Abstract
Objective:
The current research aimed at investigating growth performances and meat yield characteristics of commercial cockerels supplemented with antibiotics and probiotics to the diet.
Materials and methods:
A total of 1,350 commercial cockerels (ISA Brown) were indiscriminately distributed to 3 treatment groups, each having three replications of 150-day-old chicks based on completely randomized design. The basal diet was treated as the control, while the experimental groups receiving Enrofloxacin 1 gm/kg and Protexin 1 gm/kg feed were considered as antibiotic and probiotic groups, respectively. Bodyweight, feed intake (FI), feed conversion ratio (FCR), and other relevant characteristics were recorded weekly until 49 days of trial. In the end, similar number (10) of birds from each replicate group were slaughtered to determine the carcass characteristics.
Results:
Significantly, better results were found in the overall growth performances of the cockerels in the probiotic-fed treatment group. Highly significant differences were also found in live bodyweight, weight gain, daily gain, FI, FCR, survivability, dressing percentage, abdominal fat, breast meat, drumstick, and thigh weight in the probiotic-fed treatment group compared to the others.
Conclusion:
The results of the present study indicate that the inclusion of dietary probiotics has a superior performance to antibiotics and may have the potentiality to be used as an alternative growth enhancer in the diet of cockerels.
Keywords: Cockerel, growth performance, antibiotic alternative, probiotic
Introduction
Cockerel farming has become popular in Bangladesh in recent years due to a comparatively low chick price than that of layers or broilers. Cockerel meat has a high demand for various social and cultural occasions like marriage ceremony, anniversary programs, and reunions. It has an enormous daily demand in restaurants than that of broiler meat. The sensory characteristics like taste, flavor, juiciness, and tenderness are almost close to the indigenous chicken. Less abdominal fat, lower disease susceptibility, mortality, and morbidity are also the prime reasons as to why farmers have been showing more interest in cockerel farming in the country [1]. People usually rear broilers or layer chickens with the supplementation of feed additives like an antibiotic, prebiotic, probiotic, symbiotic, or other available commercial additives. Antibiotic and probiotic have the well-recognized capability of killing or inhibiting the development of disease-producing bacteria or microorganisms that are present in the chicken body and thus improve growth performances [2, 3].
However, the use of antibiotics as a promoter in animal and poultry feed has been barred by concerned organizations globally [4]. Researchers are suggesting probiotics as an alternative to antibiotics because they not only interrupt the performance of pathogens in the intestines but also have a potential role in increasing the bioavailability of minerals that helps in the improvement of growth and effective utilization of feeds [5]. The prevalence of diseases also reduces with the judicial application of probiotics. It also promotes the immune system and influences the morphology and function of intestines [6]. But the use of probiotics and antibiotics in rearing cockerels is still unknown. Thus, the present study was undertaken to compare and evaluate the growth performances and meat yield characteristics of commercial cockerel (ISA Brown) fed antibiotic and probiotic as feed supplementation.
Material and Methods
Ethical approval
Animal Welfare and Experimentation Ethics Committee of Bangladesh Agricultural University gave formal approval for the experiment upon birds [Reference no.: AWEEC/BAU/2017(22)].
Experimental site
This study was carried out at a farm of a local farmer of Hobirbari Union at Valuka Upazila, Mymensingh district of Bangladesh from March to April 2017.
Management of birds and design of experiment
A total of 1,350 commercial cockerel (ISA Brown), 150-day-old chicks (DOCs) having average bodyweight (BW) of about 36 gm, were taken to conduct a 49-day experiment. The ISA Brown species was chosen for the experiment because the cockerels of this strain are very popular among farmers, which might be due to its ease of availability and comparatively lower price. The DOCs were indiscriminately allocated into three groups; group-1 was considered as the control, while group-2 and group-3 were considered as antibiotic and probiotic-treated groups, respectively, having around 450 chicks per group. Each treatment group was again divided into three replications (150 chicks/replication). As no feeding standard was establish for the cockerels, considering the purpose of the current study, the basal ration was formulated as per National Research Council standard for broiler chickens [7]. The birds were provided with different forms of feed, as presented in Table 1, throughout the experiment. In group-2, commercial antibiotic (Enrofloxacin) was administrated at 1 gm/kg feed. At the same time, group-3 was supplemented with 1 gm of a commercial probiotic mixture (Protexin) in the same amount of feed for the entire period of the experiment. Enrofloxacin and Protexin were chosen in the present trial because the majority of the farmers were using these chemicals in the study location due to their availability. The birds in all the three groups received identical management, and feed and safe drinking water were supplied ad libitum basis. Newcastle, Fowl Pox, and Gumboro disease vaccines were administered to the birds.
Table 1. Composition of experimental diets.
| Nutrients/parameters | Starter (0–21 days) | Grower(22–35 days) | Finisher (36–49 days) |
|---|---|---|---|
| Moisture (%) | 11 | 11 | 11 |
| CP (%) | 21 | 20 | 19 |
| Ash (%) | 4.5 | 4.5 | 5 |
| Ca (%) | 1.00 | 0.95 | 0.90 |
| Ava. P (%) | 0.45 | 0.45 | 0.42 |
| Methionine (%) | 0.48 | 0.45 | 0.42 |
| Lysine (%) | 1.15 | 1.05 | 1.00 |
| ME (kcal/kg) | 2950 | 3000 | 3050 |
| Feed type | Mash | Crumble | Pellet |
Measurements
Individual BW, gain, feed conversion ratio (FCR), and pen-wise weekly feed intake (FI) were recorded until the end of the experiment. Mortality was adjusted from time to time to calculate the FCR. A total of 30 birds (10 from each replicate) from each treatment group were slaughtered, applying the Halal method, to determine the meat yield characteristics.
Statistical analysis
The Statistical Package for the Social Sciences version 20.0 [8] was used to analyze all the recorded data. For significant differences among groups, a p-value of < 0.05 was considered and Duncan’s Multiple Range Test [9] was used to compare the means.
Results
Overall growth performance
In the experiment conducted, the overall performances (Table 2) were found to be better in the probiotic-treated group than that in the antibiotic and control groups. The weights of the DOCs were very similar (p > 0.05) in all the three groups. Live bodyweight (LBW), weight gain (WG), average daily gain (ADG), and survivability were highly significant (p < 0.0001). At the same time, significant differences were also found in FCR (p < 0.001) and FI (p < 0.05) of cockerels.
Table 2. Growth performance of cockerels in different dietary treatments.
| Parameters | Treatments | p-value | ||
|---|---|---|---|---|
| Control | Antibiotic | Probiotic | ||
| Initial bodyweight (gm) | 36.33 ± 0.33 | 36.67±0.67 | 36.00±0.58 | 0.702 |
| Final bodyweight (gm) | 704.33 ± 6.36c | 778.0±5.77b | 820.0±4.62a | 0.0001 |
| Body WG (gm/bird) | 668.33 ± 6.36c | 742.00±5.77b | 784.00±4.61a | 0.0001 |
| Total FI (gm/bird) | 1,700.84 ± 10.66a | 1,746.18±19.34b | 1,614.88±39.89a | 0.032 |
| FCR | 2.54 ± 0.04c | 2.35±0.04b | 2.06±0.06a | 0.001 |
| ADG (gm/day) | 13.63 ± 0.13c | 15.14±0.12b | 16.00±0.09a | 0.0001 |
| Survivability (%) | 95.71 ± 0.23b | 97.76 ± 0.23a | 98.44±0.22a | 0.0001 |
Means with different superscripts in the same row are differ significantly; Values indicate mean ± SE; SE means Standard Errors.
Live bodyweight and gain
The results of the current research, shown in Table 2, indicated highly significant (p < 0.0001) LBW, WG, and ADG in the probiotic-treated group compared to the antibiotic and control groups. The ADG pattern of birds showed (Fig. 1) that the growth rate of the probiotic-treated group was almost higher throughout the experimental period compared to the other two groups.
Figure 1. ADG (gm/bird/day) of the cockerels at different ages of the experiment.

Feed intake
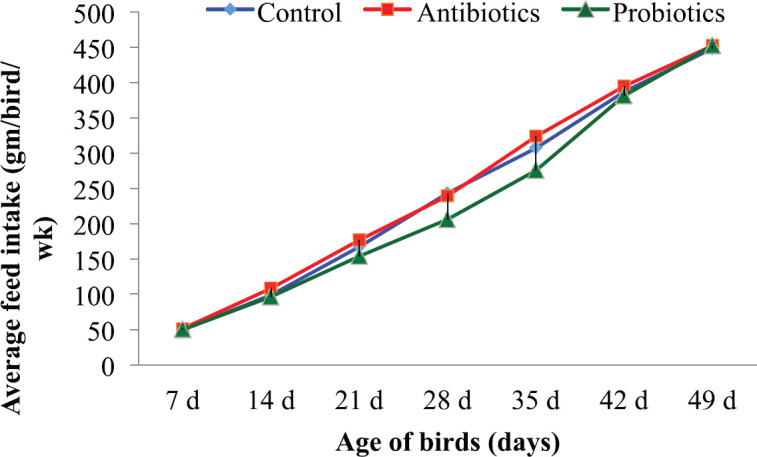
The results of the present study revealed that cockerels from the probiotic-treated group consumed a comparatively lower (p < 0.05) amount of feed (Table 2) than those in the other two groups. The highest amount of FI was observed in the antibiotic-treated group in the experiment. The weekly FI patterns of the birds (Fig. 2) represented that it was almost similar during the starting and termination period of the experiment in all three dietary groups, while a lower intake was observed in probiotic-fed birds from the second to the sixth week of age.
Figure 2. Average FI (gm/bird/week) of cockerels from beginning to end of the experiment.
Feed conversion ratio
While comparing cockerel’s FCR, a significant difference (p < 0.001) was observed in probiotic-fed birds of the present trial, followed by the antibiotic and control groups (Table 2). The weekly FCR patterns of birds (Fig. 3) indicated that it was better in the probiotic dietary treatment group from inception to termination of the experiment. At the same time, a slight fluctuation was observed in the antibiotic group.
Figure 3. Average FCR of cockerels at different ages during the experimental period.

Survivability
The survivability percentage of cockerels was found to be significantly higher (p < 0.001). The results are presented in Table 2. The mortality rate was comparatively higher in the control group, followed by the antibiotic- and probiotic-treated groups.
Carcass characteristics
The mean value of the meat yield characteristics of cockerels is shown in Table 3. High differences (p < 0.001) were found in the dressing percentage of the probiotic-fed group, followed by the antibiotic and control groups. Significant differences were also observed in blood, abdominal fat, breast meat, thigh and drumstick, gizzard, feather, skin, and heart weight among the three dietary treatment groups. But the weight of the liver, pancreas, spleen, and intestine were observed to be non-significant (p > 0.05) in all the three groups.
Table 3. Mean value of meat yield characteristics of cockerels in different weeks.
| Parameters | Treatments | p-value | ||
|---|---|---|---|---|
| Control | Antibiotic | Probiotic | ||
| Dressing percentage (% BW) | 50.10 ± 0.60c | 51.30 ± 0.61b | 54.0 ± 0.20a | 0.0001 |
| Blood weight (gm) | 3.95 ± 0.05a | 4.51 ± 0.01c | 4.35 ± 0.03b | 0.0001 |
| Breast meat weight (gm) | 10.52 ± 0.1a | 10.22 ± 0.1b | 10.73 ± 0.12a | 0.003 |
| Thigh+ Drumstick weight (gm) | 17.05 ± 0.58c | 17.83 ± 0.30b | 18.80 ± 0.26a | 0.006 |
| Digestive tract weight (gm) | 10.93 ± 0.31a | 11.6 ± 0.07b | 11.46 ± 0.06b | 0.01 |
| Skin weight (gm) | 6.05 ± 0.06bc | 5.98 ± 0.13a | 6.26 ± 0.12c | 0.044 |
| Liver weight (gm) | 2.13 ± 0.04 | 2.22 ± 0.03 | 2.17 ± 0.04 | 0.063 |
| Heart weight (gm) | 0.40 ± 0.04b | 0.50 ± 0.02a | 0.48 ± 0.04a | 0.014 |
| Gizzard weight (gm) | 1.48 ± 0.02 a | 1.4 ± 0.02 b | 1.38 ± 0.03 b | 0.004 |
| Pancreas weight (gm) | 0.21 ± 0.03 | 0.24 ± 0.03 | 0.22 ± 0.01 | 0.178 |
| Spleen weight (gm) | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.236 |
| Abdominal fat weight (gm) | 1.59 ± 0.05b | 1.79 ± 0.05a | 1.74 ± 0.05a | 0.008 |
| Intestinal weight (gm) | 2.62 ± 0.03 | 2.58 ± 0.02 | 2.72 ± 0.11 | 0.096 |
| Feather weight (gm) | 9.46 ± 0.08c | 8.52 ± 0.24a | 9.0 ± 0.1b | 0.001 |
BW means bodyweight,
means with different superscripts in the same row are differ significantly; Values indicates mean± SE; SE means Standard Errors.
Discussion
A group of researchers [6] reported improved WG, FI, and FCR in chickens fed with antibiotics. Many others [10] suggested probiotics for enhancing the performances of chickens as a replacement to the antibiotics. Final WG and FCR were found to be higher (p < 0.05) in birds feeding on probiotics than antibiotics [11]. A previous study [12] also reported that probiotic-fed chickens had better digestion, absorption, nutrients availability, and improved enzyme activities [13], which supports the results of the present study.
Although the authors found very limited research studies regarding feeding probiotics and antibiotics to cockerels or commercial egg-type male chickens, the current finding is in disagreement with a research team’s [14] outcomes, who found no significant difference in growth performance of Nigerian local white cockerels fed multi-strain probiotics. However, they found a similar ADG like the current study. Although a similar BWG and ADG has been reported in egg-type male growing chicken, no significant differences were reported between basal and low probiotics (1 gm/l) fed to crossbreds of Fayoumi and Rhode Island Red cockerels [15, 16]. Despite the few studies, the majority of the results stated that probiotics have a greater effect on BWG of the birds than that of antibiotic growth promoters [2,3,11,17–25]. This inconsistency might be due to the use of different strains of birds and a mixture of variable strains of bacteria in probiotic or antibiotic compositions added to the experimental diets. A higher growth rate in the probiotic supplemented group of the current research may be due to superior digestion, absorption, nutrients availability, and function of helpful enzymes.
The present research findings are in agreement with a previous study which reported less FI in probiotic-treated cockerel crossbreds [15], but differ from other reports [3,11,14,26], who have stated a higher significant FI (p < 0.05) in probiotic-fed broilers than that of the antibiotic and control groups. However, no influence of various probiotics on the FI of birds was reported in previous studies [25,27–30]. The variation of current research findings with past studies might be due to the differences in farming conditions, the strain of birds, types of probiotic strains, antibiotic mixture and variety of feed ingredients used to prepare experimental diets.
A research team [3] reported significantly better (p < 0.05) FCR feeding of probiotics to birds in comparison with the antibiotic and control groups, which supports the present findings. Experts [26] have also stated that supplementation of Saccharomyces cerevisiae, a probiotic to birds at three different levels (1, 1.5, and 2%), had improved FCR significantly. However, many previous studies found non-significant differences (p > 0.05) in FCR of low-level probiotic-treated cockerels (1 gm/l) [15] and antibiotic-treated broilers [10,30,31]. Variation of results in the current research might be because of the compensatory growth pattern of birds, the difference in the use of probiotics and antibiotic mixture, feeding, and management system of birds and geographical location.
Growth stimulants as feed additives have a positive effect on enhancing performance and improved production of meat [32], which supports the results of the present study. Studies have also revealed that growth enhancers had a positive influence on the performances of chicken [33]. But lower or non-significant differences in carcass yields, abdominal fat, breast and thigh weight fed antibiotic, probiotic, and symbiotic to birds was also reported [15,31,34]. Variation in the current findings with previous studies might be due to compensatory growth patterns of cockerels, varied environment, and management aspects involved during the experiment.
Conclusion
It is recommended that adding probiotics to the diet of a commercial cockerel (ISA Brown) as feed additive at 1 gm/kg may be better than that of the same level of antibiotic supplementation. Thus, it can be used as a substitute to antibiotic growth promoter for optimum production. However, repeated further studies with other probiotics and antibiotics, along with different commercial cockerel strains, are required to find out the optimum performance.
Acknowledgments
The authors acknowledge the contributions and continuous support from the Department of Animal Science and the Department Poultry Science of Bangladesh Agricultural University, Poultry Production Research Division of Bangladesh Livestock Research Institute, for the fruitful ending of the research work. The authors thank Mr. Rahim Mia, the farm owner from the Hobirbari Union at Valuka Upazila, Mymensingh district, where the experiment was conducted.
Conflict of interest
The authors declare that they have no conflicts of interest in the authorship of this article.
Authors’ contribution
MNH, MAKA, and MAGR conceptualized and designed the study. MNH conducted the experiment and collected the data. MNH, MHOR, and TY helped in collecting data and drafting this manuscript. MAKA supervised the experiment. MAGR analyzed and interpreted the data and drafted the manuscript. All authors arranged the data and prepared the manuscript. MAGR made final changes to the document as a research article and critically revised the manuscript. All the authors read and approved the final manuscript.
References
- [1].Hauqe QME, Mostari MP, Islam MR. Production and marketing age of male layer chicks in Bangladesh. In: Haque QME, editor. 4th International Poultry Show and Seminar. Dhaka, Bangladesh: 2005. pp. 70–6. [Google Scholar]
- [2].Ashayerizadeh A, Dabiri N, Ashayerizadeh O, Mirzadeh KH, Roshanfekr H, Mamooee M. Effect of dietary antibiotic, probioticand prebiotic as growth promoters, on growth performance, carcass characteristics and hematological indices of broiler chickens. Pak J Biol Sci. 2009;12(1):52–7. doi: 10.3923/pjbs.2009.52.57. https://doi.org/10.3923/pjbs.2009.52.57. [DOI] [PubMed] [Google Scholar]
- [3].Markovi R, Sefer D, Krsti M, Petrujki B. Effect of different growth promoters on broiler performance and gut morphology. Arch Med Vet. 2009;41(2):163–9. https://doi.org/10.4067/S0301-732X2009000200010. [Google Scholar]
- [4].Chattopadhyay MK. Use of antibiotics as feed additives: a burning question. Front Microbiol. 2014;5:1–3. doi: 10.3389/fmicb.2014.00334. https://doi.org/10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ferket P. Alternatives to antibiotics in poultry production: responses, practical experience and recommendations. In: Lyons TP, Jacques KA, editors. Nutritional biotechnology in the feed and food industries. Nottingham University Press; Nottingham, UK: [Nov 10;2018 ]. 2004. pp. 57–67. Available via https://www.cabdirect.org/cabdirect/abstract/20063210005 . [Google Scholar]
- [6].Yang Y, Iji PA, Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult Sci J. 2009;65(01):97–114. https://doi.org/10.1017/S0043933909000087. [Google Scholar]
- [7].NRC. Nutrient requeriments of poultry. National Academy Press; Washington DC: 1994. [Google Scholar]
- [8].SPSS I. IBM SPSS statistics for Windows, version 20.0. New York IBM Corp; Armonk, NY: 2011. [Google Scholar]
- [9].Steel R, Torrie J, Dickey D. Principles and procedures of statistics: a biometrical approach. 2nd. McGraw-Hill Book Co; New York, NY: 1980. [Google Scholar]
- [10].Toghyani M, Toghyani M, Tabeidian SA. Effect of probiotic and prebiotic as antibiotic growth promoter substitutions on productive and carcass traits of broiler chicks; 2011 International Conference on Food Engineering and Biotechnology, Bangkok, Thailand; 7–9th May 2011.pp. 82–6. [Google Scholar]
- [11].Tabidi MH, Mukhtar AM, Mohammed HI. Effects of probiotic and antibiotic on performance and growth attributes of broiler chicks. Glob J Med Plant Res. 2013;1(1):136–42. [Google Scholar]
- [12].Banday MT, Risam KS. Growth performance and carcass characteristics of broiler chicken fed with probiotics. Poult Abstr. 2002;28:388. [Google Scholar]
- [13].Edens F. An alternative for antibiotic se in poultry: probiotics. Braz J Poult Sci. 2003;5(2):75–97. https://doi.org/10.1590/S1516-635X2003000200001. [Google Scholar]
- [14].Fatufe AA, Matanmi IO. The effect of probiotics supplementation on the growth performance of two strains of cockerels. J Cent Eur Agric. 2008;9(3):405–10. [Google Scholar]
- [15].Khan SH, Rehman A, Sardar R, Khawaja T. The effect of probiotic supplementation on the growth performance, blood biochemistry and immune response of reciprocal F1 crossbred (Rhode Island Red×Fayoumi) cockerels. J Appl Anim Res. 2013;41(4):417–26. https://doi.org/10.1080/09712119.2013.792732. [Google Scholar]
- [16].Choo Y-K, Oh S-T, Lee K-W, Kang C-W, Kim H-W, Kim C-J, et al. The growth performance, carcass characteristics, and meat quality of egg-type male growing chicken and white-mini broiler in comparison with commercial broiler (Ross 308) Korean J Food Sci Anim Resour. 2014;34(5):622–9. doi: 10.5851/kosfa.2014.34.5.622. https://doi.org/10.5851/kosfa.2014.34.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Santoso U, Tanaka K, Ohtani S, Sakaida M. Effect of fermented product from Bacillus subtilis on feed conversion efficiency, lipid accumulation and ammonia production in broiler chicks. Asian-Australas J Anim Sci. 2001;14(3):333–7. https://doi.org/10.5713/ajas.2001.333. [Google Scholar]
- [18].Apata D. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture ofLactobacillus bulgaricus. J Sci Food Agric. 2008;88(7):1253–8. https://doi.org/10.1002/jsfa.3214. [Google Scholar]
- [19].Kabir SML, Rahman M, Rahman MB, Rahman MM, Ahmed SU. The dynamics of probiotics on growth performance and immune response in broilers. Int J Poult Sci. 2004;3(5):361–4. https://doi.org/10.3923/ijps.2004.361.364. [Google Scholar]
- [20].Song J, Xiao K, Ke Y, Jiao LF, Hu CH, Diao QY, et al. Effect of a probiotic mixture on intestinal microflora, morphology and barrier integrity of broilers subjected to heat stress. Am Hist Rev. 2014;119(2):581–2. doi: 10.3382/ps.2013-03455. https://doi.org/10.1093/ahr/119.2.581. [DOI] [PubMed] [Google Scholar]
- [21].Zhang ZF, Kim IH. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Am Hist Rev. 2014;119(2):364–98. doi: 10.3382/ps.2013-03314. https://doi.org/10.1093/ahr/119.2.364. [DOI] [PubMed] [Google Scholar]
- [22].Amer M, Khan S. A comparison between the effects of a probiotic and an antibiotic on the performance of Desi chickens. Vet World. 2012;5(3):160–5. https://doi.org/10.5455/vetworld.2012.160-165. [Google Scholar]
- [23].Jin LZ, Ho YW, Abdullah N, Jalaludin S. Probiotics in poultry: modes of action. Worlds Poult Sci J. 1997;53(04):351–68. https://doi.org/10.1079/WPS19970028. [Google Scholar]
- [24].Zulkifli I, Al-Aqil A, Omar AR, Sazili AQ, Rajion MA. Crating and heat stress influence blood parameters and heat shock protein 70 expression in broiler chickens showing short or long tonic immobility reactions. Poult Sci. 2009;88(3):471–6. doi: 10.3382/ps.2008-00287. https://doi.org/10.3382/ps.2008-00287. [DOI] [PubMed] [Google Scholar]
- [25].Ramasamy K, Abdullah N, Wong MCVL, Karuthan C, Ho YW. Bile salt deconjugation and cholesterol removal from media by Lactobacillus strains used as probiotics in chickens. J Sci Food Agric. 2010;90(1):65–9. doi: 10.1002/jsfa.3780. https://doi.org/10.1002/jsfa.3780. [DOI] [PubMed] [Google Scholar]
- [26].Shareef AM, Al-Dabbagh ASA. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraqi J Vet Sci. 2009;23(1):23–9. [Google Scholar]
- [27].Mahdavi AH, Rahmani HR, Pourreza J. Effect of probiotic supplements on egg quality and laying hen`s performance. Int J Poult Sci. 2005;4(7):488–92. https://doi.org/10.3923/ijps.2005.488.492. [Google Scholar]
- [28].Babazadeh D, Vahdatpour T, Nikpiran H, Jafargholipour MA, Vahdatpour S. Effects of probiotic, prebiotic and synbiotic intake on blood enzymes and performance of Japanese quails (Coturnix Japonica) Indian J Anim Sci. 2011;81(8):870–44. https://doi.org/10.1016/j.ajog.2014.10.106. [Google Scholar]
- [29].Hassanein SM, Soliman NK. Effect of probiotic (Saccharomyces cerevisae) adding to diets on intestinal microflora and performance of hy-line layer hens. J Am Sci. 2010;6(11):160–9. [Google Scholar]
- [30].Karkoodi MYK. Effect of probiotic Thepax® and Saccharomyces cerevisiae supplementation on performance and egg quality of laying Hens. Int J Poult Sci. 2007;6(1):52–4. https://doi.org/10.3923/ijps.2007.52.54. [Google Scholar]
- [31].Midilli M, Alp M, Kocabach N, Muglah OH, Turan N, Yilmaz H, et al. Effects of dietary probiotic and prebiotic supplementation on growth performance and serum IgG concentration of broilers. S Afr J Anim Sci. 2008;38(1):21–7. https://doi.org/10.4314/sajas.v38i1.4104. [Google Scholar]
- [32].Bunyan J, Jeffries L, Sayers JR, Gulliver AL, Coleman K. Antimicrobial substances and chick growth promotion: the growth-promoting activities of antimicrobial substances, including fifty-two used either in therapy or as dietary additives. Br Poult Sci. 1977;18(3):283–94. https://doi.org/10.1080/00071667708416364. [Google Scholar]
- [33].Denli M, Çelik, Okan F. Comparative effects of feeding diets containing flavomycin, Bioteksin-L and dry yeast (Saccharomyces cerevisiae) on broiler performance. J Appl Anim Res. 2003;23(2):139–44. https://doi.org/10.1080/09712119.2003.9706415. [Google Scholar]
- [34].Mokhtari R, Yazdani AR, Rezaei M, Ghorbani B. The effects of different growth promoters on performance and carcass characteristics of broiler chickens. J Anim Vet Adv. 2010;9(20):2633–9. https://doi.org/10.3923/javaa.2010.2633.2639. [Google Scholar]


