modified Eagle Medium (DMEM) | Invitrogen | Cat# 11995 |
| Fetal bovine serum (FBS) | Sigma | Cat# 12303C |
| Uridine | Sigma | Cat# U3003 |
| Glutamax™ | Gibco | Cat# 35050-061 |
| Sodium Formate | Sigma | Cat# 71539 |
| Trypsin-EDTA | Sigma | Cat# T4049 |
| Bacto™ Yeast extract | BD | Cat# 212270 |
| Bacto™ Peptone | BD | Cat# 211820 |
| Galactose | Sunrise Science | Cat# 59-23-4 |
| 6-aminocaproic acid | Sigma | Cat# A2504 |
| Bis-Tris | Sigma | Cat# B9754 |
| Protease inhibitor cocktail | Sigma | Cat# P8340 |
| Coomassie Brilliant Blue G | Bio-Rad | Cat# M2838 |
| Apoferritin | Sigma | Cat# A3660 |
| Lauryl maltose neopentyl glycol (LMNG) | Anatrace | Cat# NG310 |
| n-dodecyl-β-δ-maltoside (DDM) | Sigma | Cat# 5172 |
| Digitonin | Sigma | Cat# D141 |
| Digitonin, High Purity | EMD Millipore | Cat# 300410 |
| Isopropanol | Sigma | Cat# 24137 |
| EDTA | Sigma | Cat# EX0550 |
| Trizma® (Tris base) | Sigma | Cat# T1503 |
| NaCl | Sigma | Cat# S3014 |
| Triton X-100 | Sigma | Cat# 9002-93-1 |
| Methanol | Sigma | Cat# 646377 |
| Glycine | Sigma | Cat# G7126 |
| Iodonitrotetrazolium chloride | Sigma | Cat# 18377 |
| NADH (Nicotinic acid dinucleotide sodium salt) | Sigma | Cat# N4256 |
| Diaminobenzidine tetrahydrochloride (DAB) | Sigma | Cat# D5905 |
| Cytochrome c | Sigma | Cat# C3131 |
| Sucrose | Sigma | Cat# S8501 |
| Critical Commercial Assays | ||
| SuperSignal™ West Femto Maximum Sensitivity Substrate | ThermoFisher | Cat# 34095 |
| 20× Native PAGE™ Running Buffer | Novex-Life Technologies | Cat# BN2001 |
| 20× Native PAGE™ Cathode Buffer Additive | Novex-Life Technologies | Cat# BN2002 |
| eBlot L1 PVDF Membrane Transfer Buffer, 5× | Genscript | Cat# L00733 |
| eBlot L1 PVDF Equilibration Buffer, 10× | Genscript | Cat# L00734 |
| BCA Protein Assay kit | Sigma-Aldrich | Cat# BCA1-1KT |
| Gel Drying Solution, 1× | Bio-Rad | Cat# 1610752 |
| Experimental Models: Cell Lines | ||
| HEK293T | ATCC | Cat# CRL-3216 RRID: CVCL_0063 |
| Experimental Models: Saccharomyces cerevisiae strains | ||
| W303 I0 | (Zambrano et al., 2007) | MATa, ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1, ρ+ I0 |
| Other | ||
| Pre-cast Native PAGE 4%–16% Bis-Tris gels | Invitrogen | Cat# BN2012BX10 |
| Pre-cast Native PAGE 3%–12% Bis-Tris gels | Invitrogen | Cat# BN2011BX10 |
| eBlot™ L1 Fast Wet Transfer System for Mini Gels | Genscript | Cat# L00686 |
| Immun-Blot® PVDF membrane | Bio-Rad | Cat# 1620177 |
| BioTrace™ NT nitrocellulose | Pall Corporation | Cat# 66485 |
| VWR® Pellet Mixer | VWR | Cat# 47747-370 |
| TC20™ Automated Cell Counter | Bio-Rad | Cat# 1450102 |
| Acrylamide Drying system | Invitrogen | Cat# NI2387 |
| Scanner | HP | ENVY 5660 |
| Teflon-glass homogenizer with smooth pestle | Thomas | 3431D94 (170 mm) 3431E04 (88 mm) |
Materials and Equipment
Alternatives: We use the eBlot™ L1 Fast Wet Transfer System for Mini Gels (GenScript) for a reproducible and fast transfer of high molecular weight complexes to a membrane. However, any wet transfer system can be used, as explained in the Step-By-Step Method Details section (see Troubleshooting).
Detergent stocks
-
•
n-Dodecyl-β-D-maltoside (DDM): 10% (w/v) in H2O. Prepare and store aliquots at −20°C for up to 1 year.
-
•
Lauryl maltose neopentyl glycol (LMNG): 10% (w/v) in H2O. Store aliquots at −20°C for up to 1 year.
-
•
Digitonin ∼50% (Sigma, D141): 8 mg/mL in H2O. Prepare before use the amount needed and use it fresh to avoid its precipitation. Used in sample preparation of human whole-cell lysates.
-
•
High-purity digitonin (EMD Millipore, 300410): 10% (w/v) in H2O. Heat at 95°C to dissolve it completely for 5–10 min and use it fresh. Following reconstitution, however, it can be stored in the refrigerator (4°C). Stock solutions are stable for up to 1 week at 4°C.
Note: The concentration of digitonin ∼50% (Sigma, D141) changes lot by lot. It is essential to take into account the effective concentration and prepare the solution accordingly. The 8 mg/mL is meant for a concentration of 50%.
Note: High-purity digitonin is at least 95% pure. However, digitonin is intrinsically unstable, and upon storage, the purity will gradually decrease. We recommend to not use one year after the vial was first open.
CRITICAL: Digitonin is toxic if inhaled. Handle it in a fume hood or with personal protective equipment.
Complete DMEM
| Reagent | Final Concentration | Stock Concentration | Add to 500 mL |
|---|---|---|---|
| DMEM (high glucose, glutamine, sodium pyruvate) | n/a | n/a | 500 mL |
| FBS | 10% (v/v) | 100% (v/v) | 50 mL |
| Glutamax | 1× | 100× | 5 mL |
| Uridinea | 0.1 mg/mL | 10 mg/mL | 5 mL |
| Sodium Formatea | 3 mM | 1 M | 1.5 mL |
Store at 4°C for up to 2 months. Antibiotics and antimycotics can be added if necessary.
Supplement for MRC or OXPHOS deficient cells.
Yeast Growing Medium
| Reagent | Final Concentration | Stock Concentration | Add to 1 L |
|---|---|---|---|
| Yeast extract | 1% (w/v) | n/a | 10 g |
| Peptone | 2% (w/v) | n/a | 20 g |
| Galactose | 2% (w/v) | n/a | 20 g |
Sterilize by autoclaving. Store at 15°C–25°C for up to 1 month.
Aminocaproic Buffer
| Reagent | Final Concentration | Stock Concentration | Add to 50 mL |
|---|---|---|---|
| Aminocaproic acid | 1.5 M | n/a | 9.84 g |
| Bis-Tris pH 7 | 50 mM | 1 M | 2.5 mL |
Store, preferably at 4°C, for up to 6 months. Right before use, add 10 μL of Protease Inhibitor Cocktail per mL of buffer.
Native Loading Buffer 10×
| Reagent | Final Concentration | Stock Concentration | Add to 50 mL |
|---|---|---|---|
| Aminocaproic acid | 750 mM | n/a | 4.92 g |
| Bis-Tris pH 7 | 50 mM | 1 M | 2.5 mL |
| EDTA pH 8 | 0.5 mM | 500 mM | 50 μL |
| Coomassie Brilliant Blue G/ Serva Blue G | 5% (w/v) | n/a | 2.5 g |
Shake it at 15°C–25°C for 4–6 h until it is completely solved. Filter sterilize Native Loading buffer with a 0.2 μm pore filter, and prepare aliquots of 1 mL. Store aliquots at −20°C for up to 1 year.
eBlot L1 Transfer Buffer (GenScript) as Explained by the Supplier
| Reagent | Final Concentration | Stock Concentration | Add to 5 L |
|---|---|---|---|
| eBlot L1 PVDF Membrane Transfer buffer | 1× | 5× | 1,000 mL |
| Isopropanol | ≥99.5% (v/v) | n/a | 500 mL |
| Deionized H2O | n/a | n/a | 3,500 mL |
Store at 15°C–25°C for up to 6 months.
Transfer Buffer (Wet Transfer Standard Protocol)
| Reagent | Final Concentration | Stock Concentration | Add to 4 L |
|---|---|---|---|
| Tris base | 25 mM | n/a | 12.11 g |
| Glycine | 192 mM | n/a | 57.7 g |
| Methanol | 20% (v/v) | n/a | 800 mL |
| Deionized H2O | n/a | n/a | ~3 L |
Store at 15°C–25°C for up to 6 months.
Rinse Solution
| Reagent | Final Concentration | Stock Concentration | Add to 4 L |
|---|---|---|---|
| Tris-HCl pH 8 | 10 mM | 1 M | 40 mL |
| EDTA pH 8 | 1 mM | 0.5 M | 8 mL |
| NaCl | 150 mM | n/a | 34.8 g |
| Triton X-100 | 0.1% (v/v) | 10% (v/v) | 40 mL |
| Deionized H2O | n/a | n/a | ~3.9 L |
Store at 15°C–25°C for up to 1 year.
Coomassie Staining Solution
| Reagent | Final Concentration | Stock Concentration | Add to 10 mL |
|---|---|---|---|
| Methanol | 40% (v/v) | 100% (v/v) | 4 mL |
| Acetic acid | 7% (v/v) | 100% (v/v) | 0.7 mL |
| Coomassie brilliant blue R250 | 0.25% (w/v) | n/a | 25 mg |
Store at 15°C–25°C for up to 6 months.
Coomassie Destaining Solution
| Reagent | Final Concentration | Stock Concentration | Add to 100 mL |
|---|---|---|---|
| Methanol | 40% (v/v) | 100% (v/v) | 40 mL |
| Acetic acid | 7% (v/v) | 100% (v/v) | 7 mL |
Store at 15°C–25°C for up to 6 months.
CI activity Solution
| Reagent | Final Concentration | Stock Concentration | Add to 10 mL |
|---|---|---|---|
| Tris-HCl pH 7.4 | 2 mM | 1 M | 30 μL |
| Iodo nitro tetrazolium (red) or Nitro blue tetrazolium (blue-purple)a | 2.5 mg/mL | n/a | 25 mg |
| NADH | 0.1 mg/mL | n/a | 1 mg |
Prepare right before use, place it on a shaker at 15°C–25°C until it is dissolved, and filter it with a 0.2 μm pore filter. Use it freshly prepared, and discard any unused solution.
Note: Any reagent obtained from different suppliers must be validated.
CII activity Solution
| Reagent | Final Concentration | Stock Concentration | Add to 10 mL |
|---|---|---|---|
| Phosphate buffer pH 7.4 | 50 mM | 1M | 0.5 mL |
| Succinic acid | 84 mM | n/a | 99 mg |
| Phenazyne methasulfate | 0.2 mM | n/a | 0.6 mg |
| Nitro blue tetrazolium | 2 mg/mL | n/a | 20 mg |
| EDTA | 4.5 mM | 0.5 M | 90 μL |
| KCN | 10 mM | n/a | 6.5 mg |
Prepare right before use, place it on a shaker at 15°C–25°C until it is dissolved, and filter it with a 0.2 μm pore filter. Use it freshly prepared, and discard any unused solution.
CIV Activity Solution
| Reagent | Final Concentration | Stock Concentration | Add to 10 mL |
|---|---|---|---|
| DAB | 0.5 mg/mL | n/a | 5 mg |
| Phosphate buffer pH 7.4 | 0.05 mM | 1 M | 50 μL |
| Cytochrome c | 1 mg/mL | n/a | 10 mg |
| Sucrose | 75 mg/mL | n/a | 750 mg |
| Catalase | 0.02 mg/mL | n/a | 0.2 mg |
Prepare right before use, place it on a shaker at 15°C–25°C until it is dissolved, and filter it with a 0.2 μm pore filter. Use it freshly prepared, and discard any unused solution.
CV Activity Solution
| Reagent | Final Concentration | Stock Concentration | Add to 10 mL |
|---|---|---|---|
| Tris base | 35 mM | n/a | 42.4 mg |
| Glycine | 270 mM | n/a | 0.2 g |
| Magnesium sulfate | 14 mM | n/a | 16.85 mg |
| ATP | 5 mM | n/a | 25.36 mg |
| Lead nitrate | 0.2 % (w/v) | n/a | 20 mg |
Prepare right before use. Prepare the reaction with Tris, glycine, magnesium sulfate, and ATP in this order. Adjust pH to 7.8 and, then, add lead nitrate to the solution. Adjust pH to 8.6. The solution appears cloudy. Use it freshly prepared, and discard any unused solution.
Other Solutions
| Name | Reagents |
|---|---|
| Anode Buffer | 50 mL of 20× Native PAGE™ Running Buffer in 950 mL of deionized H2O. Store at 4°C for up to 6 months |
| Dark Blue Cathode Buffer | 50 mL of 20× Native PAGE™ Running Buffer and 50 mL of 20× Native PAGE™ Cathode Buffer Additive in 900 mL of deionized H2O. Store at 4°C for up to 6 months |
| Light Blue Cathode Buffer | 50 mL of 20× Native PAGE™ Running Buffer and 5 mL of 20× Native PAGE™ Cathode Buffer Additive in 945 mL of deionized H2O. Store at 4°C for up to 6 months |
| Equilibration Buffer | 15 mL of 10× eBlot L1 PVDF Membrane Equilibration buffer in 135 mL of deionized H2O. Store at 4°C for up to 6 months |
| Coomassie Blue Dye Washing Buffer | 62.5 mM Tris-HCl pH 6.8 and 2% SDS. Store at 15°C–25°C for up to 1 year |
Note: Any reagent obtained from different suppliers must be validated.
Step-by-Step Method Details
Sample Preparation: Day 1
Timing: 1–2 h
Following the collection of the samples, they need to be processed for analysis by BN-PAGE and immunoblotting or in gel activity assays. Sample processing includes the extraction in native conditions of the protein complexes embedded in the mitochondrial inner membrane and their preparation to be loaded onto a BN-PAGE gel.
-
1.
Thaw frozen aliquots or use freshly prepared samples.
-
2.
Re-isolate mitochondria by pelleting them by centrifugation at 14,000 × g for 10 min at 4°C. Whole-cell lysate pellets can be used directly.
-
3.
Resuspend the mitochondrial or whole-cell lysate pellets in aminocaproic acid buffer containing protease inhibitors (freshly added). Measure protein concentration at this stage, using the BCA Protein Assay kit (Sigma-Aldrich). Use 200–400 μg protein/sample in a total volume of 50–60 μL for yeast samples or 100 μL for human cells.
CRITICAL: Quantify protein concentration before adding the detergent. This will lead to reproducible results (see Troubleshooting).
Note: Digitonin-treated whole-cell lysate pellets are difficult to homogenize by pipetting. Use a pellet mixer for 3–4 s on ice to completely resuspend the pellet. If necessary, repeat until the suspension is homogeneous.
-
4.
Add the optimal amount of detergent to the sample and incubate for 10 min on ice.
Note: For the detection of individual subcomplexes (assembly intermediates) or complexes, use dodecyl maltoside (DDM). For the detection of supercomplexes, use high purity digitonin or lauryl maltose neopentyl glycol (LMNG). LMNG has been extensively used to determine the structural properties of mitochondrial membrane proteins by cryogenic electron microscopy (cryo-EM) (Parey et al., 2019; Tucker and Park, 2019), because it enhances the solubilization and stabilization of integral membrane proteins (Chae et al., 2010), facilitating crystal formation and data analysis.
Note: In the case of Saccharomyces cerevisiae respiratory complexes, individual complexes are detected upon extraction with DDM or LMNG. Otherwise, supercomplex association is preserved in the presence of digitonin or glycol-diosgenin (GDN). Both detergents have been used for yeast supercomplex structural determination by cryo-EM (Hartley et al., 2019; Rathore et al., 2019).
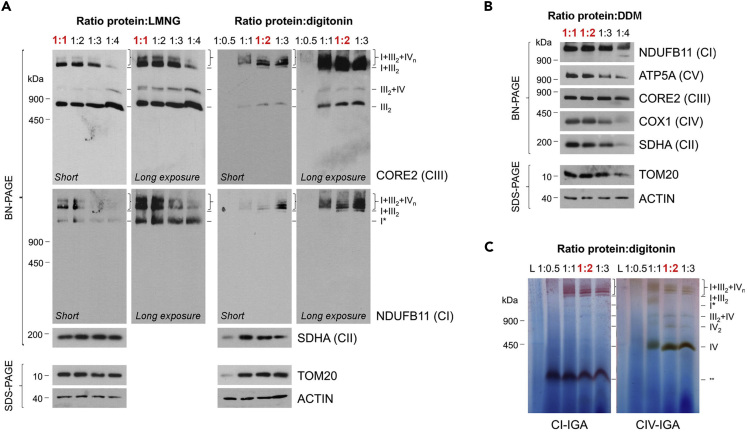
CRITICAL: The amount of detergent depends on the enzymatic complex and the cell line to be analyzed. It is recommended to start by performing a titration of the protein/detergent ratio to optimize the protocol before the analysis of a new cell line or when using a different supplier for the detergents (as in Figures 2A–2C). The amount and type of detergent need to be consistent in each replicate to be able to compare and quantify the results (see Troubleshooting).
Note: For human HEK293T cell lines, a protein/detergent ratio between 1:1–2 of DDM (Figure 2B) or 1:2–4 of high-purity digitonin (Figures 2A–2C and (Lobo-Jarne et al., 2018; Timón-Gómez et al., 2020)), and 1:1–3 of LMNG is recommended (Figure 2A). In Saccharomyces cerevisiae mitochondria, a protein/detergent ratio of 1:1–3 for DDM or digitonin is suggested (Figure 4B).
-
5.
After the incubation with the detergent, clarify the extracts by centrifuging the samples at 20,000 × g for 30 min at 4°C. Discard the pellet and carefully transfer the supernatant to a new microcentrifuge tube.
Note: If a benchtop ultracentrifuge is available, the centrifugation after incubation with the detergent can be done at 35,000 × g for 15 min at 4°C in an ultracentrifuge.
-
6.
Add 10 μL of Native loading buffer 10× to human samples. In yeast samples, add 5 μL of 50% glycerol and 2 μL of Native loading buffer 10×. Samples are ready to be loaded on to the gel.
-
7.
Load between 5–40 μL of the sample, depending on the type of analysis. Use an amount of 40–160 μg of protein for yeast samples, and 70–100 μg of protein to analyze human samples. A larger amount could be necessary for certain types of analysis, but it is necessary to take into account that excessive protein load leads to poorer resolution of high molecular weight complexes (see Troubleshooting).
Note: As a protein loading control, these samples can be separated in SDS-PAGE gels and analyzed by immunoblotting using antibodies against TOM20 or ACTIN in human (Figures 2A and 2B) or Porin in yeast. Add 1% of SDS to the native samples before loading them in SDS-PAGE gels.
Note: Samples extracted with DDM (or the remaining solution after loading the gel) can be stored at −80°C for up to 1 month for future use.
CRITICAL: Samples extracted with digitonin may undergo supercomplex disintegration and loss of in gel enzyme activities, and the BN-PAGE analysis of frozen samples may lose resolution. Thus, it is recommended to use the digitonin-treated samples freshly. Samples extracted with DDM, which does not preserve supercomplex integrity, are not affected by this step.
Figure 2.
Optimization of a BN-PAGE Experiment
(A and B) Analysis by BN-PAGE and immunoblotting of human HEK293T purified mitochondria extracted with increasing protein/detergent ratios of (A) digitonin and LMNG, and (B) DDM.
(C) CI (using iodonitrotetrazolium) and CIV in gel activity assays of purified HEK293T mitochondria extracted with increasing concentrations of digitonin.
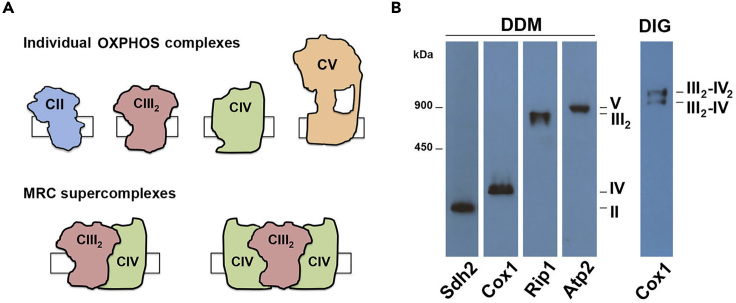
Figure 4.
Yeast Saccharomyces cerevisiae Respiratory Complexes and Supercomplexes
(A) Schematic representation of the OXPHOS individual complexes and MRC supercomplexes present in yeast mitochondria.
(B) Analysis by BN-PAGE and immunoblotting of yeast isolated mitochondria extracted with either DDM (protein/detergent ratio 1: 2) or digitonin (DIG, protein/detergent ration 1:1).
Electrophoresis: Day 1
Timing: 21 h
Mitochondrial respiratory chain complexes can be extracted as individual entities or as macromolecular associations (supercomplexes) and separated in acrylamide gradient gels for further analysis.
-
8.
Use pre-cast gels from Invitrogen. Different gradients are used according to the type of analysis:
-
a.
4%–16% Bis-Tris Gels, to visualize individual complexes and assembly intermediates after DDM extraction.
-
b.
3%–12% Bis-Tris Gels after digitonin or LMNG extraction, to analyze individual complexes and supercomplexes.
Note: Gels are available with 10 or 15 wells. 10-well gels facilitate the loading and visualization of the mitochondrial respiratory complexes.
Optional: Acrylamide gradient gels can be prepared as reported (Diaz et al., 2009), adjusting the percentages of acrylamide optimal for the different analyses.
-
9.
Fill the electrophoresis chamber with the anode buffer at the bottom and with the dark blue cathode buffer at the upper chamber.
-
10.
Before loading the samples, wash the wells (pipetting up and down with the cathode buffer) to remove traces of non-polymerized acrylamide.
-
11.
Load between 10–40 μL of sample in each well and a molecular weight marker.
Note: Commercial molecular weight markers are available depending on the size of the complexes we expect to analyze. Alternatively, use apoferritin prepared in the Native loading buffer 1×. Apoferritin, (molecular masses: monomer is 450 kDa; dimer is 900 kDa) is visible in the gel without staining, which provides a useful reference for the size of respiratory complexes and supercomplexes (Figure 2C).
-
12.
Run the gel at a constant voltage of 30 V for 1 h at 15°C–25°C. Then, change the dark blue cathode buffer for the light blue cathode buffer and run for 20 h at 30 V at 4°C (as shown in Figure 1A), to avoid overheating. The change of the buffer will help in the analysis of the samples by immunoblotting or enzymatic activity, by decreasing the amount of Coomassie blue dye in the gel.
Note: When analyzing DDM-extracted respiratory complexes, the electrophoretic voltage can be increased to 45 V for 20 h or to 70 V for 4–5 h.
Note: There is not a pause point after running the gel if BN-PAGE is combined with in gel activity assays or immunoblotting (see below). Only if it is combined with a second dimension SDS-PAGE (see Troubleshooting and Limitations sections), the cut gel strips from the first dimension (BN-PAGE) can be frozen at −20°C and stored for up to 2 weeks before running the second dimension (SDS-PAGE).
CRITICAL: It is highly recommended to run the samples until the Coomassie blue dye front runs out of the gel completely. In order to visualize small subcomplexes and the accumulation of unbound monomeric subunits, it is recommended to use a different gradient (e.g., 4%–16%), rather than running the gel for a shorter time (destaining process will be longer). It is easier to compare bands between different samples when the running time is constant. Twenty hours of electrophoresis allows for the separation of the bands corresponding to higher-order supercomplexes (CI+CIII2+CIVn). Do not leave the electrophoresis longer than 24 h to avoid compromising supercomplex stability.
Note: When using a large amount of protein (140–180 μg, depending on the cell line), it is possible to directly visualize MRC complexes and supercomplexes as Coomassie G250-stained bands migrating in the gel (as detected in Figure 2C). Alternatively, the gel can also be used for regular post-run staining with Coomassie Brilliant Blue R250.
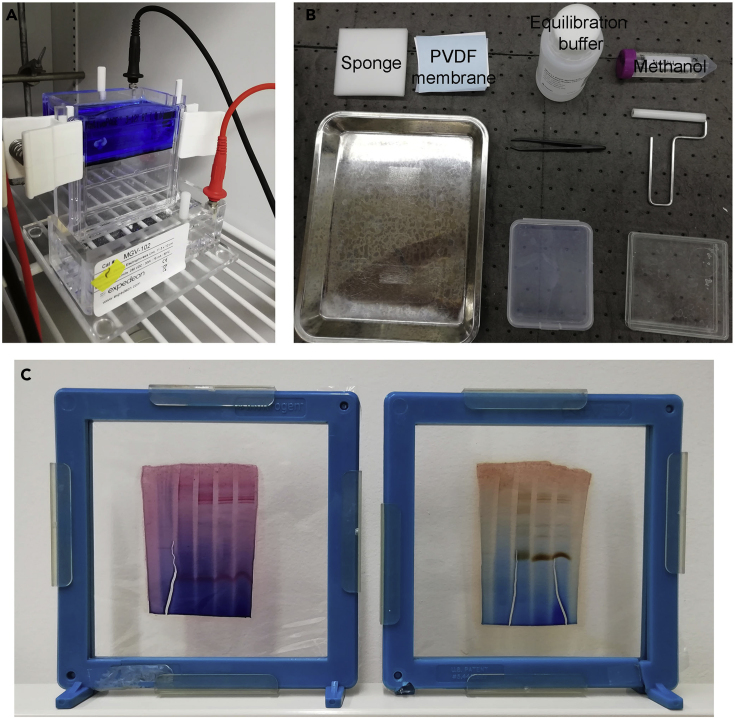
Figure 1.
Illustration of Several Steps of a BN-PAGE Experiment and Analysis
(A) Electrophoresis chamber running a 3%–12% BisTris gel at 4°C, after the removal of the dark blue cathode buffer and replacement with the light blue cathode buffer.
(B) Materials and equipment necessary for the assembling of the transfer sandwich for electroblotting.
(C) BN-PAGE gels used for in gel activity analysis of complex I (left), or complex IV (right), mounted in an acrylamide gel drying system.
Analysis of Mitochondrial Respiratory Complexes and Supercomplexes: Day 2
Timing: 4–24 h
This method allows alternatively assessing the steady-state levels and general pattern of mitochondrial respiratory complexes and suplecomplexes by Coomassie staining, their composition by immunoblotting with specific antibodies, and their activity using in gel activity stain assays.
Note: In some instances, it can be convenient to load duplicate samples in the same gel. This can, for example, allow analyzing simultaneously: (i) the activity and the composition of the respiratory complexes, (ii) the distribution of two specific proteins of different complexes which could have a similar molecular weight or be present in the same supercomplex (e.g., supercomplex I+III2 could be detected with CI and CIII antibodies).
Note: Steps 13–16 describe Analysis by Coomassie staining
-
13.
Incubate the gel in a tray containing the Coomassie staining solution and shake it gently for 30 min at 15°C–25°C.
Optional: Any commercial Coomassie staining solution should work as well.
-
14.
Remove the staining solution and replace it with Coomassie destaining solution.
Note: Coomassie staining solution can be reused multiple times and stored at 15°C–25°C. Keep the bottle sealed to prevent solvent evaporation.
-
15.
Change the destaining solution periodically with fresh solution, as it becomes blue from the dye or every 20 min until the background of the gel is transparent, and bands corresponding to MRC complexes and supercomplexes are visible. It takes 2 to 24 h.
-
16.
Take a picture or scan the gel using a plastic wrap and, for example, an HP ENVY 5660 scanner.
Note: Steps 17–26 describe analysis by immunoblotting
-
17.
When using the eBlot L1 Blotting System, immerse the gel in distilled H2O for 1 min.
-
18.
Transfer proteins to a membrane using the eBlot L1 Blotting System (GenScript). This is a wet protein transfer system that uses isopropanol instead of methanol.
Note: The use of this system allows attaining a faster and efficient transfer of high molecular bands corresponding to mitochondrial supercomplexes (see Troubleshooting).
-
19.
Use nitrocellulose (NT) or polyvinylidene difluoride (PVDF) membranes.
Note: PVDF has a higher sensitivity and protein binding capacity (170 to 200 μg/cm) than nitrocellulose. Nitrocellulose membranes, on the other hand, are more resistant and allow more antibodies to be tested sequentially with a minimal background.
-
20.
Immerse the membranes in the equilibration buffer, provided with the system (in the case of PVDF membranes, soak them first in methanol, and then in the buffer). It is essential to keep the membrane in the buffer until it is completely wet, to obtain optimal results.
-
21.
Prepare the transfer sandwich with dry sponges, membrane, and gel (Figure 1B).
-
22.
Start the program (Refer to Table 1 for program details).
Optional: Use any wet transfer system. Soak the membrane, when needed, in methanol and, then, in the transfer buffer before assembling the sandwich (usually, it also contains filter paper). Transfer at a constant 30 V for 20 h at 4°C.
CRITICAL: Do not reuse the transfer buffer and/or equilibration buffer more than five times. The recommended shelf-life of the transfer buffer is 3 weeks at 4°C.
-
23.
Air-dry the membrane completely for 1–2 h.
Pause Point: Once dried, the membrane can be kept for weeks before its reconstitution with methanol.
-
24.
Remove the dye out of the membrane at 15°C–25°C:
-
a.
In the case of PVDF membranes, the membrane needs to be completely dry to be destained. Perform a quick immersion in methanol, but do not leave the membrane for more than 5–10 s. Then, transfer the membrane immediately to the Coomassie blue dye washing buffer and incubate for 15 min. Discard the solution and incubate again for 15 min with the same solution. This step helps to expose the epitopes of the proteins that will be recognized by the antibody.
-
b.
Nitrocellulose membranes require longer destaining steps than PVDF. Incubate in abundant Coomassie blue dye washing buffer for 1–3 h, replacing the buffer every 15–30 min. Then, wash three times for 10 min in Rinse solution 3.
CRITICAL: To prevent loss of the signal, do not leave the PVDF membrane in the Coomassie blue dye washing buffer for more than 30 min in total.
-
25.
Block the membrane with 5% non-fat dry milk in Rinse solution for at least 1–3 h at 15°C–25°C (usually for 16–20 h at 4°C for nitrocellulose membranes).
-
26.
Perform a standard immunoblot protocol, using antibodies specific for mitochondrial OXPHOS complexes (see Troubleshooting).
Note: Chemiluminescent detection of high-order supercomplexes can be improved using SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo). Combine 50 μL of solution A and solution B with 1 mL of each regular chemiluminescent solution, per membrane.
Optional: To determine sub-complex, complex, and supercomplex subunit composition, a two-dimensional (2D) BN/SDS gel electrophoresis can be performed. As the first dimension BN-PAGE separates intact OXPHOS complexes, subsequent denaturing electrophoresis in the presence of the anionic detergent sodium dodecyl sulfate (SDS) resolves the individual subunits of the respective complexes and supercomplexes. In combination with immunoblotting, it offers the advantage that the signals are usually much stronger and that all proteins can be detected due to increased epitope availability (Calvaruso et al., 2008; Nijtmans et al., 2002). To perform a 2D-BN/SDS-PAGE analysis, following the first BN-PAGE dimension, a lane corresponding to a cell line or mitochondrial sample, is cut out of the gel with a razorblade and placed on a small plastic container for further processing. The gel strip can also be wrapped in plastic and frozen at −80°C for up to 2 weeks, until further use. The strip is then incubated with a denaturing solution (1% SDS and 10 mM β-mercaptoethanol) for 15 min at 20°C–25°C. The denaturing solution is removed and the strip is incubated with 1% SDS for additional 15 min at 20°C–25°C. Solution is removed as completely as possible (excess of solution is drained away using a filter paper). The gel strip is placed at the top of a glass plate and the glass sandwich to cast the SDS gel is further assembled. The first dimension gel should be thicker than the second dimension gel (1.5 mm versus 0.75 mm), to allow the gel strip to be squeezed between at the top of the glass plates. Then, a 10%–12% separation polyacrylamide separation gel is added until 5 mm below the gel strip and is overlay with water or isopropanol to create a smooth horizontal interface. When the gel is polymerized, the water/isopropanol is removed and a 4% stacking gel is added, covering the gel strip. After polymerization, electrophoresis is started at 30 V for 30 min and then continued at 80–100 V for 2–4 h until the blue dye front reaches the end of the gel. Standard immunoblotting protocols for SDS gels can then be applied.
Note: Steps 27 and 28 describe analysis by in gel activity stain assays
-
27.
After electrophoretic separation, immerse the gel directly in the desired enzymatic activity solution (Refer to Materials and Equipment). The amount of time depends on the sample and the complex activity analyzed (from 30 min for CI and CII activities to 12–24 h for CIV activity).
CRITICAL: All enzymatic activity solutions must be prepared freshly.
Note: For complex IV activity, it is recommended to incubate the gel immersed into the solution at 37°C to increase the efficiency of the enzymatic reaction. Allow between 12–48 h for optimal visualization of complex IV activity within the supercomplexes of human cultured cells, replacing CIV activity solution with newly made one every 12–24 h.
-
28.
Wrap the stained gels in plastic and capture the signals using a scanner (use a minimum resolution of 300 dpi in, for example, an HP ENVY 5660 scanner).
Optional: Dry the stained gels to get a sharper image. Soak the gel in the Gel Drying Solution (Bio-Rad) and place it between cellophane sheets, using an acrylamide drying system to assemble it (as in Figure 1C). Be careful to completely remove any air bubble to avoid the crack of the gel.
Table 1.
Detailed Program for PVDF Membrane Transfer
| Buffer 1 cycles | 4 times |
| Cycle 1 | 5 min |
| Cycle 2 | 5 min |
| Cycle 3 | 5 min |
| Cycle 4 | 5 min |
| Buffer 2 cycles | 0 times |
| Cooling cycles | 1 time |
| Cooling | 0 min |
| Cooling buffer | buffer 2 |
| First buffer | buffer 1 |
Expected Outcomes
BN-PAGE allows visualizing the mitochondrial respiratory chain (MRC) and oxidative phosphorylation (OXPHOS) system complexes and supercomplexes. As illustrated in some of the protocol variations explained here, BN-PAGE can be followed by a diversity of downstream protein detection methods, including Coomassie blue R250 or silver staining, enzymatic activity staining, immunoblot, mass spectrometry (e.g., complexome analysis), or second dimension SDS-PAGE. It can also be preceded by radioactive metabolic labeling of mtDNA-encoded proteins or the import into mitochondria of radiolabeled complex or supercomplex protein subunit or assembly factors. Therefore, by coupling BN-PAGE with upstream and downstream applications, the approach is extremely versatile.
In practical terms, successful BN-PAGE-immunoblot or BN-PAGE-enzymatic activity assays described here should yield clear and discrete bands of the analyzed subcomplexes, complexes, and supercomplexes (see Figures 3A, 3B, 4A, and 4B). The resolution of the bands depends on the amount of protein loaded, the protein/detergent ratio, the class of detergent, and the antibody used. An example of the expected band pattern using the conditions and parameters described in this protocol is shown in Figures 2, 3, and 4, with the molecular weights of the MRC and OXPHOS complexes listed in Table 2 as a guide. When using a stronger non-ionic detergent, such as DDM, we expect to observe complexes as individual entities, since their supramolecular associations are disrupted: CI (not present in yeast S. cerevisiae), CII, CIII2, CIV, and CV (Figures 2B, 4A, and 4B). Instead, when we aim to detect the complexes in their native state, both as individual and associated units, it is necessary to use a milder detergent, such as digitonin or LMNG, which will preserve the mitochondrial supercomplexes (CIII2-CIVn, CV2 and also the human respirasome CI-CIII2-CIVn and megacomplex CI2-CIII2-CIV2). However, even these milder detergents each yields a distinct banding pattern (Figure 2A), probably as a result of the micelle size they form.
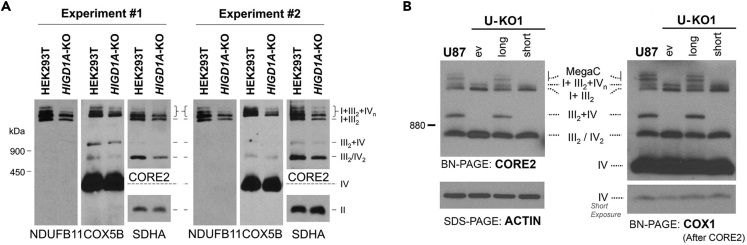
Figure 3.
Examples of BN-PAGE Experiments in Human Cell Lines
(A) Example of two replicates of an optimized BN-PAGE experiment. Mitochondria purified from wild-type, and HIGD1A-KO HEK293T cells were extracted with digitonin (protein/detergent ratio of 1:2) and analyzed by BN-PAGE and immunoblotting with the indicated antibodies. Replicates were generated for the quantification and analysis of Figures 1G and S3E in Timón-Gómez et al. (2020).
(B) Panel B of Figure 5 from (Lobo-Jarne et al., 2018). Characterization of glioblastoma U87 WT and COX7A2L-KO (U-KO1) cells. The KO cells were stably transfected with an empty vector (ev) or constructs to express the long or short versions of COX7A2L. The panel shows the BN-PAGE analysis of whole cells extracted with digitonin (protein/detergent ratio, 1:4) separated in a 3%–12% linear gradient polyacrylamide gel, followed by immunoblotting with the indicated antibodies
Table 2.
Expected Molecular Weights of Mitochondrial Respiratory Complexes and Supercomplexes
| Respiratory Complex/Supercomplex | kDa (Human) | kDa (Yeast) |
|---|---|---|
| CI | 970 | n/a |
| CII | 130 | 130 |
| CIII2 | ~500 | ~500 |
| CIV | 220 | 200 |
| CIV2 | 440 | n/a |
| CV | 600 | 600 |
| CI+CIII2 | ~1500 | n/a |
| CIII2+CIV | ~900 | ~800 |
| CIII2+CIV2 | n/a | ~1,000 |
| CI+CIII2+CIVn | ≥1,700 | n/a |
The visualization of the composition, organization, and activity of the MRC and OXPHOS system by BN-PAGE has been used for applications such as detecting differences in the steady-state levels and enzymatic activities of any complex or supercomplex in cells and tissues, dissecting the assembly pathway of individual complexes and supercomplexes, and detecting the accumulation of assembly intermediates due to alterations in the assembly pathway. Furthermore, since the BN-PAGE approach allows fast screening of biological samples for defects in the integrity of OXPHOS components is, therefore, useful for clinical diagnosis of patients suffering from mitochondrial disorders.
Limitations
The BN-PAGE approach uses the negatively charged Coomassie-dye in the presence of a neutral non-ionic detergent, which can mimic some properties of an anionic detergent, and results in the dissociation of proteins from membrane protein complexes. This has been reported to prevent the proper detection of large oligomeric states of the F1Fo-ATPase or complex V. If that is the objective of the study, then the use of clear native gels (CN-PAGE), which do not include Coomassie, are recommended (Wittig and Schägger, 2005). The use of CN-PAGE can also be recommended if the dye interferes with the detection of enzyme catalytic activities.
A major limitation for BN-PAGE coupled with immunoblotting is the relatively low number of antibodies available that can detect complexed proteins. We have provided here a list of already tested antibodies that work optimally (see Key Resources Table). As an alternative to this limitation, the BN-PAGE can be coupled to a second denaturing electrophoretic dimension (2D-BN/SDS-PAGE), which facilitates the optimal use of all available antibodies (see Troubleshooting).
The BN-PAGE approach relies on the optimal extraction from the mitochondrial membranes of native OXPHOS complexes and supercomplexes. Whereas sample preparation from cultured cells or isolated mitochondria is standardized as reported here, sample preparation from tissues and/or organoids can be more challenging and should be optimized before the assay. The use of different detergents has to be considered depending on the approach, starting material, and the results expected (e.g., in a cholesterol deficient cell line, LMNG would be a better option than digitonin, which interacts with cholesterol).
In gel activity assays are semi-quantitative. Therefore, it is recommended to confirm the results by measuring cellular respiration and respiratory substrate oxidation by polarography in freshly collected intact or permeabilized cells, respectively, as reported (Barrientos et al., 2009). The enzymatic activity of the MRC complexes should be also measured by spectrophotometry using preferentially freshly collected 3× frozen-thawed cells, although cells pellets previously frozen at −80°C for no more than one week can also be used (Barrientos et al., 2009). Independently, the co-evaluation of results obtained from the BN-PAGE analysis coupled with immunoblotting and in gel activity is an advantage to the system. It can inform about whether a reduced activity is due to decreased levels of the complex, to an abnormal composition of the complex, or to a defect in the assembly and the appearance of subassemblies. Also, when subassemblies of any complex are detected by immunoblotting, in gel activity assays will elucidate whether or not these intermediates are catalytically active.
Finally, the BN-PAGE assay provides information about the steady-state levels of mitochondrial respiratory complexes and supercomplexes in a cell line in a specific condition, but it will not readily inform about the proposed dynamics of supercomplex formation-disassociation. This is relevant because it constitutes the basis of the current model of the MRC organization, known as the plasticity model (Acin-Perez and Enriquez, 2014). Although time-course analyses of MRC organization by BN-PAGE upon nutritional or environmental stresses could be informative, we envision that quick sample processing would be paramount to obtain informative results.
Troubleshooting
Problem 1
Low detection of higher molecular weight supercomplexes by immunoblotting.
Potential Solution 1
This problem could come from different sources. The main cause would be an excess of detergent, both during membrane solubilization to obtain whole-cell lysates and during protein complexes extraction from lipidic membranes. An excess of detergent will disassociate the mitochondrial supercomplexes, and they will be obtained as individual entities, but not as macromolecular structures. A titration of the detergent is essential to optimize the signal, especially when changing the starting material (cell lines, strains, etc.) or the supplier.
Another possibility is a low efficient transfer of the proteins to the PVDF membrane. Mitochondrial respiratory supercomplexes have a molecular weight of more than 1,000 kDa. We recommend using the eBlot L1 Transfer system because it transfers protein in a reliable and reproducible manner. However, any wet transfer system should work. It is important to always perform the electrophoretic separation and the transfer of the respiratory complexes at 4°C to avoid their disruption. Particularly, CI is very labile. Extending the transfer time at low current at 4°C might improve the results.
Finally, a parameter to have into account, as mentioned earlier, is the antibody used to perform the immunoblotting. Some antibodies recognize epitopes that are embedded inside the structure of the native complexes. Therefore, their detection of the protein, even if efficient in an SDS-PAGE and immunoblotting analysis, can be compromised in native conditions. For example, an antibody to detect the protein COX1 is very useful to analyze complex IV. However, COX1 is a core protein, and within the supercomplex environment, its solvent exposure is limited. An antibody detecting a more external subunit, such as COX5B, would be more efficient in detecting CIV-containing supercomplexes. In the case our interest is on a specific protein with an embedded epitope in the structure, the alternative is to couple the BN-PAGE to a second denaturing (SDS) electrophoretic dimension (2D-BN/SDS-PAGE), as explained in the Limitations section.
Problem 2
Poor resolution of the bands.
Potential Solution 2
Bands in a gel should be clear and discrete. To discern bands corresponding to different complexes, the gradient of acrylamide in the gel can be adjusted to the optimal percentages in each case. Also, running the samples in a medium-size gel (13.3 × 8.7 cm), instead of a small one, could increase the resolution of the bands.
If a smear appears, it is often due to deficient washing after membrane solubilization with digitonin (in whole-cell lysate preparations). Additional washing steps can be performed in order to improve the resolution. A smear can also be observed when loading higher amounts of protein in the well. If it is necessary to increase the amount of protein used for this assay, we recommend using medium gels with larger wells to allow also a longer run and better resolution of the bands. However, there are multiple reasons for the appearance of a smear, e.g., the use of excessive Coomassie loading dye or running the BN-PAGE at 15°C–25°C. Each step has to be analyzed and optimized to preserve the nature of macromolecular complexes.
Problem 3
Lack of reproducibility.
Potential Solution 3
The analysis by BN-PAGE involves several critical steps, and thus, it is crucial to be consistent and accurate when performing biological replicates. The primary source of variation is in the extraction of protein complexes from the mitochondrial membrane; specifically, during the calculation of the detergent to protein ratio between the different samples. It is necessary to measure protein concentration before detergent addition.
Preparation of the starting biological material might be another source of variability. Cell lines or yeast strains need to be grown and collected in similar conditions (e.g., culture conditions, confluency or growth phase, etc.). The time of cell membrane solubilization (for whole-cell lysates) and homogenization of the cells to purify mitochondria are also critical steps to monitor for precise consistency.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Antoni Barrientos (abarrientos@med.miami.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze any dataset or code.
Acknowledgments
This research was supported by the National Institutes of Health (NIH)-R35 grant GM118141 (to A.B.), Muscular Dystrophy Association (MDA) Research Grant MDA-381828 (to A.B.), US Department of Defense Discovery Award PR180598 (to F.F.); Instituto de Salud Carlos III-MINECO/European FEDER Funds grant PI17-00048 (to C.U.), Comunidad Autónoma de Madrid/ERDF-ESF grant P2018/BAA-4403 (to C.U.), and NIH-RO1 grant GM105781 (to A.B. and C.U.).
Author Contributions
A.T.-G., F.F., and A.B. designed the presentation of the protocol. A.T.-G. collected current lab protocols and previously published protocols by our groups and generated a first draft. F.F. focused on the protocol as related to yeast mitochondrial samples. R.P.P., E.N., and C.U. discussed protocol details and edited the manuscript. All authors read, edited, and approved the final version of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Contributor Information
Alba Timón-Gómez, Email: axt809@med.miami.edu.
Flavia Fontanesi, Email: ffontanesi@med.miami.edu.
Antoni Barrientos, Email: abarrientos@med.miami.edu.
References
- Acin-Perez R., Enriquez J.A. The function of the respiratory supercomplexes: the plasticity model. Biochim. Biophys. Acta. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Barrientos A., Fontanesi F., Diaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 2009 doi: 10.1002/0471142905.hg1903s63. Chapter, Unit19.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvaruso M.A., Smeitink J., Nijtmans L. Electrophoresis techniques to investigate defects in oxidative phosphorylation. Methods. 2008;46:281–287. doi: 10.1016/j.ymeth.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Chae P.S., Rasmussen S.G., Rana R.R., Gotfryd K., Chandra R., Goren M.A., Kruse A.C., Nurva S., Loland C.J., Pierre Y. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.J., Li H.M. Stable megadalton TOC-TIC supercomplexes as major mediators of protein import into chloroplasts. Plant J. 2017;92:178–188. doi: 10.1111/tpj.13643. [DOI] [PubMed] [Google Scholar]
- Couttas T.A., Raftery M.J., Erce M.A., Wilkins M.R. Monitoring cytoplasmic protein complexes with blue native gel electrophoresis and stable isotope labelling with amino acids in cell culture: analysis of changes in the 20S proteasome. Electrophoresis. 2011;32:1819–1823. doi: 10.1002/elps.201100122. [DOI] [PubMed] [Google Scholar]
- Diaz F., Barrientos A., Fontanesi F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using blue native gel electrophoresis. Curr. Protoc. Hum. Genet. 2009 doi: 10.1002/0471142905.hg1904s63. Chapter, Unit19.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vizarra E., Ferrin G., Perez-Martos A., Fernandez-Silva P., Zeviani M., Enriquez J.A. Isolation of mitochondria for biogenetical studies: an update. Mitochondrion. 2010;10:253–262. doi: 10.1016/j.mito.2009.12.148. [DOI] [PubMed] [Google Scholar]
- Hartley A.M., Lukoyanova N., Zhang Y., Cabrera-Orefice A., Arnold S., Meunier B., Pinotsis N., Marechal A. Structure of yeast cytochrome c oxidase in a supercomplex with cytochrome bc1. Nat. Struct. Mol. Biol. 2019;26:78–83. doi: 10.1038/s41594-018-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D., Fontanesi F., Barrientos A. Exploring protein-protein interactions involving newly synthesized mitochondrial DNA-encoded proteins. Methods Mol. Biol. 2008;457:125–139. doi: 10.1007/978-1-59745-261-8_9. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Khalimonchuk O., Smith P.M., Winge D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim Biophys Acta. 2012;1823:1604–1616. doi: 10.1016/j.bbamcr.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Ackerman S.H. Characterization of mutations in the beta subunit of the mitochondrial F1-ATPase that produce defects in enzyme catalysis and assembly. J. Biol. Chem. 1996;271:26522–26528. doi: 10.1074/jbc.271.43.26522. [DOI] [PubMed] [Google Scholar]
- Lobo-Jarne T., Nyvltova E., Perez-Perez R., Timon-Gomez A., Molinie T., Choi A., Mourier A., Fontanesi F., Ugalde C., Barrientos A. Human COX7A2L regulates Complex III biogenesis and promotes supercomplex organization remodeling without affecting mitochondrial bioenergetics. Cell Rep. 2018;25:1786–1799. doi: 10.1016/j.celrep.2018.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Nijtmans L.G., Henderson N.S., Holt I.J. Blue Native electrophoresis to study mitochondrial and other protein complexes. Methods. 2002;26:327–334. doi: 10.1016/S1046-2023(02)00038-5. [DOI] [PubMed] [Google Scholar]
- Okumoto K., Tamura S., Fujiki Y. Blue Native PAGE: Applications to study peroxisome biogenesis. Methods Mol. Biol. 2017;1595:197–205. doi: 10.1007/978-1-4939-6937-1_18. [DOI] [PubMed] [Google Scholar]
- Parey K., Haapanen O., Sharma V., Köfeler H., Züllig T., Prinz S., Siegmund K., Wittig I., Mills D.J., Vonck J. High-resolution cryo-EM structures of respiratory complex I: Mechanism, assembly, and disease. Sci. Adv. 2019;5:eaax9484. doi: 10.1126/sciadv.aax9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M., Paakkarinen V., Aro E.M. Analysis of thylakoid membrane protein complexes by Blue Native Gel Electrophoresis. J. Vis. Exp. 2018;139:58369. doi: 10.3791/58369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore S., Berndtsson J., Marin-Buera L., Conrad J., Carroni M., Brzezinski P., Ott M. Cryo-EM structure of the yeast respiratory supercomplex. Nat. Struct. Mol. Biol. 2019;26:50–57. doi: 10.1038/s41594-018-0169-7. [DOI] [PubMed] [Google Scholar]
- Schagger H., von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Strogolova V., Furness A., Robb-McGrath M., Garlich J., Stuart R.A. Rcf1 and Rcf2, members of the hypoxia induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol. Cell Biol. 2012;32:1363–1373. doi: 10.1128/MCB.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timón-Gómez A., Garlich J., Stuart R.A., Ugalde C., Barrientos A. Distinct roles of mitochondrial HIGD1A and HIGD2A in respiratory complex and supercomplex biogenesis. Cell Rep. 2020;31:107607. doi: 10.1016/j.celrep.2020.107607. [DOI] [PubMed] [Google Scholar]
- Tucker K., Park E. Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution. Nat. Struct. Mol. Biol. 2019;26:1158–1166. doi: 10.1038/s41594-019-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I., Schägger H. Advantages and limitations of clear-native PAGE. Proteomics. 2005;5:4338–4346. doi: 10.1002/pmic.200500081. [DOI] [PubMed] [Google Scholar]
- Zambrano A., Fontanesi F., Solans A., de Oliveira R.L., Fox T.D., Tzagoloff A., Barrientos A. Aberrant translation of cytochrome c oxidase subunit 1 mRNA species in the absence of Mss51p in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 2007;18:523–535. doi: 10.1091/mbc.E06-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze any dataset or code.