Abstract
Objective:
This experiment was designed to assess the quality and to evaluate the feeding impact of moringa feed on intake, digestibility, rumen fermentation, methane (CH4) production, and milk yield.
Materials and methods:
According to body weight and exit–entry average daily milk production, fifteen BLRI cattle breed-1 lactating cows of 3rd or 4th stage of parturition with wk 3 and 4 of calving were selected and were equally and randomly distributed into three dietary groups. One group of cows was fed a control diet (T0) consisting of 1:1 dry matter (DM) of Napier silage and conventionally mixed concentrate. The other two groups were fed a control diet by randomly replacing i) 50% (T1) or ii) 100% (T2) of its concentrate with moringa feed. The three dietary groups were balanced nutritionally based on energy and protein following the Bangladesh Standards and Testing Institution (BSTI) standard.
Results:
The concentrate mixture was replaced with moringa feed to increase the feed efficiency and to reduce the DM or crude protein intake (p < 0.05) per 100 kg of metabolic body weight. The T2 group flourished with the highest (p < 0.05) amount of raw milk and also 4% fat-corrected milk (4.39 and 4.59 kg/day, respectively) compared to the T0 group (3.30 and 3.49 kg/day, respectively). However, it increased (p < 0.05) the concentration of total volatile fatty acid and decreased (p < 0.05) the blood and milk cholesterol, and ammonia-nitrogen (NH3-N) was reputed by adding moringa feed into the T0 group, without showing any significant (p > 0.05) change in CH4 production, fat, solid not fat, lactose or protein content of milk.
Conclusion:
Therefore, moringa feed increased the productivity in dairy cows, replacing the whole concentrate diet.
Keywords: Digestibility, intake, milk production and quality, moringa feed, rumen environment
Introduction
A high supply of macro and micro nutrients containing feeds and fodders is necessitated for intensifying the feed utilization and productive performance through intensive ruminants’ production. Nevertheless, in most developing countries, the feeding system mainly depends on high-fiber-containing crop residues and grass which are deficit in nutrients; this is a challenge for scientist and livestock experts who need to pursue alternative universally high nutrient containing animal feeds to boost the production performance [1]. The potential use of live yeast [2], herbal plants [3], phytogenic extracts [4], and essential oils [1] are alternative strategies to enhance microbial fermentation, utilization, and finally, animal productivity.
As a consequence, Moringa oleifera, originating from the Indian subcontinent, is a decent source of nutrients, like crude protein (CP) [5, 6], due to the optimally balanced composition of amino acids and fatty acid profiles and high digestible protein content [7] that can replace the soybean meal [8] and 75% of the sesame meal [6]. Moreover, saponins and tannins are the major bioactive compounds of M. oliefera [5,6,9], which provide a positive impact on feed utilization and lessen the CH4 production through antimicrobial activities [4, 5]. High nutrient containing M. oleifera in the diet was found responsive to increase in feed intake and digestibility [10] and exaggerated the milk yield, with no adverse effect on blood chemistry [6,11,12]. The feeding of Moringa as a feed for dairy cattle was explored through systematic research, but the farmers are not used to formulating the moringa feed, which is why it needs to be explored, at least to support the formulation of diets of high metabolism in lactating cows. Thus, the study aimed to reflect the quality and feeding impact of moringa feed on nutrient utilization, fermentation, and milk yield of lactating animals.
Materials and Methods
Ethical approval
The article reports on the moringa feed feeding impact on BLRI cattle breed-1 (BCB-1) dairy cows. The Research Project Evaluation Committee of BLRI accepted this research work, and the animal experimental ethics committee also approved all experimental produces through a Technical Committee by Memo No. T-4/(Part-6) 2015/1799 dated 12 December 2015 under Project No. 12.
Experimental place and agro-climate
The experiment was carried out on the experimental cattle research station of BLRI which is located at 23°42’0’’ N, 90°22’30’’ E at an altitude of 4 m above the sea level. The average day temperature and humidity during the experimental period ranged from 21°C to 35°C and 50% to 75%.
Land preparation and agronomical procedure of M. oleifera
During April 2016, the seeds of native black moringa plants containing 5%–7% moisture were sown in polythene pouches filled with sandy alluvial soil. Two seeds were put into each pouch and were kept until the age of wk 5 for raising the sapling. Before the transplantation of saplings, the predesigned land was cleaned, cultivated, and organic manure (3.0 tons/ha) and inorganic manures (90:30:15 kg/ha of N:P:K) were applied. The moringa saplings were planted with 30 cm spacing between the saplings and 45 cm between the rows. Weeds were controlled manually at about each fortnight interval period. After 120 days of the growing period from the transplantation, the 1st harvesting occurred wherein the plant was 60-cm high from the soil. Further regrowth of the shoots and twigs, with a two-month interval, was required and only 90 kg/ha urea-nitrogen was applied for each cut. The biomass was harvested six times in a year and it was processed and stored in polythene bags for further use. The annual yield of the biomass was determined by adding the total biomass of the different cuts of the moringa biomass.
Preparation of the Moringa feed
The soft twigs with leaves of the moringa plant fodder were chopped into approximately 0.5–1.0 cm pieces using an electric chopper machine, and the chopped biomass was sun-dried on a smooth concrete floor for two consecutive days. The dried biomass was milled using a 2.0-mm sieve for the production of moringa feed. The moringa feed contained acid detergent fiber (ADF) to CP of about 2:1, and it was packed in plastic bags that were sealed and kept in a well-managed storeroom.
Napier silage preparation
The experimental animals were fed with Napier silage diet. The BLRI Napier-3 was cultivated following the standard agronomical practices, followed by the BLRI. The 60–65 days old Napier fodder was harvested and cut into 6–8 cm length using an electric chopper machine and ensiled in a soil hole, following the BLRI practices of pit silage preparation.
The management of BCB-1 dairy cows
Fifteen BCB-1 dairy cows, with an average 220 kg live weight (standard deviation = 40.01) of the breeding herd of BLRI farm, of the third or fourth parity after wk 3 and 4 of their post-natal period, were used for the feeding trial. According to live weight and exit–entry milk production, the cows were randomly and equally distributed into three dietary groups. They were housed in a well-ventilated animal shed with individual concrete floor stalls fitted with individual feed and water troughs. As routine farm practices, the animals were immunized against foot and mouth and anthrax disease and were dewormed twice a year. Water was supplied ad libitum.
Diets and feeding of the BCB-1 cows
Fifteen animals were classified and equally distributed into three dietary groups, considering their live weight and exit–entry daily milk production. A group of cows were fed a control diet consisting of a 50:50 ratio [dry matter (DM) basis] of Napier silage and a concentrated mixture (Table 1). The concentrate of the control diet was replaced by moringa feed at 50%, maintaining a DM ratio of 50:25:25 of Napier silage, concentrate, and moringa feed, respectively, in diet T1 and at 100%, maintaining a ratio of 50:0:50, respectively, in diet T2. The experimental diets were fed for 75 days. All the three concentrate mixtures were iso-nitrogenous. For the contentment of the nutrient requirement of the cows, all were calculated according to the Bangladesh Standards and Testing Institution (BSTI) standard [13]. Molasses (2%) was added to the concentrate mixture with dry mash moringa feed to promote palatability.
Table 1. Feed ingredients of conventional and Moringa concentrate mixture (% fresh).
| Ingredients | T0 | T1 | T2 |
|---|---|---|---|
| Wheat bran | 40 | 20 | 0 |
| Rice bran | 25 | 12.5 | 0 |
| Soybean meal | 15 | 7.5 | 0 |
| Moringa feed | 0 | 40 | 80 |
| Crusted wheat | 16 | 16 | 16 |
| Vitamin mineral premix1) | 3 | 3 | 3 |
| Salt | 1 | 1 | 1 |
| Total | 100 | 100 | 100 |
T0, only concentrate; T1, 50% concentrate + 50% moringa feed; T2, only moringa feed.
Provided the following quantities per kg of complete diet: vitamin A, 625,000 IU; vitamin D3, 125,000 IU; vitamin E, 125 IU; vitamin K, 3.5 mg; thiamin, 4.5 mg, vitamin B12, 0.06 mg, pyridoxine, 4.3 mg; folic acid, 1.12 mg; biotin, 0.06 mg; Cu, 17.12 mg as cupper.
Napier silage was supplied twice a day to each cow at 8:00 and 16:00, and the concentrate mixture with and without moringa feed was supplied before milking time at 6:00 and 17:00. For providing the required nutrient to the lactating animals, the DM of the basal diet was evaluated twice a week through the DM incubator [14].
During the experimental period, the daily feed consumption was calculated through the conventional method variance between supply and leftover. Before the feeding time, the feeds were weighted and kept in the feed trough. On the next day, in the morning, the leftovers were collected and weighted individually and written down into the data collection sheet before offering the new feed. The DM of the leftover was determined at least once in a week except for the period of digestibility determination. Supply and refusal of Napier silage and the tested ration were collected daily for sampling and stored in a freezer (-18°C). For proximate analysis, these collected samples were mixed to produce one sample of each cow.
Hand milking was carried out twice a day, in the morning and evening. Periodically, the total milk production per cow was weighted and noted, and 100 mL of milk was kept in the milk sample bottle for storing immediately into the refrigerator and maintained at 4°C. The milk sample of a cow was mixed to have a pooled sample for the determination of milk composition.
Metabolic trial
For nutrient partitioning and digestibility, the metabolic trial was initiated ten days before the end of the research work, with three days for adjusting to the new system and seven days for data recording. Before the morning feeding, the sample collection was completed and the weight of the feed supply and leftover, cow dung, and urine at 9:00 were noted. The feces voided from each cow were kept into a large container for 24 h and encircled with the lid to avoid evaporation. Every day, the cow dung of each container was weighed and thoroughly mixed, and a 5% subsample was taken before emptying the container and was stored in the freezer. After the collection period, the total collected feces samples of each cow were melted and assorted properly, and around 300 g of mixed feces sample was taken for proximate analysis. After adding 40 ml of H2SO4, the total urine voided of each cow was collected into a container. The subsample of urine (10 ml/100 ml) was taken for the determination of nitrogen through the Kjeldahl nitrogen method. For the estimation of apparent nutrient digestibility, we used the following equation:
Analytical methods of milk
The purpose of milk constituents was analyzed for all milk samples in the BLRI dairy laboratory. According to Gerber’s method, the milk fat was determined and the lactose was assessed through the rapid method. By difference, the solid not fat (SNF) was estimated by using the following equation: fat corrected milk (FCM) = 0.4 M + 15.0 F, to measure the 4% fat corrected milk, where M = milk yield and F = fat yield.
Chemical analysis
The animal feed and refusal containing DM, CP, and ash were analyzed, following the method described by Association of Official Analytical Chemists (AOAC) [15]. Also, fiber digestion was analyzed [neutral detergent fiber (NDF) and ADF] according to the method mentioned earlier [16].
Rumen environment
After 3 hours of morning feeding, the rumen fluid was collected individually through a rubber stomach tube at the end of the digestibility trial and it was immediately cleaned through four layers of cheesecloth and straining. During the straining period, 100 ml of clean rumen liquor was collected in the sample bottle and stored for determining NH3-N and total volatile fatty acid (VFA) concentration through the steam distillation methods. Within a certain period, the pH of individual cows was determined using the Orian 680 digital pH meter.
Blood biochemical constituents
The blood samples were collected twice a week into heparinized tubes, from the external jugular vein before morning feeding, for the measurement of blood chemistry. After centrifugation (4,000 r/min), blood plasma was separated within 15 min. Immediately, the blood sugar (BS) concentration was determined for the whole blood. Total protein (TP), albumin, and globulin were also identified [17]. By determining the creatinine and blood urea through the colorimetric method, the kidney function was determined [17]. The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the blood were the main pointers measuring the liver function.
Statistical analysis
One-way analysis of variance and Duncan’s test were used to determine the feeding response of different treatments on different parameters through statistical package for social sciences -11.5 software [18]. Significance was measured at a level of 5.0% (p < 0.05).
Results
Chemical constituents, feed intake, and nutrient digestibility
The ingredients, chemical properties, feed consumption, and digestibility coefficient of Napier silage, locally mixed concentrate mixture and moringa feed are shown in Tables 1–3, respectively. No significant (Table 3; p > 0.05) differences were detected for DM intake from Napier silage and concentrate mixture among the treatment groups. Reducing (p < 0.05) the DM intake per 100 kg body weight and increasing the feed efficiency (p < 0.05) were displayed only in the T2 group (2.31 kg DM intake/day and 87.0%, respectively) compared to the T0 group (2.71 kg DM intake/day and 65.0%, respectively). With the increase of moringa feed in the control diet, no differences (p > 0.05) were observed with CP intake (gm/day), DM and CP digestibility of coefficient among the treatment groups. Only the T2 group consumed the lowest amount of CP that was 12.6 gm/kg W® compared to the T0 group (14.1 gm/kg W®), and their differences were significant (p < 0.05).
Table 3. Intake and digestibility of feed nutrient by dietary group of BCB-1 dairy cows.
| Parameters | Experimental rations | Significance | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | SEM | p-value | |
| DMI from Napier silage (kg/day) | 3.11 | 3.12 | 2.93 | 0.15 | 0.88 |
| DMI from concentrate (kg/day) | 2.67 | 2.80 | 2.67 | 0.05 | 0.51 |
| TMEI (MJ/day) | 52.1 | 52.9 | 49.9 | 1.97 | 0.84 |
| FE (%) | 65.00b | 62.00b | 87.00a | 0.04 | 0.01 |
| DMI (kg /100 kg BW) | 2.71a | 2.63ab | 2.31b | 0.06 | 0.06 |
| CP intake (gm/day) | 796.5 | 793.9 | 778.2 | 19.37 | 0.92 |
| CP intake (gm/kg) W0.75) | 14.1a | 13.5ab | 12.6b | 0.24 | 0.03 |
| DM digestibility coefficient | 0.51 | 0.54 | 0.53 | 0.01 | 0.48 |
| CP digestibility coefficient | 0.70 | 0.72 | 0.70 | 0.00 | 0.66 |
SEM, standard error of the mean; DMI, dry matter intake; TMEI, total metabolizable energy intake; FE, feed efficiency; CP, crude protein; DM, dry matter; T0, only concentrate; T1, 50% concentrate + 50% moringa feed; T2, only moringa feed.
means with different superscripts in the same row are significantly different (p < 0.05).
Table 2. Chemical composition of napier silage, conventional concentrate mixture, and Moringa feed.
| Parameters | Napier silage | Concentrate mix. | Moringa feed |
|---|---|---|---|
| DM (gm/kg fresh) | 182 | 884 | 888 |
| OM (gm/kg DM) | 915 | 898 | 901 |
| CP (gm/kg DM) | 83 | 165 | 166 |
| NDF(gm/kg DM) | 671 | 510 | 407 |
| ADF (gm/kg DM) | 455 | 208 | 328 |
| TDN ( % calculated) | - | - | 58.63 |
| GE (MJ/kg DM) | - | 10.03 | 10.01 |
DM, dry matter; OM, organic matter; CP, crude protein; NDF, neutral detergent fiber; ADF, acid detergent fiber; TDN, total digestible nutrient; GE, gross energy.
Milk production and quality
Supplementing moringa feed to the control diet, the most significant average daily fresh milk production (p < 0.05) was noted in the T2 group than that in the other two groups (Table 4). Based on 4% FCM, the T2 group produced (p < 0.05) the highest amount of milk (4.59 kg/day), whereas the lowest production of milk was observed in the T0 group (3.49 kg/day). However, no significant effect (p > 0.05) was detected for adding moringa feed on fat yield (kg/day) among the treatments.Moringa feed did not affect (p > 0.05) the milk constituents among the treatment groups (Table 4). However, the percentage of milk fat increased and it slightly reduced the protein, lactose, and SNF. The milk cholesterol also decreased significantly (from 3.81 mg/100g milk of T0 to 1.20 mg/100 gm milk of T2, respectively; p < 0.01) by the replacement of locally mixed concentrate mixture with moringa feed.
Table 4. Milk production and its quality of dairy cows fed various diets.
| Parameters | Experimental rations | Significance | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | SEM | p-value | |
| Ave. daily milk production(kg) | 3.30b | 3.31b | 4.39a | 0.29 | 0.04 |
| 4% FCM yield (kg d-1) | 3.49b | 3.41b | 4.59a | 0.34 | 0.02 |
| Fat yield (kg d-1) | 0.15 | 0.14 | 0.14 | 0.01 | 0.95 |
| Milk constituents (%) | |||||
| Fat | 4.25 | 4.27 | 4.33 | 0.14 | 0.92 |
| Protein | 3.77 | 3.90 | 3.77 | 0.04 | 0.28 |
| Lactose | 5.44 | 5.64 | 5.45 | 0.05 | 0.21 |
| SNF | 10.38 | 10.38 | 10.04 | 0.10 | 0.20 |
| Milk cholesterol (mg/100 gm) | 3.81a | 2.48ab | 1.20b | 0.02 | 0.01 |
SEM, standard error of the mean; FCM, fat corrected milk; SNF, solid not fat;
T0, only concentrate; T1, 50% concentrate + 50% moringa feed; T2, only moringa feed.
means with different superscripts in the same row are significantly different (p < 0.05).
Ruminal fermentation and CH4 production
Feeding T0, T1, and T2 diet did not affect (p > 0.05) rumen pH (Table 5). Compared with the T0 group, the concentration of NH3-N was the lowest (p < 0.05) in the T2 group that was 18.1 mg/100m/R. L and 13.4 mg/100m/R. L, respectively. But the T2 group contained the highest concentration of VFA that was 16.9 meq/100 m/R. L compared to the T1 group (14.7 meq/100 m/R. L) or the T0 group (13.4 meq/100 m/R. L), respectively, and their differences were significant (p < 0.05).
Table 5. Ruminal fermentation and methane production of different experimental diets.
| Parameters | Experimental rations | Significance | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | SEM | p-value | |
| Ph | 7.20 | 7.10 | 7.10 | 0.08 | 0.88 |
| NH3-N concentration (mg/100 m/R.L) | 18.1a | 16.0b | 13.4c | 0.69 | <0.001 |
| VFA concentration (meq/100 m/R.L) | 13.4c | 14.7b | 16.9a | 0.53 | <0.001 |
| Methane emission | |||||
| CH4 production (gm/day) | 200.1 | 204.9 | 194.3 | 6.63 | 0.83 |
| CH4 production (gm/kg DMI) | 34.6 | 34.6 | 34.6 | 0.01 | 0.77 |
| gmCH4/kg milk | 96.8 | 94.5 | 91.2 | 3.57 | 0.84 |
SEM, standard error of the mean; NH3-N, ammonia nitrogen; VFA, volatile fatty acids; CH4, methane; DMI, dry matter intake; T0, only concentrate; T1, 50% concentrate + 50% moringa feed; T2, only moringa feed.
means with different superscripts in the same row are significantly different (p < 0.05).
Supplementation of moringa feed to the concentrate mixture did not have any adverse effect (p > 0.05) on CH4 production per day (gm/day), CH4 production per kg DM intake (gm/kg DMI), and gmCH4/kg milk production. However, it reveals that the T2 group emitted the lowest amount of CH4 (194.3 gm/day, 34.6 gm/kg DMI and 91.2 gm CH4/kg milk) compared to the T1 group (204.9 gm/day, 34.6 gm/kg DMI and 94.5 gmCH4/kg milk) or the T0 group (200.1 gm/day, 34.6 gm/kg DMI and 96.8 gmCH4/kg milk), respectively.
Dietary nitrogen utilization
Table 6 presents the nitrogen utilization of different experimental diets. A result of N-retention in the T1 group was the highest, which was 74.01%, whereas the T0 group showed the lowest percentage of N-retention, which was 68.79%. The total utilization of nitrogen was not different (p > 0.05) among the treatment groups. However, the results revealed that partial or complete exchange of balance concentrate mixture by the moringa feed enhanced the percentage of dietary N-utilization significantly (p < 0.05) by nearly 60.0% in T2 group in comparison to the T0 group.
Table 6. N-utilization of production performance of BCB-1 dairy cows.
| Parameters | Experimental rations | Significance | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | SEM | p-value | |
| Total N intake, gm | 127.4 | 127.0 | 124.5 | 3.10 | 0.92 |
| Total N in feces | 35.3 | 35.1 | 37.8 | 1.50 | 0.74 |
| N out go through urine, gm | 28.9 | 23.8 | 26.2 | 1.40 | 0.37 |
| Total N-out go, gm | 64.1 | 58.6 | 64.0 | 2.49 | 0.66 |
| N-balance | 63.3 | 68.1 | 60.5 | 1.93 | 0.29 |
| Digested- N | 92.2 | 91.9 | 86.7 | 2.19 | 0.55 |
| N-retention % digestion | 68.8 | 74.0 | 69.8 | 1.27 | 0.22 |
| N-retention % intake | 49.9 | 53.6 | 48.8 | 1.22 | 0.26 |
| N-out go % total digested N | 31.2 | 25.9 | 30.2 | 1.27 | 0.22 |
| N-utilization for milk(gm) | 19.8 | 20.4 | 20.9 | 1.86 | 0.26 |
| N- utilization for meat (gm) | 7.71 | 9.17 | 11.2 | 0.77 | 0.18 |
| N- utilization for scurf and hair (gm) | 1.00 | 1.04 | 1.09 | 0.03 | 0.63 |
| Total utilization of N (gm) | 28.6 | 30.7 | 33.2 | 2.39 | 0.18 |
| % of utilization of N | 44.5b | 51.4ab | 59.8a | 2.49 | 0.04 |
SEM, standard error of the mean; N, nitrogen; T0, only concentrate; T1, 50% concentrate + 50% moringa feed; T2, only moringa feed.
means with different superscripts in the same row are significantly different (p < 0.05).
Blood biochemical constituents
No effect (p > 0.05) was observed for BS, TP, A, G, BUN, AST, ALT, and creatinine of dairy cows among the different treatment groups (Table 7). Moreover, the blood cholesterol of the T2 group reduced (p < 0.05) from the control diet (T0), which was 111.5 mg/day and 204.5 mg/day, respectively.
Table 7. Blood biochemical constituents of lactating BCB-1 cows fed experimental rations.
| Blood metabolic profile | Experimental rations | Significance | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | SEM | p-value | |
| BS (mg/day) | 51.6 | 55.3 | 51.1 | 1.52 | 0.54 |
| Cholesterol (mg/day) | 204.5b | 202.3b | 111.5a | 21.5 | 0.01 |
| TP (gm/day) | 7.52 | 8.21 | 8.00 | 0.2 | 0.30 |
| A (gm/day) | 2.93 | 3.19 | 3.31 | 0.01 | 0.24 |
| G (gm/day) | 4.61 | 5.01 | 4.80 | 0.21 | 0.75 |
| A/G ratio | 0.65 | 0.64 | 0.69 | 0.04 | 0.90 |
| BUN (mg/day) | 64.3 | 61.8 | 51.6 | 3.6 | 0.36 |
| Liver and kidney function tests | |||||
| AST (U/L) | 81.0 | 88.3 | 72.6 | 5.02 | 0.50 |
| ALT (U/L) | 21.7 | 21.3 | 21.3 | 2.80 | 0.99 |
| Creatinine (mg/day) | 0.84 | 0.99 | 0.96 | 0.05 | 0.52 |
SEM, standard error of the mean; BS, blood sugar; TP, total protein; A, albumin; G, globulin; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T0, only concentrate; T1, 50% concentrate + 50% moringa feed; T2, only moringa feed.
means with different superscripts in the same row are significantly different (p < 0.05).
Discussion
Moringa feed is the new arena of animal feedstuff that contains potent ingredients that are suitable for livestock, which improves feed efficiency, yield, and product quality. The present study reveals that the feed efficiency (%) increased significantly only for the moringa group but did not show any significant difference of DMI among the treatment groups. It is indicated to improve the microbial fermentation with feeding moringa feed that is the main reason to increase nutrient utilization with low-quality roughages [10]. Moringa feed containing phenolic compounds and other metabolites did not show any adverse effect on the ruminal environment and microbial activities. Similarly, previous studies [6,10,19] have shown that feeding moringa plant materials containing high secondary metabolites increased digestibility, maintained an excellent ruminal environment, and ensured good feeding value [12], when 18% of moringa feed was complemented in the diet.
Only the moringa feed group amplified the milk yield by 4% FCM, which was 31.51% compared to the concentrate group (Table 4). The results ascribe the application of M. oleifera as a plant material that contains high minerals [20] as vital elements for increasing animal production and quality [21,22,23]. Protein is a critical component of moringa feed that enhances the microbial protein synthesis and also increases ruminal bypass that is essential for animal productivity [8,10,19].
The replacement of feedstuff by the moringa feed in response increases the production and quality of small [7, 11] and large ruminants [12,23,24]. Adding 25% of M. oleifera leaf meal with alfalfa hay containing goat and ewes diet is also enhances milk yield, composition, and quality [7]. Organoleptic characteristics of milk, including test, smell, or color, did not differ by the supplementation of moringa feed. The present results made a positive, strong bridge with the previous finding. In lactation cows, the same results were found to be responsive to increased yield and quality [25] compared to cow fed Moringa free diet.
The percentage of total utilization of N in the T2 group was the highest (59.8%) and the lowest usage of N was shown only in the concentrate group (44.5%). Their differences were significant (p < 0.05). These results confirm the previous findings [10,11,23,26,27] that moringa foliage is a good source of protein-rich amino acids that can enhance the utilization of dietary N and can boost the production and productivity of the dairy animal.
The present study shows that NH3-N concentration in only the moringa feed group was the lowest, but their VFA concentration was the highest, which further indicated the maintenance of ruminal fermentation. Supplementation of M. oleifera decreased the ammonia-N concentration without affecting the ruminal pH and increased the propionic acid concentration [6, 7], which is supported by the present study. Moreover, a lower ruminal NH3-N and a higher VFA of only moringa feed containing ration indicated an increase in the utilization of dietary N (Table 6) and also improved the synchrony between the dietary energy and protein compared to only concentrate diet. These results are supported by the previous findings [28], which declared that moringa supplementation with low ruminal NH3-N is recognized for its low protein degradability.
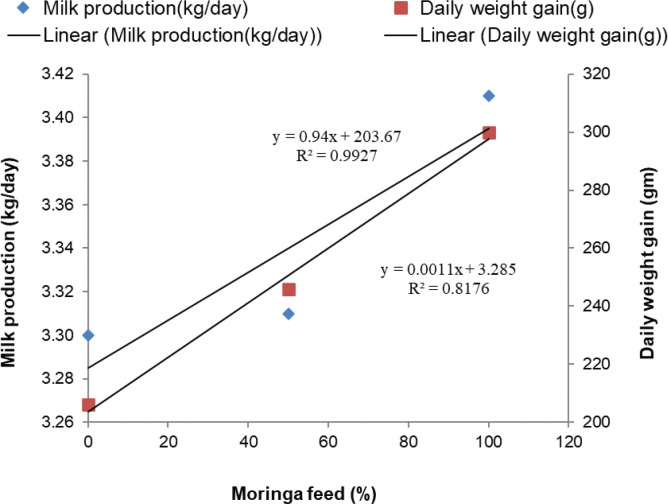
Figure 1. Moringa feed impact on milk production (kg/day) and weight gain (gm/day). The relationship between the percentage of moringa feed intake with milk yield (kg) and average daily live weight gain (gm) is shown in Figure 1. A feeding trial of 75 days showed that the average daily milk production (kg) and daily weight gain (gm) of cows increased linearly (r2 = 1, p < 0.01) with the increase of moringa feed in the concentrate mixture. It also showed that the moringa feed directly effects milk production and live weight gain.
The feed characterizations (quality and quantity) may change the blood metabolites [29]. The status of the liver determined the AST and ALT in the blood, and the blood creatinine is a good indicator of the kidney function [30] in cattle. The levels of BS, TP, A, G, BUN, AST, and ALT did not have any adverse effect on animal health (p > 0.05) by feeding of moringa feed.
The standard value of BS in dairy cows (45.0–75.0 mg/day) was supported by the moringa feed [31] that falls within the normal physiological range. This indicates that the animals were fed a sufficient amount of carbohydrates in the diet. The standard values of cholesterol and TP of healthy cattle were 65–220 mg/day and 5.7–8.1 gm/day, respectively. Supplementation of moringa feed did not affect the range of standard values of cholesterol and TP; no change was indicated in the lipid profile of the animals. Only the moringa feed group directly impacted the cholesterol level in the blood that decreased by nearly 50% compared to the concentrate group (Table 7). Moringa feed may contain phenolic compounds that might be related to reducing cholesterol. Similar results have reported that the serum cholesterol and triglycerides concentration in blood decreased [5,11,26] when the animal fed M. oleifera containing diet. The cholesterol in cows was lowered to 17.60% when the animal were fed moringa as a green fodder [10]. The standard value of creatinine in cattle has to be 1–2 mg/day [31]. The research work was reveals that moringa feed did not have any adverse effect on kidney function.
Conclusion
The results so far generated through this study reveal that, based on the nutrient intake, digestibility, nutrient utilization, reduction of enteric CH4 emission, and rumen fermentation, only the moringa feed group increased both milk production and daily gain of cows and at the same time reduced the cholesterol level in the milk. It may also be able to replace the whole concentrate in the diet. In conclusion, milk production needs to be tested further at the farmer level in order to determine the importance of moringa feed as animal feed, its cost-effectiveness, and the testing of marketing systems.
Acknowledgments
This research work was funded by the “Fodder Research and Development Project” Ministry of Fisheries and Livestock, Government of Bangladesh. The authors thank the Director General (DG) and Project Direction (PD) of Bangladesh Livestock Research Institute, Savar, and Dhaka for providing his valuable guidance, the requisite facilities, and support during the whole period of study.
Conflict of interest
The authors declare that there is no current or potential conflict of interest among the authors and other people or financial organizations about the material discussed in this manuscript.
Authors’ contribution
KSH produced the research idea. MKB designed the study, interpreted the data, and drafted the manuscript. NS helped to collect the data and also contributed to manuscript preparation. KSH and NRS reviewed the manuscript critically and discussed the results.
References
- [1].Matloup OH, Abd El Tawab AM, Hassan AA, Hadhoud FI, Khattab MSA, Khalel MS, et al. Performance of lactating Friesian cows fed a diet supplemented with coriander oil: feed intake, nutrient digestibility, ruminal fermentation, blood chemistry, and milk production. Anim Feed Sci Technol. 2017;226:88–97. https://doi.org/10.1016/j.anifeedsci.2017.02.012. [Google Scholar]
- [2].Hassan AA, Salem AZM, Kholif AE, Samir M, Yacout MH, Hafsa SA, et al. Performance of crossbred dairy Friesian calves fed two levels of Saccharomyces cerevisiae: intake, digestion, ruminal fermentation, blood parameters and faecal pathogenic bacteria. J Agric Sci Cambridge. 2016;154:1488–98. https://doi.org/10.1097/01.ccm.0000508835.79177.37. [Google Scholar]
- [3].Kholif AE, Matloup OH, Morsy TA, Abdo MM, Abu Elella AA, Anele UY, et al. Rosemary and lemongrass herbs as phytogenic feed additives to improve efficient feed utilization, manipulate rumen fermentation and elevate milk production of Damascus goats. Livest Sci. 2017;204:39–46. https://doi.org/10.1016/j.livsci.2017.08.001. [Google Scholar]
- [4].Valdes KI, Salem AZM, López S, Alonso MU, Rivero N, Elghandour MMY, et al. Influence of exogenous enzymes in presence of Salix babylonica extract on digestibility, microbial protein synthesis and performance of lambs fed maize silage. J Agric Sci Cambridge. 2015;153:732–42. https://doi.org/10.1017/S0021859614000975. [Google Scholar]
- [5].Kholif AE, Gouda GA, Morsy TA, Salem AZM, Lopez S, Kholif AM. M. oleifera leaf meal as a protein source in lactating goat’s diets: feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. Small Rumin Res. 2015;129:129–37. https://doi.org/10.1016/j.smallrumres.2015.05.007. [Google Scholar]
- [6].Kholif AE, Morsy TA, Gouda GA, Anele UY, Galyean ML. Effect of feeding diets with processed M. oleifera meal as protein source in lactating Anglo Nubian goats. Anim Feed Sci Technol. 2016;217:45–55. https://doi.org/10.1016/j.anifeedsci.2016.04.012. [Google Scholar]
- [7].Babiker EE, Juhaimia FAL, Ghafoora K, Abdoun KA. Comparative study on feeding value of Moringa leaves as a partial replacement for alfalfa hay in ewes and goats. Livestock Sci. 2017;195:21–6. https://doi.org/10.1016/j.livsci.2016.11.010. [Google Scholar]
- [8].Soliva CR, Kreuzer M, Foid N, Foid G, Machmüller A, Hess HD. Feeding value of whole and extracted M. oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim Feed Sci Technol. 2005;118:47–62. https://doi.org/10.1016/j.anifeedsci.2004.10.005. [Google Scholar]
- [9].Salem AZM, Kholif AE, Elghandour MMY, Buendía G, Mariezcurrena MD, Hernandez SR, et al. Influence of oral administration of Salix babylonica extract on milk production and composition in dairy cows. Ital J Anim Sci. 2014;13:10–4. https://doi.org/10.4081/ijas.2014.2978. [Google Scholar]
- [10].Shankhpal SS, Waghela CR, Sherasia PL, Sridhar V, Srivastava AK, Singh D. Effect of feeding Moringa (M. oleifera) as green fodder on feed intake, milk yield, microbial protein synthesis and blood profile in crossbred cows. Indian J Anim Nutr. 2019;36(3):228–34. https://doi.org/10.5958/2231-6744.2019.00038.0. [Google Scholar]
- [11].Babiker EE, Juhaimi FAL, Ghafoor K, Mohamed HE, Abdoun KA. Effect of partial replacement of alfalfa hay with Moringa species leaves on milk yield and composition of Najdi ewes. Trop Anim Health Prod. 2016;48:1427–33. doi: 10.1007/s11250-016-1111-9. https://doi.org/10.1007/s11250-016-1111-9. [DOI] [PubMed] [Google Scholar]
- [12].Cohen-Zinder M, Leibovich H, Vaknin Y, Sagi G, Shabtay A, Ben-Meir Y, et al. Effect of feeding lactation cows with ensiled mixture of M. oleifera, wheat hay and molasses, on digestibility and efficiency of milk production. Anim Feed Sci Technol. 2016;211:75–83. https://doi.org/10.1016/j.anifeedsci.2015.11.002. [Google Scholar]
- [13].BSTI. Feeds and feeding standards for farm animals and pets. Bangladesh standards and testing institution; 116-A Maan Bhaban, Tajgone Industrial Area, Dhaka, Bangladesh: 2008. [Google Scholar]
- [14].Undersander D, Mertens DR, Teix N. Forage analyses procedures. National Forage Testing Association; Omaha, NE: 2013. [Google Scholar]
- [15].AOAC. Official methods of analysis. 18th. Association of Official Analytical Chemists; Rockville, MD: 2005. [Google Scholar]
- [16].Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 2013;74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- [17].Boyd JW. Veterinary clinical pathology. Merck Sharp Dohme Corp; a subsidiary of Merck Co., Inc; Kenilworth, NJ, Wendy Sprague, Colorado, USA: 2011. The interpretation of serum biochemistry test results in domestic animals. [DOI] [PubMed] [Google Scholar]
- [18].IBM corporation. International business machines. Statistical package for social sciences (SPSS) Armonak, NY: 2013. Statistic for windows, versions 22.0. [Google Scholar]
- [19].Kholifa AE, Goudaa GA, Aneleb UY, Galyeanc ML. Extract of M. oleifera leaves improves feed utilization of lactating Nubian goats. Small Rumin Res. 2018;158:69–75. https://doi.org/10.1016/j.smallrumres.2017.10.014. [Google Scholar]
- [20].Hekmat S, Morgan K, Soltani M, Gough R. Sensory evaluation of locally grown fruits purees and inulin fibre on probiotic yogurt in Mwanza, Tanania and the microbial analysis of probiotic yogurt fortified with Moringa oleifera. J Health Population Nutr. 2015;33:60–7. [PMC free article] [PubMed] [Google Scholar]
- [21].Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Well. 2016;5(2):49–56. https://doi.org/10.1016/j.fshw.2016.04.001. [Google Scholar]
- [22].Oyeyinka AT, Oyeyinka SA. M. oleifera as a food fortificant: recent trends and prospects. J Saudi Soc Agri Sci. 2017;17(2):127–36. https://doi.org/10.1016/j.jssas.2016.02.002. [Google Scholar]
- [23].Mendieta-Araica B, Sporndly R, Reyes-Sanchez N, Sporndly E. Moringa (M. oleifera) leaf mealas a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livest Sci. 2011;137:10–7. https://doi.org/10.1016/j.livsci.2010.09.021. [Google Scholar]
- [24].Sarwatt SV, Milang’ha MS, Lekule FP, Madalla N. M. oleifera and cottonseed cake as supplements for smallholder dairy cows fed napier grass. Livest Res Rural Dev. 2004;16:38. Article # http://www.lrrd.org/lrrd16/6/sarw16038.htm . [Google Scholar]
- [25].Basitan IS, Emma GJ. Yield, quality and feed cost efficiency of milk produced by anglo-nubian goats fed different mixtures of napier (pennisetum purpureum) grass and malunggay (M. oleifera) Philip J Vet Anim Sci. 2013;39(2):193–200. [Google Scholar]
- [26].Zeng B, Sun JJ, Chen T, Sun BL, He Q, Chen XY, et al. Effects of M. oleifera silage on milk yield, nutrient digestibility and serum biochemical indexes of lactating dairy cows. J Anim Physiol Anim Nutr. 2018;102(1):75–81. doi: 10.1111/jpn.12660. https://doi.org/10.1111/jpn.12660. [DOI] [PubMed] [Google Scholar]
- [27].Nouman W, Basra SM, Siddiqui MT, Yasmeen A, Gull T, Alcayde MA. Potential of M. oleifera L. as livestock fodder crop: a review. Turk J Agric. 2014;38:1–14. https://doi.org/10.3906/tar-1211-66. [Google Scholar]
- [28].Hoffmann EM, Muetzel S, Becker K. Effect of M. oleifera seed extract on rumen fermentation in vitro. Arch Anim Nutr. 2003;57:65–81. doi: 10.1080/0003942031000086617. https://doi.org/10.1080/0003942031000086617. [DOI] [PubMed] [Google Scholar]
- [29].Ndlovu T, Chimonyo M, Okoh AI, Muchenje V, Dzama K, Dube S, et al. A comparison of nutritionally related blood metabolites among Nguni, Bonsmara and Angus steers raised on sweetveld. Vet J. 2009;179:273–81. doi: 10.1016/j.tvjl.2007.09.007. https://dayoi.org/10.1016/j.tvjl.2007.09.007 . [DOI] [PubMed] [Google Scholar]
- [30].Allen PJ. Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value. Neurosci Biobehav Rev. 2012;36(5):1442–62. doi: 10.1016/j.neubiorev.2012.03.005. https://dayoi.org/10.1016/j.neubiorev.2012.03.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Radostitis OM, Gay CC, Blood DC, Hinchcliff KW. Clinical examination farm animals. Veterinary medicine. 9th. W. B. Saunders; London, UK: 2000. pp. 1819–1822. [Google Scholar]