Abstract
Objective:
To evaluate the use of mesenchymal stem cells (MSCs) in the attenuation of canine atopic dermatitis (AD).
Materials and methods:
Sixteen dogs were selected and divided into three groups, mild, moderate, and severe, according to the Canine Atopic Dermatitis Extent and Severity Index (CADESI-4). They were evaluated for 82 days. The protocol recommended in this experiment was to inject 2 × 106/kg bodyweight of MSC’s in all groups by the intravenous route with intervals of applications of 21 days. The degree of pruritus was evaluated by examining the visual analog scale, the CADESI-4, the histopathology of the skin, hematological and biochemical parameters, the pyogenic effect of MSCs, and the thickness of the epidermis.
Results:
There was a significant difference in the reduction of epidermal thickness in the moderate and severe groups. Hematological, biochemical, and body temperature parameters remained within normal limits for the species with no side effects
Conclusion:
MSCs attenuated the clinical signs of AD.
Keywords: Dermatitis, MSC, atopic, pruritus, dogs
Introduction
Atopic dermatitis (AD) is multifactorial cutaneous hypersensitivity with environmental interactions, such as stress, aeroallergens, trophallergens, and immunological conditions, and is characterized by changes in the cutaneous barrier due to a decrease in interlamellar lipids [1].
In AD, there is an exacerbation of the immune response, while in the acute phase, there is a predominance of T helper 2 (Th2) lymphocytes with the release of proinflammatory cytokines, eosinophils, mast cell degranulation, and immunoglobulin E (IgE) [1,2]. In the chronic phase, both a predominant T lymphocyte helper 1 (Th1) response and a Th2 response are observed [3].
The currently recommended therapies for AD are oral-systemic use of immunosuppressants, such as corticosteroids, cyclosporin, and oclacitinib, associated with lotions, pipettes, and creams composed of moisturizers, emollients, and humectants to restore the skin barrier. Coadjuvant therapies with specific allergen immunotherapy help to attenuate the effects of AD on the body [4].
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can be collected and isolated from a wide variety of tissues [5]. MSCs can decrease the aggressiveness of several allergic diseases [6–8]. MSCs have been known to interact with both innate and adaptive immune systems, which results in the suppressive effect on proliferation, differentiation, and activation of immune cells. [9–12]. Several recent studies have indicated that the use of MSCs is a promising therapeutic approach for AD [13]. Shin et al. [14] found that the intravenous administration of human adipose tissue-derived MSCs alleviates AD via regulation of B lymphocyte maturation. The aim of this study was to evaluate the systemic use of allogeneic MSCs by the endovenous (EV) route as adjunctive treatment in atopic patients, as well as to demonstrate the safety and possible side effects of the treatment.
Materials and Methods
Ethical statement
The authors declare that they are aware of the contents of the resolutions of the National Council of Control of Animal Experimentation – CONCEA [15] and followed all of the guidelines. Also, all steps of the experiment, including the selection of animals, physical examination, biochemical exams, the obtaining of tissue for differentiation, the application and MSCs, and the follow-up of patients were done by veterinarians, following the guidelines of the National Council of Control of Animal Experimentation – CONCEA [15]. Likewise, all animal owners signed a veterinary responsibility term and authorization for adipose tissue collection and stem cell application.
Experimental design
Sixteen dogs with clinical diagnoses of AD (8 females and 8 males) were selected, consisting of the following breeds: French bulldog (n = 5), Jack Russell (n = 1), Maltese (n = 3), Lhasa Apso (n = 3), Shih Tzu (n = 3), and Coton de Tulear (n = 1). The dogs were aged between 1 and 12 years, with an average weight of 8.85 kg.
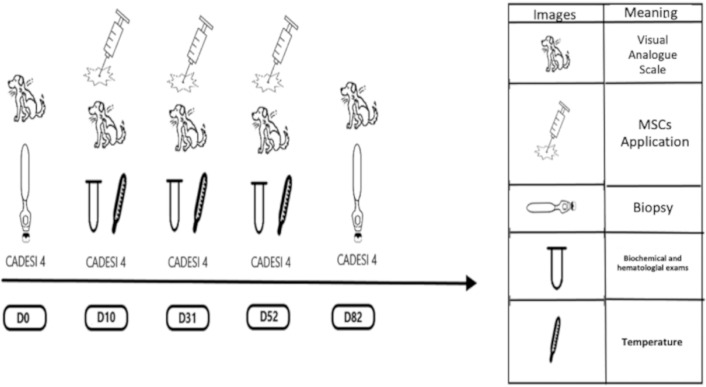
At the animal screening, skin scrapings and trichrome and skin cytology examinations were performed to rule out pruritic diseases, such as endo and ectoparasites and bacterial and yeast infections, and food restriction was established with hypoallergenic rations for 8 weeks to rule out food hypersensitivity without the use of immunosuppressive drugs for 3 months. The use of antiseptic shampoo composed of 2% miconazole nitrate and 2% chlorhexidine gluconate – Cloresten® was established for the control of secondary skin infection by bacteria and yeast. The experiment, as shown in Figure 1, started on day 0 (D0) and ended on day 82 (D82).
Figure 1. Schematic drawing of the experimental design of the treatment protocol employed.
The diagnostic evaluation of the condition was based on the Favrot criteria [16]. On D0, the patients were subjected to the evaluation of the score recommended by Canine Atopic Dermatitis Extent and Severity Index (CADESI-4) and the degree of pruritus was established by the visual analog scale (VAS). For a 6-mm punch biopsy of the skin on the right arm flexure, local anesthesia was used at a dose of 0.8 ml of 2% lidocaine hydrochloride without a constricting vessel.
All animals passed through a complete physical exam, according to the proposed of Oregon State University [17] for the physical examination for dogs. To measure organic factors over times of application of MSCs on D10, D31, and D52, blood samples from animals were harvested by venipuncture of the external jugular vein to evaluate by hemogram the liver alanine aminotransferase (ALT) and renal function (creatinine) before the EV application of MSCs. The rectal temperature of each animal in the different groups was measured before, immediately after, and 1 h after the application of MSCs. Additionally, animals were evaluated based on CADESI-4 and degree of pruritus (VAS). Dogs received 2 × 106/kg of MSCs diluted in lactated ringer serum (50 ml) on D10, D31, and D52.
On D31, four patients were excluded from the experiment; expressly, one female and one male French bulldog, one female Shih Tzu, and one male English bulldog. The first three patients presented worsening of clinical signs with secondary infection by Malassezia, and the use of systemic medications for clinical control was necessary. An English bulldog presented with osteoarthrosis; therefore, he was treated and excluded from the experiment.
On D82, 6-mm punch biopsies of the skin of the right arm flexure of each patient were administered with local anesthesia at a dose of 0.8 ml of 2% lidocaine hydrochloride without a vasoconstrictor. CADESI-4 (Table 1) and the degree of pruritus (Table 2) were also evaluated.
Table 1. Representation of the relative values (%) obtained for CADESI-4; Groups: mild, moderate, and severe; Days evaluated: D0, D10, D31, D52, and D82.
| Mild Group | D0 | D10 | D31 | D52 | D82 |
|---|---|---|---|---|---|
| N/AD | – | – | 14.29 | 42.86 | 71.42 |
| Mild | 100 | 100 | 71.42 | 57.14 | 28.58 |
| Mod | – | – | 14.29 | – | – |
| Sev | – | – | – | – | – |
| Moderate Group | D0 | D10 | D31 | D52 | D82 |
| N/AD | – | – | – | – | – |
| Mild | – | – | 66.67 | 100 | 100 |
| Mod | 100 | 100 | 33.33 | – | – |
| Sev | – | – | – | – | – |
| Severe Group | D0 | D10 | D31 | D52 | D82 |
| N/AD | – | – | – | – | – |
| Mild | – | – | – | – | – |
| Mod | – | – | – | 16.67 | 16.67 |
| Sev | 100 | 100 | 100 | 83.33 | 83.33 |
Table 2. Representation of the relative values (%) for the determination of pruritus (VAS) for the evaluated days: D0, D10, D31, D52, and D82. Groups were classified as mild, moderate, and severe.
| Mild group | D0 | D10 | D31 | D52 | D82 |
|---|---|---|---|---|---|
| ESV-10 | 14.29 | 14.29 | – | – | – |
| SV-8 | 71.42 | 71.42 | 57.14 | 57.14 | 28.57 |
| MD-6 | 14.29 | 14.29 | 42.86 | 28.58 | 42.86 |
| MI-4 | – | – | – | 14.28 | 28.57 |
| VMI-2 | – | – | – | – | – |
| NO-0 | – | – | – | – | – |
| Moderate Group | D0 | D10 | D31 | D52 | D82 |
| ESV-10 | – | – | – | – | – |
| SV-8 | 100 | 100 | 100 | 66.67 | 66.67 |
| MD-6 | – | – | – | 33.33 | 33.33 |
| MI-4 | – | – | – | – | – |
| VMI-2 | – | – | – | – | – |
| NO-0 | – | – | – | – | – |
| Severe Group | D0 | D10 | D31 | D52 | D82 |
| ESV-10 | 83.33 | 66.67 | 50 | 33.33 | 33.33 |
| SV-8 | 16.67 | 33.33 | 50 | 50 | 50 |
| MD-6 | – | – | – | 16.67 | – |
| MI-4 | – | – | – | – | 16.67 |
| VMI-2 | – | – | – | – | – |
| NO-0 | – | – | – | – | – |
Isolation, culture, immunophenotyping, and freezing of MSCs
Isolation, culture, and freezing protocols were performed at BIO CELL Laboratories and International Center for Biotechnology (CRI). All MSCs were isolated and cultured from the adipose tissue of a healthy 3-year-old Shih Tzu donor weighing 6 kg. For the collection of the adipose tissue, the donor animal was subjected to the anesthetic protocol of 0.3 mg/kg methadone and 0.02 mg/kg acepromazine via the intramuscular route. Then, an incision of approximately 2 cm was made in the lumbar region, and approximately 20 gm of adipose tissue was removed from the base of the tail. The adipose tissue was washed in 0.9% saline to remove cellular and blood residues, cut into small pieces, and then exposed to hyaluronidase to undergo enzymatic digestion. The cells were subjected to a filtration process to initiate a selection of the MSCs. Cells were then placed in culture flasks with Dulbecco’s modified Eagle medium and incubated at 37.5°C and 5% carbon dioxide. After 24 h, the medium was discarded along with the non-adherent cells, and fresh culture medium was added to the bottles. The medium was replaced once every 3 days until the cells reached 80% confluency. At that point, trypsinization was performed to remove the cells from the bottles, and the cells were quantified in the Neubauer chamber. Subsequently, the cells were mixed with dimethylsulfoxide and fetal bovine serum and packed in blisters (1 million cells/vane) for freezing in liquid nitrogen. Five vanes (5 × 106 cells) were thawed for the characterization of MSCs.
Characterization of MSCs
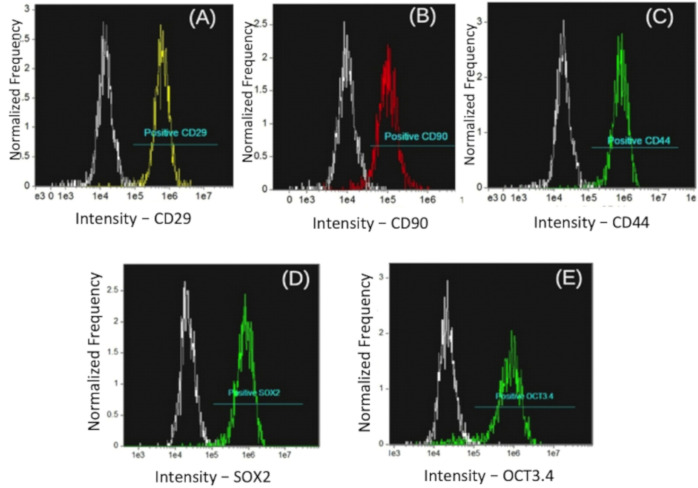
A portion of the cells was sent to the International Center for Biotechnology for molecular characterization and quantification to be carried out through Amnis® flow cytometry and to evaluate the degree of purity, functionality, and viability through the identification of molecular markers. Molecular characterization tests of MSCs were performed as determined by the International Society for Cell Therapy. A total of 1 × 106 cells were incubated with the following antibodies: anti-human mouse CD29-RD1, anti-equine rat CD44-FITC, primary CD90 anti-canine goat, and anti-goat AF594 (secondary) mouse IgM. Additionally, the rat anti-human CD34-FITC negative surface marker remained. The function of the MSCs was analyzed by the presence of SOX2 and OCT3/4, using an Amnis® Image flow cytometer (Fig. 2).
Figure 2. Expression of cell markers in immunophenotyping of MSCs from flow cytometry. (A) Positive control of CD29, (B) positive control of CD90, (C) positive control of CD44, (D) positive control SOX2, and (E) positive control OCT3/4.
The cells were evaluated for the presence of contaminants using the polymerase chain reaction (Veriti Thermal Cycler – Thermo Fisher Scientific). Besides, cell viability after thawing was evaluated by an Amnis® flow cytometer with the Alexa Fluor™ 488 annexin kit and propidium iodide (Thermo Fisher Scientific™).
Treatment of AD from the application of MSCs
The animals were treated with 2 × 106/kg of MSCs plus lactated ringer serum in 50-ml volumes at intervals of 21 days via the intravenous route.
Processing and histopathological evaluation of skin biopsies
The skin fragments after collection from the animals of each group on D0 and D82 were cleaved and fixed by immersion in 10% aqueous solution of formaldehyde. The fragments were included in histological paraffin and sectioned in a microtome with 4-μm thickness and stained with H&E. A digital camera and image analysis software (ProgResCapturePro 2.5®) coupled with a Nikon binocular microscope E200 at a 400× were used to evaluate the changes in the characterization of perivascular inflammatory infiltrate, epidermal hyperplasia (EH), superficial dermal edema (SDE), and epidermal thickness.
Statistical analysis
Data on CADESI-4 and degree of pruritus (VAS) were evaluated in relation to the frequency of occurrence in relation to the groups. The hemogram data were assessed through the application of descriptive analysis to obtain the mean and standard deviation. The descriptive analysis was applied to obtain the mean and standard deviation in relation to the quantifications of ALT, creatinine, body temperature variation, and quantification of epidermal thickness. Next, the data were also submitted to the Kolmogorov–Smirnov test to evaluate normality. Then, the ALT and temperature were evaluated using one-way analysis of variance (ANOVA) with Tukey’s multiple-way test. The creatinine was submitted to the one-way ANOVA test with Dunn’s posttest of multiple comparisons. The thickness of the epidermis was evaluated using the t-test. Sigma-Stat 3.5 software was used at p ≤ 0.05.
Results
Isolation, culture, and characterization of MSCs
A high number of MSCs with high plastic adhesion capacity were obtained after the procedure. When the cells reached 80% confluence, they had phenotypic characteristics of MSCs and were attached to the bottom of the container in a fusiform shape.
The immunophenotyping results, that is, MSC-specific molecular markers evaluated by flow cytometry, demonstrated the expression of high levels of CD29, CD44, and CD90 surface differentiation markers and low expression of CD34, a negative marker for hematopoietic cells. A high expression of the pluripotency transcription factors SOX2 and OCT3/4 was observed (Fig. 3). The donor demonstrated a high expression of CD29, CD44, and CD90 markers (approximately 90%). On the other hand, SOX2 and OCT3/4 presented 96% and 92% expression, respectively.
Figure 3. Flow cytometry analysis of MSCs after thawing: dead (A), necrotic (B), live (C), and apoptotic (D) annexin kit; 92% of the cells were alive.
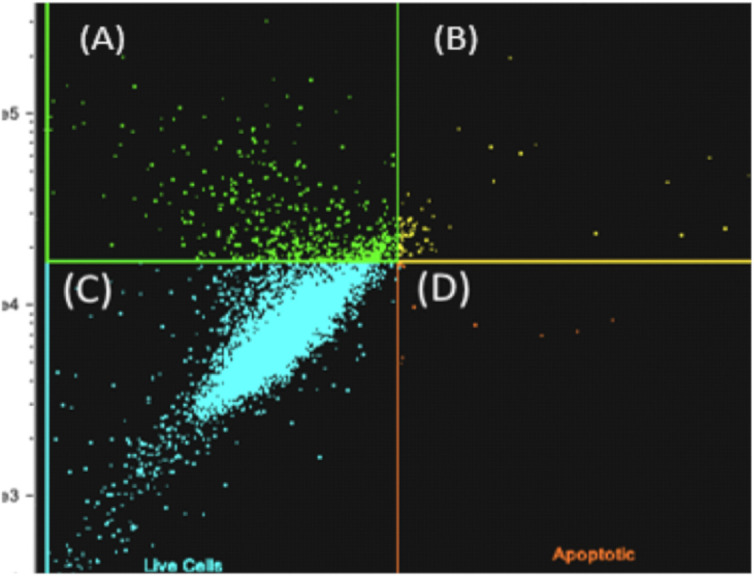
The viability analysis revealed that 92% of the cell population was alive upon being evaluated 30 min after thawing, and 8% of cells had initial apoptosis (Fig. 4).
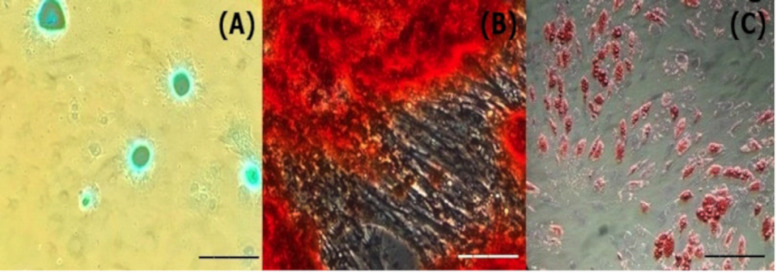
Figure 4. Images of the different stages of the differentiation process of MSCs. (A) Differentiation in chondrocytes, (B) differentiation in osteocytes, and (C) differentiation in adipocytes. Bar 10 μm, increase 400×.
The capacity of the cells for adipogenic, chondrogenic, and osteogenic differentiation (Fig. 5) was demonstrated without contaminants.
Figure 5. Clinical evaluation model based on the CADESI-4 in relation to the groups used in the experiment: mild, moderate, and severe. Bar = 10 μm.
Evaluation of CADESI-4, degree of pruritus, physical exams, hematological exams, biochemicals, and body temperature
Evaluation of CADESI-4
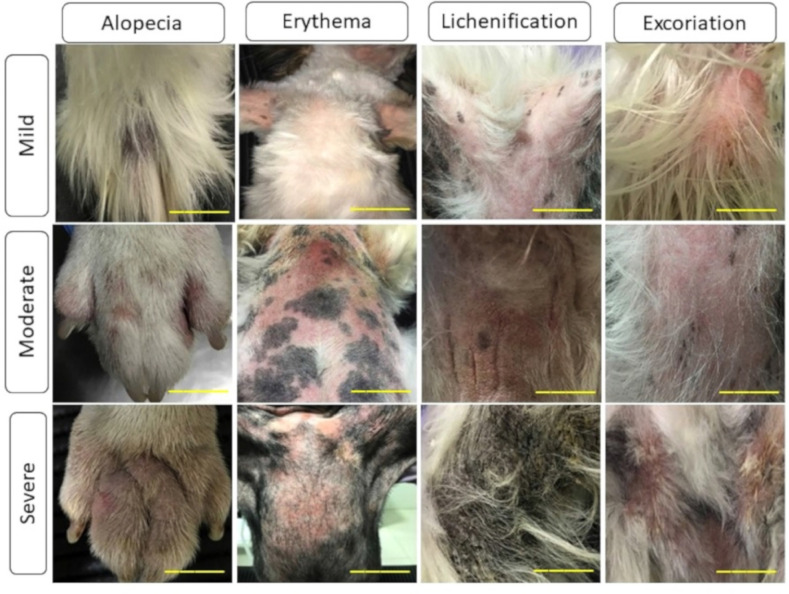
The CADESI-4 scores were evaluated for each group of animals: mild, moderate, and severe. The CADESI-4 score meaning is: N/AD = no lesion or remission; Mi = mild; Mod – moderate; and Severe = Sev.
The severity of the clinical signs, as established according to the application of the CADESI-4 criteria [16] in groups, was classified as mild (scores of 10–34), moderate (scores of 35–59), and severe (scores over 60), as shown in Figure 5.
Mild group
In the mild group, 100% of the animals showed a mild score on CADESI-4 on D0. On D10, 100% of the animals continued to present a mild score. On D31, 71.24% of the animals showed mild score, while 14.92% presented downgrade to the N/AD level, but 14.92% of the animals showed upgrade to the moderate score. On D52, 57.14% of the animals showed mild score and 42.86% showed N/AD level. On D82, 71.42% of the animals presented N/AD score, while 28.58% showed mild score. In the mild group, based on the CADESI-4 evaluation, the treatment presented positive effects by presenting 71.42% of the animals with an N/AD score.
Moderate group
In the moderate group, on D0, 100% of the animals showed a moderate score on CADESI-4. On D10, 100% of the animals still presented 100% of the moderate score. On D31, 66.67% of the animals presented downgrade from the moderate score to the mild score, while 33.33% continued to present the moderate score. On D52 and D82, 100% of the animals analyzed were with the mild score on CADESI-4. None of the animals showed a downgrade on the N/AD score in the moderate group.
Severe group
In the severe group, on D0, D10, and D31, 100% of the animals showed a severe score. On D52 and D82, 83.33% of the animals showed a severe score, while 16.77% of the animals presented a downgrade on the moderate score. None of the animals presented a downgrade on the mild or N/AD score on the severe group. The analysis of the CADESI-4 is presented in Table 1.
Analysis of VAS
The VAS was analyzed in the same way with three groups: mild, moderate, and severe. The degree of pruritus was given by: ESV-10 = extremely severe; SV-8 = severe; MD-6 = moderate; MI-4 = light; VMI-2 = very light; and NO-0 = without pruritus.
Mild group
In the mild group, on D0, 14.29% of the animals showed an extremely severe degree of pruritus, 71.42% of the animals showed a severe degree, and 14.29% of the animals showed a moderate degree. On D10, the animals still showed the same degrees as on D0. On D31, 57.14% of the animals showed a severe degree and 42.86% of the animals showed a mild degree of pruritus. On D52, 57.14% of the animals showed a severe degree of pruritus, 28.58% showed a moderate degree, and 14.28% showed a mild degree. On D82, 28.57% showed a severe degree, 42.86% showed a moderate degree, and 28.57% showed a mild degree of pruritus. None of the animals showed light, very light, or the absence of pruritus during the 82 days of treatment of the mild group.
Moderate group
In the moderate group, in D0, D10, and D31, 100% of the animals showed a severe degree of pruritus on VAS, with no modifications. On D52, 66.67% of the animals showed a severe degree, while 33.33% showed a decrease to a moderate degree. The values for D52 persisted until the analysis of D82, with no changes. None of the animals showed mild, light, very light, or the absence of pruritus.
Severe group
In the severe group, in D0, 83.33% showed an extremely severe degree, while 16.67% showed a severe degree of pruritus on VAS. On D10, 66.67% of the animals showed an extremely severe degree, while 33.33% of the animals showed a severe degree. On D31, 50% of the animals showed an extremely severe degree, while 50% showed a severe degree. On D52, 33.33% of the animals showed an extremely severe degree, 50% showed a severe degree, and 16.67% showed an increase to a moderate degree. On D82, 33.33% of the animals showed an extremely severe degree, 50% showed a severe degree, while none of the animals showed a moderate degree, and 16.67% of the animals showed an increase to the mild degree of pruritus. In the severe group, it is possible to see an improvement in the clinical status of the animals. The results of VAS can be seen in Table 2.
Physical exam and biochemicals
All animals passed through a physical exam before the evaluation on D10, D31, and D52 and did not show any type of comorbidity or collateral effects during the treatment.
In the mild and severe groups, hematological parameters and ALT and creatinine enzymes did not show significant changes (p > 0.05) on the days of MSC application. The values of hematocrit and erythrocytes in the moderate group showed a substantial difference between D10 and D52 (p < 0.05), but they were within the normality value for the species, and the lymphocyte values between D10 and D31 were within the reference value.
Body temperature was measured on D10, D31, and D52 before, immediately after, and 1 h after the application of MSCs. All animals showed temperature within the physiological pattern of the species.
Histopathological evaluation
Mild group
Perivascular infiltrate (PI)
In the mild group, it was observed that on D0, the PI was negative on 14.29% of the animals, present on a mild degree on 71.42% of the animals, and in a moderate form in 14.29% of the animals. There was no severe form present in the mild group on D0. On D82, we noted that the PI disappeared in 28.57% of the animal, which means that the frequency of the negative presence of PI had doubled. The mild presence of PI on D82 was reduced to 57.14%, while the moderate degree of the PI was maintained.
Epidermal hyperplasia
On D0, EH was negative in 28.57% of the animals, present to a mild degree in 42.86% of the animals, the moderate degree was absent, and the severe degree was present in 28.57% of the animals. On D82, we evaluated that the negative degree of EH maintained its frequency, with 28.57%, while the mild degree grew to 71.43%. The moderate degree was absent, while the severe form went to the status of absent, indicating that the severe degree form showed progression to the mild degree.
Superficial dermal edema
The SDE on D0 was negative in 28.57% of the animals, present in a mild form in 42.86% of the animals, present to a moderate way with 28.57% of frequency and absent on the severe form. On D82, SDE showed an increase in the frequency of absence (negative), with 42.86%, while the mild form grew to 57.14%. In contrast, the moderate and severe forms were absent, indicating the progression of the moderate and severe degrees to the mild degree or the absence of SDE.
Moderate group
Perivascular infiltrate
In the moderate group, on D0, none of the animals was evaluated as negative. 66.67% of the animals showed mild PI; none of the animals showed moderate PI, while 33.33% of the animals showed a severe degree of PI. On D82, none of the animals showed negative PI, whereas the frequency of the mild degree was maintained, with 66.67%, while the moderate degree of PI presented an increase to 33.33%, being the severe degree absent on D82, which indicates that the severe form progressed to the moderate degree.
Epidermal hyperplasia
On D0, EH, in the moderate group, was possible to evaluate as 33.34% of the animals showed negative EH. 33.33% showed mild EH, none of the animals showed moderate EH, and 33.33% of the animals showed severe EH. On D82, the frequency of the negative degree of EH was maintained at 33.33%, while the mild degree grew to 66.67%. On the other hand, the moderate and severe degrees were absent, which indicate that the severe degree progressed to the mild form during this period.
Superficial dermal edema
On D0, none of the animals showed a negative degree of SDE, while 66.67% showed a mild degree of SDE, 33.33% showed a moderate degree of SDE, being the moderate and severe degrees absent on D0. On D82, 100% of the animals showed a mild degree of SDE. These results indicate that the animals which presented the moderate form progressed to a mild degree.
Severe group
Perivascular infiltrate
In the severe group, on D0, none of the animals showed a negative degree for PI. 33.34% indicated a mild degree of PI, 33.33% showed a moderate degree of PI, and 33.33% showed a severe degree of PI. On D82, none of the animals showed a negative or mild degree of PI, while 66.67% showed a moderate degree of PI, and 33.33% showed a severe degree of PI.
Epidermal hyperplasia
On D0, none of the animals showed a negative degree for EH. 16.67% of the animals showed a mild degree of EH, 50% showed a moderate degree of EH, and 33.33% showed a severe degree of EH. On D82, 16.67% showed a negative degree of EH, 16.67% showed a mild degree of EH, 50% moderate degree of EH, and 16.66% severe degree of EH.
Superficial dermal edema
On D0, none of the animals presented a negative degree of SDE. 50% of the animals showed mild degrees, 33.33% showed a moderate degree, and 16.67% showed a severe degree of SDE. On D82, none of the animals showed a negative degree of SDE, while 66.67% showed a mild degree of SDE, 33.33% showed a moderate degree, and none presented a severe degree of SDE. All the data described earlier can be seen in Table 3.
Table 3. Frequency of occurrence (%) of the findings of the histopathological evaluation of the different groups before and after the application of MSCs on D0 (before) and D82 (after).
| Mild group | PI | Epidermal hyperplasia | SDE | |||
|---|---|---|---|---|---|---|
| D0 | D82 | D0 | D82 | D0 | D82 | |
| Negative (−) | 14.29 | 28.57 | 28.57 | 28.57 | 28.57 | 42.86 |
| Mild (+) | 71.42 | 57.14 | 42.86 | 71.43 | 42.86 | 57.14 |
| Moderate (++) | 14.29 | 14.29 | – | – | 28.57 | – |
| Severe (+++) | – | – | 28.57 | – | – | – |
| Moderate group | PI | Epidermal hyperplasia | SDE | |||
| D0 | D82 | D0 | D82 | D0 | D82 | |
| Negative (-) | 14.29 | 28.57 | 28.57 | 28.57 | 28.57 | 42.86 |
| Mild (+) | 71.42 | 57.14 | 42.86 | 71.43 | 42.86 | 57.14 |
| Moderate (++) | 14.29 | 14.29 | – | – | 28.57 | – |
| Severe (+++) | – | – | 28.57 | – | – | – |
| Severe group | PI | Epidermal hyperplasia | SDE | |||
| D0 | D82 | D0 | D82 | D0 | D82 | |
| Negative (-) | – | – | – | 16.67 | – | – |
| Mild (+) | 33.34 | – | 16.67 | 16.67 | 50 | 66.67 |
| Moderate (++) | 33.33 | 66.67 | 50 | 50 | 33.33 | 33.33 |
| Severe (+++) | 33.33 | 33.33 | 33.33 | 16.66 | 16.67 | – |
Measurement of the thickness of the epidermis
Mild group
In the mild group, on D0, the epidermal thickness was 37.76 ± 25.00. Thus, on D82, the value of the epidermal thickness of the mild group was smaller, with a value of 26.86 ± 16.96. Although, on the statistical analysis, the decrease of the mild group did not show statistical significance.
Moderate group
In the moderate group, the epidermal thickness on D0 was 71.03 ± 45.89. On D82, the value of the epidermal thickness was 37.33 ± 14.47, which was statistically significant.
Severe group
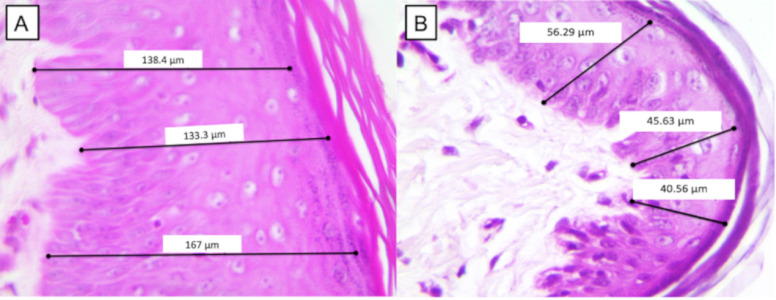
On D0, in the severe group, the epidermal thickness was 91.52 ± 31.10. On D82. The epidermal thickness of the severe group decreased to 57.21 ± 6.45, which was statistically significant. All the results described earlier and the images of the epidermal thickness measure can be seen, respectively, in Table 4 and Figure 6.
Table 4. Measurement of epidermal thickness in μm on D0 and D82 from the mild, moderate, and severe groups.
| Mild group | Moderate group | Severe group | Mild group | Moderate group | |
|---|---|---|---|---|---|
| D0 | D82 | D0 | D82 | D0 | D82 |
| 37.76 ± 25 | 26.86 ± 16.96 | 71.03 ± 45.89a | 37.33 ± 14.57 | 91.52 ± 31.10a | 57.21 ± 6.45 |
TTO = treatment.
Different letters on the same line indicate significant difference, p ≤ 0.05.
Figure 6. Measurement of the thickness of the epidermis. (A) The patient was a French bulldog, female, and in the severe group before treatment on D0. (B) The same patient on D82 presented a significant difference in relation to D0. 10-μm bar, 400×.
Discussion
AD is a growing disease since dogs are predominantly found in indoor environments with increased exposure to aeroallergens. Because AD is a disease that has no cure, treatment is based on the investigation of the underlying causes and control of secondary infections. It is recommended that the topical application of lipid compounds with ceramides, fatty acids, and cholesterols assist in the recomposition of the cutaneous barrier, as well as moisturizing and antiseptic shampoos; the control of pruritus and cutaneous lesions is achieved by the use of oral or topical glucocorticoids, oral ciclosporin, and oclacitinib (a synthetic inhibitor of JAK) with a satisfactory clinical response [4].
Specific allergen immunotherapy is used as adjunctive therapy to minimize seizures and increase tolerance to environmental allergens. To minimize the effects and the occurrence of AD complications in dogs, the efficacy and safety of the use of allogenic MSCs applied by the intravenous route in dogs with AD were evaluated. This study aimed to compare the effect and possible organic changes resulting from this therapy based on parameters related to VAS [18] CADESI-4 score and changes in the skin architecture because of changes due to the inflammatory process.
The characterization and immunophenotyping of MSCs were based on the high levels of CD29, CD44, and CD90 surface differentiation markers and expression of pluripotency transcription factors obtained by flow cytometry. They are in agreement with Ferrer et al.[19] and Villatoro et al. [20], and this standardization was fundamental for establishing adequate treatment with MSCs.
Another important factor in relation to MSCs and their activity was their ability to differentiate into osteocytes, chondrocytes, and adipocytes, similar to what was previously described [19–23]. Thus, this ability guarantees the reparative potential of MSCs, as opposed to differentiation, enabling them to minimize the effects of AD in dogs.
Regarding the route of intravenous administration of MSCs, no changes were observed in blood parameters, determinants of hygiene, or their changes due to the injection. These factors were measured to determine the efficacy of the method used to treat AD in dogs because the MSCs were expected to exhibit a chemotactic action to the inflammatory process present in the skin. At that time, MSCs did not act directly on the epidermis; instead, they performed a local paracrine action, releasing regulatory cytokines involved with the modulatory mechanism of the disease [19].
In relation to the attenuation of the problems generated by atopy, the positive action regarding the involvement of MSCs was based directly on the normality of the hematological and biochemical values of the dogs. These parameters were within the physiological patterns determined for the species for all groups, as mentioned by Ferrer et al. [19], when they reported safety regarding hematological values in the treatment of human MSCs in para-anal fistulas of canine patients. It was possible to observe that ALT values remained normal in all groups. Similarly, creatinine remained stable and within the physiological values. In keeping with Ferrer et al. [19], it was possible to establish that biochemical markers related to hepatic and renal enzymes did not change the treatment from MSCs. The body temperature of the dogs of the evaluated groups did not change after applications. This signaling was not compromised due to the nonoccurrence of oscillations of this parameter, as proposed by Sun et al. [24] when he emphasized the safety in the treatment imposed.
According to Na et al. [25], MSCs may be successful in the treatment of AD in humans. The efficacy of human umbilical cord-derived stem cell therapy was reported in mice with AD. In agreement with the mechanism of action that also occurred in the animals evaluated, Cho et al. [26] reported improvement in AD symptoms, such as decreased inflammation of the skin and pruritus in mice with the systemic use of human MSC exosomes responsible for the regulation of the inflammatory response and expression of the proinflammatory cytokines.
Based on CADESI-4 data, the CADESI-4 score was considered a parameter of great diagnostic importance since it established the locations and severity of AD, high sensitivity, and specificity for effect, as well as in the evaluation of the clinical maintenance of the patient [18]. Clinical improvement and decrease in the CADESI-4 score were observed in the three groups in the experiment. This effect was in contrast with the findings of Villatoro et al. [20], who verified improvement of this parameter in atopic dogs treated with allogeneic MSCs, administered intravenously at 1.5 × 106/kg.
The degree of pruritus exhibited a more significant decrease between the mild and moderate groups, while in the severe group, the pruritus response was not as pronounced. However, for a patient with AD, even on a smaller scale, the decrease in the degree of pruritus had already represented a better quality of life, coinciding with the findings of Villatoro et al. [20] when they observed improvement in the degree of pruritus.
In AD, pruritus is a predominant factor and is mediated by the interaction of cutaneous barrier dysfunction and inflammatory activators [27]. Kim et al.[28] observed an improvement in the degree of pruritus in human patients with moderate and severe AD when they used allogeneic MSCs derived from the umbilical cord, which promoted a decrease in eczema, pruritus, and serum IgE levels without triggering adverse effects in the patients treated. Therefore, the use of MSCs in the animals of this study was effective in the presence of interference, and there was a decrease in this parameter.
Cellular alterations were determined for the characterization of the response modulation before MSC application in patients with AD, especially when the alterations reveal, in association with the inflammatory process, changes resulting from the treatment. Fragments of skin revealed decreased epidermal hyperplasia and superficial edema in the groups. Partly in keeping with these results, in mice with AD, there was a decrease in epidermal hyperplasia and dermal inflammation after treatment with MSC [25]. Therefore, these epidermal changes were characteristic of individuals with AD, as determined by the chronicity of the local inflammatory process, thereby revealing the importance of the histological evaluation of the skin in association with the clinical evaluation when employing allogenic MSCs in atopic patients.
In relation to the thickness of the epidermis, mainly between the moderate and severe groups, it was observed that patients with AD presented hyperplasia of the epidermis with an increase in the PI and that in more chronic cases, hyperkeratosis could occur. Above all, the decrease in the thickness of the epidermis was possibly related to the reduction of the inflammatory pattern and the hyperplasia of the epidermis, considering the action of the treatment imposed. Therefore, this parameter was fundamental to evaluate the improvement in cellular levels of the alterations resulting from AD, still revealing itself as a kind of biomarker responsible for the signaling of the paracrine action promoted by MSCs in the regulatory response to inflammation.
The occurrence or nonoccurrence of adverse effects was evaluated in animals subjected to AD treatment from the application of MSCs. Dogs that received MSCs had no side effects during the applications or 30 days after the last application. In keeping with it, clinical improvement occurred after the application of human stem cells of embryogenic origin in the treatment of perianal fistulas in dogs [18]. The safety of the treatment was ascertained by Harman et al. [23]. They did not find side effects in relation to the placebo group after 60 days of the application of MSCs in dogs with osteoarthritis. In human patients, it was mentioned that the treatment of AD was well-tolerated [27]. Such safety can be established by the understanding of the mechanisms involved in attenuation of the affection when the MSCs acted in the modulation of the cellular response from the release of cytokines locally, thereby ensuring less systemic interference, which could trigger unwanted reactions in the treatment of atopic disease.
In allergic diseases [8], MSCs were responsible for triggering a specific immunomodulatory capacity. The MSCs derived from canine adipose tissue were able to interfere with the production and release of IL10 and TGFβ1, which are immunoregulatory cytokines, and the cells thus effectively improved AD in dogs [29]. MSCs could act on dendritic cells, not allowing their maturation; therefore, all activation and presentation to the TCD4+ lymphocyte would be blocked, and the cascade of the release of proinflammatory interleukins would be attenuated by this immunoregulatory effect [30]. These findings demonstrate the promise of using MSC therapy for this condition.
As a positive effect of using MSCs to treat dogs with AD, clinical improvement of the patients concerning the degree of pruritus, CADESI-4 score, inflammatory tissue changes, and epidermal thickness decreases were verified, all of which were related to the minimization of inflammatory patterns imposed by AD. Otherwise, it is suggested that proinflammatory and regulatory cytokines, as well as tissue IgE, may be complementary factors to reinforce the paracrine action imposed by MSCs in atopic dogs. There was inhibition of T, CD4+, and CD8+ lymphocyte proliferation in dogs with anal fistula treated with human MSCs [19].
Conclusion
Systemic treatment with allogeneic MSCs has been suggested to be a promising option for the attenuation of canine AD. This treatment is able to promote a clinical improvement in the period between D0 and D82 and is important because it does not trigger side effects. However, it is important to keep researching in this field.
Acknowledgment
Nothing to disclose.
Conflict of interests
The authors declare that there is no conflict of interest.
Authors’ contributions
Fernanda Oliveira Ramos (FOR) conducted the experiments and wrote the article. Patricia Furtado Malard (PFM) participated in the experiments. Hilana dos Santos Sena Brunel (HSSB) participated in the experiments. Giane Regina Paludo (GRP) conducted the clinical analysis. Márcio Botelho de Castro (MBC) conducted the histopathological analysis. Paulo Henrique Sampaio da Silva (PHSS) made the tables, schematical drawings, and part of the results. André Rodrigues da Cunha Barreto Vianna (ARCBV) made the tables, schematical drawings, and part of the discussion. Eduardo Maurício Mendes de Lima (EMML) made statistical analysis, discussion, and results.
References
- [1].Pucheu-Haston CM, Bizikova P, Marsella R, Santoro D, Nuttall T, Eisenschenk MN. Lymphocytes, cytokines, chemokines and the T-helper 1–T-helper 2 balance in canine atopic dermatitis. Vet Dermatol. 2015;26:124–32. doi: 10.1111/vde.12205. https://doi.org/10.1111/vde.12205. [DOI] [PubMed] [Google Scholar]
- [2].Majewska A, Gajewska M, Dembele K, Maciejewski H, Prostek A, Jank M. Lymphocytic, cytokine and transcriptomic profiles in peripheral blood of dogs with atopic dermatitis. BMC Vet Res. 2016;12(1):174. doi: 10.1186/s12917-016-0805-6. https://doi.org/10.1186/s12917-016-0805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Olivry T, Mayhew D, Paps JS, Linder KE, Peredo C, Rajpal D, et al. Early activation of Th2/Th22 inflammatory and pruritogenic pathways in acute canine atopic dermatitis skin lesions. J Invest Dermatol. 2016;136:1961–9. doi: 10.1016/j.jid.2016.05.117. https://doi.org/10.1016/j.jid.2016.05.117. [DOI] [PubMed] [Google Scholar]
- [4].Olivry T, DeBoer DJ, Favrot C, Jackson HA, Mueller RS, Nuttall T, et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA) BMC Vet Res. 2015;11:210. doi: 10.1186/s12917-015-0514-6. https://doi.org/10.1186/s12917-015-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS, Kim TY. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: phase I/IIa studies. Stem Cells. 2017;35:248–55. doi: 10.1002/stem.2401. https://doi.org/10.1002/stem.2401. [DOI] [PubMed] [Google Scholar]
- [6].Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107(12):5652–7. doi: 10.1073/pnas.0910720107. https://doi.org/10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–31. doi: 10.1111/j.1398-9995.2010.02509.x. https://doi.org/10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- [8].Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Ramesshwar P. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy–induced asthma. J Allergy Clin Immunol. 2012;129:1094–101. doi: 10.1016/j.jaci.2011.10.048. https://doi.org/10.1016/j.jaci.2011.10.048. [DOI] [PubMed] [Google Scholar]
- [9].Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604–15. doi: 10.1016/j.exphem.2009.01.005. https://doi.org/10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27:693–702. doi: 10.1634/stemcells.2008-0687. https://doi.org/10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- [11].Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. https://doi.org/10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [12].Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46–57. doi: 10.1182/blood-2008-04-154138. https://doi.org/10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- [13].Song JY, Kang HJ, Ju HM, Park A, Park H, Hong JS, et al. Umbilical cord-derived mesenchymal stem cell extracts ameliorate atopic dermatitis in mice by reducing the T cell responses. Sci Rep. 2019;9(1):6623. doi: 10.1038/s41598-019-42964-7. https://doi.org/10.1038/s41598-019-42964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shin T, Lee B, Won Choi S, Shin J, Kang I, Young Lee J, et al. Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget. 2016;8:512–22. doi: 10.18632/oncotarget.13473. https://doi.org/10.18632/oncotarget.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].BRASIL. Ministério da Ciência, Tecnologia, Inovações e Comunicações/Gabinete do Ministro. [Nov 10;2019 ];Resolução Normativa Número 39, de 20 de junho de 2018. 120:1. 7. Available via http://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/27128118/do1-2018-06-25-resolucao-normativa-n-39-de-20-de-junho-de-2018-27128107 . [Google Scholar]
- [16].Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res. 2015;11:196. doi: 10.1186/s12917-015-0515-5. https://doi.org/10.1186/s12917-015-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oregon State University. Small animals physical exams: physical examinations of dogs and cats. [Oct 20;2019 ]; Available via https://stuorgs.oregonstate.edu/ivsa/nicaragua-veterinary-brigade/clinic-documents/small-animal-physical-exams .
- [18].Olivry T, Saridomichelakis M, Nuttall T, Bensignor E, Griffin CE, Hill PB. Validation of the canine atopic dermatitis extent and severity index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet Dermatol. 2014;25:77–e25. doi: 10.1111/vde.12107. https://doi.org/10.1111/vde.12107. [DOI] [PubMed] [Google Scholar]
- [19].Ferrer L, Kimbrel EA, Lam A, Falk EB, Zewe C, Juopperi T, et al. Treatment of perianal fistulas with human embryonic stem cell-derived mesenchymal stem cells: a canine model of human fistulizing Crohn’s disease. Regen Med. 2016;11:33–43. doi: 10.2217/rme.15.69. https://doi.org/10.2217/rme.15.69. [DOI] [PubMed] [Google Scholar]
- [20].Villatoro AJ, Hermida-Prieto M, Fernández V, Fariñas F, Alcocholado C, Rodríguez-García MI, et al. Allogeneic adipose-derived mesenchymal stem cell therapy in dogs with refractory atopic dermatitis: clinical efficacy and safety. Vet Rec. 2018;183:654. doi: 10.1136/vr.104867. https://doi.org/10.1136/vr.104867. [DOI] [PubMed] [Google Scholar]
- [21].Marx C, Silveira MD, Beyer NN. Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev. 2015;24:803–13. doi: 10.1089/scd.2014.0407. https://doi.org/10.1089/scd.2014.0407. [DOI] [PubMed] [Google Scholar]
- [22].Pérez-Merino E, Usón-Casaús J, Duque-Carrasco J, Zaragoza-Boyle C, Mariñas-Pardo L, Hermida-Prieto M, et al. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: endoscopic and histological outcomes. Vet J. 2015;206:391–7. doi: 10.1016/j.tvjl.2015.07.023. https://doi.org/10.1016/j.tvjl.2015.07.023. [DOI] [PubMed] [Google Scholar]
- [23].Harman R, Carlson K, Gaynor J, Gustafson S, Dhupa S, Clement K, et al. A prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front Vet Sci. 2016;3:81. doi: 10.3389/fvets.2016.00081. https://doi.org/10.3389/fvets.2016.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, et al. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413–22. doi: 10.1016/j.jcyt.2015.11.018. https://doi.org/10.1016/j.jcyt.2015.11.018. [DOI] [PubMed] [Google Scholar]
- [25].Na K, Yoo H, Zhang Y, Choi MS, Lee K, Yi TG, et al. Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell death Dis. 2014;5:e1345. doi: 10.1038/cddis.2014.299. https://doi.org/10.1038/cddis.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187. doi: 10.1186/s13287-018-0939-5. https://doi.org/10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51:263–92. doi: 10.1007/s12016-015-8488-5. https://doi.org/10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- [28].Kim HS, Yun JW, Shi TH, Lee SH, Lee BC, Yu KR, et al. Human umbilical cord blood mesenchymal stem cell-derived PGE 2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem cells. 2015;33:1254–66. doi: 10.1002/stem.1913. https://doi.org/10.1002/stem.1913. [DOI] [PubMed] [Google Scholar]
- [29].Jee M, Im Y, Choi J, Kang SK. Compensation of cATSCs-derived TGFβ1 and IL10 expressions was effectively modulated atopic dermatitis. Cell Death Dis. 2013;4:e497. doi: 10.1038/cddis.2013.4. https://doi.org/10.1038/cddis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–8. doi: 10.1016/j.imlet.2007.10.002. https://doi.org/10.1016/j.tvjl.2015.08.003. [DOI] [PubMed] [Google Scholar]