Abstract
Aims:
The primary aim of this study is to characterize hepatocellular malignant neoplasm, NOS (HEMNOS), a new provisional entity describing a subset of paediatric hepatocellular tumours, which have histological features of neither typical hepatoblastoma (HB) nor hepatocellular carcinoma (HCC).
Methods and results:
The clinicopathological features of 11 patients with HEMNOS were analysed retrospectively. The median age and serum alpha-fetoprotein level at diagnosis was 7 years and 182 000 ng/ml, respectively. Ten patients presented with pretreatment extent of disease (PRETEXT) stages III/IV multifocal tumours, eight with major vascular involvement, three with lung metastases and three with extrahepatic extension. The original pathology diagnoses were: HB in seven patients, HCC in two and HEMNOS in two. Our pathology review of pre-chemotherapy specimens showed that six tumours had equivocal/overlapping histological features of HB and HCC, four had predominant HB histology along with focal HCC-like histology and one had HB histology. Seven of nine post-chemotherapy resection specimens showed predominant HCC-like histology. Beta-catenin, glypican 3 and spalt-like transcription factor 4 immunostaining showed that all the tumours had a mixed HB/HCC immunophenotype. Telomerase reverse transcriptase immunostaining showed nuclear staining in nine of the 11 tumours. All patients received chemotherapy and achieved gross total primary tumour resection. Nine of the 11 patients were treated with established HB chemotherapy regimens. After a median follow-up of 6.1 years (range: 1.2–11.8 years), all patients were in remission.
Conclusions:
HEMNOS is a subtype of HB with focal HCC-like histology, a high-risk clinical profile but favourable outcome following chemotherapy and complete tumour resection.
Keywords: hepatoblastoma, hepatocellular carcinoma, hepatocellular malignant neoplasm, telomerase reverse transcriptase, transitional liver cell tumour
Introduction
Paediatric malignant epithelial liver tumours are rare, and are divided into two major histological subgroups: hepatoblastoma (HB) and hepatocellular carcinoma (HCC).1 HB occurs mainly in infants and young children between the ages of 6 months and 3 years, with only 9% of cases occurring in children older than 4 years of age. In contrast, paediatric HCC usually affects older children and adolescents, with approximately 13% seen in children under the age of 5 years.2 Hepatocellular malignant neoplasm, NOS (HEMNOS) is a new provisional entity describing a small subset of paediatric malignant hepatocellular tumours that demonstrate complex morphologies and, in some cases, an admixture of histological patterns typical of both HB and HCC in the same tumour, precluding their exact classification.3 In 2002, Prokurat and colleagues first reported on seven malignant hepatocellular tumours that developed in older children and adolescents and showed histological features that were not typical of HB or HCC.4 They proposed the term transitional liver cell tumour (TLCT) based on the hypothesis that these tumours may represent a new entity different from both HB and HCC. In that series, only three patients had pre-chemotherapy biopsies, all of which were suggestive of HB. All other histological and immunohistochemical features described were from post-chemotherapy specimens. It is well known that some post-chemotherapy HB specimens may have pleomorphic and or anaplastic areas, resembling HCC.5 Therefore, controversy exists as to whether these tumours are HBs with chemotherapy-induced HCC-like histology, clonally progressed HBs or a true new entity. As such, in 2014, an international paediatric liver tumour consensus classification abandoned the term TLCT in favour of HEMNOS.3 An accurate diagnosis of HEMNOS is critical for the selection of appropriate treatment, but remains challenging due to its rarity and limited knowledge of its clinicopathological features. Therefore, to characterize this entity more clearly, we analysed retrospectively the clinicopathological features of 11 patients with HEMNOS.
Materials and methods
CASE SELECTION
After approval by the institutional review board (CHLA-16-00115), the surgical pathology archives of Children’s Hospital Los Angeles were searched for patients who were at least 3 years of age, and were diagnosed with malignant epithelial liver tumours during the time-period between 1 January 2000 and 28 February 2016. All patients selected had slides available for histopathological verification. The slides from liver biopsy, tumour resections and/or liver explants from 41 patients were reviewed. Seventeen patients with typical HB histology, eight patients with classic HCC histology and five patients with fibrolamellar HCC histology were excluded from further analysis. Eleven patients with either pre- or post-chemotherapy specimens showing either equivocal/overlapping histological features of HB and HCC or both HB and HCC-like (reminiscent of HCC) histology, consistent with HEMNOS/TLCT,3,4 were subjected to detailed clinicopathological evaluation. HCC-like histology was defined as large atypical hepatocyte-like cells with prominent nucleoli, abundant cytoplasm and a high mitotic activity, resembling HCC.
EVALUATION OF CLINICAL PARAMETERS
Demographic data (age and gender), tumour location and tumour size were extracted from surgical pathology reports. Clinical information including staging, treatment and follow-up was collected by retrospective medical record review. Tumours were staged using the pretreatment extent of disease (PRETEXT) staging system.6
HISTOPATHOLOGICAL ANALYSIS
All slides from the 11 cases were re-evaluated by two authors (S.Z. and L.W.) for histological features of the tumours and the presence or absence of vascular invasion.
IMMUNOHISTOCHEMICAL STAINING
One to two representative formalin-fixed, paraffin-embedded blocks from each case were selected for immunohistochemical staining. Automated immunohistochemical staining was performed with a Leica Bond Max Instrument (Leica, Buffalo Grove, IL, USA). Tissue sections (4 μm) were deparaffinized, rehydrated and treated with 3% hydrogen peroxide for 15 min to quench endogenous peroxidase. Antigen retrieval was performed using a bond epitope retrieval solution [an ethylenediamine tetraacetic acid (EDTA)-based buffer and surfactant] at pH 9.0 for 20 min. The slides were first incubated with one of the following primary antibodies: beta-catenin (3.5 mg/l, Leica), glypican-3 (1:50; Biocare, Concord, CA, USA), spalt-like transcription factor 4 (SALL4) (ready to use; clone 6E3; Cell Marque, Rocklin, CA, USA) and telomerase reverse transcriptase (TERT) (A-6) (1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), following the manufacturers’ instructions. After incubation with either an antimouse or antirabbit secondary antibody, a Bond Polymer Refine Detection system (Leica) was used for single brown colour staining with 3,3′ diaminobenzidine (DAB) chromogen. Appropriate positive and negative controls were included and evaluated with the specimens tested.
Results
CLINICAL FINDINGS
The clinical features are summarized in Table 1. There were 10 males and one female. The median age at diagnosis was 7 years (range: 4 – 15 years). The median alpha fetoprotein (AFP) level was 182 000 ng/ml (range: 617–1 280 000 ng/ml). The majority of patients presented with right upper quadrant abdominal pain and three patients (cases 1, 6 and 11) presented with an acute abdomen due to tumour rupture. Ten of the 11 tumours (eight in the right lobe, one in the left and two involving both lobes) showed multifocal nodules on diagnostic radiographic imaging studies. PRETEXT stage was II in one patient, III in seven patients and IV in three patients. Seven patients had portal vein involvement, and one had inferior vena cava involvement. Three patients (cases 3, 5 and 7) had lung metastases and three (cases 1, 2 and 11) had extrahepatic extension. Patients 4 and 6 had a history of prematurity.
Table 1.
Clinical features and follow-up of 11 patients with HEMNOS
| Case no. | Age (years)/sex | AFP ng/ml | Size (cm) | Pretext stage | Chemo | Surgery | Vital status (years) |
|---|---|---|---|---|---|---|---|
| 1 | 4.5/M | 25 K | 11.3 | II (E2, F1, H1) | C5V × 4 | L | NED (11.8) |
| 2 | 12.5/M | 980 K | 10† | III (E1, F1, P1a) | C5V × 6, ID × 5 | L | NED (8.8) |
| 3 | 4/M | 301 K | 5† | IV (F1, M1, P1a) | C5V × 4 | L | NED (7.5) |
| 4 | 9/M | 493 K | 4.5† | III (F1, P1a) | PIAF × 5, IROX, S, IROX | L | NED (9.0) |
| 5 | 5/M | 80 K | 9† | III (F1, M1, P1a) | C5VD × 3, IROX, ID | L + Trans | NED (7.9) |
| 6 | 7/M | 70 K | 4.3† | IV (F1, H1) | PLADO × 6 | Trans | NED (6.1) |
| 7 | 4/M | 182 K | 12.1† | III (F1, M1) | C5VD × 4, IROX × 4 | L | NED (4.6) |
| 8 | 15/F | 617 | 10† | III (F1, V3a) | PLADO × 2, PLADO/S × 4, TACE × 2, PLADO × 1, GO × 6 | Trise | NED (4.5) |
| 9 | 14/M | 198 K | 4.5† | III (F1, P1a) | PLADO × 2, PLADO/S × 2, TACE × 1 | Trans | NED (5.1) |
| 10 | 7/M | 1280 K | 6† | IV (F1, P1a) | C5VD × 5 | Trans | NED (2.4) |
| 11 | 11/M | 26.9 K | 11.3† | III (C1, E2, F1, H1, P1a) | CDDP/Dox × 5, GO × 2, ICE × 5 | Trise + Trans | NED (1.2) |
HEMNOS, Hepatocellular malignant neoplasm, NOS; AFP, Alpha fetoprotein; K, Thousand; C1, Tumour involving the caudate lobe; E1, Direct extension of tumour into diaphragm; E2, Peritoneal nodules; F1, Patient with two or more discrete tumours; H1, Tumour rupture; M1, Pulmonary metastasis; P1a, Intravascular tumour is present in portal vein; V3a, Intravascular tumour is present in inferior vena cava; C5V, Cisplatin + 5-fluorouracil + vincristine; ID, Ifosfamide + doxorubicin; PIAF, Cisplatin + interferon alpha-2b + doxorubicin + 5-fluorouracil; IROX, Irinotecan + oxaliplatin; S, Sorafenib; C5VD, Cisplatin + 5-fluorouracil + vincristine + doxorubicin; PLADO, Cisplatin + doxorubicin; TACE, Transarterial chemoembolization; GO, Gemcitabine + oxaliplatin; CDDP/Dox, Cisplatin + doxorubicin; ICE, Ifosfamide, carboplatin and etoposide; L, Liver lobectomy; Trise, Liver trisegmentectomy; Trans, Liver transplantation; NED, No evidence of disease.
Multifocal tumour, the diameter of the biggest mass.
HISTOLOGICAL FINDINGS
All patients had a liver biopsy or primary tumour resection before chemotherapy. The original pathology diagnoses were: HB in seven patients, HCC in two, TLCT in one and HEMNOS in one. After chemotherapy, pathology diagnosis was changed from HB to TLCT in one case and from HCC to HB in another. Extensive vascular invasion was noted in 10 of the 11 tumours. Neither small-cell undifferentiated nor mesenchymal components were seen in any patient.
Pathology review performed as part of this study revealed six pre-chemotherapy tumours with equivocal/overlapping histological features of HB and HCC (Figures 1A and 2A,B), four with predominant HB histology along with focal HCC-like histology (Figure 3A,B) and one with classic fetal HB histology only (Figure 4A). Seven of nine post-chemotherapy resection specimens showed predominant HCC-like histology in viable tumours (Figures 1C, 2C, 3C,D and 4B). Focal HB histology was also present in five of the seven tumours. One post-chemotherapy specimen showed predominant HB histology and one had complete tumour necrosis. In addition, focal cytoplasm vacuolation, nuclear clearing and syncytial multinucleated hepatocyte-like giant cells were also seen occasionally. Table 2 depicts a summary of pathology features of all 11 patients with HEMNOS. Histological examination of uninvolved liver revealed no significant pathological abnormalities in all cases.
Figure 1.
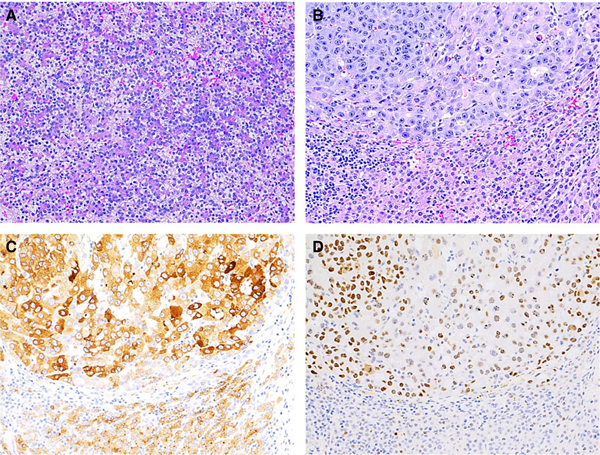
Case 2. A, Representative pre-chemotherapy wedge biopsy of liver tumour showed two cell populations, one with round vesicular nuclei and abundant clear to eosinophilic cytoplasm, morphologically suggestive of pleomorphic fetal hepatoblastoma (HB) (right lower); the other characterized by large atypical hepatocyte-like cells with prominent nucleoli, abundant cytoplasm and a high mitotic activity (left upper), resembling hepatocellular carcinoma (HCC) histology (insert, higher magnification of left upper part). B, Predominantly weak membranous staining in fetal HB histology and strong mixed membranous and cytoplasmic staining with focal nuclear staining in HCC-like histology. The post-chemotherapy resection specimen showed a treated tumour with areas of necrosis and fibrosis admixed with large areas of viable HCC-like histology (C) and focal fetal HB-like histology (D). The former was characterized by large, anaplastic cells with abundant eosinophilic cytoplasm, numerous mitoses and focal prominent formation of eosinophilic cytoplasmic globules (A,C,D, haematoxylin and eosin stain; B, beta-catenin staining).
Figure 2.
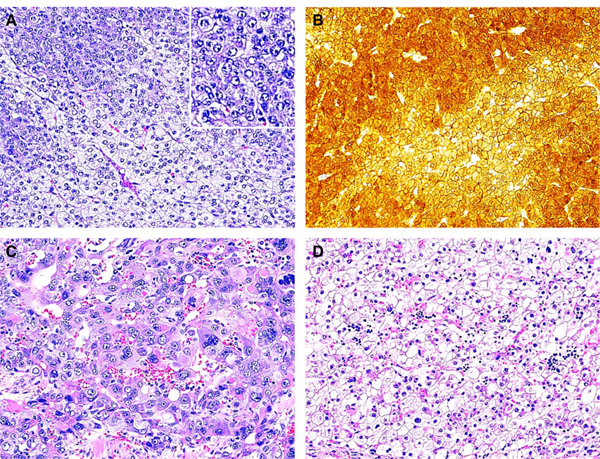
Case 6. The patient presented with tumour rupture. The pre-chemotherapy wedge biopsy of liver tumour showed a heterogenous malignant tumour with equivocal histological features. There were areas suggestive of atypical embryonal hepatoblastoma (HB) (A) and areas reminiscent of hepatocellular carcinoma (HCC) (B). The post-chemotherapy liver explant showed that the viable tumour cells were composed morphologically of two types of cells. The predominant cells contained variably sized cells including large, anaplastic cells with numerous mitoses and apoptosis and cytoplasmic vacuolation, resembling HCC-like histology (C), and the other showing small round nuclei and abundant eosinophilic to clear cytoplasm, consistent with fetal HB histology (D) (haematoxylin and eosin stain).
Figure 3.
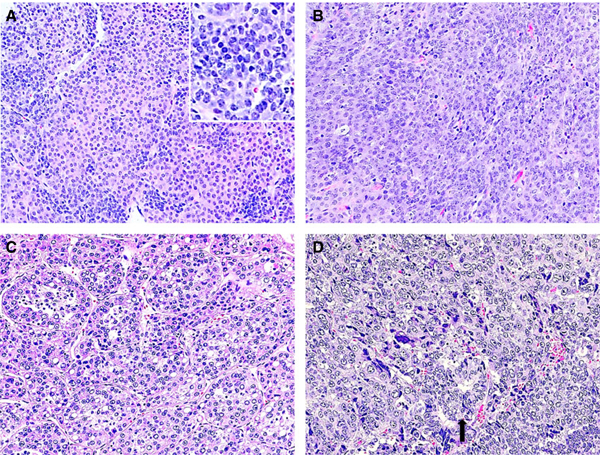
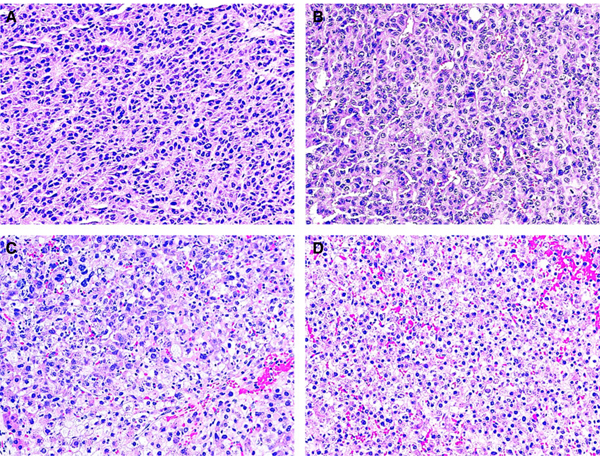
Case 5. The pre-chemotherapy needle biopsy of liver tumour showed two distinct groups of cells, one morphologically consistent with fetal/embryonal hepatoblastoma (HB) (A) (insert, higher magnification of left middle part showing malignant epithelial cells), and the other with larger hyperchromatic nuclei, coarse chromatin, eosinophilic cytoplasm, and frequent mitosis, suggestive of hepatocellular carcinoma (HCC)-like histology (B). Both post-chemotherapy resection (C) and recurrent tumours (D) showed a malignant hepatocellular tumour, resembling HCC with focal glandular structures (D, arrow). No definite HB histology was seen in tumour resections (haematoxylin and eosin stain).
Figure 4.
Case 7. A, The pre-chemotherapy needle biopsy of liver tumour showed a uniform population of cells with abundant clear (light area) or eosinophilic (dark area) cytoplasm, consistent with typical fetal hepatoblastoma (HB). B, The post-chemotherapy resection specimen demonstrated focal viable tumour with two distinct cell populations, one was consistent with fetal HB histology (lower part) and the other hepatocellular carcinoma (HCC)-like histology (upper part) characterized by large cells with prominent nucleoli; abundant eosinophilic granular cytoplasm; and increased mitotic activity. Of note, there was no transitional zone between these two histological patterns. C, Glypican 3 staining was positive in both populations of cells with much stronger cytoplasmic staining in HCC-like histology than in fetal HB histology. D, spalt-like transcription factor 4 (SALL4) staining showed moderate to strong positive in HCC-like histology and a focal weak positive manner in fetal HB histology (A,B, haematoxylin and eosin stain; C, glypican 3; D, SALL4).
Table 2.
Pathology features of 11 patients with HEMNOS
| Dx |
Pathology review |
|||
|---|---|---|---|---|
| Case no. | Pre-chemo | Post-chemo | Pre-chemo | Post-chemo |
| 1 | HB | NA | HB + focal HCC-like | NA |
| 2 | HB | TLCT | Equivocal | HCC-like + focal HB |
| 3 | HB | HB | Equivocal | HCC-like |
| 4 | HCC | HB | Equivocal | HB |
| 5 | HB | HB | HB + focal HCC-like | HCC-like |
| 6 | TLCT | TLCT | Equivocal | HCC-like + focal HB |
| 7 | HB | HB | HB | HCC-like + focal HB |
| 8 | HCC | HCC | HB + focal HCC-like | HCC-like + HB |
| 9 | HB | HB | Equivocal | HCC-like + focal HB |
| 10 | HB | HB | HB + focal HCC-like | NA (not viable) |
| 11 | HEMNOS | NA | Equivocal | NA |
HEMNOS, Hepatocellular malignant neoplasm, NOS; Dx, Diagnosis; HB, Hepatoblastoma; HCC, Hepatocellular carcinoma; TLCT, Transitional liver cell tumour; NA, Not available.
IMMUNOPHENOTYPICAL FINDINGS
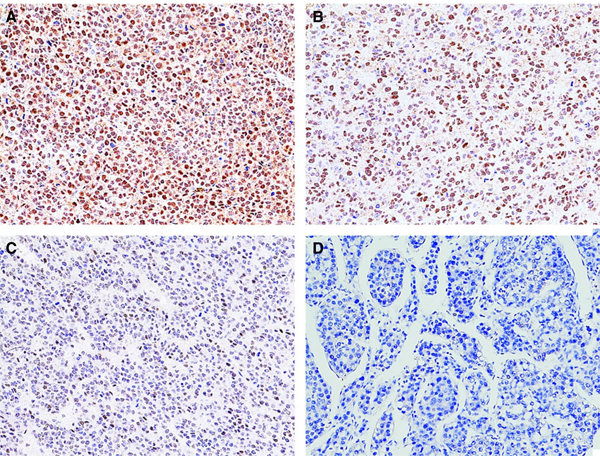
All tumours had mixed beta-catenin immunostaining patterns corresponding to their histological features: predominantly weak membranous staining in fetal HB histology; predominantly strong cytoplasmic staining in embryonal HB histology; and strong mixed membranous and cytoplasmic staining with focal nuclear staining in HCC-like and equivocal histology (Figure 1B). All tumours were positive for glypican 3 with a predominantly finely granular canalicular pattern in fetal HB histology, and often strong diffuse cytoplasmic and granular canalicular staining patterns in other histologies (Figure 4C). SALL4 was also positive in every case with variable nuclear expression; a negative to focal weak positive staining in fetal HB histology; frequently extensive positive staining in embryonal HB histology; and focal weak to moderate positive staining in HCC-like and equivocal histology (Figure 4D). Nine of 11 tumours showed nuclear staining of TERT: strong and diffuse positive in five tumours (Figure 5A); moderate positive in one tumour (Figure 5B, case 2); weak positive in three tumours (Figure 5C, cases 4, 7 and 9); and negative in two tumours (Figure 5D, cases 5 and 8) and surrounding uninvolved normal liver. Immunophenotypical features from patients 2 and 5 were presented in part in another study.7
Figure 5.
Representative telomerase reverse transcriptase (TERT) staining patterns. A, strong diffuse nuclear staining; B, moderate nuclear staining; C, weak nuclear staining; D, complete negative nuclear staining.
TREATMENT
Two patients (cases 1 and 11) with tumour rupture underwent gross total primary tumour resection at diagnosis. Patient 11 had microscopic residual disease in the liver. The remaining nine patients received neoadjuvant chemotherapy. Seven of the nine patients (cases 3–7, 9 and 10) had a partial radiographic response to neoadjuvant chemotherapy and a decline in serum AFP levels from diagnosis. After neoadjuvant therapy, five patients (cases 2–5 and 7) underwent hepatic lobectomies and two of them (cases 3 and 7) also had their lung metastases resected. Four of these five patients achieved complete tumour resection and one patient has focal involvement of the surgical margin microscopically. One patient (case 8) underwent hepatic trisegmentectomy and had a positive microscopic surgical margin. Three primary tumours (cases 6, 9 and 10) remained unresectable following neoadjuvant chemotherapy. However, these three patients achieved complete remission following orthotopic cadaveric liver transplantation. All but two patients (cases 4 and 11) were treated using established HB chemotherapy regimens with the majority receiving cisplatin and doxorubicin.
OUTCOME
Three patients experienced local relapse. Patient 4 had three local recurrences, which were treated with chemotherapy and surgical resections. Patient 5 experienced two local recurrences and was treated with chemotherapy and tumour resection followed by a cadaveric liver transplant. Patient 11 had one local recurrence and was treated with surgical resection and chemotherapy followed by a cadaveric liver transplant. After a median follow-up of 6.1 years (range: 1.2–11.8 years), all patients were in complete radiographic remission. Ten patients maintained normal serum AFP levels and one patient (case 11) had falling AFP levels 6 weeks following liver transplant.
Discussion
This study represents a case series of HEMNOS/TLCT diagnosed and treated at a single institution. Each of our patients had a pre-chemotherapy tumour specimen to evaluate histology and complete clinical information. As the majority of HBs occur in patients younger than 3 years, we included only patients older than 3 years in this study. The median age of 7 years (range: 4 – 15 years) in our series is similar to the previous series.4 The male to female ratio is 1.5:1 for HB8 and 3:1 for paediatric HCC.9 Interestingly, the male to female ratio in the current series was 10:1. This may be due to a relatively small sample size. At diagnosis, serum AFP is elevated in more than 90% of HB patients10–13 and in approximately 70% of paediatric HCC patients,14,15 although AFP levels are typically lower in HCC compared to in HB. All our patients had elevated AFP levels with a median level of 182 000 ng/ml. Similar to the Prokurat et al. series,4 we found that the tumours occurred predominantly in the right lobe of liver. The majority of our patients had advanced tumours and all patients except for patients 4 and 9 would have been considered high-risk patients according to the SIOPEL (Société Internationale d’Oncologie Pédiatrique – Epithelial Liver Tumour Study Group) risk stratification.6
Patients with HEMNOS in this study had complex tumour histologies with six of the 11 pre-chemotherapy biopsies/resections showing equivocal/overlapping histological features of HB and HCC, four with predominant HB histology along with focal HCC-like histology and one with classic fetal HB histology only. The latter might not have been representative of the whole tumour, as the resection specimen of the same tumour following neoadjuvant chemotherapy clearly showed both fetal HB and HCC-like histology. Post-chemotherapy resection specimens showed predominant HCC-like histology in seven of the 9 cases. Focal HB histology was also present in five of the 7 cases. The coexistence of both HB and HCC-like histology raises the intriguing possibility that HEMNOS is a clonally progressed HB with focal HCC-like histology. Mixed HB and HCC histology has been described rarely in mixed hepatoblastoma.16–21 Consistent with previous observations,4,5 we found that post-chemotherapy specimens had more HCC-like histology than pre-chemotherapy specimens. Moreover, two post-chemotherapy specimens showed only HCC-like histology, which suggest that without pre-chemotherapy biopsy, these cases might have been misdiagnosed as HCC. A small core needle biopsy may result in a sampling error and thus fail to reveal the underlying true histological phenotype of the hepatic neoplasm. Therefore, in order to diagnose liver tumours appropriately in children older than 3 years of age, an open pre-chemotherapy wedge biopsy should be considered.
Prokurat et al.4 found that three of seven TLCT tumours exhibited predominantly membranous staining pattern for beta-catenin, and the remaining four tumours showed predominantly cytoplasmic staining with variable nuclear staining. In this study, we observed mixed beta-catenin immunostaining patterns in all tumours. Glypican 3 is an oncofetal protein that is expressed in both HB22 and HCC.23 All tumours included in this study were positive for glypican 3, with variable staining patterns corresponding to their histological features. SALL4, a regulator of embryonal development, is expressed in fetal liver cells, silenced in fully differentiated hepatocytes and reactivated in a subset of adult HCC24–26 and some HBs.27 In a recent study,28 we found that SALL4 was expressed highly in embryonal HB, whereas fetal HB showed a negative or relatively weak focal punctate/clumped nuclear staining pattern. Our unpublished data show that SALL4 staining is mild (punctuate/clumped pattern) to marked (diffuse pattern) positive in classic paediatric HCCs. In this series, variable SALL4 staining patterns were seen in different regions of the same tumour. Overall, the immunophenotypical features of HEMNOS are consistent with a mixed HB/HCC immunophenotype.
Telomerase is known to be silenced in normal somatic cells due to the down-regulation of TERT after birth and reactivated by activating TERT promoter mutations in some malignant cells.29–31 Eichenmuller et al.32 reported that TERT promoter mutations were identified in two of three patients with TLCT but not in any of 33 typical HBs. Recently, Sumazin et al.33 reported that only two of 88 HBs had somatic point mutations at the TERT promotor. Interestingly, these two patients were the oldest children in their series (aged 6 and 8 years). One of them was diagnosed as HEMNOS. These findings suggest that the TERT promoter mutation might be associated with HEMNOS. In line with the above findings, we found that TERT expression was present in nine of the 11 tumours studied, and not in the surrounding uninvolved liver, indicating increased telomerase activity in the majority of HEMNOS.
With increased complete remission rates achieved by liver transplantation and refinement of chemotherapy, the 3-year overall survival of high risk HB patients has improved steadily during the last three decades (53% in SIOPEL 2,34 69% in SIOPEL 313 and 83% in SIOPEL 435). In contrast, the prognosis of paediatric HCC remains extremely poor, with the overall 5-year survival approximately 22–28%.15,36,37 Although the majority of our patients were high-risk patients, all our patients survived with a median follow-up of 6.1 years. This finding is in contrast to the Prokurat et al. series,4 in which four of the 7 patients died of disease after a median follow-up of 22 months (range: 0.5–60 months). It is plausible that the four tumours not biopsied at original diagnosis might be HCC instead of TLCT, thus explaining the poorer prognosis. Additionally, in the same series, complete surgical resection followed by normalization of serum AFP was achieved in only one patient. Most of our patients were diagnosed with HB prior to this review; nine of the 11 patients received established HB chemotherapy, with the majority receiving cisplatin and doxorubicin. More importantly, in spite of advanced stage, gross total tumour resection was achieved in all our patients. Patient 11 was treated initially with standard HB chemotherapy but had local tumour recurrence with progressive disease on first salvage chemotherapy. However, this patient had multifocal disease limited to the liver and was able to achieve complete radiographic remission following a recent cadaveric liver transplant, and had declining serum AFP levels. At the end of follow-up, all patients were in complete remission. The high survival rate of our series support further that HEMNOS is best considered as a subtype of HB.
The pathogenesis of HEMNOS/TLCT is largely unknown. Eichenmuller et al.32 found that TLCT tumours share the common CTNNB1 mutation with HBs, but additionally demonstrated chromosomal instability due to deletions of the genome guardians RAD17 and TP53, and TERT promoter mutations. They also found that TLCT had 27 mutations/case, higher than HB (2.9 mutations/case), but lower than adult HCC (45 mutations/case).38 Based on the above findings, they concluded that TLCT is a genetically derailed progeny of HB. The overall clinicopathological features of our serials support that HEMNOS/TLCT may begin with HB, acquiring more mutations along the differentiation pathway resulting in aggressive HB with HCC-like features. Further studies looking at the molecular pathways involved in this group of tumours are necessary.
The limitations of our study include a relatively small number of cases, its retrospective nature and variations in treatments. Nevertheless, to the best of our knowledge, our study represents the largest case series on HEMNOS/TLCT to date. The results of our study support that HEMNOS is a subtype of HB with focal HCC-like histology, a high-risk clinical profile but favourable outcome following chemotherapy and complete surgical resection. Reclassification of this tumour as a subtype of HB might facilitate appropriate risk stratification and treatment.
Acknowledgements
The authors would like to thank Fusheng Yang and Brian Cooper for their assistance with immunohistochemistry staining.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ferris ITJ, Ortega Garcia JA, Garcia ICJ, Lopez Andreu JA, RibesKoninckx C, Berbel Tornero O. Risk factors for paediatric malignant liver tumours. An. Pediatr. (Barc.) 2008; 68; 377–384. [DOI] [PubMed] [Google Scholar]
- 2.Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. Children. Hepatology 2003; 38; 560–566. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Terrada D, Alaggio R, de Davila MT et al. Towards an international paediatric liver tumour consensus classification: Proceedings of the Los Angeles Cog Liver Tumours Symposium. Mod. Pathol 2014; 27; 472–491. [DOI] [PubMed] [Google Scholar]
- 4.Prokurat A, Kluge P, Kosciesza A, Perek D, Kappeler A, Zimmermann A. Transitional liver cell tumours (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumours expressing beta-catenin. Med. Paediatr. Oncol 2002; 39; 510–518. [DOI] [PubMed] [Google Scholar]
- 5.Wang LL, Filippi RZ, Zurakowski D et al. Effects of neoadjuvant chemotherapy on hepatoblastoma: a morphologic and immunohistochemical study. Am. J. Surg. Pathol 2010; 34; 287–299. [DOI] [PubMed] [Google Scholar]
- 6.Roebuck DJ, Aronson D, Clapuyt P et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Paediatr. Radiol 2007; 37; 123–132; quiz 249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S, Parham DM, Yung E, Pattengale P, Wang L. Quantification of glypican 3, beta-catenin and claudin-1 protein expression in hepatoblastoma and paediatric hepatocellular carcinoma by color deconvolution. Histopathology 2015; 67; 905–913. [DOI] [PubMed] [Google Scholar]
- 8.Geramizadeh B, Bahador A, Foroutan HR, Banani A, Nikeghbalian S, Malek-Hosseini SA. Pathology of paediatric liver tumours, a single center experience from south of Iran. Indian J. Pathol. Microbiol. 2010; 53; 422–426. [DOI] [PubMed] [Google Scholar]
- 9.Lau CS, Mahendraraj K, Chamberlain RS. Hepatocellular carcinoma in the paediatric population: a population based clinical outcomes study involving 257 patients from the Surveillance, Epidemiology, and End Result (SEER) database (1973–2011). HPB Surg. 2015; 2015; 670–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Schweinitz D, Hecker H, Schmidt-von-Arndt G, Harms D. Prognostic factors and staging systems in childhood hepatoblastoma. Int. J. Cancer 1997; 74; 593–599. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs J, Rydzynski J, Von Schweinitz D et al. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Paediatric Liver Tumour Study HB 94. Cancer 2002; 95; 172–182. [DOI] [PubMed] [Google Scholar]
- 12.Perilongo G, Maibach R, Shafford E et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N. Engl. J. Med 2009; 361; 1662–1670. [DOI] [PubMed] [Google Scholar]
- 13.Zsiros J, Maibach R, Shafford E et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3 hr study. J. Clin. Oncol 2010; 28; 2584–2590. [DOI] [PubMed] [Google Scholar]
- 14.Katzenstein HM, Krailo MD, Malogolowkin MH et al. Hepatocellular carcinoma in children and adolescents: results from the paediatric oncology group and the children’s cancer group intergroup study. J. Clin. Oncol 2002; 20; 2789–2797. [DOI] [PubMed] [Google Scholar]
- 15.Czauderna P, Mackinlay G, Perilongo G et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Paediatric Oncology group. J. Clin. Oncol 2002; 20; 2798–2804. [DOI] [PubMed] [Google Scholar]
- 16.Honan RP, Haqqani MT. Mixed hepatoblastoma in the adult: case report and review of the literature. J. Clin. Pathol 1980; 33; 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postovsky S, Elhasid R, Otte GB, Ben Itzhak O, Gaitini D, BenArush MW. Late recurrence of combined hepatocellular carcinoma and hepatoblastoma in a child: case report and review of the literature. Eur. J. Paediatr. Surg 2001; 11; 61–65. [DOI] [PubMed] [Google Scholar]
- 18.Cho MS, Lee SN, Sung SH, Han WS. Sarcomatoid hepatocellular carcinoma with hepatoblastoma-like features in an adult. Pathol. Int 2004; 54; 446–450. [DOI] [PubMed] [Google Scholar]
- 19.Canberk S, Uludokumaci A, Sonmez C, Cakalir C, Gulsen F,Ozbay G. Mixed hepatocellular carcinoma and hepatoblastoma: cytohistopathologic findings and differential diagnosis. Acta Cytol. 2013; 57; 91–95. [DOI] [PubMed] [Google Scholar]
- 20.Park KW, Seo CJ, Yun DY et al. A case of hepatoblastoma misdiagnosed as combined hepatocellular carcinoma and cholangiocarcinoma in an adult. Clin. Mol. Hepatol 2015; 21; 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ertel AE, Fu B, Shah SA. Mixed transitional liver cell tumourin a 23-year-old female: a case report. Semin. Roentgenol 2016; 51; 123–125. [DOI] [PubMed] [Google Scholar]
- 22.Zynger DL, Gupta A, Luan C, Chou PM, Yang GY, Yang XJ.Expression of glypican 3 in hepatoblastoma: an immunohistochemical study of 65 cases. Hum. Pathol 2008; 39; 224–230. [DOI] [PubMed] [Google Scholar]
- 23.Honsova E, Lodererova A, Frankova S, Oliverius M, Trunecka P. Glypican-3 immunostaining significantly improves histological diagnosis of hepatocellular carcinoma. Cas. Lek. Cesk 2011; 150; 37–40. [PubMed] [Google Scholar]
- 24.Yong KJ, Gao C, Lim JS et al. Oncofetal gene sall4 in aggressive hepatocellular carcinoma. N. Engl. J. Med 2013; 368; 2266–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han SX, Wang JL, Guo XJ et al. Serum SALL4 is a novel prognosis biomarker with tumour recurrence and poor survival of patients in hepatocellular carcinoma. J. Immunol. Res 2014; 2014; 262385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu TC, Vachharajani N, Chapman WC, Brunt EM. SALL4 immunoreactivity predicts prognosis in western hepatocellular carcinoma patients but is a rare event: a study of 236 cases. Am. J. Surg. Pathol 2014; 38; 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnemmi V, Leteurtre E, Sudour-Bonnange H et al. SALL4 is a marker of the embryonal subtype of hepatoblastoma. Histopathology 2013; 63; 425–428. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Venkatramani R, Gomulia E, Shillingford N, Wang L. The diagnostic and prognostic value of SALL4 in hepatoblastoma. Histopathology 2016; 69; 822–830. [DOI] [PubMed] [Google Scholar]
- 29.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA Highly recurrent tert promoter mutations in human melanoma. Science 2013; 339; 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn S, Figl A, Rachakonda PS et al. Tert promoter mutations in familial and sporadic melanoma. Science 2013; 339; 959–961. [DOI] [PubMed] [Google Scholar]
- 31.Weinhold N, Jacobsen A, Schultz N, Sander C, Lee W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat. Genet 2014; 46; 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eichenmuller M, Trippel F, Kreuder M et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J. Hepatol 2014; 61; 1312–1320. [DOI] [PubMed] [Google Scholar]
- 33.Sumazin P, Chen Y, Trevino LR et al. Genomic analysis of hepatoblastoma identifies distinct molecular and prognostic subgroups. Hepatology 2017; 65; 104–121. [DOI] [PubMed] [Google Scholar]
- 34.Perilongo G, Shafford E, Maibach R et al. Risk-adapted treatment for childhood hepatoblastoma. Final report of the second study of the international society of paediatric oncology – SIOPEL 2. Eur. J. Cancer 2004; 40; 411–421. [DOI] [PubMed] [Google Scholar]
- 35.Zsiros J, Brugieres L, Brock P et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol. 2013; 14; 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murawski M, Weeda VB, Maibach R et al. Hepatocellular carcinoma in children: does modified platinum- and doxorubicin-based chemotherapy increase tumour resectability and change outcome? Lessons learned from the SIOPEL 2 and 3 studies. J. Clin. Oncol 2016; 34; 1050–1056. [DOI] [PubMed] [Google Scholar]
- 37.Allan BJ, Wang B, Davis JS et al. A review of 218 paediatric cases of hepatocellular carcinoma. J. Paediatr. Surg 2014; 49; 166–171; discussion 171. [DOI] [PubMed] [Google Scholar]
- 38.Cleary SP, Jeck WR, Zhao X et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology 2013; 58; 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]