Abstract
Objective:
This study was performed to probe the antimicrobial resistance and virulence genes profiling in Pseudomonas aeruginosa recovered from the cases of pericarditis in broiler chickens.
Materials and methods:
The samples (n = 250) collected from the cases of pericarditis in broiler chickens were bacteriologically examined. Antimicrobial susceptibility was tested by disc diffusion technique. The isolates were genotypically studied for the presence of antimicrobial resistance and virulence gene traits. Finally, the nucleotide sequence of representative resistance gene (mexR gene) and virulence genes (toxA and lasI genes) was analyzed.
Results:
P. aeruginosa was isolated from 45 samples (18%). Antimicrobial susceptibility testing revealed multidrug resistance in most of the recovered P. aeruginosa isolates, whereas colistin and imipenem were the furthermost in vitro-sensitive antibiotics. Antimicrobial resistance genes, such as blaCTX, fox, and mexR, were prevalent in 100%, 80%, and 100% of the isolates, respectively. PCR confirmed virulence genes such as toxA, exoY, lasB, and lasI in 100%, 60%, 80%, and 80% of the isolates, respectively. Nucleotide sequence analysis of representative resistance gene (mexR gene) and virulence genes (toxA and lasI genes) revealed a high correlation between P. aeruginosa recovered from pericarditis in broiler chickens in the present study with PAO1 (reference strain) and with other sequences published on the GenBank representing different localities worldwide.
Conclusion:
It could be concluded that P. aeruginosa recovered from pericarditis in broiler chickens in the current study is highly virulent bacteria, resisting most of the therapeutic agents which not only bear hazards for poultry industry but also represent a public health concern.
Keywords: MDR, P. aeruginosa, PCR, Resistance, Sequence, Virulence
Introduction
Poultry farms are wide open to many bacterial species infections resulting in the different clinical sequel, which affects the investments in the poultry industry badly thus, decreasing the total amount of produced protein and/or transmitting pathogens of zoonotic importance to human [1, 2]. Pseudomonas infection in birds is an essential opportunistic bacterial disease which may spread promptly through poultry flocks, triggering mortality rates and producing different and often distinctive postmortem lesion [3].
Pseudomonas aeruginosa has numerous virulence elements, either encoded on plasmids or chromosomal genes [4]. Exotoxin A (ETA) and elastase are of the major virulence factors of P. aeruginosa and are of great significance; ETA is an important ADP-ribosyltransferase toxin that encoded by toxA gene [5, 6]. Elastase is a protease that can reduce host defense, immunoregulatory proteins, and impaired epithelia, which encoded by lasB gene [7]. LasI gene (auto-inducer) is responsible for the secretion of some virulence tools of P. aeruginosa as elastase and pyocyanin [8]. Exotoxin Y is a type III secretion system effector that is a dual soluble adenylyl and guanylyl cyclase, resulting in intracellular cAMP and cGMP accumulation and the creation of interendothelial cell gaps and increased macromolecular permeability [9].
Antimicrobial agents are needed to treat different types of diseases and to save healthy and productive birds, and antimicrobial agents used in livestock production have been measured as a risk aspect in the evolution and spreading of drug resistance in livestock production farms [10]. Food-producing animals and their surrounding environments act as a reservoir for both resistant bacteria and resistance genes that could be indeed transmitted to humans either by direct contact between humans and animals or indirectly through the food production chain [11].
Antimicrobial resistance is of significance in poultry bacterial infections as it may change treatable diseases into untreatable ones and act as an essential factor for the transmission of resistance to human flora or other bacterial pathogens. Beta-lactams are a significant antibiotic group used to treat infections of humans and animals and to prevent diseases [12]. Their uses have always been followed by the development of resistance, most commonly caused by beta-lactamases [13]. Extended-spectrum beta-lactamases and plasmid-mediated AmpC beta-lactamase are important causes behind the antimicrobial resistance [14]. Therefore, this study was aimed to investigate the antimicrobial resistance, virulence, and resistance genes of P. aeruginosa isolated from pericarditis in diseased broiler chickens.
Materials and Methods
Sample collection
Pericardial sac and heart blood specimens were collected aseptically according to the OIE protocol for a sample collection from 250 slaughtered diseased and freshly dead broiler cases of 1–5 weeks old (each one broiler chicken represented one farm) in Beni-Suef and Fayoum Governorate, Egypt, in the period from January to June 2018. These chickens were suffered from respiratory manifestations and/or signs of septicemia.
Isolation and biochemical identification of P. aeruginosa
Nutrient broth, nutrient agar, and cetrimide agar medium were used for primary isolation and further cultivation. The identification analysis such as Gram stain, motility, oxidase, catalase, pigment production, growth at 42°C, hemolysis on blood agar, gelatin hydrolysis, and nitrate reduction tests was done as per the standard techniques [15]. The identified isolates were stored at –70° in 20% glycerol stocks until studied.
Antimicrobial susceptibility testing
The antibiogram of identified isolates was tested by Kirby–Bauer disc diffusion technique against 22 antimicrobial agents comprising 11 different antimicrobial classes of both human and veterinary significance. The suspensions of the isolates equivalent to McFarland turbidity standard tube No. 0.5 were prepared, and the Mueller–Hinton agar plates were inoculated. Antimicrobial discs (oxoid) of amikacin (30 μg), ampicillin (10 μg), amoxicillin/clavulanic acid (30 μg), apramycin (15 μg), aztreonam (30 μg), cefepime (30 μg), cefotaxime (30 μg), cefoxitin (30 μg), ceftazidime (30 μg), colistin (10 μg), doxycycline HCL (30 μg), enrofloxacin (5 μg), erythromycin (15 μg), florfenicol (30 μg), gentamicin (10 μg), imipenem (10 μg), lincomycin (10 μg), piperacillin (100 μg), polymyxin-B (300 IU), spectinomycin (100 μg), spiramycin (100 μg), and sulfamethoxazole-trimethoprim (23.75 + 1.25) were applied on the plates. The plates were then incubated at 37°C for 24 hours, and the detected inhibition zones were analyzed according to the guidelines of the Clinical and Laboratory Standards Institute [16]. Multidrug-resistant isolates (MDR) were determined by resistance to at least one agent in three or more antimicrobials of different tested classes [17].
Identification of virulence and resistance genes
Polymerase chain reaction (PCR) was performed for the recognition of four virulence (toxA, exoY, lasB, and lasI) and three resistance (blactx, fox, and mexR) genes. The extraction of genomic DNA by QIAamp DNA Extraction Miniprep Kit from confirmed cultures is done according to the manufacturer’s instructions. Extracted DNA was kept at −80°C till used in PCR amplification. Positive control DNA was obtained from confirmed positive P. aueroginosa field isolate in RLQP (Reference laboratory for veterinary quality control on poultry production, Dokki, Giza, Egypt). On the contrary, a negative control is a PCR mixture free from the DNA template. The sequences of primers, sizes of amplified segments, and their references are mentioned in Table 1. PCR conditions were conducted as described previously by listed authors.
Table 1. Oligonucleotide primers sequences for different antimicrobial resistance and virulence genes of P. aeruginosa.
| Target gene | Primers direction | Primers sequences (5’-3’) | Amplified product size (bp) | Reference |
|---|---|---|---|---|
| blaCTX | F | ATGTGCAGYACCAGTAARGTKATGGC | 593 | [39] |
| R | TGGGTRAARTARGTSACCAGAAYCAGCGG | |||
| Fox | F | CAAAGCGCGTAACCGGATTGG | 190 | [40] |
| R | CAAAGCGCGTAACCGGATTGG | |||
| mexR | F | GCGCCATGGCCCATATTCAGG | 637 | [41] |
| R | GCATTCGCCAGTAAGCGG | |||
| toxA | F | GACAACGCCCTCAGCATCACCAGC | 396 | [42] |
| R | CGCTGGCCCATTCGCTCCAGCGCT | |||
| exoY | F | CGGATTCTATGGCAGGGAGG | 289 | [43] |
| R | GCCCTTGATGCACTCGACCA | |||
| lasB | F | ACAGGTAGAACGCACGGTTG | 1,220 | [44] |
| R | GATCGACGTGTCCAAACTCC | |||
| lasI | F | ATGATCGTACAAATTGGTCGGC | 606 | [8] |
| R | GTCATGAAACCGCCAGTCG |
F = forward; R = reverse; bp = base pair.
Gene sequencing and sequence analysis
The amplified products of mexR, lasI, and toxA genes were purified from the gel using QIAquick Gel Extraction Kits (Qiagen). All steps of purification were carried following the manufacturer’s instructions using reagents provided in the kit and then sequenced directly using the ABI Prism 3,100 automated sequencing machine (Applied Biosystems, Foster City, CA), and a nucleotide BLAST search (http://www.ncbi.nlm.nih.gov/BLAST) was carried for each sequence. Sequence comparisons and maximum likelihood phylogeny testing through a bootstrap of 1,000 trials were determined with MEGA X software [18] after the Clustal W alignment algorithm. Nucleotide and amino acid identities were determined using Geneious®7.1.3, Build 2014-03-17, Java Version 1.7, Copyright® 2005–2014 Biomatters Ltd.
Results
Isolation of P. aeruginosa from broiler chickens
Bacteriological examination revealed that of 250 examined cases, 45 were positive for P. aeruginosa isolation with a percentage of 18%.
Antimicrobial susceptibility testing of P. aeruginosa isolates
The results shown in Table 2 revealed that the most sensitive antimicrobials were colistin and imipenem (100% each), amikacin (92%), ceftazidime (92%), polymyxin-B (77.7%), gentamicin (75.5%), cefepime (71.1%), and piperacillin (68.9%). In comparison, there was a lower degree of sensitivity to enrofloxacin (53.3%), aztreonam (44.4%), and spectinomycin (33.3%). On the other hand, all tested isolates were resistant (100%) to ampicillin, doxycycline, cefoxitin, florfenicol, lincomycin, and sulfamethoxazole-trimethoprim, followed by 95.5% and 91.2% of the isolates which were resistant to erythromycin and cefotaxime, respectively. All P. aeruginosa isolates under test showed MDR pattern as they resisted at least one member of three different classes and extended to 14 of the tested antimicrobial classes within five (11.11%) of the tested isolates.
Table 2. Antimicrobial susceptibility of P. aeruginosa isolated from broiler chicken.
| Antimicrobial group | Antimicrobial Agents | Disk content (μg/disk ) | P. aeruginosa (n = 45) | |||||
|---|---|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||||||
| No. | % | No. | % | No. | % | |||
| β-Lactams (including combination) | Ampicillin | 10 | 0 | 0 | 0 | 0.0 | 45 | 100 |
| Amoxicillin + Clavulanic acid | 30 | 20 | 44.4 | 0 | 0 | 25 | 55.6 | |
| Pippracillin | 100 | 31 | 68.9 | 6 | 13.4 | 8 | 17.7 | |
| Cefepime | 30 | 32 | 71.1 | 2 | 4.4 | 11 | 24.4 | |
| Cefotaxime (ш) | 30 | 0 | 0.0 | 4 | 8.8 | 41 | 91.2 | |
| Cefoxitin (п) | 30 | 0 | 0.0 | 0 | 0.0 | 45 | 100 | |
| Ceftazidime (ш) | 30 | 41 | 92 | 3 | 6.6 | 1 | 2.2 | |
| Carbapenems (Imipenem) | 10 | 45 | 100 | 0 | 0 | 0 | 0 | |
| Aminoglycosides | Amikacin | 30 | 41 | 92 | 2 | 4.4 | 2 | 4.4 |
| Apramycin | 15 | 4 | 8.8 | 23 | 51.2 | 18 | 40 | |
| Gentamicin | 10 | 34 | 75.5 | 9 | 20 | 2 | 4.5 | |
| Spiramycin | 100 | 5 | 11.1 | 9 | 20 | 31 | 68.9 | |
| Polypeptides | Polymyxin- B | 300-U | 35 | 77.7 | 0 | 0 | 10 | 223 |
| Colistinsulphate | 10 | 45 | 100 | 0 | 0 | 0 | 0 | |
| Monobactam | Aztreonam | 30 | 2 | 4.4 | 23 | 51.1 | 20 | 44.4 |
| Fluoroquinolones | Enrofloxacin | 5 | 21 | 46.7 | 0 | 0 | 24 | 53.3 |
| Lincosamides | Lincomycin | 10 | 0 | 0.0 | 0 | 0 | 45 | 100 |
| Macrolides | Erythromycin | 15 | 2 | 4.5 | 0 | 0 | 43 | 95.5 |
| Phenicol | Florofenicol | 30 | 0 | 0 | 0 | 0 | 45 | 100 |
| Aminocyclitol | Spectinomycin | 100 | 15 | 33.3 | 16 | 35.5 | 14 | 31.2 |
| Tetracycline | Doxycycline HCL | 30 | 0 | 0.0 | 0 | 0.0 | 45 | 100 |
| Sulfonamides | Sulfamethoxazole-trimethoprim | 23.75 + 1.25 | 0 | 0 | 0 | 0 | 45 | 100 |
% = percentage was calculated in relation to the total number of isolates.
Distribution of antimicrobial resistance genes
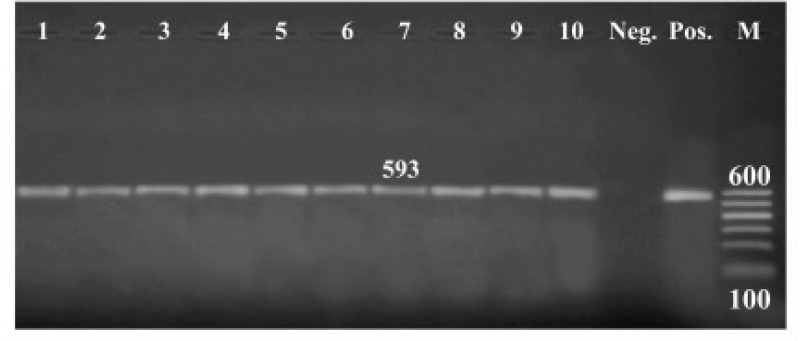
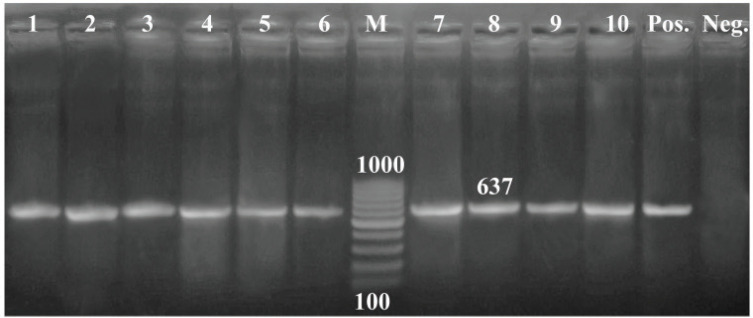
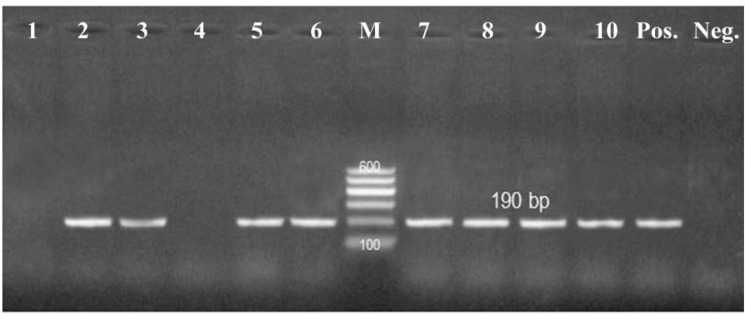
All the tested isolates (100%) were positive to blactx and mexR, whereas 36 of 45 isolates (80%) were positive to fox gene (Figs. 1–3).
Figure 1. PCR amplification of the blaCTX gene at 593-bp. Lane (1–10): Showed +Ve isolates. M = DNA ladder; Pos = Control positive; Neg = Control negative.
Figure 3. PCR amplification of the mexR gene at 637-bp. Lane (1–10): Showed +Ve isolates. M = 100 bp DNA ladder; Pos = Control positive; Neg = Control negative.
Distribution of virulence genes
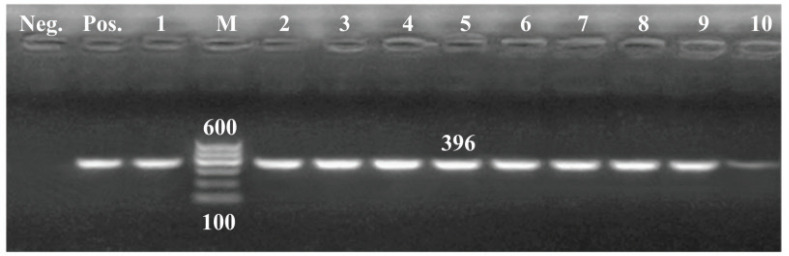
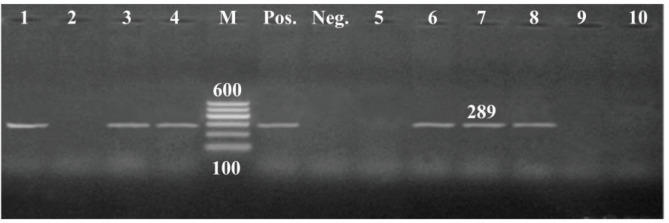
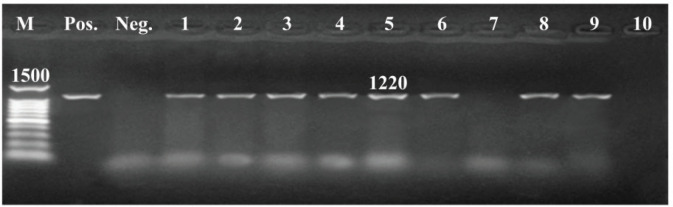
All tested isolates harbored toxA gene, whereas, out of them, 36 carried both lasB and lasI genes with 80%, and 27 isolates (60%) confirmed positive to exoY gene (Figs. 4–7).
Figure 4. PCR amplification of toxA gene of 396pb fragment. Lane (1–10): Showed +Ve isolates. M = 100 bp DNA ladder; Pos = Control positive; Neg = Control negative.
Figure 7. PCR amplification of lasIgene of 606bp fragment. Lane (1,2,3,4,5,6,7,9): Showed +Ve isolates, Lane (8,10): Showed -Ve isolates, M = 100 bp DNA ladder; Pos = Control positive; Neg = Control negative.
Sequence analysis
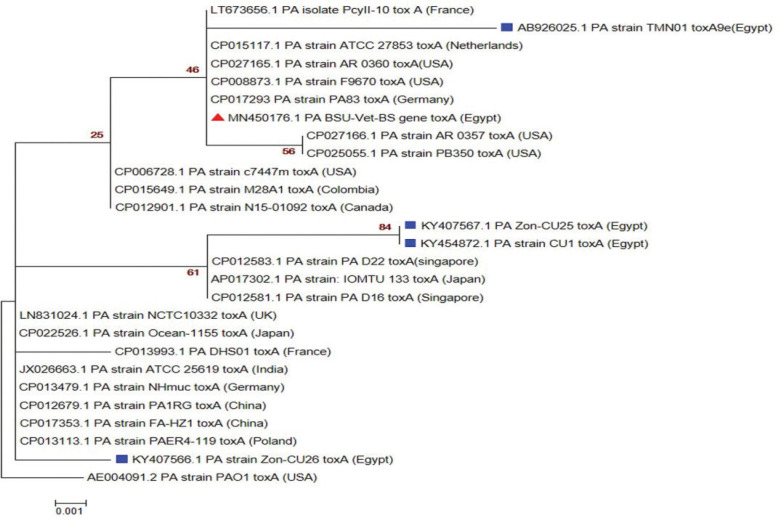
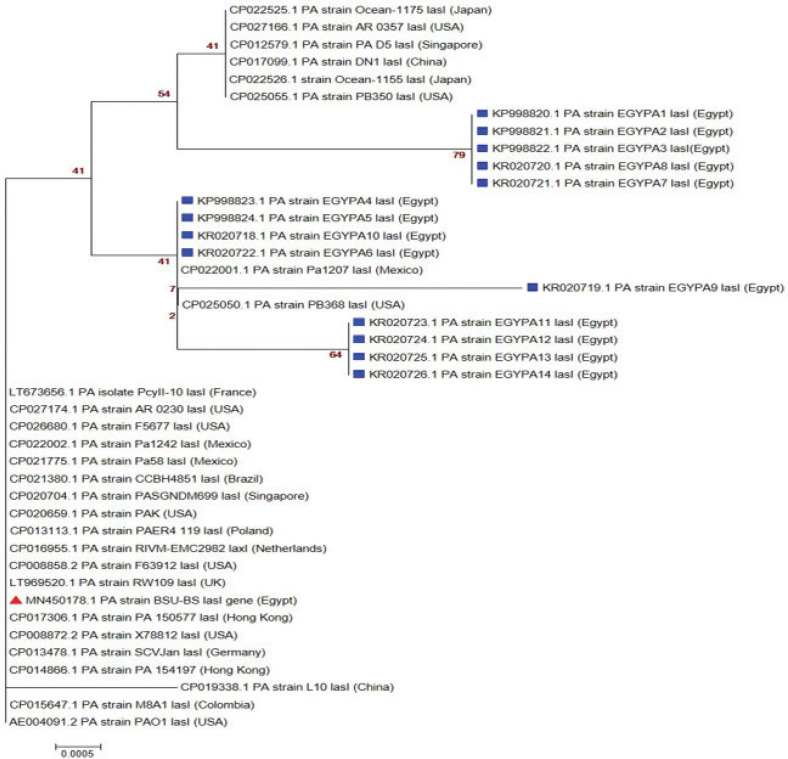
The sequences of representative amplicons of toxA, mexR, and lasIgenes have been deposited to the GenBank and have approved GenBank accession numbers MN450176: MN450178; respectively. Amino acid and nucleotide sequence analysis (as shown in Tables 3–5) revealed a high correlation most of the time with other P. aeruginosa sequences published on the GenBank representing different clinical lesions, hosts, and localities worldwide, especially with P. aeruginosa reference strain (PAO1). Phylogenetic trees (Figs. 8–10) explained the correlation between the sequences of the current study with other sequences.
Table 3. Nucleotides and amino acids identity of toxA gene sequences o f P. aeruginosa.
| Strains | Nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| AE004091.2 (USA) | 1 | 99.58 | 99.24 | 98.02 | 99.07 | 98.02 | 95.42 | 98.75 | 99.22 | 98.96 | 99.16 | |
| LN831024.1 (UK) | 2 | 99.84 | 99.50 | 98.31 | 99.38 | 98.31 | 95.69 | 98.64 | 99.53 | 98.85 | 99.27 | |
| MN450176.1 (This study) | 3 | 100.0 | 100.0 | 98.58 | 99.06 | 98.58 | 96.23 | 100.0 | 99.50 | 99.5 | 99.75 | |
| KY407567.1 (Egypt) | 4 | 99.15 | 99.15 | 99.15 | 98.44 | 100.0 | 97.46 | 98.31 | 98.31 | 98.31 | 98.59 | |
| KY407566.1 (Egypt) | 5 | 99.06 | 99.06 | 99.06 | 99.06 | 98.44 | 97.82 | 98.75 | 99.38 | 99.38 | 99.07 | |
| KY454872.1 (Egypt) | 6 | 99.15 | 99.15 | 99.15 | 100.0 | 99.06 | 97.46 | 98.31 | 98.31 | 98.31 | 98.59 | |
| AB926025.1 (Egypt) | 7 | 80.00 | 80.00 | 80.00 | 82.91 | 90.57 | 82.91 | 96.23 | 95.69 | 95.69 | 95.96 | |
| CP017293.1 (Germany) | 8 | 98.43 | 98.59 | 100.0 | 99.15 | 99.06 | 99.15 | 80.00 | 98.28 | 99.06 | 98.54 | |
| CP013113.1 (Poland) | 9 | 99.53 | 99.69 | 100.0 | 99.15 | 99.06 | 99.15 | 80.00 | 98.28 | 98.59 | 99.11 | |
| CP022526.1 (Japan) | 10 | 99.06 | 99.22 | 100.0 | 99.15 | 99.06 | 99.15 | 80.00 | 99.06 | 98.90 | 98.64 | |
| CP012901.1 (Canada) | 11 | 99.22 | 99.37 | 100.0 | 99.15 | 99.06 | 99.15 | 80.00 | 98.28 | 99.37 | 98.90 | |
| Aminoacids | ||||||||||||
Table 5. Nucleotides and amino acids identity of lasI gene sequences of P. aeruginosa.
| Strains | Nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| AE004091.2 (USA) | 1 | 100.0 | 100.0 | 99.47 | 99.30 | 99.30 | 99.65 | 99.65 | 99.48 | 100.0 | 100.0 | |
| LT969520.1 (UK) | 2 | 100.0 | 100.0 | 99.47 | 98.94 | 96.82 | 94.70 | 93.11 | 92.58 | 91.52 | 90.99 | |
| MN450178.1 (This study) | 3 | 100.0 | 100.0 | 99.43 | 99.25 | 99.25 | 99.62 | 99.62 | 99.44 | 100.0 | 100.0 | |
| KP998820.1 Egypt) | 4 | 100.0 | 100.0 | 100.0 | 100.0 | 99.64 | 99.64 | 99.47 | 99.47 | 99.47 | 99.47 | |
| KP998821.1 (Egypt) | 5 | 99.47 | 99.47 | 99.44 | 100.0 | 99.3 | 99.3 | 99.47 | 99.48 | 99.30 | 99.30 | |
| KR020718.1 (Egypt) | 6 | 98.95 | 98.95 | 98.88 | 100.0 | 99.47 | 99.65 | 99.82 | 99.48 | 99.30 | 99.30 | |
| KR020722.1 (Egypt) | 7 | 99.47 | 99.47 | 99.44 | 100.0 | 99.47 | 99.47 | 99.82 | 99.47 | 99.65 | 99.65 | |
| KR020725.1 (Egypt) | 8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 99.65 | 99.65 | |
| KR020726.1 (Egypt) | 9 | 99.48 | 99.48 | 99.44 | 100.0 | 100.0 | 99.47 | 99.47 | 100.0 | 99.48 | 99.48 | |
| CP015647.1 (Colombia) | 10 | 100.0 | 100.0 | 100.0 | 100.0 | 99.47 | 98.95 | 99.47 | 100.0 | 99.48 | 100.0 | |
| CP016955.1 (Netherlands) | 11 | 100.0 | 100.0 | 100.0 | 100.0 | 99.47 | 98.95 | 99.47 | 100.0 | 99.48 | 100.0 | |
| Aminoacids | ||||||||||||
Figure 8. Phylogenetic analysis of P. aeuroginosa toxA gene. Maximum likelihood tree of 1,000 bootstrap value was conducted after the Clustal W alignment algorithm using MEGA X program. Strain presented in this study is labeled with red triangle while other Egyptian strains are marked with blue square.
Figure 10. Phylogenetic analysis of P. aeuroginosa lasI gene. The maximum likelihood tree of 1,000 bootstrap value was conducted after the Clustal W alignment algorithm using the MEGA X program. Strain presented in this study is labeled with a red triangle while other Egyptian strains are labeled with blue square.
Discussion
In this study, trials were made to examine 250 specimens from the pericardial sac of broiler chickens with lesions of pericarditis for the isolation of P. aeruginosa. Forty-five isolates were obtained out of the studied cases with an occurrence of 18%. This prevalence was relatively higher than that obtained by Abed [19], who recovered 15 isolates of P. aeruginosa with a prevalence of 5% from the pericardial sac of broiler chickens with pericarditis and [20] who recovered seven isolates of P. aeruginosa with a prevalence of 3.5% from the heart blood of broiler chickens with congestion and pericarditis.
Figure 2. PCR amplification of the fox gene at 190-bp. Lane(2,3,5,6,7,8,9,10): Showed +Ve isolates, Lane (1,4): Showd -Ve isolates, M = DNA ladder; Pos = Control positive; Neg = Control negative.
Figure 5. PCR amplification of exoY gene of 289bp fragment. Lane (1,3,4,6,7,8): Showed +Ve isolates, Lane (2,5,9,10): Showed -Ve isolates, M = 100 bp DNA ladder; Pos = Control positive; Neg = Control negative.
Figure 6. PCR amplification of lasB gene of 1220 bp fragment, Lane (1,2,3,4,5,6,8,9): Showed +Ve isolates, Lane (7,10) : Showed -Ve isolates, M = DNA ladder; Pos = Control positive; Neg = Control negative.
An in vitro susceptibility testing was applied against 22 antimicrobial agents, and the most effective antibiotics were colistin sulfate and imipenem (100%), amikacin and ceftazidime (92%), followed by polymyxin-B (77.7%), gentamicin (75.5%), and cefepime (71.1%). Gill et al. [21] mentioned that colistin is a bactericidal antibiotic well thought out as a critical option for treating infections with MDR-resistant P. aeruginosa. The high sensitivity of the studied isolates to imipenem agreed with that of Viedma et al. [22]. Carbapenems, especially meropenem and imipenem, are a crucial group of β-lactams used for the treatment of pseudomonas infections due to their stability to most β-lactamases. The observed sensitivity of the tested isolates to amikacin could be supported by a former study used amikacin as monotherapy for sepsis caused by pan-resistant P. aeruginosa [23]. P. aeruginosa strains showed a high degree of sensitivity to polymyxin-B, and this result coincides the previous record [24]. A previous study [25] recommended cefepime for the treatment of P. aeruginosa infections that agree with the current results. On the other hand, all the tested isolates are entirely resistant to various antimicrobial agents (ampicillin, doxycycline, cefoxitin, florfenicol, lincomycin, and sulfamethoxazole/trimethoprim), followed by erythromycin (95.5%) and cefotaxime (91.2%). All P. aeruginosa isolates under test showed an MDR pattern as they resisted at least a single agent from four classes and extended to 14 of the tested antimicrobial classes within 5 (11.11%) of the isolates under test. Multidrug-resistant bacteria are deliberated now as an authentic hazard in human and/or veterinary medicine [26]. P. aeruginosa is naturally resistant to most antimicrobials and has numerous intrinsic mechanisms to decrease its susceptibility to them as well; it commonly attains added resistance mechanisms and usually develops multidrug resistance during a treatment regimen [27].
Table 4. Nucleotides and amino acids identity ofmexR gene sequences of P. aeruginosa.
| Strains | Nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| AE004091.2 (USA) | 1 | 100.0 | 100.0 | 82.23 | 83.22 | 83.11 | 99.77 | 99.55 | 99.77 | 99.77 | 99.55 | |
| LT969520.1 (UK) | 2 | 100.0 | 100.0 | 82.23 | 83.22 | 83.11 | 99.77 | 99.55 | 99.77 | 99.77 | 99.55 | |
| MN450177.1 (This study) | 3 | 100.0 | 100.0 | 82.23 | 83.22 | 83.11 | 99.77 | 99.55 | 99.77 | 99.77 | 99.55 | |
| KU597741.1 (Egypt) | 4 | 99.20 | 99.20 | 99.20 | 99.47 | 99.47 | 81.96 | 82.49 | 81.96 | 81.96 | 82.23 | |
| KU597742.1 (Egypt) | 5 | 100.0 | 100.0 | 100.0 | 99.20 | 100.0 | 82.99 | 82.77 | 82.99 | 82.99 | 82.77 | |
| KU597743.1 (Egypt) | 6 | 100.0 | 100.0 | 100.0 | 99.20 | 100.0 | 82.88 | 82.66 | 82.88 | 82.88 | 82.66 | |
| CP013479.1 (Germany) | 7 | 100.0 | 100.0 | 100.0 | 99.20 | 100.0 | 100.0 | 99.32 | 100 | 99.55 | 99.32 | |
| AY899300.1 (China) | 8 | 99.32 | 99.32 | 99.32 | 100.0 | 99.32 | 99.32 | 99.32 | 99.32 | 99.32 | 99.55 | |
| CP012901.1 (Canada) | 9 | 100.0 | 100.0 | 100.0 | 99.20 | 100.0 | 100.0 | 100.0 | 99.32 | 99.55 | 99.32 | |
| CP015001.1 (Brazil) | 10 | 99.32 | 99.32 | 99.32 | 98.40 | 99.32 | 99.32 | 99.32 | 98.64 | 99.32 | 99.32 | |
| CP015649.1 (Colombia) | 11 | 100.0 | 100.0 | 100.0 | 99.20 | 100.0 | 100.0 | 100.0 | 99.32 | 100.0 | 99.32 | |
| Aminoacids | ||||||||||||
The genotypic investigation of antimicrobial resistance and virulence genes of P. aeruginosa of poultry origin is scanty, so one of these studies aims was to detect the occurrence of antimicrobial resistance genes such as blaCTX, fox, and mexR genes and virulence genes such as toxA, exoY, lasB, and lasI genes among P. aeruginosa isolates. Concerning resistance genes, blaCTX, was detected in 100% of the examined P. aeruginosa isolates although the lower prevalence was recorded by others [28, 29]. The detection of blaCTX gene among all the tested isolates could explain the growing resistance pattern of P. aeruginosa, which reached to complete resistance against the third generation of cephalosporins represented by cefotaxime. In relation to fox gene, it was noticed in 80% of the isolates, but this result was higher than previously reported by Voolaid et al. [30], who detected the fox gene in 25% of the tested P. aeruginosa isolates. This high prevalence of fox gene in this work may elucidate the rising resistance behavior, which reached to complete resistance against the second-generation cephalosporins represented by cefoxitin. Regarding mexR gene, it was identified in 100% of the isolates, and this may explain why all the recovered P. aeruginosa isolates in the present work showed a multidrug-resistant pattern, which represents a high risk in the treatment of pseudomonal infection not only in poultry diseases but also with human infection. Quorum sensing is a bacterial way of communication through specific chemical molecules to regulate their action against hosts through the production of virulence factors and antimicrobial-resistant determinants [31]. One of the most critical virulence genes is toxA, which found in 100% of the studied isolates, and this result coincides with several studies [24,32,33]. ToxA gene can be used as a marker to identify P. aeruginosa by using PCR [32]. The existence of toxA gene among P. aruginosa clinical isolates follows the essential role of this virulence factor in chicken respiratory affections [34]. ExoY gene was detected in 60% of the investigated isolates; a higher prevalence was recorded [9] with exoenzyme-Y, which is being implicated in the pathogenesis of P. aeruginosa. Among the tested isolates, lasB gene was detected in 80%, and this prevalence is nearly close to that previously described by Benie et al. [35]. Elastase (LasB) is an extracellularly secreted endopeptidase which regarded as an important virulence factor of P. aeruginosa [36]. Finally, lasI gene (autoinducer) was perceived in 80% of the isolates, which is responsible for the secretion of some virulence tools of P. aeruginosa as elastase and pyocyanin [8].
Figure 9. Phylogenetic analysis of P. aeuroginosa mexR gene. The maximum likelihood tree of 1,000 bootstrap value was conducted after the Clustal W alignment algorithm using MEGA X program. Strain presented in this study is labeled with a red triangle, while other Egyptian strains are labeled with blue square.
Amino acid and nucleotide sequence analysis revealed a high correlation most of the time with other P. aeruginosa sequences published on the GeneBank representing different clinical lesions, hosts, and localities, worldwide, especially with P. aeruginosa reference strain (PAO1). These findings were explained by Klockgether et al. [37]. They clarified that the core genome of P. aeruginosa is exceptionally conserved, and the relationships of strains from the same clonal complex, nevertheless discrete geographic origin, illustrate a low substitution rate.
P. aeruginosa can induce variable clinical lesions in different localization sites due to its inhibitory effect on the cytokines production that reduces the host’s ability to clear infection [38]. Therefore, the probability of transferring the highly virulent strains of this microorganism from broilers to human with zoonotic losses represents public health hazard.
Conclusion
Pseudomonas aeruginosa that was isolated from diseased broilers makes available proof that poultry environment could constitute a public health risk due to the existence of multiple antibiotic resistances by several strains in this study, in addition to the distribution of both virulence and resistance genes among them.
Acknowledgment
Nothing to disclose.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
First and the last author designed the work, second and last author carried the practical part, the third author carried out the sequencing part, the last author has written the manuscript, and other authors reviewed the writing.
References
- [1].Astill J, Dara RA, Fraser EDG, Sharif S. Detecting and predicting emerging disease in poultry with the implementation of new technologies and big data: a focus on avian influenza virus. Front Vet Sci. 2018;5:263. doi: 10.3389/fvets.2018.00263. https://doi.org/10.3389/fvets.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang Z, Zuo J, Gong J, Hu J, Jiang W, Mi R, et al. Development of a multiplex PCR assay for the simultaneous and rapid detection of six pathogenic bacteria in poultry. AMB Express. 2019;9(1):185. doi: 10.1186/s13568-019-0908-0. https://doi.org/10.1186/s13568-019-0908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walker SE, Sander JE, Cline JL, Helton JS. Characterization of Pseudomonas aeruginosa isolates associated with mortality in broiler chicks. Avian Dis. 2002;46(4):1045–50. doi: 10.1637/0005-2086(2002)046[1045:COPAIA]2.0.CO;2. https://doi.org/10.1637/0005-2086(2002)046[1045:COPAIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [4].Morita Y, Tomida J, Kawamura Y. Efflux-mediated fluoroquinolone resistance in the multidrug-resistant Pseudomonas aeruginosa clinical isolate PA7: identification of a novel MexS variant involved in upregulation of the mexEF-oprN multi-drug efflux operon. Front Microbiol. 2015;6:8. doi: 10.3389/fmicb.2015.00008. https://doi.org/10.3389/fmicb.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu D. Molecular detection of foodborne pathogens. 1st. Taylor & Francis CRC Press; Boca Raton, FL: 2009. Available via https://doi.org/10.1201/9781420076448. [Google Scholar]
- [6].Khosravi AD, Shafie F, Abbasi Montazeri E, Rostami S. The frequency of genes encoding exotoxin A and exoenzyme S in Pseudomonas aeruginosa strains isolated from burn patients. Burns. 2016;42(5):1116–20. doi: 10.1016/j.burns.2016.02.012. https://doi.org/10.1016/j.burns.2016.02.012. [DOI] [PubMed] [Google Scholar]
- [7].Dulon S, Leduc D, Cottrell GS, D’Alayer J, Hansen KK, Bunnett NW, et al. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2005;32(5):411–9. doi: 10.1165/rcmb.2004-0274OC. https://doi.org/10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- [8].Bratu S, Gupta J, Quale J. Expression of the las and rhl quorum-sensing systems in clinical isolates of Pseudomonas aeruginosa does not correlate with efflux pump expression or antimicrobial resistance. J Antimicrob Chemother. 2006;58(6):1250–3. doi: 10.1093/jac/dkl407. https://doi.org/10.1093/jac/dkl407. [DOI] [PubMed] [Google Scholar]
- [9].Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem. 2012;287(30):25407–18. doi: 10.1074/jbc.M111.301440. https://doi.org/10.1074/jbc.M111.301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ghosh S, LaPara TM. The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J. 2007;1(3):191–203. doi: 10.1038/ismej.2007.31. https://doi.org/10.1038/ismej.2007.31. [DOI] [PubMed] [Google Scholar]
- [11].Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res. 2017;13(1):211. doi: 10.1186/s12917-017-1131-3. https://doi.org/10.1186/s12917-017-1131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64(Suppl 1):i3–10. doi: 10.1093/jac/dkp256. https://doi.org/10.1093/jac/dkp256. [DOI] [PubMed] [Google Scholar]
- [13].Bush K, Bradford PA. Beta-Lactams and beta-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8):a025247. doi: 10.1101/cshperspect.a025247. https://doi.org/10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dame-Korevaar A, Fischer EAJ, van der Goot J, Stegeman A, Mevius D. Transmission routes of ESBL/pAmpC producing bacteria in the broiler production pyramid, a literature review. Prev Vet Med. 2019;162:136–50. doi: 10.1016/j.prevetmed.2018.12.002. https://doi.org/10.1016/j.prevetmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- [15].Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, Fitzpatrick ES. Veterinary microbiology and microbial disease. 2nd. Wiley-Blackwell; Hoboken, NJ: 2011. [Google Scholar]
- [16].CLSI. (Clinical and laboratory Standards Institute) Performance standards for antimicrobial susceptibility testing. 28th. Wayne; PA: 2018. [Google Scholar]
- [17].Gill JS, Arora S, Khanna SP, Kumar KH. Prevalence of multidrug-resistant, extensively drug-resistant, and pandrug-resistant Pseudomonas aeruginosa from a tertiary level intensive care unit. J Glob Infect Dis. 2016;8(4):155–9. doi: 10.4103/0974-777X.192962. https://doi.org/10.4103/0974-777X.192962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. doi: 10.1093/molbev/msy096. https://doi.org/10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abed AH. A contribution towards the bacterial pathogens associated with respiratory problems in broiler chickens. Beni-Suef University; Beni Suef, Egypt: 2007. (Unpublished master’s thesis) [Google Scholar]
- [20].Hassan HRM. Characterization of Pseudomonas aeruginosa isolated from different pathological lesions in chickens. Beni-Suef University; Beni Suef, Egypt: 2013. (Unpublished master’s thesis) [Google Scholar]
- [21].Gill MM, Rao JU, Kaleem F, Hassan A, Khalid A, Anjum R. In vitro efficacy of colistin against multi-drug resistant Pseudomonas aeruginosa by minimum inhibitory concentration. Pak J Pharm Sci. 2013;26(1):7–10. [PubMed] [Google Scholar]
- [22].Viedma E, Juan C, Villa J, Barrado L, Orellana MA, Sanz F, et al. VIM-2-producing multidrug-resistant Pseudomonas aeruginosa ST175 clone, Spain. Emerg Infect Dis. 2012;18(8):1235–41. doi: 10.3201/eid1808.111234. https://doi.org/10.3201/eid1808.111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Layeux B, Taccone FS, Fagnoul D, Vincent JL, Jacobs F. Amikacin monotherapy for sepsis caused by panresistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54(11):4939–41. doi: 10.1128/AAC.00441-10. https://doi.org/10.1128/AAC.00441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morales-Espinosa R, Delgado G, Espinosa LF, Isselo D, Mendez JL, Rodriguez C, et al. Fingerprint analysis and identification of strains ST309 as a potential high risk clone in a Pseudomonas aeruginosa population isolated from children with bacteremia in Mexico City. Front Microbiol. 2017;8:313. doi: 10.3389/fmicb.2017.00313. https://doi.org/10.3389/fmicb.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paul M, Yahav D, Bivas A, Fraser A, Leibovici L. Anti-pseudomonal beta-lactams for the initial, empirical, treatment of febrile neutropenia: comparison of beta-lactams. Cochrane Database Syst Rev. 2010;11:CD005197. doi: 10.1002/14651858.CD005197.pub3. https://doi.org/10.1002/14651858.CD005197.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karp BE, Tate H, Plumblee JR, Dessai U, Whichard JM, Thacker EL, et al. National antimicrobial resistance monitoring system: two decades of advancing public health through integrated surveillance of antimicrobial resistance. Foodborne Pathog Dis. 2017;14(10):545–57. doi: 10.1089/fpd.2017.2283. https://doi.org/10.1089/fpd.2017.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177–92. doi: 10.1016/j.biotechadv.2018.11.013. https://doi.org/10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- [28].Polotto M, Casella T, de Lucca Oliveira MG, Rubio FG, Nogueira ML, de Almeida MT, et al. Detection of P. aeruginosa harboring bla CTX-M-2, bla GES-1 and bla GES-5, bla IMP-1 and bla SPM-1 causing infections in Brazilian tertiary-care hospital. BMC Infect Dis. 2012;12:176. doi: 10.1186/1471-2334-12-176. https://doi.org/10.1186/1471-2334-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Peymani A, Naserpour-Farivar T, Zare E, Azarhoosh KH. Distribution of blaTEM, blaSHV, and blaCTX-M genes among ESBL-producing P. aeruginosa isolated from Qazvin and Tehran hospitals, Iran. J Prev Med Hyg. 2017;58(2):E155–60. [PMC free article] [PubMed] [Google Scholar]
- [30].Voolaid V, Tenson T, Kisand V. Aeromonas and Pseudomonas species carriers of ampC FOX genes in aquatic environments. Appl Environ Microbiol. 2013;79(3):1055–7. doi: 10.1128/AEM.03171-12. https://doi.org/10.1128/AEM.03171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Asfour HZ. Anti-quorum sensing natural compounds. J Microsc Ultrastruct. 2018;6(1):1–10. doi: 10.4103/JMAU.JMAU_10_18. https://doi.org/10.4103/JMAU.JMAU_10_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Auda IG, Al-Kadmy IMS, Ali ANM, Muslim SN, Salman IMA. ToxA gene as a chromosomal marker for rapid identification of Otitis media Pseudomonas aeruginosa. Trans Sci Technol. 2015;2(2):6–10. https://doi.org/10.15242/IJACEBS.C0815022. [Google Scholar]
- [33].Elsayed MSA, Ammar AM, Al shehri ZS, Abd-El Rahman H, Abd-El Rahman NA. Virulence repertoire of Pseudomonas aeruginosa from some poultry farms with detection of resistance to various antimicrobials and plant extracts. Cell Mol Biol. 2016;62(1):124–9. [Google Scholar]
- [34].Tartor YH, El-Naenaeey EY. RT-PCR detection of exotoxin genes expression in multi-drug resistant Pseudomonas aeruginosa. Cell Mol Biol (Noisy-le-grand) 2016;62(1):56–62. [PubMed] [Google Scholar]
- [35].Benie CKD, Dadié A, Guessennd N, DésiréKouame N, NadègeAhou N, Kouadio NAN, et al. Molecular identification and virulence factors of Pseudomonas aeruginosa strains isolated from animal products. J Bacteriol Mycol. 2017;4(3):91–6. https://doi.org/10.15406/jbmoa.2017.04.00094. [Google Scholar]
- [36].Caballero AR, Moreau JM, Engel LS, Marquart ME, Hill JM, O’Callaghan RJ. Pseudomonas aeruginosa protease IV enzyme assays and comparison to other Pseudomonas proteases. Anal Biochem. 2001;290(2):330–7. doi: 10.1006/abio.2001.4999. https://doi.org/10.1006/abio.2001.4999. [DOI] [PubMed] [Google Scholar]
- [37].Klockgether J, Cramer N, Wiehlmann L, Davenport CF, Tummler B. Pseudomonas aeruginosa genomic structure and diversity. Front Microbiol. 2011;2:150. doi: 10.3389/fmicb.2011.00150. https://doi.org/10.3389/fmicb.2011.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Weber A, Zimmermann C, Mausberg AK, Dehmel T, Kieseier BC, Hartung HP, et al. Pseudomonas aeruginosa and its bacterial components influence the cytokine response in thymocytes and splenocytes. Infect Immun. 2016;84(5):1413–23. doi: 10.1128/IAI.00905-15. https://doi.org/10.1128/IAI.00905-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Archambault M, Petrov P, Hendriksen RS, Asseva G, Bangtrakulnonth A, Hasman H, et al. Molecular characterization and occurrence of extended-spectrum beta-lactamase resistance genes among Salmonella enterica serovar Corvallis from Thailand, Bulgaria, and Denmark. Microb Drug Resist (Larchmont, NY) 2006;12(3):192–8. doi: 10.1089/mdr.2006.12.192. https://doi.org/10.1089/mdr.2006.12.192. [DOI] [PubMed] [Google Scholar]
- [40].Perez-Perez FJ, Hanson ND. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40(6):2153–62. doi: 10.1128/JCM.40.6.2153-2162.2002. https://doi.org/10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sanchez P, Linares JF, Ruiz-Diez B, Campanario E, Navas A, Baquero F, et al. Fitness of in vitro selected Pseudomonas aeruginosa nalB and nfxB multi-drug resistant mutants. J Antimicrob Chemother. 2002;50(5):657–64. doi: 10.1093/jac/dkf185. https://doi.org/10.1093/jac/dkf185. [DOI] [PubMed] [Google Scholar]
- [42].Matar GM, Ramlawi F, Hijazi N, Khneisser I, Abdelnoor AM. Transcription levels of Pseudomonas aeruginosa exotoxin a gene and severity of symptoms in patients with otitis externa. Curr Microbiol. 2002;45(5):350–4. doi: 10.1007/s00284-002-3703-z. https://doi.org/10.1007/s00284-002-3703-z. [DOI] [PubMed] [Google Scholar]
- [43].Winstanley C, Kaye SB, Neal TJ, Chilton HJ, Miksch S, Hart CA, et al. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J Med Microbiol. 2005;54(Pt 6):519–26. doi: 10.1099/jmm.0.46005-0. https://doi.org/10.1099/jmm.0.46005-0. [DOI] [PubMed] [Google Scholar]
- [44].Finnan S, Morrissey JP, O’Gara F, Boyd EF. Genome diversity of Pseudomonas aeruginosa isolates from cystic fibrosis patients and the hospital environment. J Clin Microbiol. 2004;42(12):5783–92. doi: 10.1128/JCM.42.12.5783-5792.2004. https://doi.org/10.1128/JCM.42.12.5783-5792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]